Abstract
Six new triterpene saponins (1–5,7) and 3 known saponins (6,8,9) were isolated from MeOH extracts of the cactus Stenocereus pruinosus. The structures of the isolated saponins were elucidated using MS, IR, and comprehensive NMR measurements. To develop drugs for treating Alzheimer’s disease (AD) on the basis of the amyloid cascade hypothesis, the isolated saponins were evaluated for inhibition of BACE1 activity and amyloid beta (Aβ) aggregation using thioflavin-T assay, and triterpenes as an aglycone moiety and an alkaline hydrolysate of the saponins were also evaluated. One saponin, stenoside A (7), exhibited inhibitory activity related to Aβ aggregation and its degree of Aβ aggregation was 40.6% at 100 μM.
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s11418-020-01463-0) contains supplementary material, which is available to authorized users.
Keywords: Cactus, Stenocereus pruinosus, Saponins, Alzheimer’s disease, Amyloid beta, BACE1
Introduction
Cacti range mainly throughout South America to the southern areas of North America, regions that have two seasons clearly distinguishable as rainy and dry. Cactus plants are divided into primarily three forms, known as arborescent cacti, columnar cacti, and globular cacti. Stenocereus pruinosus (Otto) Buxb. belongs to columnar cacti widely distributed in semi-arid areas of the South East of Mexico, intensely managed in Central Mexico to gather its fruits, and sometimes cultivated as home garden [1]. The height of S. pruinosus is up to 8 m, blanches are green with 5–8 ribs, and flowers are infundibuliform 7–10 cm in length growing in the blanch apexes with green-brownish external tepals and white internal tepals which are produced 2 or 3 years after being planted [1, 2]. The constituents of cacti have been investigated by Djerassi and co-workers, who reported a lot of triterpenoid sapogenins in an acid-hydrolyzed saponin-rich extract from many cacti and one of their works revealed S. pruinosus contained oleanolic acid [3]. Considering those reports, we had been further investigating triterpene sapogenins from many cacti and discovered that S. pruinosus contained erythrodiol, longispinogenin and 3β-hydroxy-11α,12α-epoxyolean-28,13β-olide in addition to oleanolic acid from hydrolysate of MeOH extract of S. pruinosus [4]. Now, we have been investigating triterpene saponins from cacti for several decades [5–12] and reported the identification of numerous saponins exhibiting bioactivities such as anti-type I allergy activity [7], inhibition or promotion of melanin synthesis [5], inhibition of amyloid β (Aβ) aggregation, and protective effects on SH-SY5Y cells against Aβ-associated toxicity [6].
Saponins are well-known phytochemicals that are comprised of two parts, an aglycone moiety and a sugar moiety. The activities of saponins have been well studied and include anti-inflammatory, anti-tumor, anti-obesity, anti-angiogenesis, anti-allergic, anti-microbial, and anti-Alzheimer’s disease (AD) [13, 14]. In particular, our group found that saponins from cacti have unique structures [5–12] that have not been adequately investigated.
AD is a globally significant disease that involves cognitive and locomotor disorders resulting from progressive neurodegeneration. It is frequently said that AD has a complex pathogenesis that involves several key steps: Aβ production, Aβ aggregation, and neural cell death. Aβ production is caused by the cleavage of amyloid precursor protein (APP) by the action of the enzymes β-secretase and γ-secretase [15, 16]. As the concentration of Aβ increases, it begins to aggregate and form Aβ-oligomers, Aβ-fibrils, and in most cases, senile plaques. Once Aβ aggregation occurs, neural cell death gradually begins [17, 18]. This step also involves complex pathways that consist of synaptic and neuritic injury, altered ionic homeostasis, oxidative damage, altered kinase/phosphatase activity, neurofibrillary tangles, and microglial and astrocytic activation [19]. The prevention of neural cell death is clearly important, but Aβ production and aggregation are also important for the Aβ-associated steps that represent the upstream pathways of AD pathogenesis. As such, many groups have explored numerous candidate phytochemicals from natural resources for inhibition of Aβ production and aggregation [19–22]. A recent report identified saponins as a class of candidate phytochemicals [23, 24].
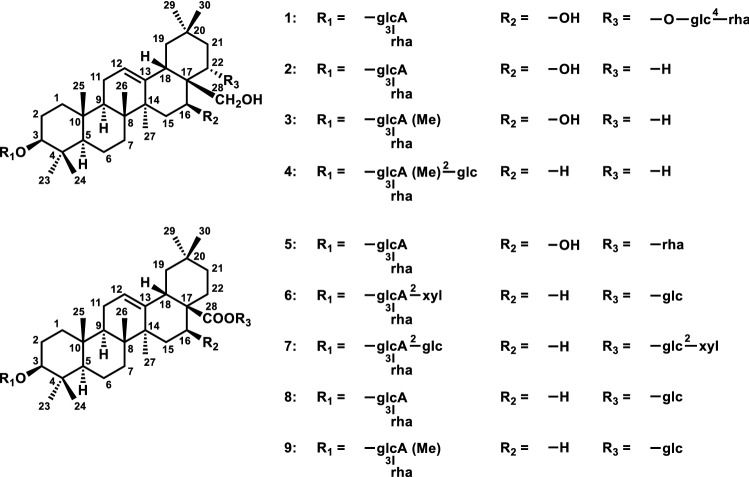
Here, we report the isolation and structure elucidation of various saponins (1–9), including 6 new compounds (1–5,7) and 3 known saponins (6,8–9), isolated from the cactus Stenocereus pruinosus (Otto) Buxb. (Fig. 1). We also evaluated the activity of these compounds in terms of inhibition of Aβ aggregation and BACE1 activity. Our data revealed two particularly remarkable findings. First, compounds 1 and 6–7 have a rare linkage of a sugar unit. Compound 1 has a glucopyranosyl unit that binds to the C-22 region of its aglycone, whereas 6 has a xylopyranosyl unit that binds to the C-3′ of glucuronic acid, and 7 also has a xylopyranosyl unit that binds to the C-2′′′ of glucose binding to C-28 of its aglycone. Second, compounds 2–4 have rare triterpenes for cactus plants, longispinogenin and erythrodiol, as aglycones. These are the first reports of isolation of such saponins from columnar cactus plants.
Fig. 1.
Structures of compounds (1–9) Isolated from Stenocereus pruinosus
Results and discussion
Dried Stenocereus pruinosus was extracted repeatedly with chloroform and then with methanol. The methanol extract was separated by silica gel and octadecyl silyl silica gel (ODS) column chromatography, yielding chichipenoside D (1), longispinoside A (2), longispinoside A methyl ester (3), erythronoside A (4), cochalinoside C (5), oleanolic acid 3-O-β-d-xylopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (6), Stenoside A (7), oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (8) and oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-6′-O-methyl glucuronopyranosyl-28-O-β-d-glucopyranoside (9) (Fig. 1). The structures of compounds 1–9 were determined by mass and NMR spectroscopy using 1H- and 13C-NMR, DEPT, HMQC, HMBC, 1H-1H COSY or DQF-COSY, HSQC-TOCSY, phase-sensitive TOCSY and phase-sensitive NOESY experiments.
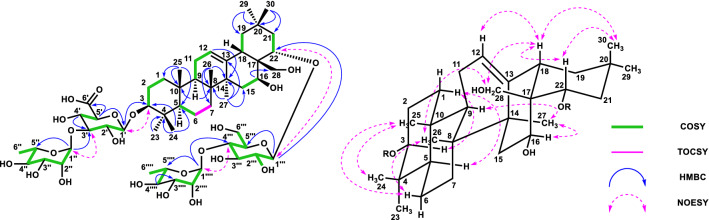
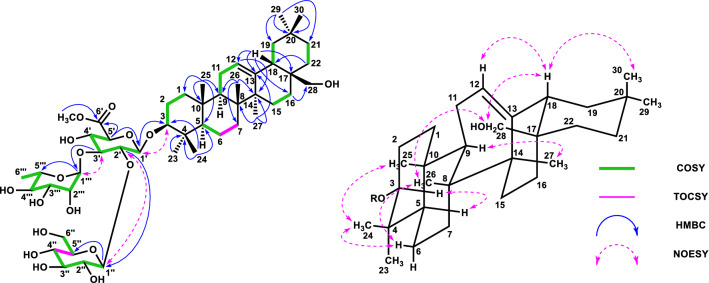
Compound 1 (16.7 mg, 0.003%) was obtained as a colorless amorphous powder, and its optical rotation value was elucidated as [α]21D-28.0 (c 0.25, MeOH). The molecular formula of 1 was elucidated to be C54H88O23 using negative HRFABMS (m/z 1103.5640, [M-H]−). The IR spectrum of 1 showed absorptions at 3385 cm− 1 (hydroxy) and 1714 cm− 1 (carbonyl). The signal patterns of the 1H- and 13C-NMR spectral data indicated that the aglycone moiety was an oleanane-type triterpene. The 1H- and 13C-NMR spectral data indicated the presence of seven methyl groups in the aglycone, characterized by signals at [δH 0.76, 0.87, 0.90, 0.96 and 1.16 (each 3H, s), and δH 0.88 (6H, s)], and 15.3, 16.4, 16.6, 24.4, 26.8, 27.6, and 32.8 (each CH3). The presence of signals at δC 64.5 (CH) and δH 4.42 (1H, br s), δC 77.9 (CH) and δH 4.25 (1H, dd, J = 12.6, 4.5 Hz), δC 56.7 (CH2) and [δH 3.33 (1H, o), and δH 3.77 (1H, m)] indicated that the aglycone had three hydroxyl groups, except in the C-3 region [δC 88.1 (CH) and δH 3.02 (1H, dd, J = 11.3, 3.2 Hz)], and the stereochemistry of 3-OH was determined as a β-configuration by its 3JH2/H3 coupling constants of 11.3 Hz. Further analysis of the aglycone of 1 primarily using HMQC, HMBC, DQF-COSY, phase-sensitive NOESY spectral correlations, and complementarily using phase-sensitive TOCSY spectral correlations, revealed four hydroxyl groups assigned at C-3 (δC 88.1), C-16 (δC 64.5), C-22 (δC 77.9), and C-28 (δC 56.7). The NOESY correlations between δH 1.16 (H3-27) and δH 4.42 (H-16), δH 3.33, 3.77 (H-28) and δH 0.90 (H-26), and δH 4.25 (H-22) and δH 2.50 (H-18) supported the stereochemistries of 16-OH and 22-OH as β-configuration and α-configuration, respectively (Fig. 2). The signals at δH 1.56 (1H, br t, J = 12.6 Hz, H-21α), δH 1.68 (1H, br d, J = 12.6 Hz, H-21β) and δH 4.25 (1H, dd, J = 12.6, 4.5 Hz, H-22) also supported the stereochemistry of 22-OH as α-configuration by its large 3JH21α/H22 coupling constants of 11.3 Hz and small 3JH21β/H22 coupling constants of 4.5 Hz. These data confirmed that the aglycone of 1 was chichipegenin. The 1H- and 13C-NMR spectra exhibited four anomeric proton signals [δH 4.17 (1H, d, J = 7.7 Hz), δH 4.33 (1H, d, J = 7.8 Hz), δH 4.69 (1H, br s), δH 5.04 (1H, br s)] and carbon signals [δC 100.2 (CH), δC 100.5 (CH), δC 103.3 (CH), δC 105.1 (CH)]. Detailed analysis of the sugar moiety of 1 using HMQC, HMBC, DQF-COSY, HSQC-TOCSY, phase-sensitive TOCSY, and phase-sensitive NOESY experiments revealed the presence of one β-glucuronic acid, one β-glucose, and two α-rhamnose units. The linkage of each sugar was determined by HMBC correlations. The correlation between H-1′ of the glucuronic acid (δH 4.17) and C-3 of the aglycone (δC 88.1) showed that the glucuronic acid-1 unit binds at C-3 of the aglycone moiety. Similarly, the correlation between H-1′′ of rhamnose (δH 5.04) and C-3′ of glucuronic acid (δC 80.3) showed that the rhamnose-1 unit binds at C-3′ of the glucuronic acid unit. In contrast, the correlation between H-1′′′ of glucose (δH 4.33) and C-22 of the aglycone (δC 77.9) showed that the glucose-1 unit binds at C-22 of the aglycone moiety. Considering the chemical shift of C-22 (δC 67.4) of chichipenoside C [5], this deshielding of C-22 (δC 77.9) of the aglycone also supported the binding of the glucose-1 unit at C-22 of the aglycone. In addition, the correlation between H-1′′′′ of the second rhamnose (δH 4.69) and C-4′′′ of glucose (δC 76.6) indicated that the rhamnose-1 unit binds at C-4′′′ of the glucose unit. These correlations are shown in Fig. 2. The absolute configuration of each sugar unit was determined by measuring the ODS HPLC retention times of the derivatives from d-glucuronic acid, d-glucose, and l-rhamnose [25]. Whereas the anomeric proton on the glucuronopyranosyl and glucopyranosyl units were determined to have a β-configuration based on the 3JH1-H2 values (7.7 and 7.8 Hz, respectively), the anomeric protons on the two rhamnopyranosyl units were determined to have a α-configuration based on 1JC1-H1 value (171.7 and 169.4 Hz) [26] and three-bond strong HMBC correlations from H-1′′ to C-3′′ and C-5′′, and H-1′′′′ to C-3′′′′ and C-5′′′′. Its three-bond strong HMBC correlations were attributed to the dihedral angles between H-1 and C-3, H-1 and C-5 of rhamnose about 180° [27]. Thus, compound 1 was determined chichipegenin 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-22-O-α-l-rhamnopyranosyl-(1 → 4)-β-d-glucopyranoside and named as chichipenoside D (Fig. 2).
Fig. 2.
HMBC, COSY, and Key NOESY correlations of 1
Compound 2 (16.0 mg, 0.003%) was obtained as a colorless amorphous powder, and its optical rotation value was elucidated as [α]18D-52.0 (c 0.2 MeOH). The molecular formula of 2 was elucidated to be C42H68O13 using positive HRFABMS (m/z 803.4546, [M + Na]+). The IR spectrum of 2 showed absorptions at 3330 cm− 1 (hydroxy) and 1740 cm− 1 (carbonyl). The 1H- and 13C-NMR spectral data indicated the presence of seven methyl groups in its aglycone, characterized by signals at [δH 0.75, 0.87, 0.90, 0.96 and 1.14 (each 3H, s), and 0.84 (6H, s)] and 15.3, 16.3 16.5, 23.7, 26.6, 27.5, and 33.1 (each CH3). The signal patterns of the 1H- and 13C-NMR spectral data indicated that the aglycone moiety was an oleanane-type triterpene. The presence of three sets of signals at δC 64.5 (CH) and δH 4.06 (1H, dd, J = 12.4, 4.4 Hz), δC 65.2 (CH2) and δH 3.12 (1H, o), 3.51 (1H, d, J = 10.2 Hz) and δC 88.3 (CH) and δH 3.05 (1H, dd, J = 11.3, 4.0 Hz) indicated that the aglycone of 2 had three hydroxyl groups. Further analysis of the aglycone of 2 using HMQC, HMBC, DQF-COSY, and phase-sensitive NOESY spectral correlations assigned the three hydroxyl groups at C-3, C-16, and C-28. The NOESY correlation between δH 1.14 (H3-27) and δH 4.06 (H-16) and the 3JH15/H16 coupling constant of 12.4 Hz revealed the stereochemistry of the 16-OH was β-configuration and confirmed that the aglycone of 2 was longispinogenin (Fig. 3). The 1H- and 13C-NMR spectra about sugar units showed partially similar signal patterns to those of 1. Additionally considering ODS HPLC data [25] and comprehensive 2D-NMR analysis including strong three-bond HMBC correlation [27], it was revealed that the presence of two sugars, β-d-glucuronic acid and α-l-rhamnose. It could not be performed to measure the 1JC1-H1 value about the anomeric protons on the rhamnopyranosyl units contained in new saponins (2–5,7) from S. pruinosus, the rhamnopyranosyl units presumably had α-configuration because of our previous reports [5–12], their multiplicity (“br s” or “d” with small coupling constant) and their strong three-bond HMBC correlations [27]. Further analysis confirmed that compound 2 was longispinogenin 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranoside and named as longispinoside A (Fig. 3).
Fig. 3.
HMBC, COSY, and Key NOESY correlations of 2
Compound 3 (24.2 mg, 0.004%) was obtained as a colorless amorphous powder, and its optical rotation value was elucidated as [α]19D-117.3 (c 0.1 MeOH). The molecular formula of 3 was elucidated to be C43H70O13 using positive HRFABMS (m/z 795.4900, [M + H]+). The IR spectrum of 3 showed absorptions at 3363 cm− 1 (hydroxy) and 1746 cm− 1 (carbonyl). The 1H- and 13C-NMR spectral data were almost the same with 2 except for methyl signal [δC 51.9 (CH3) and δH 3.65 (3H, s)] and C-6′ of glucuronic acid [δC 169.5 (C)]. This differences and further analysis confirmed that compound 3 was longispinogenin 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranoside methyl ester and named as longispinoside A methyl ester (Fig. S1).
Compound 4 (9.3 mg, 0.001%) was obtained as a colorless amorphous powder, and its optical rotation value was elucidated as [α]19D-4.1 (c 0.1 MeOH). The molecular formula of 4 was elucidated to be C49H80O17 using positive HRFABMS (m/z 941.5481, [M + H]+). The IR spectrum of 4 showed absorptions at 3370 cm− 1 (hydroxy) and 1743 cm− 1 (carbonyl). The 1H- and 13C-NMR spectral data indicated the presence of seven methyl groups in its aglycone, characterized by signals at [δH 0.74, 0.82, 0.84, 0.95 and 1.10 (each 3H, s), and 0.87 (6H, s)], and 15.3, 16.1, 16.4, 23.5, 25.6, 27.5, and 33.1 (each CH3). The signal patterns of the 1H- and 13C-NMR spectral data indicated that the aglycone moiety was an oleanane-type triterpene. The presence of two sets of signals at [δC 67.4 (CH2) and δH 2.93 (1H, o) and 3.29 (1H, dd, J = 9.6, 4.8 Hz)] and [δC 88.6 (CH) and δH 3.04 (1H, dd, J = 11.3, 4.0 Hz)] indicated that the aglycone of 4 had two hydroxyl groups in the C-3 region. Further analysis of the aglycone of 4 using HMQC, HMBC, DQF-COSY, phase-sensitive TOCSY, and phase-sensitive NOESY spectral correlations assigned the two hydroxy groups at C-3 and C-28, which indicated that the aglycone of 4 was erythrodiol (Fig. 4). The 1H- and 13C-NMR spectra showed three anomeric proton signals [δH 4.43 (1H, d, J = 7.9 Hz), δH 4.52 (1H, d, J = 8.3 Hz), δH 4.94 (1H, br s)] and carbon signals [δC 100.5 (CH), δC 101.6 (CH), δC 103.1 (CH)]. Detailed analysis of the sugar moiety of 4 using HMQC, HMBC, DQF-COSY, HSQC-TOCSY, phase-sensitive TOCSY, and phase-sensitive NOESY experiments revealed the presence of one β-glucuronic acid, one β-glucose, and one α-rhamnose unit, and the signals at δH 3.65 (3H, s) and δC 169.5 indicated the presence of glucuronic acid methyl ester instead of glucuronic acid. The linkage of each sugar was determined by HMBC correlations and the correlations indicated that the glucuronic acid-1 unit binds at C-3 of the aglycone moiety, the glucose unit binds at C-2′ of the glucuronic acid methyl ester, and the rhamnose-1 unit binds at C-3′ of the glucuronic acid methyl ester (Fig. 4). The sugar units were determined as β-d-glucuronic acid, α-l-rhamnose, and β-d-glucose by the same method as compound 2 [25, 27]. Thus, compound 4 was determined erythrodiol 3-O-β-d-glucopyranosyl-(1 → 2)-[α-l-rhamnopyranosyl-(1 → 3)]-β-d-glucuronopyranoside methyl ester and named as erythronoside A methyl ester (Fig. 4).
Fig. 4.
HMBC, COSY, and Key NOESY correlations of 4
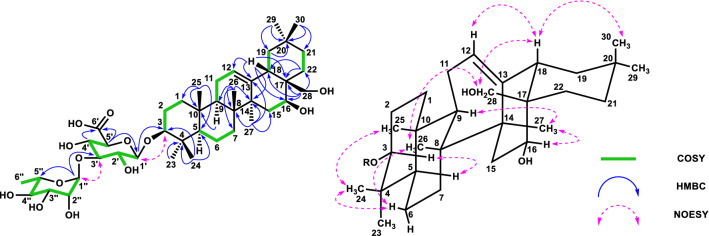
Compound 5 (27.5 mg, 0.004%) was obtained as a colorless amorphous powder, and its optical rotation value was elucidated as [α]22D-28.8 (c 0.25 MeOH). The molecular formula of 5 was elucidated to be C48H76O18 using negative HRFABMS (m/z 939.4952, [M-H]−). The IR spectrum of 5 showed absorptions at 3370 cm− 1 (hydroxy) and 1710 cm− 1 (carbonyl). The 1H- and 13C-NMR spectral data indicated the presence of seven methyl groups in its aglycone, characterized by signals at δH 0.69, 0.75, 0.84, 0.90, 0.92, 0.95, and 1.13 (each 3H, s) and δC 15.1, 16.4, 16.8, 23.7, 26.7, 27.5, and 32.7 (each CH3). The signal patterns of the 1H- and 13C-NMR spectral data indicated that the aglycone moiety was an oleanane-type triterpene. The presence of a signal at δC 62.7 (CH) and δH 4.06 (1H, t-like, J = 11.8 Hz) indicated that the aglycone of 5 had one hydroxyl group excepting the C-3 region [δC 88.1 (CH) and δH 3.03 (1H, dd, J = 9.9, 3.8 Hz)]. Further analysis of the aglycone of 5 using HMQC, HMBC, DQF-COSY, phase-sensitive TOCSY, and phase-sensitive NOESY spectral correlations assigned the two hydroxy groups at C-3 and C-16. The stereochemistry of 16-OH was revealed as β-configuration by NOESY correlations and its 3JH15/H16 coupling constant of 12.6 Hz, which indicated the aglycone of 5 was cochalic acid (Fig. 5). The 1H- and 13C-NMR spectra further indicated the presence of the same sugar moiety with 2 and another anomeric signals [δH 5.74 (1H, d, 1.1) and δC 93.3 (CH)]. Further analysis revealed the presence of another rhamnose unit binding at C-28 of the aglycone. The sugar units were determined as β-d-glucuronic acid and α-l-rhamnose by the same method as compound 2 [25, 27]. Thus, compound 5 was determined cochalic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-α-l-rhamnopyranoside and named as cochalinoside C (Fig. 5).
Fig. 5.
HMBC, COSY, and Key NOESY correlations of 5
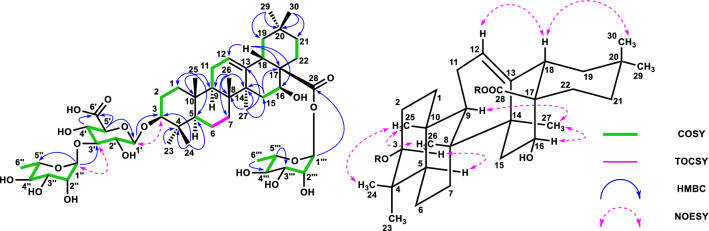
Compound 7 (28.9 mg, 0.005%) was obtained as a colorless amorphous powder, and its optical rotation value was elucidated as [α]19D -8.6 (c 0.1 MeOH). The molecular formula of 7 was elucidated to be C59H94O27 using positive HRFABMS (m/z 1273.5620, [M + K]+). The IR spectrum of 7 showed absorptions at 3372 cm−1 (hydroxy) and 1732 cm−1 (carbonyl). The 1H- and 13C-NMR spectral data indicated that the aglycone was oleanolic acid, the presence of the same sugar moiety with 4 except for methyl ester, and the presence of two other sugar units. Further analysis revealed the presence of one glucose unit binding at C-28 of the aglycone and one xylose unit binding at C-2′′′′ of the glucose unit (Fig. S3). The sugar units were determined as β-d-glucuronic acid, α-l-rhamnose, β-d-glucose and β-d-xylose by the same method as compound 2 [25, 27]. Thus, compound 7 was determined oleanolic acid 3-O-β-d-glucopyranosyl-(1 → 2)-[α-l-rhamnopyranosyl-(1 → 3)]-β-d-glucuronopyranosyl-28-O-β-d-xylopyranosyl-(1 → 2)-β-d-glucopyranoside and named as stenoside A (Fig. S3).
Compound 6 was analyzed in the same way as described above and elucidated as known one, oleanolic acid 3-O-β-d-xylopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside [28]. The 2D-correlations and completed assignment were shown in Fig. S2 and Table S1.
Compounds 8 and 9 were identified as oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (8) and oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-6′-O-methyl glucuronopyranosyl-28-O-β-d-glucopyranoside (9) by comparing their spectroscopic data with published data [29, 30].
Some of isolated saponins (1–4,7–8), hydrolysate of 8 (8a), and oleanolic acid as the aglycone moiety of 6–9 were evaluated the inhibition of Aβ aggregation using a Th-T assay and inhibition of BACE1 activity (Table 1). A newly isolated saponin (7) exhibited Aβ aggregation inhibitory activity (40.6% at 100 μM), and compounds 1 and 4 exhibited moderate activity (66.4% and 70.0%, respectively, at 100 μM). The activity of 8a was almost same level as 8 at 100 μM.
Table 1.
Assay results of the isolated and associated compounds against Aβ aggregation and BACE1 activity
| Compound | Conc. (μM) | Aβ aggregation (%) | BACE1 inhibition (%) |
|---|---|---|---|
| 1 | 100 | 66.4 ± 6.4 | NAb |
| 2 | 100 | 92.5 ± 7.3 | NAb |
| 3 | 100 | 77.3 ± 4.1 | NAb |
| 4 | 100 | 70.0 ± 0.7 | NAb |
| 5 | 100 | NDa | NDa |
| 6 | 100 | NDa | NDa |
| 7 | 100 | 40.6 ± 5.8 | NAb |
| 8 | 100 | 81.4 ± 3.7 | NAb |
| 9 | 100 | NDa | NDa |
| 8a | 100 | 95.4 ± 15.4 | NAb |
| Oleanolic acid | 100 | 78.8 ± 7.8 | 45.3 ± 23.2 |
| Cochalic acid | 100 | NDa | 35.8 ± 8.8 |
| Chichipegenin | 100 | NDa | NAb |
| Myricetin | 25 | 23.4 ± 2.2 | |
| Inhibitor IV | 10 | 69.1 ± 13.7 |
Data are shown as mean with standard errors (n = 3)
aND = no data
bNA = not active
A preliminary SAR suggested that glycosylations at C-28 position of the oleanolic acid like 7 increased its inhibitory activity comparing with a glycosylation at C-28 position of the oleanolic acid like 8. In addition to this, glycosylations at C-3 position of the oleanolic acid hardly had any effects on its activity in the case of 8a. According to these results, glycosylations at C-28 might be important for Aβ aggregation inhibitory activity.
Chichipenoside C, chichipegenin 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranosyl-(1 → 2)-β-d-glucuronopyranoside, which has already isolated from Polaskia chichipe [5], exhibited Aβ aggregation inhibitory activity at the same level as chichipegenin (chichpenoside C; 43.8%, chichipegenin; 39.2%, respectively, at 25 μM) [6], while chichipenoside D exhibited weaker activity than that of chichipenoside C and chichipegenin. Its SAR suggested that glycosylations at C-22 position of the chichipegenin attenuated Aβ aggregation inhibitory activity. It would be expected that saponins mono-glycosylated at C-22 position of the chichipegenin are isolated and tested for inhibitory activity related to Aβ aggregation.
In contrast, saponins (1–4,7–8) and hydrolysate of 8 (8a) did not exhibit any BACE1 inhibitory activities at 100 μM, while oleanolic acid exhibited moderate activity (45.3% at 100 μM). Its IC50 value was previously reported [31]. Considering oleanolic acid, cochalic acid and chichipegenin, the presence of a carboxyl group at C-28 region of the oleanane-type triterpenes might be effective for BACE1 inhibitory activity and the oleanane-type triterpenoid saponins might have less ability for inhibiting BACE1 activity.
In summary, six new saponins (1–5,7) were isolated from Stenocereus pruinosus, and their structures were elucidated along with known saponins (6,8,9). The structure of 1 has chichipegenin as the aglycone, in which C-3 and C-22 are glycosylated. Although saponins that have a sugar unit at C-22 to some extent have been identified [32–34], this is the first report in the case of chichipegenin. Compounds 2–3 and 4 have longispinogenin or erythrodiol as the aglycone moiety. Some saponins that have longispinogenin or erythrodiol as the aglycone have already been reported [35–42], but this is the first report of their isolation from cacti. Saponins 5 and 6–7 have cochalic acid or oleanolic acid as the aglycone. These types of saponin has been also isolated from another cactus, Polaskia chichipe [6], but the sugar unit differs from known ones. With respect to the sugar unit, 6 has a unique sugar chain for cactus plants. There are some reports related to that type of sugar chain [28, 43–47], as it is not a common sugar unit, and this is the first report of its isolation from cacti. Paying attention to glucuronic acid, some compounds (3, 4, 9) were isolated as glucuronic acid methyl esters. As compound 3 and 9 appeared to be derivatives of 2 and 8, compounds 3, 4 and 9 could be artifacts caused by methanol extraction, which thought to be similar case to our previous report [5]. Some of the isolated saponins (1–4,7–8) and hydrolysate of 8 (8a) were tested for their ability to inhibit Aβ aggregation and BACE1 activity. Compound 7 was most effective at inhibiting Aβ aggregation, followed by compounds 1 and 4. Oleanolic acid exhibited less of an effect on Aβ aggregation, as the structure consisting of oleanolic acid and sugar units is important for attenuating Aβ aggregation. Results observed with compound 7 also suggested that glycosylations at C-28 of oleanolic acid is important for attenuating Aβ aggregation. In the BACE1 assay, tested compounds exhibited no inhibitory effect on BACE1 activity except for oleanolic acid and cochalic acid. Although six new saponins were described, more isolations from cacti should be pursued because of their unique structures, and additional SARs should be conducted to characterize the relationship to inhibition of Aβ aggregation and BACE1 activity.
Experimental
General experimental procedures
Optical rotation values were recorded with a Horiba SEPA-300 polarimeter. IR spectra were measured with a Thermo FT-IR Nicolet iS5 spectrometer (ATR). 1H- and 13C-NMR spectra were recorded using a JNM LA-500 spectrometer. HRFABMS spectra were obtained using a JEOL JMS-700 spectrometer. Column chromatography was carried out with silica gel 60 N (63–210 μm) from Kanto Chemical, ODS silica gel YMC-GEL ODS-A from YMC Co. Ltd., and Diaion HP-20 from Mitsubishi Chemical Co. Ion-exchange chromatography was performed using Dowex 50 W-X8 resin (50–100 mesh, H-form) from Sigma-Aldrich. Thin-layer chromatography was carried out using TLC silica gel 60F254 and RP-18 F254S plates from Merck. Inhibition of Aβ aggregation was assayed using a Synergy HTX Multi-Mode Reader (BioTek, Winooski, Vermont, USA), Aβ40 (Peptide Institute, Osaka, Japan), and thioflavin-T (Fujifilm-Wako).
Plant material
Stenocereus pruinosus (Otto) Buxb. was purchased from Hokoen (Iga City, Mie, Japan). A voucher specimen is deposited at our laboratory.
Extraction and isolation
Aerial parts of S. pruinosus were dried, and the dry powder (639.1 g) was extracted three times with CHCl3 and then extracted three times with MeOH. The MeOH extract (144.2 g) was applied to a Diaion HP-20 column, which was successively eluted with MeOH–H2O mixture to give 4 fractions [H2O-eluted fraction (disposal), 30% MeOH-eluted fraction (15.0 g), 70% MeOH-eluted fraction (60.3 g), and 100% MeOH-eluted fraction (106.5 g)], respectively.
The 70% MeOH-eluted fraction (60.3 g) was subjected to silica gel column chromatography (Si. C. C.) using a CHCl3–MeOH–H2O mixture to give 10 fractions (A–J). Fr. H (10.3 g) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to give 3 fractions (Ha–Hc). Fr. Hb (7.5 g) was subjected to Si. C. C. using a CHCl3-MeOH-H2O mixture to give 5 fractions (Hb1–Hb5). Fr. Hb4 (5.0 g) was subjected to Si. C. C. using a CHCl3-MeOH-H2O mixture to give 3 fractions (Hb4-1–Hb4-3). Fr. Hb4-2 (4.1 g) was subjected to octadecyl-silylated silica gel column chromatography (ODS C. C.) using a MeOH–H2O mixture to give 5 fractions (Hb4-2a–Hb4-2e). Fr. Hb4-2b (93.6 mg) was subjected to ODS C. C. using a MeOH-H2O mixture to give 3 fractions (Hb4-2b-1–Hb4-2b-3). Fr. Hb4-2b-2 (48.3 mg) was further separated by Si. C. C. using a CHCl3–MeOH–H2O mixture to afford chichipenoside D (1, 16.7 mg, 0.003%, Fr. Hb4-2b-2d).
Fr. Hb4-2d (1.0 g) was subjected to ODS C. C. using a MeOH–H2O mixture to give 3 fractions (Hb4-2d-1–Hb4-2d-3). Fr. Hb4-2d-2 (905.5 mg) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to give 3 fractions (Hb4-2d-2a–Hb4-2d-2c). Fr. Hb4-2d-2b (202.3 mg) was further separated by ODS C. C. using a MeCN–H2O mixture to afford oleanolic acid 3-O-β-d-xylopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 3)-β- d-glucuronopyranosyl-28-O-β-d-glucopyranoside (6, 72.0 mg, 0.011%, Fr. Hb4-2d-2b-9).
Fr. Hb2 (853.5 mg) was subjected to ODS C. C. using a MeOH–H2O mixture to afford 5 fractions (Hb2-1–Hb2-5) and oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (8, 440.3 mg, 0.069%, Fr. Hb2-4).
Fr. Hb2-2 (86.5 mg) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to afford cochalinoside C (5, 27.5 mg, 0.004%, Fr. Hb2-2e).
The 100% MeOH-eluted fraction (94.5 g) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to give 8 fractions (1–8). Fr. 4 (1.9 g) was subjected to ODS C. C. using a MeCN–H2O mixture to give 3 fractions (4A–4C). Fr. 4B (1.2 g) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to afford oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-6′-O-methyl-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (9, 40.5 mg, 0.006%, Fr. 4B-2).
Fr. 5 (15.9 g) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to give 6 fractions (5A–5F). Fr. 5E (300.8 mg) was subjected to ODS C. C. using a MeOH–H2O mixture to give 2 fractions (Fr. 5E-1–5E-2). Fr. 5E-1 (235.2 mg) were partially (46.0 mg) subjected to ODS C. C. using a MeCN–H2O mixture to afford longispinoside A (2, 16.0 mg, 0.003%, Fr. 5E-1e).
Fr. 5B (186.1 mg) was subjected to ODS C. C. using a MeCN–H2O mixture to give 3 fractions (5B-1–5B-3). Fr. 5B-2 (51.4 mg) was subjected to ODS C. C. using a MeOH–H2O mixture to afford longispinoside A methyl ester (3, 24.2 mg, 0.004%, Fr. 5B-2c).
Fr. 5D (391.3 mg) was subjected to ODS C. C. using a MeOH–H2O mixture to give 3 fractions (5D-1–5D-3). Fr. 5D-2 (54.3 mg) was subjected to ODS C. C. using a MeOH–H2O mixture to give 2 fractions (5D-2a–5D-2b). Fr. 5D-2b (40.3 mg) was subjected to ODS C. C. using a MeCN–H2O mixture to afford erythronoside A methyl ester (4, 9.3 mg, 0.001%, Fr. 5D-2b-6).
Fr. 8 (6.1 g) was subjected to Si. C. C. using a CHCl3–MeOH–H2O mixture to give 2 fractions (8A–8B). Fr. 8B (1.6 g) was subjected to ODS C. C. using a MeOH–H2O mixture to afford stenoside A (7, 28.9 mg, 0.005%, Fr. 8B-3).
Chichipenoside D (1): colorless amorphous powder; [α]21D -28.0 (c 0.25, MeOH); IR (ATR) νmax: 3385, 2939, 1714, 1602, 1417, 1376, 1293, 1041 cm−1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; negative HRFABMS m/z: 1103.5640 [M-H]− (calcd for C54H87O23, 1103.5640).
Table 2.
1H-NMR spectroscopic data for compounds 1–5,7 (500 MHz, in DMSO-d6)
| Position | 1 | 2 | 3 | 4 | 5 | 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α | 0.88 (o) | α | 0.87 (o) | α | 0.88 (o) | α | 0.84 (o) | α | 0.86 (o) | α | 0.88 (o) |
| β | 1.49 (o) | β | 1.47 (o) | β | 1.49 (o) | β | 1.49 (o) | β | 1.48 (o) | β | 1.47 (o) | |
| 2 | α | 1.88 (brd, 10.8) | α | 1.73 (brd, 10.2) | α | 1.62 (o) | α | 1.68 (o) | α | 1.88 (brd, 9.9) | α | 1.92 (o) |
| β | 1.49 (o) | β | 1.52 (o) | β | 1.52 (o) | β | 1.53 (o) | β | 1.48 (o) | β | 1.51 (o) | |
| 3 | 3.02 (dd, 11.3, 3.2) | 3.05 (dd, 11.3, 4.0) | 3.05 (dd, 11.5, 4.4) | 3.04 (dd, 11.3, 4.0) | 3.03 (dd, 9.9, 3.8) | 2.99 (o) | ||||||
| 5 | 0.71 (brd, 11.3) | 0.71 (brd, 11.6) | 0.71 (brd, 11.6) | 0.69 (brd, 11.5) | 0.71 (o) | 0.68 (o) | ||||||
| 6 | α | 1.51 (o) | α | 1.49 (o) | α | 1.49 (o) | α | 1.47 (o) | α | 1.48 (o) | α | 1.47 (o) |
| β | 1.35 (o) | β | 1.33 (o) | β | 1.32 (o) | β | 1.33 (brt, 11.5) | β | 1.32 (o) | β | 1.29 (o) | |
| 7 | α | 1.48 (o) | α | 1.47 (o) | α | 1.47 (o) | α | 1.47 (o) | α | 1.42 (o) | α | 1.37 (o) |
| β | 1.24 (o) | β | 1.25 (o) | β | 1.26 (o) | β | 1.26 (o) | β | 1.23 (o) | β | 1.24 (o) | |
| 9 | 1.47 (o) | 1.45 (o) | 1.45 (o) | 1.49 (o) | 1.43 (o) | 1.46 (o) | ||||||
| 11 | 1.81 (brs) | 1.78 (o) | 1.78 (o) | 1.78 (o) | 1.74 (o) | 1.78 (o) | ||||||
| 12 | 5.21 (brs) | 5.14 (t-like, 3.2) | 5.14 (t-like, 3.4) | 5.10 (t-like, 3.3) | 5.20 (brs) | 5.14 (brs) | ||||||
| 15 | α | 1.12 (o) | α | 1.20 (dd, 12.4, 4.4) | α | 1.20 (dd, 13.0, 4.8) | α | 0.84 (o) | α | 1.26 (o) | α | 0.88 (o) |
| β | 1.47 (o) | β | 1.61 (t, 12.4) | β | 1.61 (t, 13.0) | β | 1.69 (o) | β | 1.83 (t, 12.9) | β | 1.71 (brt, 12.9) | |
| 16 | 4.42 (m) | 4.06 (dd, 12.4, 4.4) | 4.06 (m) | α | 1.72 (o) | 4.06 (t-like, 12.9) | α | 1.89 (o) | ||||
| β | 1.10 (o) | β | 1.61 (o) | |||||||||
| 16-OH | 4.38 (d, 6.2) | |||||||||||
| 18 | 2.50 (m) | 2.21 (dd, 13.3, 4.3) | 2.22 (dd, 12.0, 3.5) | 1.92 (dd, 13.1, 3.8) | 2.89 (dd, 13.4, 3.8) | 2.71 (dd, 13.0, 2.8) | ||||||
| 19 | α | 1.68 (t, 13.1) | α | 1.61 (t, 13.3) | α | 1.61 (o) | α | 1.66 (t, 13.1) | α | 1.63 (t, 13.4) | α | 1.62 (o) |
| β | 0.93 (o) | β | 0.96 (o) | β | 0.98 (o) | β | 0.96 (o) | β | 1.05 (o) | β | 1.05 (o) | |
| 21 | α | 1.56 (brt, 12.6) | α | 1.30 (o) | α | 1.35 (o) | α | 1.22 (o) | α | 1.42 (o) | α | 1.34 (o) |
| β | 1.68 (brd, 12.6) | β | 1.08 (brd, 9.1) | β | 1.08 (o) | β | 1.07 (o) | β | 1.13 (o) | β | 1.13 (brd, 8.2) | |
| 22 | 4.25 (dd, 12.6, 4.5) | α | 1.88 (brd, 13.5) | α | 1.88 (dt, 13.6, 3.0) | α | 1.44 (o) | α | 2.10 (brd, 12.2) | α | 1.58 (o) | |
| β | 1.37 (td, 13.5, 3.4) | β | 1.38 (td, 13.6, 3.4) | β | 1.25 (o) | β | 1.37 (o) | β | 1.43 (o) | |||
| 23 | 0.96 (s) | 0.96 (s) | 0.96 (s) | 0.95 (s) | 0.95 (s) | 0.96 (s) | ||||||
| 24 | 0.76 (s) | 0.75 (s) | 0.75 (s) | 0.74 (s) | 0.75 (s) | 0.74 (s) | ||||||
| 25 | 0.88 (s) | 0.87(s) | 0.87 (s) | 0.87 (s) | 0.84 (s) | 0.85 (s) | ||||||
| 26 | 0.90 (s) | 0.90 (s) | 0.90 (s) | 0.87 (s) | 0.69 (s) | 0.66 (s) | ||||||
| 27 | 1.16 (s) | 1.14 (s) | 1.14 (s) | 1.10 (s) | 1.13 (s) | 1.06 (s) | ||||||
| 28 | 3.33 (o) | 3.12 (o) | 3.13 (o) | 2.93 (o) | ||||||||
| 3.77 (m) | 3.51 (d, 10.2) | 3.51 (dd, 10.5, 4.0) | 3.29 (dd, 9.6, 4.8) | |||||||||
| 28-OH | 3.46 (o) | 4.28 (t, 4.8) | ||||||||||
| 29 | 0.88 (s) | 0.84 (s) | 0.85 (s) | 0.84 (s) | 0.90 (s) | 0.86 (s) | ||||||
| 30 | 0.87 (s) | 0.84 (s) | 0.85 (s) | 0.82 (s) | 0.92 (s) | 0.85 (s) | ||||||
| GlcA | GlcA | GlcA (Me) | GlcA (Me) | GlcA | GlcA | |||||||
| 1′ | 4.17 (d, 7.7) | 4.25 (d, 7.7) | 4.31 (d, 7.9) | 4.52 (d, 8.3) | 4.16 (d, 7.6) | 4.30 (d, 7.4) | ||||||
| 2′ | 3.08 (o) | 3.11 (o) | 3.12 (o) | 3.66 (t, 8.3) | 3.08 (q, 7.6) | 3.58 (o) | ||||||
| 2′-OH | 5.17 (d, 7.6) | |||||||||||
| 3′ | 3.36 (o) | 3.38 (t, 9.3) | 3.40 (t, 8.9) | 3.58 (t, 8.3) | 3.36 (o) | 3.50 (t, 8.9) | ||||||
| 4′ | 3.17 (o) | 3.29 (t, 9.3) | 3.32 (o) | 3.51 (td, 8.3, 6.2) | 3.19 (t, 7.6) | 3.25 (t, 8.9) | ||||||
| 4′-OH | 5.22 (d, 6.2) | |||||||||||
| 5′ | 3.31 (o) | 3.57 (d, 9.3) | 3.80 (d, 9.4) | 3.88 (d, 8.3) | 3.33 (d, 7.6) | 3.35 (o) | ||||||
| 6′-OCH3 | 3.65 (s) | 3.65 (s) | ||||||||||
| Rha-1 | Rha | Rha | Glc | Rha-1 | Glc-1 | |||||||
| 1′′ | 5.04 (brs) | 5.02 (brs) | 5.00 (d, 1.4) | 4.43 (d, 7.9) | 5.04 (brs) | 4.46 (d, 9.1) | ||||||
| 2′′ | 3.67 (o) | 3.69 (brs) | 3.69 (td, 4.0, 1.4) | 2.98 (o) | 3.67 (brs) | 2.95 (o) | ||||||
| 2′′-OH | 4.55 (d, 4.0) | 4.86 (d, 4.3) | ||||||||||
| 3′′ | 3.45 (o) | 3.47 (dd, 9.4, 3.1) | 3.46 (m) | 3.13 (t, 8.9) | 3.45 (dd, 9.8, 3.0) | 3.13 (o) | ||||||
| 3′′-OH | 5.05 (brs) | |||||||||||
| 4′′ | 3.15 (o) | 3.16 (t, 9.4) | 3.16 (td, 9.6, 4.7) | 2.96 (o) | 3.15 (t, 9.8) | 2.96 (o) | ||||||
| 4′′-OH | 4.55 (d, 4.7) | 4.96 (d, 4.5) | ||||||||||
| 5′′ | 3.96 (dq, 9.4, 6.1) | 3.88 (dq, 9.4, 6.2) | 3.85 (dq, 9.6, 6.2) | 3.03 (o) | 3.96 (9.8, 6.2) | 3.03 (o) | ||||||
| 6′′ | 1.05 (d, 6.1) | 1.06 (d, 6.2) | 1.06 (d, 6.2) | 3.39 (m) | 1.05 (d, 6.2) | 3.38 (o) | ||||||
| 3.66 (o) | 3.65 (dd, 9.4, 5.4) | |||||||||||
| 6′′-OH | 5.01 (o) | |||||||||||
| Glc | Rha | Rha-2 | Rha | |||||||||
| 1′′′ | 4.33 (d, 7.8) | 4.94 (brs) | 5.74 (d, 1.1) | 5.01 (brs) | ||||||||
| 2′′′ | 2.94 (td, 7.8, 3.3) | 3.75 (t-like, 3.3) | 3.55 (brs) | 3.75 (brs) | ||||||||
| 2′′′-OH | 5.26 (d, 3.3) | |||||||||||
| 3′′′ | 3.21 (o) | 3.45 (brd, 10.2) | 3.21 (o) | 3.44 (o) | ||||||||
| 4′′′ | 3.36 (o) | 3.18 (td, 10.2, 4.5) | 3.21 (o) | 3.16 (o) | ||||||||
| 4′′′-OH | 4.61 (d, 4.5) | |||||||||||
| 5′′′ | 3.11 (m) | 3.80 (dq, 10.2, 6.2) | 3.50 (o) | 3.96 (dq, 9.1, 6.0) | ||||||||
| 6′′′ | 3.45 (o) | 1.07 (d, 6.2) | 1.08 (d, 6.2) | 1.05 (d, 6.0) | ||||||||
| 3.57 (brd, 11.6) | ||||||||||||
| 6′′′-OH | ||||||||||||
| Rha-2 | Glc-2 | |||||||||||
| 1′′′′ | 4.69 (brs) | 5.32 (d, 7.9) | ||||||||||
| 2′′′′ | 3.59 (brs) | 3.40 (o) | ||||||||||
| 3′′′′ | 3.39 (o) | 3.45 (o) | ||||||||||
| 4′′′′ | 3.17 (o) | 3.16 (o) | ||||||||||
| 4′′′′-OH | 5.14 (o) | |||||||||||
| 5′′′′ | 3.83 (dq, 9.2, 6.1) | 3.18 (o) | ||||||||||
| 6′′′′ | 1.08 (d, 6.1) | 3.42 (o) | ||||||||||
| 3.59 (o) | ||||||||||||
| Xyl | ||||||||||||
| 1′′′′′ | 4.48 (d, 7.9) | |||||||||||
| 2′′′′′ | 2.93 (o) | |||||||||||
| 2′′′′′-OH | 5.30 (d, 4.0) | |||||||||||
| 3′′′′′ | 3.07 (td, 9.5, 3.9) | |||||||||||
| 3′′′′′-OH | 5.08 (d, 3.9) | |||||||||||
| 4′′′′′ | 3.18 (o) | |||||||||||
| 5′′′′′ | α | 2.98 (o) | ||||||||||
| β | 3.57 (o) | |||||||||||
Table 3.
13C-NMR spectroscopic data for compounds 1–5,7 (125 MHz in DMSO-d6)
| Position | 1 | 2 | 3 | 4 | 5 | 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38.3 | CH2 | 38.2 | CH2 | 38.1 | CH2 | 38.1 | CH2 | 38.2 | CH2 | 38.3 | CH2 |
| 2 | 25.4 | CH2 | 25.6 | CH2 | 25.6 | CH2 | 25.4 | CH2 | 25.4 | CH2 | 25.4 | CH2 |
| 3 | 88.1 | CH | 88.3 | CH | 88.4 | CH | 88.6 | CH | 88.1 | CH | 88.7 | CH |
| 4 | 38.7 | C | 38.7 | C | 38.7 | C | 38.6 | C | 38.7 | C | 38.7 | C |
| 5 | 54.8 | C | 54.8 | CH | 54.8 | CH | 54.8 | CH | 55.0 | CH | 55.2 | CH |
| 6 | 17.7 | CH2 | 17.8 | CH2 | 17.7 | CH2 | 17.8 | CH2 | 17.7 | CH2 | 17.9 | CH2 |
| 7 | 32.1 | CH2 | 32.2 | CH2 | 32.1 | CH2 | 32.1 | CH2 | 32.4 | CH2 | 32.3 | CH2 |
| 8 | 39.4 | C | 39.2 | C | 39.1 | C | 39.9 | C | 39.2 | C | 39.1 | C |
| 9 | 46.2 | CH | 46.2 | CH | 46.2 | CH | 47.0 | CH | 46.2 | CH | 47.1 | CH |
| 10 | 36.1 | C | 36.1 | C | 36.1 | C | 36.1 | C | 36.2 | C | 36.3 | C |
| 11 | 23.1 | CH2 | 23.0 | CH2 | 23.0 | CH2 | 23.0 | CH2 | 23.0 | CH2 | 23.0 | CH2 |
| 12 | 122.4 | CH | 121.4 | CH | 121.6 | CH | 121.5 | CH | 122.2 | CH | 121.7 | CH |
| 13 | 141.9 | C | 143.3 | C | 143.3 | C | 144.3 | C | 142.4 | C | 143.5 | C |
| 14 | 41.7 | C | 42.9 | C | 42.9 | C | 41.2 | C | 43.3 | C | 41.2 | C |
| 15 | 34.8 | CH2 | 35.2 | CH2 | 35.2 | CH2 | 25.1 | CH2 | 36.5 | CH2 | 27.6 | CH2 |
| 16 | 64.5 | CH | 64.5 | CH | 64.5 | CH | 21.7 | CH2 | 62.7 | CH | 22.1 | CH2 |
| 17 | 44.6 | C | 40.1 | C | 40.1 | C | 36.5 | C | 50.1 | C | 46.0 | C |
| 18 | 42.0 | CH | 42.6 | CH | 42.6 | CH | 41.8 | CH | 43.3 | CH | 40.8 | CH |
| 19 | 45.5 | CH2 | 46.4 | CH2 | 46.3 | CH2 | 46.3 | CH2 | 45.1 | CH2 | 45.5 | CH2 |
| 20 | 31.4 | C | 30.5 | C | 30.5 | C | 30.7 | C | 30.2 | C | 30.3 | C |
| 21 | 41.0 | CH2 | 33.5 | CH2 | 33.5 | CH2 | 33.8 | CH2 | 32.4 | CH2 | 33.3 | CH2 |
| 22 | 77.9 | CH | 23.8 | CH2 | 23.8 | CH2 | 30.9 | CH2 | 25.9 | CH2 | 31.5 | CH2 |
| 23 | 27.6 | CH3 | 27.5 | CH3 | 27.4 | CH3 | 27.5 | CH3 | 27.5 | CH3 | 27.6 | CH3 |
| 24 | 16.4 | CH3 | 16.3 | CH3 | 16.3 | CH3 | 16.1 | CH3 | 16.4 | CH3 | 16.2 | CH3 |
| 25 | 15.3 | CH3 | 15.3 | CH3 | 15.3 | CH3 | 15.3 | CH3 | 15.1 | CH3 | 15.2 | CH3 |
| 26 | 16.6 | CH3 | 16.5 | CH3 | 16.5 | CH3 | 16.4 | CH3 | 16.8 | CH3 | 16.7 | CH3 |
| 27 | 26.8 | CH3 | 26.6 | CH3 | 26.6 | CH3 | 25.6 | CH3 | 26.7 | CH3 | 25.6 | CH3 |
| 28 | 56.7 | CH2 | 65.2 | CH2 | 65.0 | CH2 | 67.4 | CH2 | 174.5 | C | 175.3 | C |
| 29 | 32.8 | CH3 | 33.1 | CH3 | 33.1 | CH3 | 33.1 | CH3 | 32.7 | CH3 | 32.8 | CH3 |
| 30 | 24.4 | CH3 | 23.7 | CH3 | 23.7 | CH3 | 23.5 | CH3 | 23.7 | CH3 | 23.4 | CH3 |
| GlcA | GlcA | GlcA (Me) | GlcA (Me) | GlcA | GlcA | |||||||
| 1′ | 105.1 | CH | 105.2 | CH | 105.2 | CH | 103.1 | CH | 105.2 | CH | 103.6 | CH |
| 2′ | 74.6 | CH | 74.3 | CH | 74.2 | CH | 77.0 | CH | 74.5 | CH | 77.7 | CH |
| 3′ | 80.3 | CH | 80.4 | CH | 80.1 | CH | 81.1 | CH | 80.3 | CH | 81.6 | CH |
| 4′ | 70.6 | CH | 70.2 | CH | 70.1 | CH | 69.9 | CH | 70.3 | CH | 70.9 | CH |
| 5′ | 74.2 | CH | 75.1 | CH | 75.2 | CH | 74.8 | CH | 74.1 | CH | 74.0 | CH |
| 6′ | 173.1 | C | 171.3 | C | 169.5 | C | 169.5 | C | 173.5 | C | 173.7 | C |
| 6′-OCH3 | 51.9 | CH3 | 51.9 | CH3 | ||||||||
| Rha-1 | Rha | Rha | Glc | Rha-1 | Glc-1 | |||||||
| 1′′ | 100.2 | CH | 100.5 | CH | 100.7 | CH | 101.6 | CH | 100.3 | CH | 101.6 | CH |
| 2′′ | 70.6 | CH | 70.6 | CH | 70.5 | CH | 70.4 | CH | 70.6* | CH | 70.4 | CH |
| 3′′ | 70.7 | CH | 70.6 | CH | 70.5 | CH | 76.6 | CH | 70.6 | CH | 76.6 | CH |
| 4′′ | 72.1 | CH | 72.1 | CH | 72.0 | CH | 74.2 | CH | 72.1 | CH | 74.6 | CH |
| 5′′ | 67.8 | CH | 68.0 | CH | 68.1 | CH | 77.0 | CH | 67.8 | CH | 77.0 | CH |
| 6′′ | 17.9 | CH3 | 17.9 | CH3 | 17.8 | CH3 | 61.4 | CH2 | 17.9 | CH3 | 61.4 | CH2 |
| Glc | Rha | Rha-2 | Rha | |||||||||
| 1′′′ | 103.3 | CH | 100.5 | CH | 93.3 | CH | 100.6 | CH | ||||
| 2′′′ | 74.1 | CH | 70.6 | CH | 69.6 | CH | 70.6 | CH | ||||
| 3′′′ | 75.3 | CH | 70.6 | CH | 71.5 | CH | 70.8 | CH | ||||
| 4′′′ | 76.6 | CH | 71.9 | CH | 71.3 | CH | 72.1 | CH | ||||
| 5′′′ | 75.5 | CH | 68.6 | CH | 70.7 | CH | 68.2 | CH | ||||
| 6′′′ | 60.1 | CH2 | 17.8 | CH3 | 17.9 | CH3 | 17.9 | CH3 | ||||
| Rha-2 | Glc-2 | |||||||||||
| 1′′′′ | 100.5 | CH | 91.9 | CH | ||||||||
| 2′′′′ | 70.7 | CH | 78.8 | CH | ||||||||
| 3′′′′ | 70.6 | CH | 76.9 | CH | ||||||||
| 4′′′′ | 71.9 | CH | 77.6 | CH | ||||||||
| 5′′′′ | 68.6 | CH | 69.2 | CH | ||||||||
| 6′′′′ | 17.7 | CH3 | 60.6 | CH2 | ||||||||
| Xyl | ||||||||||||
| 1′′′′ | 104.0 | CH | ||||||||||
| 2′′′′ | 74.2 | CH | ||||||||||
| 3′′′′ | 76.5 | CH | ||||||||||
| 4′′′′ | 69.5 | CH | ||||||||||
| 5′′′′ | 65.8 | CH2 | ||||||||||
Longispinoside A (2): colorless amorphous powder, [α]18D-52.0 (c 0.2 MeOH); IR (ATR) νmax: 3330, 2940, 1740, 1450, 1020 cm− 1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; positive HRFABMS m/z: 803.4546 [M + Na]+ (calcd for C42H68O13Na, 803.4548).
Longispinoside A methyl ester (3): colorless amorphous powder, [α]19D -117.3 (c 0.1 MeOH); IR (ATR) νmax: 3363, 2945, 1746, 1442, 1024 cm− 1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; positive HRFABMS m/z: 795.4900 [M + H]+ (calcd for C43H71O13, 795.4895).
Erythronoside A methyl ester (4): colorless amorphous powder, [α]19D-4.1 (c 0.1 MeOH); IR (ATR) νmax: 3330, 2970, 1752, 1040 cm− 1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; positive HRFABMS m/z: 941.5481 [M + H]+ (calcd for C43H71O13, 941.5474).
Cochalinoside C (5): colorless amorphous powder, [α]22D-28.8 (c 0.25 MeOH); IR (ATR) νmax: 3370, 2970, 2940, 1710, 1610, 1420, 1390, 1240, 1140, 1050 cm−1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; negative HRFABMS m/z: 939.4952 [M-H]− (calcd for C48H75O18, 939.4962).
Oleanolic acid 3-O-β-d-xylopyranosyl-(1 → 2)-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (6): colorless amorphous powder, [α]23D -4.9 (c 0.3 MeOH); IR (ATR) νmax: 3370, 2970, 2930, 1730, 1460, 1390, 1260, 1230, 1020 cm− 1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; negative HRFABMS m/z: 1071.5386 [M-H]− (calcd for C53H83O22, 1071.5309).
Stenoside A (7): colorless amorphous powder, [α]19D -8.6 (c 0.1 MeOH); IR (ATR) νmax: 3372, 2927, 1732, 1602, 1417, 1260, 1074, 1041 cm− 1; 1H- and 13C-NMR (DMSO-d6, 500 and 125 MHz), see Tables 2 and 3; positive HRFABMS m/z: 1273.5642979.4658 [M + K]+ (calcd for C59H94O27K, 1273.5620).
Alkaline hydrolysis of 8
Oleanolic acid 3-O-α-l-rhamnopyranosyl-(1 → 3)-β-d-glucuronopyranosyl-28-O-β-d-glucopyranoside (8) (10.1 mg) was dissolved in 4 mL of MeOH and hydrolyzed with 0.4 mL of 5% K2CO3 aq. solution under reflux for 4 h at 110 ℃. After the reaction, the mixture was subjected to DOWEX 50 W-X8 to remove potassium ions. After washing with plenty of MeOH, the eluent was evaporated to dryness. The residue was chromatographed over ODS C. C. using a stepwise gradient (80% MeOH → 100% MeOH) to afford hydrolysate (8a) (2.0 mg), the 28-O-deglycosylated saponin of 8.
Determination of sugar configuration
Sugar configuration was determined according to a previously reported procedure [25]. Saponins (1–7) (each 2 mg) were dissolved in 1 M HCl (0.4 mL) and then heated at 110 ℃ for 2 h. Following neutralization with 1 M NaOH, the reaction mixture was evaporated under reduced pressure to dryness, and then the residue was dissolved in pyridine (0.4 mL) containing l-cysteine methyl ester hydrochloride (2 mg) and heated at 60 ℃ for 1 h. O-Tolylisothiocyanate (2 μL) was then added to the mixture, and it was heated at 60 ℃ for 1 h. The reaction mixture was analyzed by HPLC using a system equipped with a PU-2089 pump, UV-2075 UV/VIS detector (Nippon Bunko Co.), and Senshu Pak PEGASIL 4.6 ϕ × 250-mm HPLC column (temp, 35 ℃; flow, 0.8 mL/min; elution buffer, MeCN–H2O 25:75 containing 50 mM H3PO4). The HPLC column was washed with MeOH after each injection. The reaction conditions for d, l-glucose, d-glucuronic acid, d, l-xylose, and l-rhamnose were the same as described above except for the mass, authentic sugar (5 mg), and l-cysteine methyl ester hydrochloride (5 mg), respectively. l-Glucuronic acid and d-rhamnose derivatives were synthesized from d-glucuronic acid, l-rhamnose (each 5 mg), and d-cysteine methyl ester hydrochloride (5 mg). These derivatives were used for determining the retention time. The retention times (min.) for the authentic sugar derivatives were d-glucose (23.56), l-glucose (21.89), d-glucuronic acid (24.37), l-glucuronic acid (derived from d-glucuronic acid and d-cysteine methyl ester, 23.38), d-rhamnose (derived from l-rhamnose and d-cysteine methylester, 18.36), l-rhamnose (40.19), d-xylose (22.17), and l-xylose (20.14). These retention times were compared with the retention times of each saponin (1–7) in the reaction mixtures. The peaks at 23.55, 24.25, and 40.18 min of the sugar derivatives from 1 coincided with the derivatives of d-glucose, d-glucuronic acid, and l-rhamnose, respectively. The other saponins (2–7) provided the same results as 1.
Th-T assay
The rate of Aβ aggregation was evaluated using a slightly modified thioflavin-T (Th-T) method described in a previous report [6]. The Th-T method was originally developed by Naiki and co-workers [48]. The rate of Aβ aggregation was calculated by comparing the fluorescence intensity of each sample with that of a control (25 μM of Aβ40 and DMSO containing no test sample). The aggregation rate (%) was calculated using the following formula: , (S, fluorescence of Th-T solution incubated with Aβ40 and sample; C, fluorescence of Th-T solution incubated with Aβ40 and DMSO; B, fluorescence of Th-T solution not incubated with Aβ40 and DMSO). Myricetin (Fujifilm-Wako) was used as a positive control at 25 μM as the final concentration, and that of test samples (1–4,7–8) was 100 μM.
BACE1 FRET assay
BACE1 assays were performed in 384-well black plates using a BACE1 FRET assay kit, Red (Thermo Fisher Scientific, USA). The assay was carried out according to the supplied manual, with some modifications. Samples were dissolved in the assay buffer (50 mM sodium acetate, pH 4.5) with DMSO (final concentrations were 10%). Next, 9 μL of test sample, 9 μL of BACE1 substrate (750 nM Rh-EVNLDAEFK-Quencher, in 50 mM ammonium bicarbonate), and 9 μL of BACE1 enzyme (1.0 U/mL) were mixed in the wells and incubated 60 min in the dark at room temperature. The fluorescence intensity of each well was measured using a SYNERGY HTX multimode reader (BioTek, USA), with excitation at 540 nm and emission at 590 nm. The inhibition ratio was calculated by the following formula: , where C represents the fluorescence of a control [enzyme, substrate, and assay buffer concentration with DMSO (final concentrations 10%)] after 60 min of incubation, C0 represents the initial fluorescence of a control [enzyme, substrate, and assay buffer concentration with DMSO (final concentrations 10%)], B represents the fluorescence of a control [substrate and assay buffer concentration with DMSO (final concentrations 10%)] after 60 min of incubation, B0 represents the initial fluorescence of a control [substrate and assay buffer concentration with DMSO (final concentrations were 10%)], S represents the fluorescence of the tested sample (enzyme, sample solution, and substrate) after 60 min of incubation, and S0 represents the initial fluorescence of the tested sample (enzyme, sample solution, and substrate). β-Secretase inhibitor IV (Merck, Germany) was used as a positive control at 100 μM. Isolated compounds (1–4,7–8) were tested for their BACE1 inhibitory activity at 100 μM (final concentration).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Ms. Tamami Koseki (MS spectra) and Ms. Shoko Yamada (NMR spectra), Instrumental analysis center in Meiji Pharmaceutical University for measuring the MS or NMR spectra.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parra F, Pérez-Nasser N, Lira R, et al. Population genetics and process of domestication of Stenocereus pruinosus (Cactaceae) in the Tehuacán Valley, México. J Arid Environ. 2008;72:1997–2010. doi: 10.1016/j.jaridenv.2008.06.007. [DOI] [Google Scholar]
- 2.Casas A, Caballero-Mellado J, Valiente-Banuet A. Use, management and domestication of columnar cacti in South-central Mexico: a historical perspective. J Ethnobiol. 1999;19:71–95. [Google Scholar]
- 3.Djerassi C, Liu LH, Farkas E, et al. Terpenoids. XI.1 investigation of nine cactus species. Isolation of two new triterpenes, stellatogenin and machaeric acid2. J Am Chem Soc. 1955;77:1200–1203. doi: 10.1021/ja01610a033. [DOI] [Google Scholar]
- 4.Kinoshita K, Akiba M, Saitoh M, et al. Antinociceptive effect of triterpenes from cacti. Pharm Biol. 1998;36:50–55. doi: 10.1076/phbi.36.1.50.4614. [DOI] [Google Scholar]
- 5.Fujihara K, Takahashi K, Koyama K, Kinoshita K. Triterpenoid saponins from Polaskia chichipe Backbg. and their inhibitory or promotional effects on the melanogenesis of B16 melanoma cells. J Nat Med. 2017 doi: 10.1007/s11418-017-1082-9. [DOI] [PubMed] [Google Scholar]
- 6.Fujihara K, Koike S, Ogasawara Y, et al. Inhibition of amyloid β aggregation and protective effect on SH-SY5Y cells by triterpenoid saponins from the cactus Polaskia chichipe. Bioorg Med Chem. 2017;25:3377–3383. doi: 10.1016/j.bmc.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Kakuta K, Baba M, Ito S, et al. New triterpenoid saponins from cacti and anti-type i allergy activity of saponins from cactus. Bioorg Med Chem Lett. 2012;22:4793–4800. doi: 10.1016/j.bmcl.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 8.Kakuta K, Koike T, Kinoshita K, et al. New triterpenoid saponins from Stenocereus eruca. Heterocycles. 2012;85:1377–1392. doi: 10.3987/COM-12-12458. [DOI] [Google Scholar]
- 9.Okazaki S, Kinoshita K, Ito S, et al. Triterpenoid saponins from Echinopsis macrogona (Cactaceae) Phytochemistry. 2011;72:136–146. doi: 10.1016/j.phytochem.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki S, Kinoshita K, Koyama K, et al. New triterpene saponins from Stenocereus eruca (Cactaceae) J Nat Med. 2007;61:24–29. doi: 10.1007/s11418-006-0014-x. [DOI] [Google Scholar]
- 11.Imai T, Okazaki S, Kinoshita K, et al. Triterpenoid saponins from cultural plants of Stenocereus stellatus (Cactaceae) J Nat Med. 2006;60:49–53. doi: 10.1007/s11418-005-0004-4. [DOI] [Google Scholar]
- 12.Kinoshita K, Koyama K, Takahashi K, et al. A new triterpenoid saponin from Isolatocereus dumortieri. J Nat Prod. 2000;63:701–703. doi: 10.1021/np9903907. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Zhang S, Yang Y, et al. Natural barrigenol–like triterpenoids: a comprehensive review of their contributions to medicinal chemistry. Phytochemistry. 2019;161:41–74. doi: 10.1016/j.phytochem.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari P, Sendri N, Devidas SB. Dammarane triterpenoid glycosides in Bacopa monnieri: a review on chemical diversity and bioactivity. Phytochemistry. 2020;172:112276. doi: 10.1016/j.phytochem.2020.112276. [DOI] [PubMed] [Google Scholar]
- 15.Tagawa K, Kunishita T, Maruyama K, et al. Alzheimer’s disease amyloid β-clipping enzyme (APP secretase): identification, purification, and characterization of the enzyme. Biochem Biophys Res Commun. 1991;177:377–387. doi: 10.1016/0006-291X(91)91994-N. [DOI] [PubMed] [Google Scholar]
- 16.Tischer E, Cordell B. β-amyloid precursor protein. J Biol Chem. 1996;271:21914–21919. doi: 10.1074/jbc.271.36.21914. [DOI] [PubMed] [Google Scholar]
- 17.Sandberg A, Luheshi LM, Söllvander S, et al. Stabilization of neurotoxic Alzheimer amyloid-β oligomers by protein engineering. Proc Natl Acad Sci USA. 2010;107:15595–15600. doi: 10.1073/pnas.1001740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthi M, Singh S, Pandey VP, Dwivedi UN. Alzheimer’s disease: an overview of amyloid beta dependent pathogenesis and its therapeutic implications along with in silico approaches emphasizing the role of natural products. J Neurol Sci. 2016;361:256–271. doi: 10.1016/j.jns.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Velander P, Wu L, Henderson F, et al. Natural product-based amyloid inhibitors HHS public access author manuscript. Biochem Pharmacol. 2017;139:40–55. doi: 10.1016/j.bcp.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshpande P, Gogia N, Singh A. Exploring the efficacy of natural products in alleviating Alzheimer’s disease. Neural Regen Res. 2019;14:1321–1329. doi: 10.4103/1673-5374.253509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le BuX, Rao PPN, Wang YJ. Anti-amyloid aggregation activity of natural compounds: implications for Alzheimer’s drug discovery. Mol Neurobiol. 2016;53:3565–3575. doi: 10.1007/s12035-015-9301-4. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Cui J, Yu Y, et al. Traditional Chinese nootropic medicine Radix Polygalae and its active constituent onjisaponin B reduce β-amyloid production and improve cognitive impairments. PLoS ONE. 2016;11:1–19. doi: 10.1371/journal.pone.0151147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan MI, Shin JH, Kim MY, et al. Green tea seed isolated theasaponin E1 ameliorates AD Promoting neurotoxic pathogenesis by attenuating Aβ peptide levels in SweAPP N2a cells. Molecules. 2020;25:2334. doi: 10.3390/molecules25102334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka T, Nakashima T, Ueda T, et al. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem Pharm Bull. 2007;55:899–901. doi: 10.1248/cpb.55.899. [DOI] [PubMed] [Google Scholar]
- 26.Bock K, Lundt I, Pedersen C. Assignment of anomeric structure to carbohydrates through geminal13C-H coupling constants. Tetrahedron Lett. 1973;14:1037–1040. doi: 10.1016/S0040-4039(01)95898-8. [DOI] [Google Scholar]
- 27.Jia Z, Koike K, Nikaido T. Major triterpenoid saponins from Saponaria officinalis. J Nat Prod. 1998;61:1368–1373. doi: 10.1021/np980167u. [DOI] [PubMed] [Google Scholar]
- 28.Borel C, Gupta MP, Hostettmann K. Molluscicidal saponins from Swartzia simplex. Phytochemistry. 1987;26:2685–2689. doi: 10.1016/S0031-9422(00)83572-4. [DOI] [Google Scholar]
- 29.Kunert O, Haslinger E, Schmid MG, et al. Three saponins, a steroid, and a flavanol glycoside from Achyrantes aspera. Monatshefte fur Chemie. 2000;131:195–204. doi: 10.1007/PL00010306. [DOI] [Google Scholar]
- 30.Melek FR, Miyase T, El-Gindi OD, et al. Saponins from Fangonia mollis. Phytochemistry. 1996;42:1405–1407. doi: 10.1016/0031-9422(95)00087-9. [DOI] [Google Scholar]
- 31.Wang X, Perumalsamy H, Kwon HW, et al. Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci Rep. 2015 doi: 10.1038/srep16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemertelidze EP, Skhirtladze AV, Ganzera M. Steroidal and Triterpenoid Glycosides from Roots of Digitalis ciliata. Chem Nat Compd. 2017;53:492–496. doi: 10.1007/s10600-017-2029-9. [DOI] [Google Scholar]
- 33.Li Q, Cao J, Yuan W, et al. New triterpene saponins from flowers of Impatiens balsamina L. and their anti-hepatic fibrosis activity. J Funct Foods. 2017;33:188–193. doi: 10.1016/j.jff.2017.03.033. [DOI] [Google Scholar]
- 34.Rengifo Carrillo M, Mitaine-Offer AC, Miyamoto T, et al. Oleanane-type glycosides from Pittosporum tenuifolium “variegatum” and P. tenuifolium “gold star”. Phytochemistry. 2017;140:166–173. doi: 10.1016/j.phytochem.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Ye W, Liu X, Zhang Q, et al. Antisweet saponins from Gymnema sylvestre. J Nat Prod. 2001;64:232–235. doi: 10.1021/np0004451. [DOI] [PubMed] [Google Scholar]
- 36.Rasoanaivo P, Multari G, Federici E, Galeffi C. Triterpenoid diglucoside of Enterospermum pruinosum. Phytochemistry. 1995;39:251–253. doi: 10.1016/0031-9422(94)00859-R. [DOI] [Google Scholar]
- 37.Harinantenaina L, Brodie PJ, Callmander MW, et al. Two antiproliferative saponins of Tarenna grevei from the Madagascar dry forest [1] Nat Prod Commun. 2012;7:705–708. doi: 10.1177/1934578x1200700604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaheen U, Ragab EA, Abdalla AN, Bader A. Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry. 2018;145:168–178. doi: 10.1016/j.phytochem.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Hariharan V, Rangaswami S. Structure of saponins A and B from the seeds of achyranthes aspera. Phytochemistry. 1970;9:409–414. doi: 10.1016/S0031-9422(00)85154-7. [DOI] [Google Scholar]
- 40.Ito A, Chai HB, Kardono LBS, et al. Saponins from the Bark of Nephelium maingayi. J Nat Prod. 2004;67:201–205. doi: 10.1021/np030389e. [DOI] [PubMed] [Google Scholar]
- 41.Ye WC, Zhang QW, Liu X, et al. Oleanane saponins from Gymnema sylvestre Wen-Cai. Phytochemistry. 2001;53:893–899. doi: 10.1021/np0004451. [DOI] [PubMed] [Google Scholar]
- 42.Wu HB, Liu TT, Wang WS, et al. Oleanane-type saponins from the roots of ligulariopsis shichuana and their α-glucosidase inhibitory activities. Molecules. 2017;22:4–11. doi: 10.3390/molecules22111981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinjo J, Suyama K, Nohara T. Triterpenoidal saponins from dumasia truncata. Phytochemistry. 1995;40:1765–1767. doi: 10.1016/0031-9422(95)00545-I. [DOI] [PubMed] [Google Scholar]
- 44.Voutquenne L, Guinot P, Froissard C, et al. Haemolytic acylated triterpenoid saponins from Harpullia austro-caledonica. Phytochemistry. 2005;66:825–835. doi: 10.1016/j.phytochem.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Zebiri I, Haddad M, Duca L, et al. Zebiriosides A-L, oleanane saponins from the roots of Dendrobangia boliviana. Phytochemistry. 2016;130:262–272. doi: 10.1016/j.phytochem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Dizes C, Gerald F, Lavaud C, et al. Harpuloside a triterpenoid saponin from harpullia ramiflora. Phytochemistry. 1998;48:1229–1232. doi: 10.1016/S0031-9422(98)00166-6. [DOI] [Google Scholar]
- 47.Stevenson PC, Nyirenda SP, Veitch NC. Highly glycosylated flavonoids from the pods of Bobgunnia madagascariensis. Tetrahedron Lett. 2010;51:4727–4730. doi: 10.1016/j.tetlet.2010.07.013. [DOI] [Google Scholar]
- 48.Naiki H, Gejyo F. [20] kinetic analysis of amyloid fibril formarion. Methods Enzymol. 1999;309:305–318. doi: 10.1016/S0076-6879(99)09022-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.