Abstract
Subdural effusion (SDE) is a common complication secondary to decompressive craniectomy (DC). This current case report describes a patient with contralateral SDE with a typical clinical course. Initially, he made a good recovery following a head trauma that caused a loss of consciousness and was treated with decompressive craniectomy. However, he only achieved temporary relief after each percutaneous fluid aspiration from an Ommaya reservoir implanted into the cavity of the SDE. He was eventually transferred to the authors’ hospital where he underwent cranioplasty, which finally lead to the reduction and disappearance of his contralateral SDE. Unexpectedly, his clinical condition deteriorated again 2 weeks after the cranioplasty with symptoms of an uncontrolled bladder. A subsequent CT scan found the apparent expansion of the whole cerebral ventricular system, indicating symptomatic communicating hydrocephalus. He then underwent a ventriculoperitoneal shunt procedure, which resulted in a favourable outcome and he was discharged 2 weeks later. A review of the current literature identified only 14 cases of contralateral SDE that were cured by cranioplasty alone. The mechanism of contralateral SDE has been widely discussed. Although the exact mechanism of contralateral SDE and why cranioplasty is effective remain unclear, cranioplasty could be an alternative treatment option for contralateral SDE.
Keywords: Contralateral subdural effusion, decompressive craniectomy, cranioplasty
Introduction
Contralateral subdural effusion (SDE) is frequently found after decompressive craniectomy (DC).1,2 Although most contralateral SDE is stable and even disappears spontaneously,1 patients with clinical deterioration due to contralateral SDE should be treated actively. Treatment options for contralateral SDE include cranial strapping, bur-hole drainage, Ommaya reservoir implantation, temporal muscle sticking and subduroperitoneal shunt with or without cranioplasty.2–5 Cranioplasty solely for contralateral SDE has been reported in recent years,6,7 although only in short case series. This current case report first describes a patient with contralateral SDE that was successfully treated using cranioplasty and then it reviews the literature regarding the mechanism of contralateral SDE and why cranioplasty could resolve this complication.
Case report
A 47-year-old male patient was admitted to the Department of Neurosurgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China in November 2016, 1 month after a previous head trauma that caused a loss of consciousness and was treated with decompressive craniectomy, which was initially followed by a good recovery. The earlier head trauma was caused by a fall injury and the patient had become unconsciousness immediately. He was sent to the Sir Run Run Shaw Hospital, Zhejiang University School of Medicine with the right pupil dilated and fixed. An immediate craniocerebral computed tomography (CT) scan found an epidural haematoma in the right temporal area and a severe right-to-left midline shift. A right fronto-temporo-parietal craniotomy was performed and the haematoma was evacuated. The bone flap was not replaced in case of delayed cerebral oedema. The patient recovered his consciousness after the operation and had a favourable prognosis during the subsequent 3 weeks of uneventful clinical recovery, although paralysis in the left upper limb was evident. However, his conscious state deteriorated again in postoperative week 4 and he had a cranial defect bulge. The following CT scan found a contralateral subdural effusion with the midline shifted more than 1 cm to the right side. He underwent a burr-hole drainage operation to evacuate the subdural effusion while his attending physicians implanted an Ommaya reservoir in case of the recurrence of the effusion. His condition recovered after the operation but deteriorated again after the drainage was removed. His condition only improved during short periods after each percutaneous fluid aspiration of the Ommaya reservoir. He was transferred to the Department of Emergency and Trauma Centre, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China 1 week after the second surgery with the hope of him achieving a full recovery.
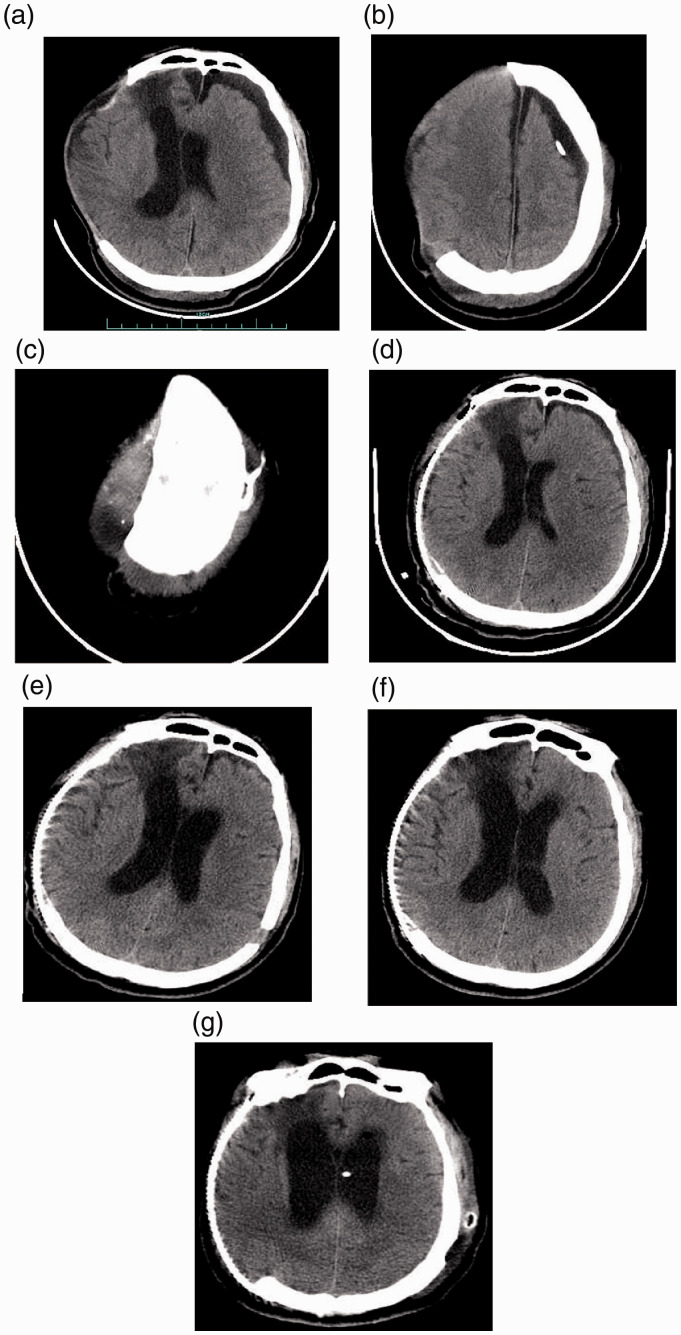
When the patient was admitted the Department of Emergency and Trauma Centre, The First Affiliated Hospital, he had a Glascow Coma Score of 4 + T + 5 and bilateral pupil reactive sensitively. A thin-layer cranial CT scan was undertaken (Figure 1). A three-dimensional designed titanium plate suitable for his cranial defect was ordered. He underwent cranioplasty with the titanium plate as soon as possible. His consciousness improved immediately after cranioplasty and the following CT scan indicated a significant reduction of the contralateral SDE, which had completely disappeared in the CT scan 9 days later (Figure 1). Unexpectedly, his clinical condition deteriorated again 2 weeks after the cranioplasty with symptoms of an uncontrolled bladder and a subsequent CT scan found the apparent expansion of the whole cerebral ventricular system, indicating symptomatic communicating hydrocephalus (Figure 1). He then underwent a ventriculoperitoneal shunt procedure, which resulted in a favourable outcome and he was discharged 2 weeks later.
Figure 1.
Representative computed tomography (CT) scans of a 47-year-old male patient that was admitted to hospital 1 month after a previous head trauma with decompressive craniectomy and deterioration of conscious state following an apparent good recovery. (a) CT scan after the patient was admitted to hospital showing a contralateral subdural effusion (SDE) and midline shift to the side of the cranial defect, with brain tissue herniation. (b & c) CT scans after the patient was admitted to hospital showing the Ommaya reservoir that had been implanted during week 4 after the initial head injury. (d) CT scan after the cranioplasty showing the reduced contralateral SDE. (e) CT scan 9 days after the cranioplasty showing that the contralateral SDE had totally disappeared. In this image, there are also signs of hydrocephalus with mild interstitial oedema appearing around the anterior horn of the left lateral ventricle; but the patient appeared to be well without any symptoms of hydrocephalus. (f) CT scan 2 weeks after the cranioplasty showing apparent hydrocephalus. The patient had the symptoms of an uncontrolled bladder. (g) CT scan after the successful ventriculoperitoneal shunt procedure.
Discussion
Subdural effusion is a common complication secondary to DC, with an incidence of 11.0–57.4%.8–10 A previous report of 108 cases of DC after traumatic brain injury from the same clinical centre presented an incidence of SDE of 21.3%.10 Contralateral SDE is a special type of SDE and its incidence has been reported to be 4.4–7.3%.1,2,9 Although it is not generally considered to be a serious complication, SDE with mass effects and associated clinical symptoms can have a negative impact on the prognosis and outcomes of patients with traumatic brain injury due to the lack of effective treatment and the high rate of recurrence.
There are several theories to explain the formation of SDE; the most acceptable one being the formation of an arachnoid valve following tearing of the arachnoid membrane by shear stress during the trauma or injury during surgery, which creates a unidirectional membrane valve that allows cerebral spinal fluid (CSF) to only flow into the subdural space, maintaining the fluid thus collected.2,7 The fact that contralateral SDE rarely occurs after DC in stroke patients also suggests that there needs to be an initial arachnoid rupture caused by trauma.11,12 It has been proposed that during the DC procedure, the rapid decrease in intracranial pressure (ICP) and the outward herniation of brain tissue would generate a pressure gradient between the bilateral hemispheres and then enlarge the contralateral subdural space, leading to CSF effusion.2 This theory is widely accepted. Other theories, such as the disturbance of CSF circulation and flattening of the dicrotic ICP waveform, are also important hypotheses for SDE.6,13 A previous study reported the normal dicrotic ICP waveform was flattened after hemicraniectomy.14 As the arachnoid granulations function as pressure-dependent one-way valves,15 the decreased pulse of the ICP waveform secondary to DC could result in decreased CSF absorption and subsequently lead to SDE.6,7,13,14 However, this theory could be the explanation for both SDE and post-traumatic hydrocephalus, and not an exclusive explanation of contralateral SDE. In addition, it is believed that shrinkage of the ipsilateral brain due to tissue retraction during the DC or pressing by the haematoma and inability to reshape would also cause a pressure gradient between the two hemispheres and brain shift as well, which would finally lead to enlargement of the contralateral subdural space and the accumulation of subdural effusions.2,12
Patients with clinical deterioration due to contralateral SDE should be treated actively. Treatment options for contralateral SDE include cranial strapping or bandaging, bur-hole drainage, Ommaya reservoir implantation, temporal muscle sticking, subduroperitoneal shunt with or without cranioplasty and cranioplasty alone.2–7 However, studies about these methods are limited to individual case reports or short case series, so consensus on the best treatment of contralateral SDE has yet to be established. Cranioplasty as a new treatment option for contralateral SDE has been reported in recent years. As shown in Table 1,3,6,7,12 16 cases have been reported whose treatment included cranioplasty. If the two cases that received subduroperitoneal shunt and cranioplasty simultaneously are eliminated,3,12 only 14 reported cases of contralateral SDE were cured by cranioplasty alone.6,7 In one study, five patients with contralateral SDE received bur-hole drainage first and it lead to transient improvements of their clinical symptoms.7 After their symptoms deteriorated again, all five patients underwent cranioplasty and the contralateral SDE finally disappeared. The other two patients underwent cranioplasty directly and the contralateral SDE resolved quickly after the surgery. In another study, after all 13 patients were dissatisfied with their conservative treatment, including compression cranial bandaging, head-down bed rest, lumbar drainage and Ommaya reservoir implantation, six patients underwent temporal muscle sticking and the other seven underwent early cranioplasty.6 Contralateral SDE in these 13 patients completely resolved or was significantly reduced in the following 1 month and no recurrence was observed in the 6-month follow-up period.6 Patients that underwent early cranioplasty also experienced benefits compared with those that underwent temporal muscle sticking, including having at least one surgery less, a shorter hospital stay and lower medical costs.6 These previous studies influenced the decision of the attending physicians treating the current case to perform cranioplasty within 2 months after the DC and this treatment achieved a favourable outcome because the contralateral SDE dissipated quickly after the surgery.3,6,7,12 It should be noted that after cranioplasty, hydrocephalus may occur in some patients after their contralateral SDE has resolved and a further shunt implantation might be necessary. This was observed in three out of seven patients in one of the previous studies as well as in the current case.7
Table 1.
Studies relevant to cranioplasty as the treatment for contralateral subdural effusion (SDE).3,6,7,12
| Study | Case number | Male:female | Age, years | Primary disease | Preoperative duration | Treatment and outcome |
|---|---|---|---|---|---|---|
| Wan et al. 20166 | 13 | 7:6 | Mean of 32.1 (range, 21–35) | Severe traumatic brain injury | Within 1 week | Six patients underwent temporal muscle sticking and seven underwent early cranioplasty. The contralateral SDE in all 13 patients was successfully resolved. |
| Salunke et al. 20157 | 7 | 6:1 | Mean of 32.1 (range, 27–52) | Six traumatic brain injuries and one middle cerebral artery infarction and aneurismal subarachnoid haemorrhage | Mean of 27.4 days (range, 5–62 days) | Five patients underwent burr-hole drainage that failed after temporary improvements and then cranioplasty was performed. Two patients received cranioplasty directly. SDE in the seven patients resolved eventually, but three patients subsequently developed hydrocephalus and ventriculoperitoneal shunt procedures were then performed. |
| Su et al. 20113 | 13 | 11:2 | Mean of 45.7 (range, 29–75) | Traumatic brain injury | Mean of 13 days (range, 3–22 days) | Six patients were treated conservatively. Six patients received burr-hole craniectomy to evacuate the SDE; of which, four patients were successfully treated and the other two patients subsequently received a subduroperitoneal shunt. In one patient, subduroperitoneal shunt and cranioplasty were simultaneously performed. |
| Kilincer et al. 200512 | 1 | 1:0 | 56 | Aneurismal subarachnoid haemorrhage and middle cerebral artery infarction | 1 week | Burr-hole drainage was firstly used but the contralateral SDE quickly recurred. Then complete resolution of the effusion was achieved through a cranioplasty and subduroperitoneal shunt procedure. |
The authors of two of the previous case reports were of the opinion that early cranioplasty could restore the normal dicrotic ICP waveform, which is necessary for the absorption of CSF and becomes flattened by the cranial defect.6,7 As stated previously, this theory could explain both SDE and post-traumatic hydrocephalus, and might not be an exclusive explanation of contralateral SDE. A previous case study reported treating a patient with recurrent, symptomatic, delayed, contralateral subdural fluid after decompressive craniectomy by limiting the protrusion of the brain tissue from the cranial defect and by reducing the relative negative pressure space in the contralateral subdural space, ultimately achieving similar results to cranioplasty.5 The mechanism by which cranioplasty can effectively treat contralateral SDE needs further investigation. However, if hydrocephalus develops after cranioplasty, especially after the disappearance of the contralateral SDE, it should then be assumed that the hydrocephalus is the result of a circulatory disorder of the CSF rather than a cranial defect and shunt implantation is necessary.
In conclusion, contralateral SDE is a common complication secondary to DC. Cranioplasty is an effective treatment option, especially for intractable cases. As there are only case reports and short case series reported in the literature, well-designed clinical case–control studies are needed to evaluate the efficacy of cranioplasty in contralateral SDE. In addition, the exact mechanism of contralateral SDE and why cranioplasty is effective deserve further investigation.
Footnotes
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Hao Wang https://orcid.org/0000-0001-8686-6777
Zuobing Chen https://orcid.org/0000-0001-6356-3255
References
- 1.Wang HK, Lu K, Liang CL, et al. Contralateral subdural effusion related to decompressive craniectomy performed in patients with severe traumatic brain injury. Injury 2012; 43: 594–597. [DOI] [PubMed] [Google Scholar]
- 2.Yang XF, Wen L, Li G, et al. Contralateral subdural effusion secondary to decompressive craniectomy performed in patients with severe traumatic brain injury: incidence, clinical presentations, treatment and outcome. Med Princ Pract 2009; 18: 16–20. [DOI] [PubMed] [Google Scholar]
- 3.Su TM, Lee TH, Huang YH, et al. Contralateral subdural effusion after decompressive craniectomy in patients with severe traumatic brain injury: clinical features and outcome. J Trauma 2011; 71: 833–837. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Chu S, Feng DF. Ommaya reservoir implantation for the treatment of contralateral progressive traumatic subdural effusion secondary to decompressive craniectomy: a case report. Br J Neurosurg 2017; 31: 628–629. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan P, Roy Chowdhury S. Recurrent, symptomatic, late-onset, contralateral subdural effusion following decompressive craniectomy treated by cranial strapping. Br J Neurosurg 2015; 29: 730–732. [DOI] [PubMed] [Google Scholar]
- 6.Wan Y, Shi L, Wang Z, et al. Effective treatment via early cranioplasty for intractable contralateral subdural effusion after standard decompressive craniectomy in patients with severe traumatic brain injury. Clin Neurol Neurosurg 2016; 149: 87–93. [DOI] [PubMed] [Google Scholar]
- 7.Salunke P, Garg R, Kapoor A, et al. Symptomatic contralateral subdural hygromas after decompressive craniectomy: plausible causes and management protocols. J Neurosurg 2015; 122: 602–609. [DOI] [PubMed] [Google Scholar]
- 8.Grille P, Tommasino N. Decompressive craniectomy in severe traumatic brain injury: prognostic factors and complications. Rev Bras Ter Intensiva 2015; 27: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarabi B, Chesler D, Maulucci C, et al. Dynamics of subdural hygroma following decompressive craniectomy: a comparative study. Neurosurg Focus 2009; 26: E8. [DOI] [PubMed] [Google Scholar]
- 10.Yang XF, Wen L, Shen F, et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir (Wien) 2008; 150: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 11.Kilincer C, Hamamcioglu MK. Contralateral subdural effusion secondary to decompressive craniectomy: differences in patients with large hemispheric infarctions and traumatic brain injury. Med Princ Pract 2010; 19: 499. [DOI] [PubMed] [Google Scholar]
- 12.Kilincer C, Simsek O, Hamamcioglu MK, et al. Contralateral subdural effusion after aneurysm surgery and decompressive craniectomy: case report and review of the literature. Clin Neurol Neurosurg 2005; 107: 412–416. [DOI] [PubMed] [Google Scholar]
- 13.Paredes I, Cicuendez M, Delgado MA, et al. Normal pressure subdural hygroma with mass effect as a complication of decompressive craniectomy. Surg Neurol Int 2011; 2: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waziri A, Fusco D, Mayer SA, et al. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery 2007; 61: 489–493. [DOI] [PubMed] [Google Scholar]
- 15.Upton ML, Weller RO. The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg 1985; 63: 867–875. [DOI] [PubMed] [Google Scholar]