Abstract
Objective
Pulmonary artery hypertension (PAH) is a severe complication of congenital heart disease (CHD). Monitoring of pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR) is essential during follow-up. This retrospective study aimed to examine carcinoembryonic antigen (CEA) as an additional marker for evaluation by investigating the correlation between CEA levels and hemodynamics in CHD-PAH.
Methods
Seventy-six patients with CHD-PAH (mean PAP [mPAP] >25 mmHg and PVR >3 Wood units, group A), 71 patients with CHD and pulmonary hypertension (CHD-PH, mPAP >25 mmHg and PVR ≤3 Wood units, group B), and 102 patients with CHD without PH (mPAP ≤25 mmHg, group C) were enrolled. Serum CEA levels and the relationships between CEA levels and hemodynamic data were assessed.
Results
Mean serum CEA levels were 1.99±1.61, 2.44±1.82, and 1.58±1.07 ng/mL, mPAP was 58.66±20.21, 30.2±4.83, and 17.31±4.51 mmHg, and PVR was 10.12±7.01, 2.19±0.56, and 2.2±1.1 Wood units in groups A, B, and C, respectively. Mean pulmonary output (PO) was 7.24±3.07, 15.79±5.49, 10.18±4.72 L/minute, respectively. CEA levels were positively correlated with PO and negatively correlated with PVR in all of the patients.
Conclusion
CEA levels are increased with PO and decreased with PVR in CHD-PH.
Keywords: Congenital heart disease, pulmonary hypertension, carcinoembryonic antigen, vascular resistance, hemodynamics, right heart catheterization
Introduction
Congenital heart disease (CHD) is a common cause of congenital malformation with an incidence of approximately 0.8% in newborns.1 Pulmonary arterial hypertension (PAH), which is one of the most severe complications of CHD, affects the quality of life of patients and can be a contraindication for repair surgery for CHD. These patients may require PAH decompression therapy, as well as close follow-up before further intervention. Ultrasound, cardiac magnetic resonance imaging, and N-terminal pro-brain natriuretic peptide (BNP) level measurement remain the most effective noninvasive method for estimating the pulmonary pressure and disease stage.2 Although cystatin C,3 C-reactive protein,4 uric acid, and the 6-minute walking test5 partially indicate heart function, additional parameters are required for better assessment.
Carcinoembryonic antigen (CEA) was first discovered in 1965 by Gold and Freedman.6 Serum CEA levels are currently widely used for detecting tumors and assessment of therapeutic effects.7–9 CEA also alters endothelial cell behavior, including adhesion, spreading, proliferation, and migration in vitro and in vivo.10–12
In PAH, proliferation and migration of endothelial cells are one of the most important pathological changes in progression of the disease.13 Moreover, cancer-like growth of lung tissue and inhibition of apoptosis occur in patients with PAH.14,15 These findings indicate a possible association between CEA levels and PAH. Therefore, this study aimed to examine the relationship between circulating serum levels of CAE and progression of PAH in patients with CHD.
Methods
Patients
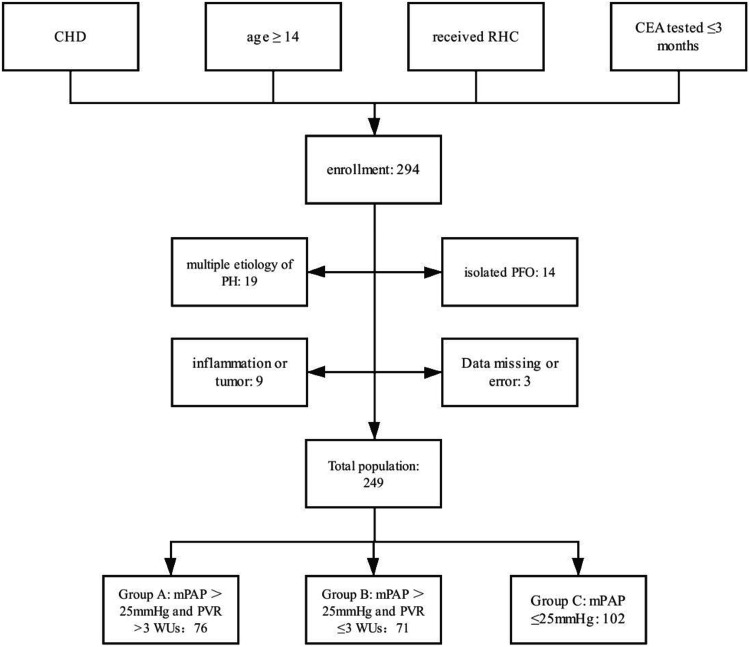
We conducted this retrospective study in the Department of Adult Congenital Heart Disease, Guangdong Provincial People’s Hospital. All of the patients who were hospitalized in the Department of Grown-up Congenital Heart Disease between January 2013 and January 2018 and met the following criteria were initially included: 1) established diagnosis of CHD with shunt(s), corrected or uncorrected; 2) age ≥14 years; 3) received right heart catheterization (RHC) and 4) had a blood test for CEA levels within 3 months before RHC. Exclusion criteria included the following: 1) presence of etiologies of pulmonary hypertension (PH), 2) isolated patent foramen ovale with or without PH, 3) established diagnosis of inflammatory diseases or any type of tumor, and 4) missing data or errors due to technical reasons. The original list of patients with CHD who received RHC was provided by the hospital before statistical analysis. This list was then screened for enrollment and exclusion by two medical students with cross checking. The sample list was then re-checked and de-identified by the authors for further analysis (Figure 1).
Figure 1.
Flowchart of patients’ enrollment.
CHD, congenital heart disease; RHC, right heart catheterization; CEA, carcinoembryonic antigen; PH, pulmonary hypertension; PFO, patent foramen ovale; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; WU, Wood units.
Patients were classified in accordance with guidelines for the diagnosis and treatment of PH.16 PH was defined as mean pulmonary arterial pressure (mPAP) >25 mmHg as measured by RHC at sea level and PAH was defined with an additional criteria of pulmonary vascular resistance (PVR) >3 Wood units (WU). The patients were divided into the three following groups: 1) group A, CHD-PAH (mPAP >25 mmHg and PVR >3 WU); 2) group B, CHD-PH (mPAP >25 mmHg and PVR ≤3 WU); and 3) group C, control (mPAP ≤25 mmHg).16 Patients in group C without PVR data were considered as having PVR ≤3 WU and the PVR data were not analyzed further.
RHC
Before RHC, weight and height were routinely measured and recorded. RHC was performed in the catheter laboratory under electrocardiographic monitoring. After local anesthesia, a 6-French MPA 2 catheter (Cordis Inc., Miami, FL, USA) was advanced into the right heart system through the right femoral vein by placement of a 6-French vascular sheath. The catheter was manipulated to the correct position under fluoroscopy. We measured pressure in the right atrium, pulmonary artery, and wedged pulmonary artery. In patients with atrial septal defect, mean pressure of the pulmonary vein was used instead of pulmonary arterial wedged pressure. Before measurement of pressure, transducers were calibrated to zero at atmospheric pressure. Pressure of the systemic circulation was measured with an arm-cuff electronic sphygmomanometer (M3 type; EDAN Inc., Shenzhen, China). After these procedures, patients with suspicion of PAH received complete hemodynamic measure. Blood from the superior vena cava, inferior vena cava, pulmonary artery, and systemic circulation was aspirated for blood gas assay. After oxygen saturation was measured, PVR, pulmonary output (PO), the pulmonary index, and the ratio of blood flow volume between the pulmonary and systemic circulations (Qp/Qs) was calculated using an estimated metabolic equation called the Fick method17 and a resistance equation (see the Appendix).
Quantification of serum CEA levels
Blood samples were taken for quantifying serum CEA levels. Blood samples were stored in a gel and clot activator tube at room temperature. CEA levels were quantified using the Access CEA Reagent Pack (Cat. No. 33200; Beckman Coulter, Inc., Brea, CA, USA) in the hospital central laboratory.
In Guangdong Provincial People’s Hospital, the CEA test is part of a routine test and it has a low cost (¥63.48 as of 12 August 2020). Patients were able to consult with their clinician to add or refuse a certain test, which included the CEA test.
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 20 software (IBM Corp., Armonk, NY, USA). Descriptive data analyses were conducted to examine the distribution of the data. Proportions were compared using the chi-square test, the Mann–Whitney U test, and the Kruskal–Wallis test. Correlations were calculated using the Spearman method and linear regression. Statistical significance was set as P<0.05.
Ethics
This study was approved by the Research Ethics Committee of Guangdong General Hospital, Guangdong, China on 24 July 2015 (No. GDREC2015254H(R1)). Data from all participants were de-identified and informed consent was waived for this study.
Results
Clinical characteristics of the participants
The study sample consisted of 73 men and 176 women. The numbers of patients in groups A, B, and C were 76, 71, and 102, respectively. The types of CHD after surgery included atrial septal defect, ventricular septal defect, patent ductus arteriosus, and other CHDs. These other CHD included atrioventricular canal defect (n=3, 1.2%), tetralogy of Fallot (n=2, 0.8%), single ventricle (n=1, 0.4%), an abnormal pulmonary venous connection with or without atrial septal defect (n=7, 2.8%), and more than one simple CHD (n=9, 3.6%). The types of CHD were significantly different among the groups (χ2=19.96, P=0.03) (Table 1).
Table 1.
Types of CHD in participants.
| Types of CHD* | Group A | Group B | Group C |
|---|---|---|---|
| After surgery | 3 | 1 | 4 |
| ASD | 36 | 48 | 65 |
| PDA | 11 | 12 | 13 |
| VSD | 18 | 2 | 14 |
| Others† | 8 | 8 | 6 |
*P=0.03 for comparison of types of CHD among the groups; †atrioventricular canal defect, tetralogy of Fallot, single ventricle, abnormal pulmonary venous connection with or without ASD, and more than one simple CHD.
CHD, congenital heart disease; ASD, atrial septal defect; PDA, patent ductus arteriosus; VSD, ventricular septal defect.
The clinical characteristics of the patients, including hemodynamic data, are shown in Table 2. The mean CEA level was significantly different among the whole sample (χ2=12.53, P=0.02). When examining differences between each group, the mean CEA level was significantly higher in group B than in group C (Z=−3.584, P<0.001).
Table 2.
Clinical characteristics.
| Group A (n=76) | Group B (n=71) | Group C (n=102) | |
|---|---|---|---|
| Men | 26 (34.2) | 16 (22.5%) | 31 (30.6%) |
| Women | 50 (65.8%) | 55 (77.5%) | 71 (69.4%) |
| Age (years)# | 40.33±13.7 | 49.14±13.97 | 41.62±14.55 |
| CEA (ng/mL)# | 1.99±1.61 | 2.44±1.82 | 1.58±1.07 |
| CREA (µmol/L) | 73.28±18.94 | 68.49±19.58 | 68.85±17.15 |
| CK (U/L)* | 71.28±48.93 | 92.38±59.93 | 93.18±78.44 |
| CK-MB (U/L) | 12.95±13.31 | 11.52±5.12 | 11.37±9.68 |
| UA (µmol/L)* | 442.84±166.1 | 362.71±113.43 | 388.74±96.89 |
| ALT (U/L)* | 23.33±26.86 | 21.23±15.82 | 18.05±15.9 |
| AST (U/L)* | 25.64±10.42 | 27.75±15.41 | 21.48±6.6 |
| HGB (g/L)* | 145.82±30.12 | 135.9±30.31 | 132.85±17.26 |
| WBC count (×109/L)* | 7.22±2.17 | 6.35±1.52 | 6.31±1.44 |
| BNP (pg/mL)* | 1355.66±1651.82 | 704.89±999.03 | 238.83±504.62 |
| TNT (ng/mL)# | 11.12±9.66 | 51.93±249.56 | 5.32±3.94 |
| sPAP (mmHg)# | 96.21±31.59 | 49.68±11.06 | 31.72±8.16 |
| dPAP (mmHg)# | 36.67±16.24 | 17.08±5.57 | 9.11±3.75 |
| mPAP (mmHg)# | 58.66±20.21 | 30.2±4.83 | 17.31±4.51 |
| PVR (Wood units)# | 10.12±7.01 | 2.19±0.56 | 2.2±1.1 |
| PO (L/minute)# | 7.24±3.07 | 15.79±5.49 | 10.18±4.72 |
| PI (L/(minutes×m2))# | 4.82±2.15 | 10.68±4.66 | 6.54±3.19 |
| Qp/Qs# | 1.61±0.96 | 3.22±1.5 | 1.9±0.87 |
Values are n (%) or mean ± standard deviation.
*P<0.05, #P<0.01.
CEA, carcinoembryonic antigen; CREA, creatinine, CK, creatine kinase; UA, uric acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HGB, hemoglobin; WBC, white blood cell; BNP, N-terminal pro-brain natriuretic peptide; TNT, troponin T; sPAP, systolic pulmonary arterial pressure; dPAP, diastolic pulmonary arterial pressure; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; PO, pulmonary output; PI, pulmonary index; Qp/Qs, ratio of blood flow quantity between the pulmonary and systemic circulations.
Pulmonary arterial pressure (PAP) was significantly different among the groups, with the highest mean value in group A, followed by groups B and C (all P<0.01). Among them, 32 patients in group B and 34 patients in group C had valid complete hemodynamic data. Mean PO was significantly different among the groups, with the highest mean value in group B (P<0.01). All hemodynamic data were significantly different among the groups (P<0.01). Laboratory test results showed that other variables were significantly different among the groups (all P<0.05), except for creatinine and creatinine kinase-MB.
Correlations of CEA levels with clinical characteristics
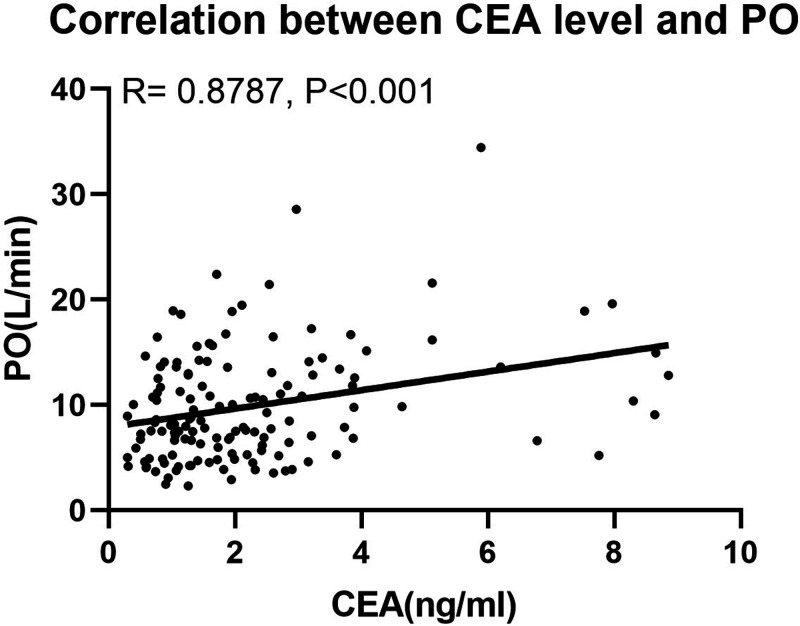
We analyzed the correlations of CEA levels with clinical characteristics using correlation and linear regression analyses (Table 3). In group A, CEA was negatively correlated with PAP and PVR and positively correlated with Qp/Qs (all P<0.01). However, no correlations were found between CEA levels and hemodynamic data in group B or C, except for systolic PAP in Group B (P<0.01). In the combined groups, the correlations between CEA levels and hemodynamics were negative when with PVR and positive with systolic PAP, PO (Figure 2), the pulmonary index, and Qp/Qs (Table 3). CEA levels were also significantly positively lineally correlated with aspartate aminotransferase, BNP, and troponin T levels, which are common markers of PAH, in each group and the combined groups, except for BNP in group C (all P<0.05).
Table 3.
Linear regression analysis between CEA levels and other clinical characteristics.
| Group A | Group B | Group C | Total | |
|---|---|---|---|---|
| Age | 0.470# | 0.060 | 0.256# | 0.293# |
| CREA | 0.125 | 0.347# | 0.326# | 0.258# |
| CK | 0.017 | 0.250* | 0.365# | 0.211# |
| CK-MB | 0.327# | 0.189 | 0.194 | 0.239** |
| UA | 0.231 | 0.234 | 0.119 | 0.174* |
| ALT | 0.268* | 0.380# | 0.210* | 0.301# |
| AST | 0.409# | 0.451# | 0.263# | 0.388# |
| HGB | −0.045 | 0.179 | 0.122 | 0.083 |
| WBC count | −0.084 | −0.061 | −0.093 | −0.087 |
| BNP | 0.291* | 0.368# | 0.124 | 0.300# |
| TNT | 0.317* | 0.449* | 0.389# | 0.387# |
| sPAP | −0.228* | 0.359# | 0.106 | 0.134* |
| dPAP | −0.330# | −0.012 | 0.017 | 0.039 |
| mPAP | −0.296# | 0.197 | 0.091 | 0.111 |
| PVR* | −0.304# | −0.042 | 0.052 | −0.207* |
| PO | 0.174 | 0.142 | 0.054 | 0.255# |
| PI | 0.164 | 0.07 | 0.003 | 0.236# |
| Qp/Qs | 0.369# | 0.224 | −0.082 | 0.346# |
Data are shown as R values.
*P<0.05, #P<0.01.
CREA, creatinine; CK, creatine kinase; UA, uric acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HGB, hemoglobin; WBC, white blood cell; BNP, N-terminal pro-brain natriuretic peptide; TNT, troponin T; sPAP, systolic pulmonary arterial pressure; dPAP, diastolic pulmonary arterial pressure; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; PO, pulmonary output; PI, pulmonary index; Qp/Qs, ratio of blood flow quantity between the pulmonary and systemic circulations.
Figure 2.
Correlation between CEA levels and PO.
CEA, carcinoembryonic antigen; PO, pulmonary output.
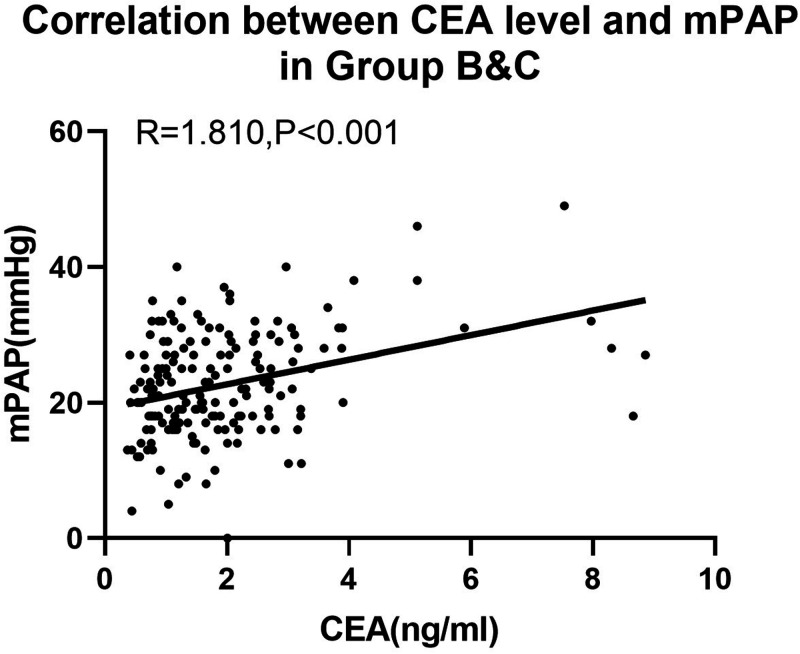
We also analyzed these correlations after eliminating group A from the total sample because PAP and PO can decrease in severe PAH. In groups B and C, PAP (Figure 3), PO, and Qp/Qs were positively correlated with CEA levels (all P<0.05, Table 4).
Figure 3.
Correlation between CEA levels and mPAP.
CEA, carcinoembryonic antigen; mPAP, mean pulmonary arterial pressure.
Table 4.
Linear regression analysis between carcinoembryonic antigen levels and other clinical characteristics in groups B and C.
| R value | |
|---|---|
| sPAP | 0.317# |
| dPAP | 0.172* |
| mPAP | 0.293# |
| PVR | 0.024 |
| PO | 0.276* |
| PI | 0.235 |
| Qp/Qs | 0.271* |
*P<0.05, #P<0.01.
sPAP, systolic pulmonary arterial pressure; dPAP, diastolic pulmonary arterial pressure; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; PO, pulmonary output; PI, pulmonary index; Qp/Qs, ratio of blood flow quantity between the pulmonary and systemic circulations.
Significance of CEA for predicting PH or PAH
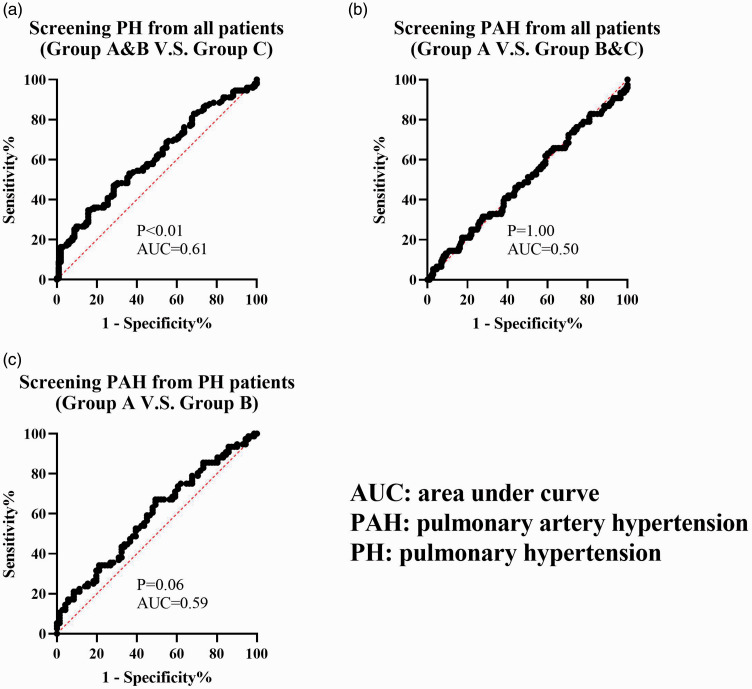
We constructed three receiver operating characteristic curves of CEA levels for predicting PH or PAH (Figure 4). The screening value only reached statistical significance when predicting PH (groups A and B vs group C) (P=0.003). However, the area under curve of this subgroup was only 0.61. The screening value for predicting PAH did not reach significance from the total sample (group A vs groups B and C) or from patients with PH (group A vs group B).
Figure 4.
Receiver operating characteristic curve of CEA levels.
CEA, carcinoembryonic antigen; PH, pulmonary hypertension; PAH, pulmonary arterial hypertension; AUC, area under the curve.
Discussion
Possible role of CEA in patients with PAH
In healthy adults, CEA is mainly expressed in the tongue, digestive system, trachea, and at low levels in the lungs.18,19 CEA also plays a role in cell adhesion, signal transduction, and innate immunity.20 However, if the balance in differentiation is lost, CEA can also help abnormal tissue transform malignantly and metastasize.21 These malignant changes also occur in development of PAH.13 We hypothesize that CEA also participates in pulmonary endothelial cell proliferation and migration in patients with PAH.
Furthermore, while in the progression stage of PAH, transforming growth factor β (TGF-β) is implicated in proliferation, migration, and differentiation of pericytes,22 and endothelial-to-mesenchymal transition23,24 and smooth muscle cell proliferation,25 which lead to vascular media thickening. TGF-β and CEA have an interactive effect with each other. CEA can activate the nuclear factor kappa-B pathway by combining with the TGF-β receptor.26 More importantly, CEA gene expression is induced through the TGF-β signal pathway.27 These findings suggest that CEA levels could be upregulated during the progressive stage of PAH owing to upstream or direct gene activation.
Hemodynamic changes and CEA levels
In this study, we found that CEA levels were significantly correlated with PVR, PO, the pulmonary index, and Qp/Qs, but not with mPAP, in the total sample. However, in groups B and C, PAP, PO, and Qp/Qs were significantly correlated with CEA levels. These findings might be due to two reasons. First, this was a retrospective study with a small sample size. Therefore, underlying bias might have led to unreliable results. Another reason might be that only PVR changes linearly in CHD-PH. During the dynamic stage of PH, vascular pathological changes are not significant and PAP increases with increased PO due to a shunt(s). This situation might represent groups B and C. However, group A represents the stage of PAH, and PAP increases while PO declines when PVR increases because of vascular remodeling. This situation might explain the positive correlation of CEA levels with PAP in groups B and C, and the negative correlation in group A. This situation could also explain the finding that CEA levels were correlated with PO and PVR in the combined groups.
Predictive value of CEA levels in PAH
In this study, we found that CEA levels increased with PO and decreased with PVR. However, the areas under the ROC curve of CEA were all <0.7 (Figure 4), which suggested that measurement of CEA levels might be more practical for following up than for screening of PH or PAH.
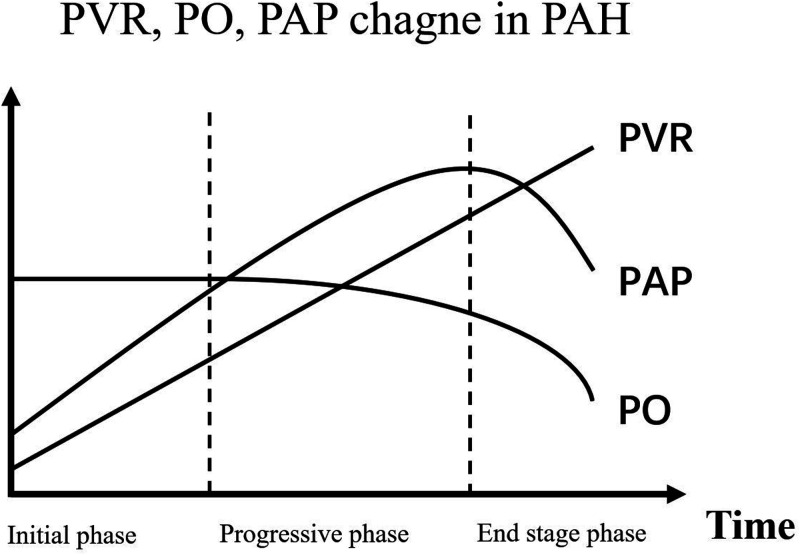
In clinical practice, patients with CHD-PAH have close follow-up visits, irrespective of whether cardiac malformation is corrected. However, acquiring precise hemodynamic data by RHC in each visit is costly and impractical. Although ultrasound and cardiac magnetic resonance can estimate systolic PAP and right heart function, assessment of PAH could still be confusing because systolic PAP does not change linearly in development of PAH (Figure 5). Therefore, more predictive markers of PAH are required. Because we found that CEA levels were related to PO and PVR in this study, this might still be the case in follow-up of each individual patient. If this hypothesis is verified, assessment of PH would be more accurate with assessment of CEA levels than without CEA levels. Especially when patients have elevated estimated systolic PAP after therapy, clinicians can better judge the actual therapeutic effects by combining ultrasound, physical signs, and blood tests, such as measurement of BNP and CEA. Importantly, CEA might be an additional parameter, but not a substitute, of the traditional method. To verify this hypothesis, more research, such as examining changes in CEA levels during follow up of patients with CHD, is required.
Figure 5.
Changes in PVR, PO, and PAP in pulmonary arterial hypertension.
PVR, pulmonary vascular resistance; PO, pulmonary output; PAP, pulmonary arterial pressure.
Limitations
This study has some limitations, which include not using healthy people as a control group and no follow-up test of CEA levels during treatment of PAH or after corrective surgery. If changes in CEA levels in each individual patient could be obtained, the correlation may be more convincing. Our sample size was limited owing to the small population of adult patients with CHD who had CEA levels measured. Smoking was not taken into consideration during data collection, which might be a possible bias.28
Conclusion
This is the first study to show the possible relationship between CEA levels and hemodynamics in CHD-PH. CEA levels increase with PO and decrease with PVR in CHD-PH. This finding might help to better evaluate progression of the disease, the therapeutic effect, and the prognosis of patients with CHD-PH.
Acknowledgements
We sincerely thank Dr. Fan Yang for her generous help in writing and polishing the manuscript.
Appendix: calculation method.
CO, cardiac output; SaO2, saturation of arterial oxygen; Hb, hemoglobin; SvO2, saturation of venous oxygen; PO, pulmonary output; SpvO2, saturation of pulmonary venous oxygen; SpaO2, saturation of pulmonary arterial oxygen; PVR, pulmonary vascular resistance; mPAP, mean pulmonary arterial pressure; SVR, systemic vascular resistance; MAP, mean arterial pressure; Qp/Qs, ratio of blood flow volume between the pulmonary and systemic circulations; PI, pulmonary index; BSA, body surface area.
Footnotes
Author contributions: This study was mainly undertaken by Yang Ziyang and Zhao Kaixun, and supervised by Zhang Caojin. Luo Dongling, Zhou Chengbin, and Chen Jimei provided help in data analyses. Yin Zhou helped in collecting the data.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National key Research and Development Program (2018YFC1002600), the Science and Technology Department of Guangdong Province (No. 2014A050503048, 2017A070701013, 2017B090904034, and 2017030314109), and the National Nature Science Foundation of China (No. U1401255).
ORCID iD: Yang Zi-yang https://orcid.org/0000-0002-6605-3987
References
- 1.Dolk H, Loane M, Garne E, et al. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation 2011; 123: 841–849. DOI: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 2.Schuuring MJ, Van Riel AC, Vis JC, et al. New predictors of mortality in adults with congenital heart disease and pulmonary hypertension: Midterm outcome of a prospective study. Int J Cardiol 2015; 181: 270–276. DOI: 10.1016/j.ijcard.2014.11.222. [DOI] [PubMed] [Google Scholar]
- 3.Blok IM, Van Riel AC, Schuuring MJ, et al. The role of cystatin C as a biomarker for prognosis in pulmonary arterial hypertension due to congenital heart disease. Int J Cardiol 2016; 209: 242–247. DOI: 10.1016/j.ijcard.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Scognamiglio G, Kempny A, Price LC, et al. C-reactive protein in adults with pulmonary arterial hypertension associated with congenital heart disease and its prognostic value. Heart 2014; 100: 1335–1341. DOI: 10.1136/heartjnl-2014-305494. [DOI] [PubMed] [Google Scholar]
- 5.Kula S, Canbeyli F, Atasayan V, et al. A retrospective study on children with pulmonary arterial hypertension: A single-center experience. Anatol J Cardiol 2018; 20: 41–47. DOI: 10.14744/AnatolJCardiol.2018.78370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965; 122: 467–481. DOI: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012; 76: 138–143. [DOI] [PubMed] [Google Scholar]
- 8.Das V, Kalita J, Pal M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges . Biomed Pharmacother 2017; 87: 8–19. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa M, Morine Y, Ikemoto T, et al. Elevated Preoperative Serum CEA Level Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma Through the Epithelial-Mesenchymal Transition. Anticancer Res 2017; 37: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 10.Eidelman FJ, Fuks A, DeMarte L, et al. Human carcinoembryonic antigen, an intercellular adhesion molecule, blocks fusion and differentiation of rat myoblasts. J Cell Biol 1993; 123: 467–475. DOI: 10.1083/jcb.123.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordoñez C, Screaton RA, Ilantzis C, et al. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 2000; 60: 3419–3424. [PubMed] [Google Scholar]
- 12.Soeth E, Wirth T, List HJ, et al. Controlled ribozyme targeting demonstrates an antiapoptotic effect of carcinoembryonic antigen in HT29 colon cancer cells. Clin Cancer Res 2001; 7: 2022–2030. [PubMed] [Google Scholar]
- 13.Hemnes AR, Humbert M. Pathobiology of pulmonary arterial hypertension: understanding the roads less travelled. Eur Respir Rev 2017; 26: 170093. DOI: 10.1183/16000617.0093-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldred MA, Comhair SA, Varella-Garcia M, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 1153–1160. DOI: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeager ME, Halley GR, Golpon HA, et al. Microsatellite Instability of Endothelial Cell Growth and Apoptosis Genes Within Plexiform Lesions in Primary Pulmonary Hypertension. Circ Res 2001; 88: E2–E11. DOI: 10.1161/01.RES.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 16.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. DOI: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 17.Fick A. Über die Messung des Blutquantums in den Herzventrikeln. Sitzungsberichte der Physiologisch-Medizinischen Gesellschaft zu Würzburg 1870; 2: 16. [Google Scholar]
- 18.Nap M, Mollgard K, Burtin P, et al. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol 1988; 9: 145–153. [DOI] [PubMed] [Google Scholar]
- 19.Eades-Perner AM, Van Der Putten H, Hirth A, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res 1994; 54: 4169–4176. [PubMed] [Google Scholar]
- 20.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 1999; 9: 67–81. DOI: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 21.Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013; 32: 643–671. DOI: 10.1007/s10555-013-9444-6. [DOI] [PubMed] [Google Scholar]
- 22.Ricard N, Tu L, Le Hiress M, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 2014; 129: 1586–1597. DOI: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark KR, Frid M, Perros F. Endothelial-to-Mesenchymal Transition: An Evolving Paradigm and a Promising Therapeutic Target in PAH. Circulation 2016; 133: 1734–1737. DOI: 10.1161/CIRCULATIONAHA.116.022479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooley BC, Nevado J, Mellad J, et al. TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med 2014; 6: 227ra234. DOI: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Zhang X, Li X, et al. TGF-beta1 inhibits the apoptosis of pulmonary arterial smooth muscle cells and contributes to pulmonary vascular medial thickening via the PI3K/Akt pathway. Mol Med Rep 2016; 13: 2751–2756. DOI: 10.3892/mmr.2016.4874. [DOI] [PubMed] [Google Scholar]
- 26.Jensen-Jarolim E, Fazekas J, Singer J, et al. Crosstalk of carcinoembryonic antigen and transforming growth factor-beta via their receptors: comparing human and canine cancer. Cancer Immunol Immunother 2015; 64: 531–537. DOI: 10.1007/s00262-015-1684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han SU, Kwak TH, Her KH, et al. CEACAM5 and CEACAM6 are major target genes for Smad3-mediated TGF-beta signaling. Oncogene 2008; 27: 675–683. DOI: 10.1038/sj.onc.1210686. [DOI] [PubMed] [Google Scholar]
- 28.Sajid KM, Parveen R, Sabih D, et al. Carcinoembryonic antigen (CEA) levels in hookah smokers, cigarette smokers and non-smokers. J Pak Med Assoc 2007; 57: 595–599. [PubMed] [Google Scholar]