Abstract
With the widespreading use of biologic drugs, reports of renal injury are increasing, most of which belong to the spectrum of secondary autoimmune syndromes. We present the case of a young man affected by Ankylosing Spondylitis, treated with tumor necrosis factor alpha inhibitors (Anti-TNF) that develop a peculiar renal damage: a coexistence of 2 glomerulonephritis due to different noxae, an IgA nephropaty with a Membranous nephropathy. The first one probably related to the rheumatologic disease, the second one related to Anti-TNF. Despite the underlying mechanisms, the renal involvement both related to Ankylosing Spondylitis and secondary to biologic treatment are currently rare and not predictable. Regular control of renal function and urinalysis during treatment with anti-TNF is mandatory. A concomitant treatment with Disease Modifying Anti Rheumatic Drugs or eventually a low dose of steroids may prevent the formation of anti-drug antibodies and could limit the renal damage related to this phenomenon.
Keywords: Biologic drugs, secondary autoimmune syndromes, Ankylosing Spondylitis, glomerulonephritis
Introduction
Ankylosing spondylitis (AS) is a chronic, progressive inflammatory arthritis primarily affecting the axial skeleton, presenting at the beginning as chronic back pain and elevated acute phase response, typically in men before the age of 45. Most patients are positive for disease-associated human leukocyte antigen B27 (HLA-B27) alleles (>85%, in a recent review1), and a number of inflammatory pathways have been implicated in its pathogenesis. AS has several common extra-axial manifestations and associated complications—all of which increase patient morbidity.1
Renal involvement is rare but possible and may include type AA amyloidosis, interstitial nephritis secondary to Non-Steroidal Anti-Inflammatory Drugs (NSAID) use or various forms of glomerulonephritis, mainly IgA nephropathy.2
Over the past decade, tumor necrosis factor alpha inhibitors (Anti-TNF) have become the cornerstone for therapy in improving functional outcomes, and decreasing disease activity in patients with a marginal benefit from non-steroidal anti-inflammatory (NSAID) therapy.3
Anti-TNF represent the longest-lived class of biologics. Patients with a clinical diagnosis of AS are considered for biologic treatment when pathology is active for more than 4 weeks despite therapy with at least 2 different NSAIDs.4 Given that fact, the vast majority of patients with diagnosis of AS underwent biologic therapy during the follow up.5 Currently, among the biologics therapies, Anti-TNF and Interleukin 17—blocking agents (Anti IL-17) have indication for AS but the last one is much more recent and its use, although well established in terms of efficacy and safety, is less diffuse than the first category.6 Patients who do not respond to or do not tolerate one anti-TNF agent may respond to an alternative anti-TNF.7
With the widespreading use of biologic agents, reports of renal injury are increasing and most of them belong to the spectrum of secondary autoimmune syndromes. The highest number of reports on the development of these syndromes is related to anti-TNF partially due to their largest established use but also to specific immunoregolatory role of TNF.7,8
The largest series on biologic renal toxicity, by Piga and coworkers, identified 29 cases subdivided into primary renal immunologic pathologies (glomerular or interstitial), systemic vasculitis with renal involvement and lupus-like syndromes. In the absence of differential histopathological findings from the primitive disorders, the diagnosis is mainly clinical and anamnestic, based on temporal association with drug administration, absence of other potential causes and response to drug withdrawal and when documented reinduction of disease with the reintroduction of the therapy.9
Case Report
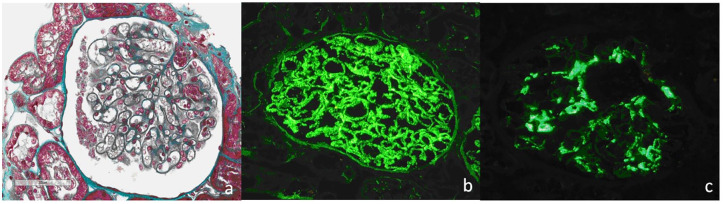
Here we present the case of a 27 years old man, with diagnosis of AS since 2011, according to ASAS (Assessment of Spondyloarthritis International Society) classification criteria; from 2012 he underwent therapy with Anti-TNF, firstly Golimumab (50 mg/monthly) until december 2016 when it was substituted with Adalimumab (40 mg/2 weeks) for inefficacy. At this time AS was active with sacroileitis, incomplete pain control and mild elevation of inflammation index (C reactive protein and velocity of erythrocyte sedimentation rate). The clinimetric scores documented high disease activity: Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 6,1. Bath Ankylosing Spondylitis Functional Index (BASFI): 4,9. Global Health 70. In January 2017 there is the first evidence of albuminuria at urinalysis (20 mg/dl) to the following further exam, there is evidence of proteinuria (0.66 g/day) without other anomalies to urinalysis and normal serum creatinine. Subsequently a progressive worsening of proteinuria was observed until reaching nephrotic range (5.36 g/day) in the absence of clinical findings or other laboratory signs of nephrotic syndrome. At the second level urinalysis with sediment there was evidence of mild cilindruria and moderate microematuria with a 40% of dismorfic red cells. Qualitative typization of urinary proteins showed glomerular type of proteinuria. There was no complement alteration neither abnormalities in the autoimmunity. Hepatitis markers were negative. There were no hematologic abnormalities. There were no infection signs or symptoms and the patient had not developed any infectious disease in the previous period. Serum amyloid A was normal. In the following weeks Adalimumab was discontinued and a renal biopsy was performed. The histology showed a picture of double glomerulonephritis: IgA nephropathy with minimal mesangial deposits and Membranous Nephropathy (Figure 1). Serum-Antibodies against phospholipase A2 receptor (Ab Anti-PLA2R) were negatives. Malignancy was excluded with further examinations. Patients’ main laboratory values course is reported in Table 1.
Figure 1.
Renal histology of case presented. (a) Masson trichrome original magnification (270×) demonstrated diffuse mild thickening of glomerular basement membranes with slight dilatation of the capillary lumens, Mesangial proliferation was not evident on light microscopy. Immunofluorescence showed diffuse and global finely granular deposits of IgG (+++) (b) and C3 (+) along outer aspect of the glomerular basement membranes. There was also a bright mesangial positivity for IgA (+++) and less intense for C3 (++) (c).
Table 1.
Patients’ main laboratory values course.
| Jan 2017 | Apr 2017 | Oct 2017 | Feb 2018 | Jul 2018 | Nov 2018 | Feb 2019 | |
|---|---|---|---|---|---|---|---|
| Proteinuria 24 h (g/day) | 0.30 | 0.66 | 0.80 | 5.36 | 2.70 | 1.60 | 0.50 |
| Hematuria (urine dipstick) | − | ++ | ++ | ++ | + | + | − |
| Leucocyturia (urine dipstick) | − | + | − | − | − | + | − |
| s-creatinine (mg/dl) | 0.76 | 0.90 | 0.85 | 0.78 | 0.83 | 0.92 | 0.88 |
| ANA | <1/80 | <1/80 | <1/80 | <1/80 | <1/80 | <1/80 | <1/80 |
| Anti-dsDNA | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| C3–C4 | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| s-Anti Phospholipid | − | − | Neg | Neg | − | − | Neg |
| s-ANCA | − | Neg | − | Neg | − | Neg | Neg |
| s-AntiPLA2R | − | − | − | Neg | − | − | Neg |
| Amyloid A | − | Neg | − | − | Neg | − | Neg |
Abbreviations: ANA, Antinuclear Antibodies; ANCA, antineutrophil cytoplasmic antibodies; Anti-dsDNA, Anti double-strend DNA antibodies; Anti-PLA2R, Serum anti-phospholipase A2 receptor antibody; Apr, April; Feb, February; Jan, January; Jul, July; Nov, November; Oct, October.
The subsequent control of proteinuria showed an initial decrease (2.7 g/die) while a therapy with low dose of steroids (Prednisone 7.5 mg daily) and Ace-Inhibitors was prescribed.
At the moment, 13 months after biopsy, proteinuria continued to decrease (last control was 0.5 g/day) without other nephrologic abnormalities (hematuria was no longer present). In consideration of incomplete pain control and radiologic signs of AS activity the initiation of a new biologic agent against IL-17 was hypothesized but not yet implemented. Nephrological trend was depicted in Figure 2.
Figure 2.
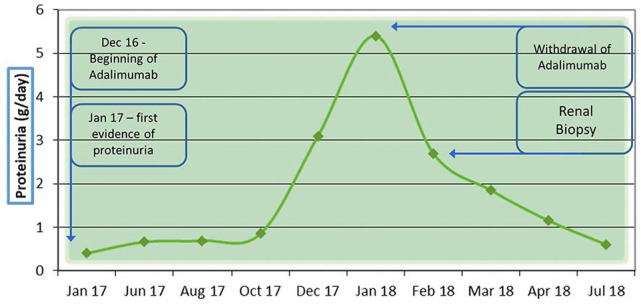
Nephrological evolution of case reported.
Discussion
The association between IgA nephropathy and AS is known,10,11 although not frequent: in a recent study by Lee and coworkers, 2 cases of IgA nephropathy at renal biopsy were detected among 681 patients (0.3%) in a context of about 8% of renal involvement. In the same series of patients no cases of Membranous nephropathy were highlighted.2
Irrespective of any rheumatological pathology in the general population there are few cases in literature of Membranous nephropathy concomitant with IgA nephropaty,.12,13 As mentioned in the past by Kobayashi et al,14 IgA-GNM should be considered as a separate entity but this definition is still debated. In a recent series of 9 patients, only 38% of patient had positivity of Ab Anti-PLA2R thus supporting the hypotesis of another possible pathogenetic mechanism.13
As discussed earlier, there are growing evidences of possible renal damage related to Anti-TNF even in the form of glomerulonephritis. In this regard only one case of membranous nephropathy associated to Adalimumab15 was reported as well as one case of IgA nephropathy with the same drug. However, in the latter case, it is probably an exacerbation of a pre-existing nephropathy in a patient with psoriasis16 rather than an initial glomerulonephritis due to Anti-TNF.
To our best knowledge, the clinical case we report is the first case of double glomerulonephritis with coexistence of a rheumatologic disease. In this complex scenario, the most likely hypotesis is the coexistence of 2 glomerulonephritis due to different noxae. On one hand, IgA nephropathy is probably related to the rheumatologic disease as reported in literature and also the moderate to high activity of AS leaning towards this hypotesis. On the other hand, Membranous nephropathy should is most probably related to Anti-TNF; the absence of Ab anti-PLA2R with the exclusion of other comorbidities, infectious diseases, malignancies, the absence of other potentially involved drugs and the spontaneous reduction of the proteinuria after discontinuation of the biologic therapy accords to this hypotesis. The hypothesis is also confirmed by use of Naranjo scale with a score of 7. It means that the reaction followed a reasonable temporal sequence after a drug, followed a recognized response to the suspected drug, was confirmed by withdrawal, and could not be reasonably explained by other known characteristics of the patient’s clinical state.17
The mechanisms involved in anti-TNF induced nephritis are uncertain. Guillevin and Mouthon suggested that anti-TNF drugs may form immune complexes, activate complement, elevate apoptosis by binding to TNF alfa on plasma membrane of immune cells stimulating production of Anti dsDNA and mediate inflammation by switching from T helper type 1 to type 2 cytokine response, thus upregulating antibody production.18-20 It is known that prolonged treatment with Anti-TNF therapy induces autoantibodies, including antinuclear antibody and anti-double stranded DNA although usually these remain clinically silent and disappear after discontinuation of therapy.21 In the specific case of membranous nephropathy TNF alfa produced by the glomerular visceral epithelial cells could interact with infused anti-TNF forming immune complexes. Moreover, renal disease should be also caused by formation of antibodies against the drug itself.15 A limitation of this study is the absence of determination of antibodies against Anti-TNF.
Despite the underlying mechanism, is likely that the biologic drug plays a central role in the pathogenetic mechanisms. Therefore perform regular control of renal function and urinalysis during treatment with anti TNF alpha antibodies is mandatory, even in patients without previous history of nephrologic disease. Moreover, a recent study showed that HLA B27 negative AS patients are more prone to develop extraarticular involvement and have worse response rate to Anti-TNF.22
It should be underlined that renal toxicity incidence of these drugs is rare and lower of that related to traditional Disease Modifying Anti-Rheumatic Drugs to now there are a lot of evidences that favor their utilization in rheumatologic patients even with severe impairment of renal function.23
These case raise the question on how to treat at the same time renal pathology and underlying rheumatic disease. As today the renal involvement secondary to biologic treatment is rare and not predictable.
Many authors support the hypothesis that the concomitant treatment with Disease Modifying Anti Rheumatic Drugs or eventually a low dose of steroids prevent the formation of anti-drug antibodies and could limit the renal damage related to this phenomenon.
Our choice was to discontinue biologic treatment, adding a low dose of steroid and monitoring proteinuria and renal function without underwent other Immunosuppressive therapy. If persistent activity of AS suggest to use biologic therapy to better control clinical and radiological features the use of another class of biologic drugs non anti-TNF (eg, Anti IL17) could be a safer choice.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: DD and MP conceived and designed the study and drafted the manuscript. LC and SP performed clinical assessments. BA performed histologic analysis and experiments and contributed to data interpretation. EF and LB contributed to data interpretation and critically revised the manuscript. All authors take part in manuscript development for intellectual content and final approved the manuscript.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standard of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
ORCID iD: Priora Marta  https://orcid.org/0000-0002-9194-9179
https://orcid.org/0000-0002-9194-9179
References
- 1. Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis. 2002;61(suppl 3):iii8-iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee S-H, Lee EJ, Chung SW, et al. Renal involvement in ankylosing spondylitis: prevalence, pathology, response to TNF-a blocker. Rheumatol Int. 2013;33:1689-1692. [DOI] [PubMed] [Google Scholar]
- 3. Callhoff J, Sieper J, Weiß A, Zink A, Listing J. Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis. 2015;74:1241-1248. [DOI] [PubMed] [Google Scholar]
- 4. Van der Heijde D, Sieper J, Maksymowych WP, et al. 2010 update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:905-908. [DOI] [PubMed] [Google Scholar]
- 5. Osman MS, Maksymowych WP. An update on the use of tumor necrosis factor alpha inhibitors in the treatment of ankylosing spondylitis. Expert Rev Clin Immunol. 2017;13:125-131. [DOI] [PubMed] [Google Scholar]
- 6. Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2013;382:1705-1713. [DOI] [PubMed] [Google Scholar]
- 7. Rudwaleit M, Van den Bosch F, Kron M, Kary S, Kupper H. Effectiveness and safety of adalimumab in patients with ankylosing spondylitis or psoriatic arthritis and history of anti-tumor necrosis factor therapy. Arthritis Res Ther. 2010;12:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ernandez T, Mayadas T. Immunoregulatory role of TNFα in inflammatory kidney diseases. Kidney Int. 2009;76:262-276. [DOI] [PubMed] [Google Scholar]
- 9. Piga M, Chessa E, Ibba V, et al. Biologics-induced autoimmune renal disorders in chronic inflammatory rheumatic diseases: systematic literature review and analysis of a monocentric cohort. Autoimmun Rev. 2014;13:873-879. [DOI] [PubMed] [Google Scholar]
- 10. Champtiaux N, Lioté F, El Karoui K, et al. Spondyloarthritis-associated IgA nephropathy. Kidney Int Rep. 2020;5:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricardo Pires J, Tavares Valadão Barcelos A. IgA nephropathy-ankylosing spondylitis-associated or adalimumab-induced? J Clin Rheumatol. Published online December 24, 2019. doi: 10.1097/rhu.0000000000001249 [DOI] [PubMed] [Google Scholar]
- 12. Hu R, Xing G, Wu H, Zhang Z. Clinicopathological features of idiopathic membranous nephropathy combined with IgA nephropathy: a retrospective analysis of 9 cases. Diagn Pathol. 2016;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen P, Shi S-F, Qu Z, et al. Characteristics of patients with coexisting IgA nephropathy and membranous nephropathy. Ren Fail. 2018;40:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi Y, Fujii K, Hiki Y, Chen XM. Coexistence of IgA nephropathy and membranous nephropathy. Acta Pathol Jpn. 1985;35:1293-1299. [DOI] [PubMed] [Google Scholar]
- 15. Gupta A, Pendyala P, Arora P, Sitrin MD. Development of the nephrotic syndrome during treatment of Crohn’s disease with adalimumab. J Clin Gastroenterol. 2011;45:e30-e33. [DOI] [PubMed] [Google Scholar]
- 16. Wei SS, Sinniah R. Adalimumab (TNF α inhibitor) therapy exacerbates IgA glomerulonephritis acute renal injury and induces lupus autoantibodies in a psoriasis patient. Case Rep Nephrol. 2013;2013:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [DOI] [PubMed] [Google Scholar]
- 18. Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet Lond Engl. 2013;382:819-831. [DOI] [PubMed] [Google Scholar]
- 19. Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625. [DOI] [PubMed] [Google Scholar]
- 21. Little MA, Bhangal G, Smyth CL, et al. Therapeutic effect of anti-TNF-α antibodies in an experimental model of anti-neutrophil cytoplasm antibody-associated systemic vasculitis. J Am Soc Nephrol. 2006;17:160-169. [DOI] [PubMed] [Google Scholar]
- 22. Akkoç N, Yarkan H, Kenar G, Khan MA. Ankylosing spondylitis: HLA-B*27-positive versus HLA-B*27-negative disease. Curr Rheumatol Rep. 2017;19:26. [DOI] [PubMed] [Google Scholar]
- 23. Kim HW, Lee C-K, Cha H-S, Choe J-Y, Park E-J, Kim J. Effect of anti-tumor necrosis factor alpha treatment of rheumatoid arthritis and chronic kidney disease. Rheumatol Int. 2015;35:727-734. [DOI] [PubMed] [Google Scholar]