Abstract
Cognitive impairments are pervasive and disabling features of schizophrenia. Targeted cognitive training (TCT) is a “bottom-up” cognitive remediation intervention with efficacy for neurocognitive outcomes in schizophrenia, yet individual responses are variable. Gamma oscillatory measures are leading candidate biomarkers in the development of biologically informed pro-cognitive therapeutics. Forty-two schizophrenia patients were recruited from a long-term residential treatment facility. Participants were randomized to receive either 1 h of cognitive training (TCT, n = 21) or computer games (TAU, n = 21). All participants received standard-of-care treatment; the TCT group additionally completed 30 h of cognitive training. The auditory steady-state response paradigm was used to elicit gamma oscillatory power and synchrony during electroencephalogram recordings. Detailed clinical and cognitive assessments were collected at baseline and after completion of the study. Baseline gamma power predicted cognitive gains after a full course of TCT (MCCB, R2 = 0.31). A change in gamma power after 1-h TCT exposure predicted improvement in both positive (SAPS, R2 = 0.40) and negative (SANS, R2 = 0.30) symptoms. These relationships were not observed in the TAU group (MCCB, SAPS, and SANS, all R2 < 0.06). The results indicate that the capacity to support gamma oscillations, as well as the plasticity of the underlying ASSR circuitry after acute exposure to 1 h of TCT, reflect neural mechanisms underlying the efficacy of TCT, and may be used to predict individualized treatment outcomes. These findings suggest that gamma oscillatory biomarkers applied within the context of experimental medicine designs can be used to personalize individual treatment options for pro-cognitive interventions in patients with schizophrenia.
Subject terms: Predictive markers, Schizophrenia
Introduction
Disruptions in neural oscillatory dynamics are thought to underlie the perceptual and cognitive impairment associated with schizophrenia (SZ)1,2. Gamma oscillations (30–80 Hz) have received much interest in preclinical and translational studies of SZ, given their role in local and interregional information flow critical for cognition and perception3,4. Gamma-band abnormalities in SZ are thought to arise from a disturbance in the activity of fast-spiking, parvalbumin-positive interneurons, and the consequent effects on the dynamic balance of excitation and inhibition in cortical microcircuits5. The resulting “mesoscale” abnormalities in gamma-band oscillations and synchrony have been proposed as pathophysiologic mechanisms underlying the cognitive and clinical symptoms of SZ1,6. Clinical studies in SZ patients have corroborated preclinical findings and have linked gamma-band abnormalities with disturbances in low-level sensory and perceptual processes7–10, psychopathological domains (e.g., hallucinations, disorganization, and thought disorder)9–12, and higher-order neurocognitive functions (e.g., working memory, executive function, verbal learning, and memory)13–15.
While evidence of aberrant gamma oscillations and synchrony in SZ has been documented extensively across a broad range of experimental paradigms, including task-based and resting-state conditions12,15–18, the heterogeneity of gamma-band abnormalities seen across experimental paradigms has limited the translation of these findings into clinical settings. From this perspective, the 40-Hz auditory steady-state response (ASSR) paradigm has emerged as a leading candidate biomarker in clinical and translational neuroscience, given its ability to provide robust measures of the brain’s capacity to support gamma oscillations under-optimized, stimulus-driven conditions19–21. In the ASSR paradigm, stimulus trains of amplitude-modulated tones or clicks presented at a rate of 40-Hz-drive oscillatory power and synchrony across a distributed thalamocortical network22–25. ASSR deficits in gamma oscillatory power and synchrony are well characterized in SZ16,17,19 and have been linked to pathophysiologic and phenomenological dimensions of psychotic disorders12,22,26–30 and other neuropsychiatric conditions.
Auditory-based targeted cognitive training (TCT) is a “bottom-up” cognitive remediation intervention designed to improve cognitive function by stimulating low-level perceptual networks presumed to mediate higher-order cognition, e.g., verbal memory and executive function. TCT has demonstrated efficacy in SZ and psychosis-spectrum disorders31–34 with enduring benefits in cognitive and functional outcomes32,35. Recent meta-analysis confirms the efficacy of TCT and other related cognitive remediation interventions on cognitive, clinical, and functional outcomes36–38. Despite the abundance of evidence demonstrating the feasibility, tolerability, and efficacy of TCT and other cognitive remediation interventions in psychotic disorders, the adoption of TCT in community settings remains stagnant (cf. Thomas et al.33). The variability of patient responses to TCT remains a significant barrier to the broader implementation of TCT and other pro-cognitive interventions in broader community settings. While TCT is efficacious at the group level, individual responses to TCT are variable with only subsets of patients showing clinically meaningful cognitive gains.
Electroencephalographic (EEG) biomarkers of early auditory information processing (EAIP) are promising tools in experimental medicine, which may help parse the heterogeneity of responses to TCT and other pro-cognitive interventions39,40. We have demonstrated the utility of EAIP biomarkers, including ASSR, as sensitive measures of target engagement and therapeutic sensitivity in neural mechanisms relevant for learning, memory, and cognitive rehabilitation40–43. Findings from these experimental medicine studies suggest that assessments of baseline (e.g., event-related activity recorded prior to the initiation of TCT or other pro-cognitive interventions) and the capacity for change or “malleability” of the underlying neural circuitry in response to acute or limited “doses” of TCT may predict future TCT-related outcomes44,45. We recently completed a “proof-of-concept” experimental medicine trial assessing the utility of EAIP biomarkers as predictors of cognitive and clinical response to TCT33,46. In Thomas et al.33, we report the main clinical outcomes from this trial, including improvement in verbal learning and reduction in positive symptoms in the TCT group relative to the treatment as the usual group. Consistent with the predictions of our experimental medicine framework, we found that both baseline and the magnitude of change in our primary EAIP biomarkers after 1 h of TCT or “EAIP malleability,” predicted improvement in cognition after a full (30-h) course of TCT in this cohort of chronic SZ patients46,47.
Despite the widespread use of ASSR in the clinical and translational literature, its use as a predictive biomarker of pro-cognitive response to TCT or other cognitive remediation interventions has not been evaluated. Here we present findings from our analysis of ASSR measures in a cohort of patients with chronic psychotic disorders previously characterized as part of our “proof-of-concept” experimental medicine study assessing the utility of EAIP biomarkers as predictors of TCT-related cognitive and clinical outcomes33,46,47. Given the capacity of ASSR measures to detect plasticity in cortical mechanisms of interest to cognitive remediation, we hypothesized that EEG measures of gamma oscillatory power and synchrony collected at the outset of treatment (e.g., “baseline” and “malleability” after 1 h of TCT) would predict pro-cognitive and clinical responses to TCT in refractory patients with chronic psychotic disorders.
Materials and methods
Participants and study design
Forty-two patients with treatment-refractory SZ or schizoaffective disorder were recruited from a community-based inpatient-treatment program following an extended acute-care hospitalization. Sample-size calculations were made to ensure adequate power to detect effect sizes (d ≈ 0.8) previously reported in Fisher et al.31 for primary outcomes in this trial. Details of the recruitment and ascertainment procedures in this cohort were previously reported33,46. Participants were enrolled in the study after they were determined to be clinically stable by the treatment team. All participants were under public conservatorship by the San Diego or Los Angeles Counties. The diagnosis was verified using an abbreviated version of the Structured Clinical Interview for DSM-IV-TR48. Exclusion criteria included premorbid intellectual disability (e.g., wide range achievement test (WRAT) reading subtest below 70), inability to provide informed consent, limited English proficiency, history of significant neurological illness or head injury, severe systemic illness, or current mania. All participants were engaged in rehabilitative programming as part of their standard of care or “treatment as usual” (TAU), including medication management, individual and group therapy, and participation in structured social activities. After initial screening and assessments, enrolled participants were randomized to receive either 1 h of cognitive training (TCT, n = 21) or 1 h of computer games (TAU, n = 21) using a parallel design with stratified random assignment by sex, age, and ethnicity. Participants randomized to TAU continued to receive standard-of-care treatment. The TCT group additionally completed 1 h of TCT, ~3–5 days per week, for 30 h. Neurophysiologic recordings were obtained at baseline (T0) and after participants underwent 1 h (T1) of TCT or 1 h of computer games (TAU). Structured clinical and cognitive assessments were collected at baseline (T0) and at the end of the study (T2, approximately 10–12 weeks later). All subjects provided written informed consent. The Institutional Review Board of the University of California, San Diego, approved all experimental procedures (IRB#130874).
Targeted cognitive training
TCT was administered on individual laptop computers with headphones. We previously reported full details of the cognitive training exercises applied in TCT33,46. Briefly, six training exercises by BrainHQ (Posit Science Corporation, San Francisco, CA) were administered. Training exercises were designed to engage neuroplasticity mechanisms in auditory networks of auditory perception and processing speed (Sound Sweeps, Fine Tuning) and auditory memory (Syllable Stacks, Memory Grid, To-Do List Training, and Rhythm Recall). Exercises applied an n-up/m-down algorithm to participant responses to estimate thresholds, ensuring that participants were engaging in the exercises and were continuously challenged at an appropriate level (~80% accuracy) as their abilities improved.
Clinical and cognitive assessments
Clinical and cognitive outcomes in the TCT vs. TAU groups from this cohort were previously reported33. Participants were assessed on measures of cognition and clinical symptoms at baseline (T0) and at the end of treatment (T2). Cognition was assessed using age- and gender-corrected T scores from the MATRICS consensus cognitive battery (MCCB)49. MCCB measures seven cognitive domains of particular relevance to SZ: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, and reasoning, and problem-solving. The battery was designed for use as a repeated measure in clinical trials of pro-cognitive therapeutics. The MCCB scoring program yields individual domain scores (speed of processing, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving) and a composite score. Clinical symptoms were assessed with the scale for the assessment of positive symptoms (SAPS)50 and negative symptoms (SANS)51. SAPS comprises individual symptom ratings that are divided among four subdomains (i.e., hallucinations, delusions, bizarre behavior, and formal thought disorder), each subdomain is also given a separate global symptom-severity score. SANS symptom ratings are divided among five subdomains (i.e., affective flattening/blunting, alogia, avolition–apathy, anhedonia–asociality, and attention), and are also given separate global symptom-severity ratings. Individual subdomains, and the composite and global scores for both SAPS and SANS, were calculated52. Motivation and pleasure (MAP) and expressive (EXP) dimensions of negative symptoms53 were quantified by averaging SANS subscales; MAP is the mean of Anhedonia and Apathy subscales, and EXP is the mean of Affective blunting and Alogia subscales.
EEG paradigm, acquisition, and processing
The ASSR paradigm utilized 500-ms trains of 85-dB clicks (1-ms duration each) presented at a frequency of 40 Hz. A total number of 250 click trains were played with an intertrain interval of 0.5 s. The auditory stimuli were delivered through insert earphones. Participants were instructed to ignore auditory stimuli while watching a silent movie. ASSR recordings lasted approximately 4 min. EEG data were continuously recorded with a 64-channel BioSemi ActiveTwo system at a sampling rate of 8192 Hz. Data processing were performed offline using a precoded pipeline and applied to all subjects in an automated manner on BrainVision Analyzer as per established methods17,43. Briefly, data were downsampled to 1000 Hz. A robust average reference was applied to the EEG recordings, and eye-movement artifacts were corrected using independent component analysis. Continuous data were segmented relative to the onset of the stimuli (−500 to 500 ms), and each epoch was baseline-corrected relative to the 100-ms prestimulus interval. Epochs containing ±70 μV were automatically rejected. Gamma-evoked power (γEP) and phase locking (γPL) were averaged across frontocentral electrodes (C1, C2, Cz, F1, F2, Fz, FC1, FC2, and FCz) to create a composite frontocentral measure (FC Comp). γEP and γPL were calculated on wavelet coefficients obtained from the Morlet wavelet transformation of the segmented data (representing the 1–50-Hz frequency range, with a total number of 50 frequency layers using a Morlet parameter of 10). γPL quantifies the consistency of the oscillatory phase across individual trials, ranging from 0 (purely non-phase-locked activity) to 1 (fully phase-locked activity). γPL was estimated by averaging across the 36–44-Hz frequency layers. Mean values obtained for each of the six 100-ms time windows from −100 to 500 ms relative to stimulus onset and the mean of the entire 500-ms post-stimulus interval were used in separate analyses described below.
Statistical analyses
We first assessed whether 1 h of TCT or computer games had any temporal effects on ASSR activity (e.g., early vs. late) similar to what was seen in our previous studies17,43. Linear mixed-effect models were used to analyze the effects of 1 h of TCT on gamma oscillatory activity (γEP and γPL). Six 100-ms time windows from the –100- to 500-ms ASSR segment were used as dependent variables and were regressed onto contrast-coded treatment (TAU and TCT), time (T0 and T1), and interaction terms modeled as fixed effects. All models included centered fixed effects and subject intercepts as random effects. In contrast to our expectations, analysis with linear mixed-effect models did not reveal any significant treatment × time interactions on ASSR activity; hence, subsequent analyses were conducted using the mean response from the 1–500-msec post-stimulus window.
Analyses were focused on a priori-defined spatiotemporal ASSR measure (e.g., the 1–500-msec post-stimulus window derived from only the frontocentral composite electrode) and a change in key clinical and cognitive outcomes to reduce the number of statistical comparisons and the likelihood of type I error. We used linear regression models to assess the relationship between gamma oscillatory activity and TCT outcomes, where primary outcome variables were regressed onto ASSR measures of gamma oscillatory activity (e.g., baseline or “malleability” indices of γEP and γPL), treatment (TAU and TCT) group, and their interaction (e.g., ASSR predictor × treatment interaction). Primary outcome variables were: (1) a change in neurocognitive composite T scores (MCCB-NC), change in (2) SAPS, and (3) SANS composite measures. A change in primary outcome variables was calculated as a “T2 minus T0” difference score. “Malleability” of ASSR predictors was calculated as a “T1 minus T0” difference score over the 1–500-ms post-stimulus interval. Rank-based inverse normal transformations were applied to dependent variables when fitted-model residuals were non-normally distributed54. Only interaction terms were examined for significance and are reported as delta R2 (ΔR2). Delta R2 represents the improvement in R2 gained by the introduction of an interaction term in a regression model (i.e., the R2 for the model with the main effects of ASSR predictor, treatment, and their interaction minus the R2 for the model with the main effects of ASSR predictor and treatment alone). Significant ASSR × treatment interactions were decomposed via post hoc analysis of secondary outcome measures (e.g., subscales/subdomains of primary outcomes) and by follow-up regressions for each treatment group, separately. In other words, a significant ASSR predictor × treatment interaction on MCCB-NC would be followed up by (1) analyzing ASSR predictor × treatment interactions on MCCB subscales (e.g., attention/vigilance, working memory, etc.) and (2) by assessing the effect size (i.e., R2) of the ASSR predictor on primary and secondary outcome measures in the treatment groups, separately. Statistical analyses were implemented using the “lme4” package55 and built-in functions in R.
Results
Demographic and clinical features
The demographic and clinical characteristics of this sample were previously reported33,46 and are shown in Table 1. There were no significant differences in any demographic (e.g., age, age of onset, sex, education, and WRAT) or clinical variable (e.g., SANS, SAPS, chlorpromazine equivalents (CPZ), and global assessment of function (GAF)) between the treatment groups.
Table 1.
Baseline demographic and clinical characteristics.
| TAU (n = 21) mean (sem) | TCT (n = 21) mean (sem) | p | |
|---|---|---|---|
| Age (years) | 33.2 (2.4) | 35.6 (2.6) | n.s. |
| Sex (F:M) | 11:10 | 11:10 | n.s. |
| Age of onset (years) | 21.0 (1.1) | 18.6 (1.1) | n.s. |
| Education (years) | 11.7 (0.5) | 12.4 (0.4) | n.s. |
| WRAT | 92.4 (3.0) | 91.4 (3.0) | n.s. |
| Scale for assessment of positive symptoms | |||
| Hallucinations | 4.2 (1.5) | 3.7 (1.1) | n.s. |
| Delusions | 4.0 (1.3) | 7.2 (1.8) | n.s. |
| Bizarre behavior | 0.8 (0.3) | 0.5 (0.2) | n.s. |
| Thought disorder | 5.2 (1.5) | 4.5 (1.4) | n.s. |
| Composite | 14.2 (3.8) | 15.8 (3.3) | n.s. |
| Global | 4.8 (1.1) | 5.0 (0.9) | n.s. |
| Scale for assessment of negative symptoms | |||
| Affective blunting | 7.4 (1.4) | 8.4 (1.6) | n.s. |
| Alogia | 2.5 (0.7) | 2.4 (0.5) | n.s. |
| Apathy | 2.0 (0.5) | 2.0 (0.5) | n.s. |
| Anhedonia | 3.7 (0.7) | 3.4 (0.6) | n.s. |
| Attention | 3.8 (0.7) | 2.4 (0.5) | n.s. |
| Composite | 19.3 (2.7) | 18.7 (2.7) | n.s. |
| Global | 6.9 (0.9) | 7.5 (1.0) | n.s. |
| Motivation and pleasure | 2.8 (0.4) | 2.7 (0.5) | n.s. |
| Expressive symptoms | 5.0 (0.9) | 5.4 (0.9) | n.s. |
| GAF | 30.5 (1.1) | 31.1 (1.5) | n.s. |
| Chlorpromazine equivalents | 946.7 (176.8) | 1154.8 (245.2) | n.s. |
Demographics and clinical symptoms.
ASSR biomarkers do not significantly change after 1 h of TCT or computer games
Linear mixed-effect analysis did not find any significant treatment × time interactions on γEP (β = 0.01, SE = 0.03, t = 0.47, p > 0.5) or γPL (β = –0.02, SE = 0.04, t = –0.69, and p = 0.50) at the group level.
Baseline γEP predicts cognitive improvement
Linear regression analysis revealed a significant interaction between baseline γEP and improvement in overall neurocognitive functioning in the TCT group relative to the TAU group (MCCB-NC; ΔR2 = 0.16, β = 0.40, SE = 0.15, and p = 0.012, Fig. 1). Secondary analyses on MCCB subscales revealed that this omnibus cognition effect was likely driven by significant interactions between baseline γEP and treatment group for the attention and vigilance (ΔR2 = 0.18, β = 0.38, SE = 0.15, and p = 0.015) and working memory (ΔR2 = 0.17, β = 0.31, SE = 0.15, and p = 0.050) subdomains. No significant interactions were found between baseline γEP and treatment on either clinical symptom outcomes or baseline γPL and treatment on any primary cognitive or clinical outcome variables. Table 2 summarizes the results of ASSR × treatment interactions and follow-up analyses of primary outcomes. Supplementary Table 1 summarizes the results of ASSR × treatment interactions and follow-up analyses of secondary outcomes.
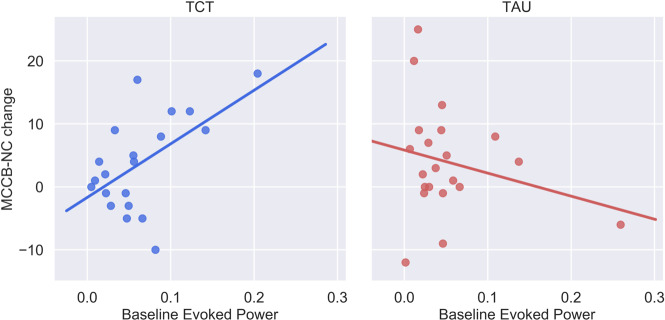
Fig. 1. Baseline-evoked gamma power predicts overall TCT-related cognitive improvement.
The significant interaction between baseline-evoked power and treatment on change in MCCB-NC (ΔR2 = 0.16, p = 0.01), revealed that the effect was driven by the TCT group (R2 = 0.31), but not the TAU group (R2 = 0.06).
Table 2.
Relationships between gamma oscillatory biomarkers and TCT-related change in cognitive and clinical outcomes.
| TCT R2 | TAU R2 | ASSR × treatment interaction | |||||
|---|---|---|---|---|---|---|---|
| ΔR2 | β | SE | t | p | |||
| Baseline gamma power | |||||||
| Δ MCCB-NC | 0.31 | 0.06 | 0.16 | 0.40 | 0.15 | 2.64 | 0.012 |
| Δ SANS composite | 0.03 | 0.01 | 0.02 | –0.15 | 0.17 | –0.87 | 0.390 |
| Δ SAPS composite | 0.16 | 0.00 | 0.00 | –0.06 | 0.16 | –0.39 | 0.699 |
| Baseline gamma phase-locking | |||||||
| Δ MCCB-NC | 0.15 | 0.05 | 0.08 | 0.30 | 0.16 | 1.83 | 0.075 |
| Δ SANS composite | 0.02 | 0.01 | 0.01 | –0.13 | 0.18 | –0.73 | 0.473 |
| Δ SAPS composite | 0.09 | 0.02 | 0.00 | –0.06 | 0.16 | –0.38 | 0.703 |
| 1-h Δ Gamma power | |||||||
| Δ MCCB-NC | 0.19 | 0.01 | 0.07 | –0.26 | 0.16 | –1.62 | 0.115 |
| Δ SANS composite | 0.30 | 0.04 | 0.15 | –0.39 | 0.15 | –2.53 | 0.016 |
| Δ SAPS composite | 0.40 | 0.04 | 0.14 | 0.38 | 0.15 | 2.59 | 0.014 |
| 1-h Δ Gamma phase locking | |||||||
| Δ MCCB-NC | 0.00 | 0.06 | 0.02 | 0.14 | 0.17 | 0.86 | 0.396 |
| Δ SANS composite | 0.20 | 0.15 | 0.00 | –0.03 | 0.16 | –0.16 | 0.871 |
| Δ SAPS composite | 0.20 | 0.24 | 0.24 | 0.50 | 0.14 | 3.52 | 0.001 |
The primary analysis focused on elucidating significant ASSR biomarker × treatment interactions on TCT-related change in cognitive and clinical outcomes using linear regressions. To further clarify any significant interactions, R2 values are also provided for linear regressions run in TCT and TAU groups, separately.
“Malleability” indices predict improvement in clinical symptoms
The magnitude of change in gamma power after 1 h of TCT (ΔγEP) significantly predicted clinical improvement in overall positive (SAPS composite: ΔR2 = 0.14, β = 0.38, SE = 0.15, and p = 0.014) and negative (SANS composite: ΔR2 = 0.19, β = –0.38, SE = 0.15, and p = 0.016, Fig. 2) symptoms at the end of the trial in the TCT group relative to TAU. To follow up on the primary findings on overall psychopathology, secondary analyses of individual SAPS and SANS subscales were conducted. This omnibus effect of ΔγEP and SAPS was likely driven by changes in Hallucinations (ΔR2 = 0.14, β = 0.36, SE = 0.14, and p = 0.017) and global symptom severity (ΔR2 = 0.19, β = 0.44, SE = 0.13, and p = 0.002, Fig. 3). ΔγEP also predicted a change in individual SANS subdomains, including anhedonia (ΔR2 = 0.10, β = –0.32, SE = 0.16, and p = 0.05) and EXP-negative symptoms (i.e., the mean of affective blunting and alogia subscales53) (ΔR2 = 0.11, β = –0.34, SE = 0.16, and p = 0.038). ΔγEP did not interact significantly with any primary cognitive outcomes.
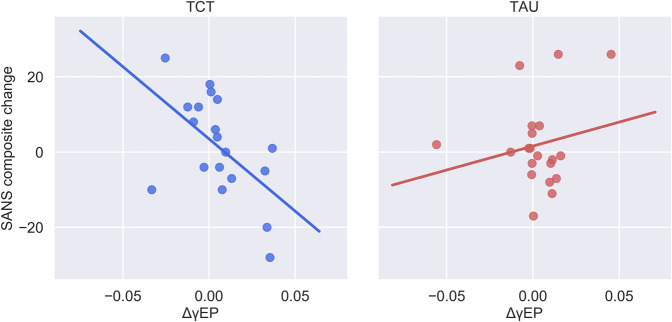
Fig. 2. Malleability of gamma-evoked power after 1 h of TCT predicts overall improvement in negative symptoms.
Decomposing the significant ΔγEP × treatment interaction (ΔR2 = 0.15, p = 0.01) on change in SANS symptoms revealed that the effect was largely driven by the TCT group (R2 = 0.30), but not the TAU group (R2 = 0.04).
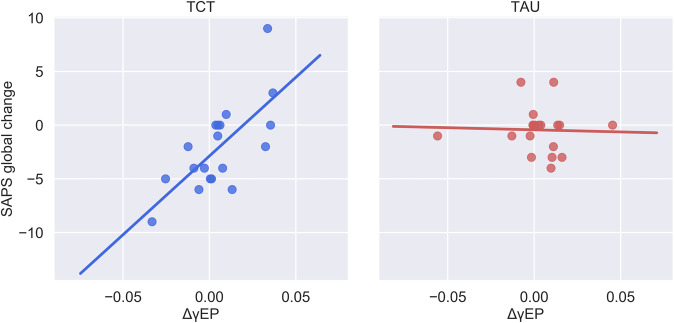
Fig. 3. Malleability of gamma-evoked power after 1 h of TCT predicts overall improvement in global positive symptom severity.
Decomposing the significant ΔγEP × treatment interaction on change in SAPS Global (ΔR2 = 0.10, p = 0.002) revealed that the effect was largely driven by the TCT group (R2 = 0.49) and was absent in the TAU group (R2 < 0.01).
The magnitude of change in gamma phase-locking after 1 h of TCT (ΔγPL) significantly predicted clinical improvement in overall positive symptoms (SAPS composite: ΔR2 = 0.24, β = 0.50, SE = 0.14, and p = 0.001) in the TCT group relative to TAU. ΔγPL was also found to predict changes in SAPS subscales, including a change in Hallucinations (ΔR2 = 0.10, β = 0.29, SE = 0.14, and p = 0.048), Delusions (ΔR2 =0.11, β = 0.34, SE = 0.14, and p = 0.019), Thought Disorder (ΔR2 = 0.15, β = 0.40, SE = 0.16, and p = 0.017), and Global Symptom Severity (ΔR2 = 0.15, β = 0.39, SE = 0.14, and p = 0.010). ΔγPL did not interact significantly with primary cognitive or negative-symptom outcome measures.
Discussion
Developing effective treatments for the hallmark cognitive symptoms of SZ continues to be one of the more daunting challenges in psychiatric neuroscience. While antipsychotic medications provide modest relief of the more dramatic, but “secondary” symptoms (e.g., hallucinations and delusions) of SZ56, the core cognitive symptoms persist and cause profound disability and loss of functioning for many patients30,57,58. Auditory-based TCT is a promising approach to cognitive remediation, although considerable interindividual variability in treatment response has limited the translation of TCT from academic laboratories to real-world community settings. The present study assessed whether ASSR measures of gamma power and synchrony collected at the outset of treatment would predict the cognitive and clinical benefits of TCT in a cohort of SZ patients receiving long-term care in a community-based residential treatment facility. Specifically, the findings suggest that baseline γEP is a significant predictor of TCT-induced cognitive enhancement and that the “malleability” or plasticity of gamma power and synchronization immediately following acute exposure to the initial 1-h “dose” of TCT robustly predicts changes in psychopathological dimensions.
Currently, there is a dearth of reliable biomarkers that can predict future cognitive training-related gains either at baseline or early in the treatment course47,59,60. Hence, a significant proportion of patients who are treated with TCT do not demonstrate any meaningful improvement in cognitive functioning or clinical symptoms. The relationship between baseline γEP and improvement in global cognition after 30 h of TCT suggests that a brief (i.e., 4–5 min) ASSR assessment can predict the extent of pro-cognitive gains from a full (30 h) course of TCT. While these data are insufficient for providing a mechanistic understanding of the changes in brain function attributable to TCT, this particular form of cognitive remediation is designed to leverage intact neuroplasticity mechanisms in “spared” neural circuitry rather than promoting compensatory strategies for working around their cognitive impairments. Baseline gamma-evoked power may be an index of the brain’s overall “adaptive integrity“ in these lower-level perceptual networks as the patients with the largest ASSR responses had more robust cognitive gains in response to TCT39,44,61.
Previous studies support the notion that auditory-based TCT mediates its therapeutic effects on neurocognition by inducing neuroplastic changes in distributed frontal–temporal and thalamocortical circuits25,62–65. Indeed, training-induced changes in oscillatory activity and event-related potentials have been shown to correlate with TCT-induced cognitive enhancements62,65. These training-related effects on gamma activity (i.e., the pre–post difference scores after 30–50 hours of TCT) have been shown to correlate with improvements in executive function and other cognitive domains62,65. Similar patterns of adaptive neuroplasticity have been reported for EAIP biomarkers in response to as little as 1 h of TCT and acute pharmacologic challenges41,42,66. Previous studies using mismatch negativity (MMN) provide empirical support for the utility of EAIP biomarkers in the prediction of TCT-related cognitive outcomes46,47. While baseline MMN measures alone do not appear to be particularly sensitive in detecting the pro-cognitive effects of TCT in this cohort of patients47,60,67, the “malleability” of MMN responses after 1 h of TCT were sensitive to the acute learning effects of TCT and response to a full (30-h) course of TCT46. It is conceivable that the neuroplasticity mechanisms indexed by ASSR and other EAIP biomarkers may be used synergistically to predict future benefits in the overall neurocognitive and clinical status and/or gains in specific neurocognitive and clinical domains.
Interestingly, though 1 h of TCT did not produce any statistically significant changes in evoked gamma power and synchrony at the group level in frontocentral electrodes, we found that changes after 1 h of training at the individual level explained significant amounts of variance in the change in symptom domains (see Table 2 and Supplemental Table 1). Specifically, ΔγEP explained ~30–50% of the variance of the change in multiple SAPS subdomains after a full course of TCT. Measures of ΔγPL also predicted a change in SAPS and subdomains of positive symptoms, but not as robustly as ΔγEP. Similarly, the malleability of evoked gamma power predicted an overall change in negative- symptom severity. Two independent meta-analyses of cognitive remediation trials have reported small effect sizes (g ≈0.1–0.3) on negative symptoms36,38, suggesting that subsets of patients may experience modest clinical improvement in this symptom domain. While TCT did not produce a significant improvement in negative symptoms at the group level in this cohort of patients33, these findings suggest that ASSR biomarkers may predict beneficial treatment response in terms of negative symptoms in subsets of patients who undergo TCT. These results extend our previous findings of EAIP malleability as predictors of TCT-related change in positive-symptom severity in this same cohort of patients46 to include ASSR-evoked gamma oscillatory biomarkers as robust predictors of clinical outcomes in response to TCT. These patterns of ASSR malleability predicting improvement in psychopathological dimensions are complex and should be interpreted cautiously. Further studies incorporating ASSR biomarkers in experimental medicine trials of TCT and other pro-cognitive interventions are necessary to replicate and extend the present findings.
Caveats and limitations
Findings from the current study should be considered in the context of several important limitations. First, the sample sizes of this “proof-of-concept” cognitive remediation trial were modest. While this sample was sufficiently powered to detect moderate-to-large effect sizes33, the overall sample size was not powered to identify other mediators or moderators of therapeutic gains, or to tease apart complex multivariate relationships in this data set. Although the large effect sizes of the findings in the TCT group warrant further investigation and replication, it is curious that ASSR biomarkers were not robust predictors of target engagement for computer games or general outcomes associated with TAU. This pattern of findings supports the interpretation that ASSR is a sensitive and specific biomarker of TCT response. Second, medication status was not experimentally controlled; patients were receiving complex medication regimens per “standard of care” community-based practices for treatment-refractory psychosis. While we cannot rule out the potential for medications to enhance or blunt gains from cognitive training, our previous studies from this sample suggest that the pro-cognitive benefits of TCT may offset the adverse cognitive effects of antipsychotic polypharmacy and anticholinergic burden68. Third, this study was conducted in patients with refractory psychotic disorders with well-established illness; therefore, the findings may not generalize to at-risk, early illness, or other clinical populations28,69. Fourth, no group-level differences were detected in ASSR measures after 1 h of TCT. While these results were unexpected, it is possible that the “malleability” of ASSR responses may not be detectable at the level of scalp electrodes. Scalp-level ASSR responses are generated by dynamic interactions from a distributed network of frontotemporal sources;25 it is conceivable that 1 h of TCT may induce plasticity in discrete sources and/or their dynamic connectivity patterns, which may not be readily apparent when collapsing across the full 1–500-ms response window at a frontocentral composite electrode. Fifth, even with the emphasis on distilled composite measures, relationships among ASSR measures and TCT-related outcomes are still complex. Future larger-scale clinical trials are needed to better characterize the neural substrates of ASSR biomarkers and the relationships with TCT-related pro-cognitive and clinical outcomes.
In this context, another limitation of relevance to clinical and translational studies incorporating high-density EEG (or other high-dimensional brain phenotypes) as predictive biomarkers of treatment outcomes in clinical trials is the need to rely upon relatively circumspect and conservative (e.g., frequentist) analytic approaches. Analyses in this proof-of-concept study were constrained to a few a priori predictors and outcomes in order to minimize the risk of type 1 errors. Despite this conservative statistical framework, multiple “signals” and potentially clinically relevant associations were detected, most of which were specific to the TCT group. It is possible (and likely) that while attempting to mitigate the risk of type 1 error, many associations between ASSR variables (e.g., at discrete time windows or other electrode sites) and clinically and cognitively relevant outcomes were missed (i.e., type 2 error). As high-dimensional neurobiological measures are increasingly applied for use as predictive biomarkers of therapeutic outcomes in clinical trials, novel statistical methods that allow for the detection of meaningful associations embedded within multidimensional clinical datasets that emphasize effect sizes and appropriately balance the risk of type 1 and type 2 errors are necessary. These methodological developments will be critical to the advancement of biologically informed treatments for SZ and other neuropsychiatric disorders.
Conclusions
To our knowledge, this is the first study to demonstrate the feasibility of ASSR biomarkers as clinically relevant predictors of cognitive and clinical outcomes in community settings. These findings underscore the utility of incorporating neurophysiologic biomarkers of gamma-evoked power and synchronization in the development of pro-cognitive therapeutics and their potential application in routine clinical practice. It is conceivable that the coupling of validated behavioral40,70–72 and neurophysiologic measures of target engagement46,47,59,60 may enhance the sensitivity and accuracy of predictive algorithms for TCT-related cognitive and clinical outcomes. These findings thus support future personalized medicine trials of pro-cognitive therapeutics, where individuals may be stratified to receive effective interventions based on their unique neurophysiologic profiles and their likelihood of response.
Supplementary information
Acknowledgements
This work was supported by the Sidney R Baer, Jr Research Foundation, the Brain and Behavior Research Foundation, the National Institute of Mental Health (MH059803, MH094320, and MH101072), the VISN-22 Mental Illness Research, Education, and Clinical Center (MIRECC), the Department of Veterans Affairs Rehabilitation Research and Development Service (IK2 RX003395), the American Psychiatric Association Foundation/Substance Abuse and Mental Health Service Administration Minority Fellowship Program, and the Japan Society for the Promotion of Science Overseas Research Fellowships.
Author contributions
J. Nungaray, L. Cardoso, J. Sprock, and G. Light collected the data. J. Molina, M. Thomas, and G. Light analyzed the data. J. Molina, M. Thomas, Y. Joshi D., W. Hochberger, D. Koshiyama, D. Braff, N. Swerdlow, and G. Light interpreted the results. J. Molina and G. Light designed the study. G. Light supervised all aspects of collection, analysis, and interpretation of the data. J. Molina and G. Light wrote the original paper. M. Thomas, Y. Joshi, W. Hochberger, D. Koshiyama, J. Sprock, D. Braff, and N. Swerdlow reviewed and edited the paper. All authors contributed to and approved the final paper.
Conflict of interest
G. Light has served as a consultant for Astellas, NeuroSig, and Sosei-Heptares. The remaining authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-01089-6).
References
- 1.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 2.Moran LV, Hong LE. High vs low frequency neural oscillations in schizophrenia. Schizophr. Bull. 2011;37:659–663. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen O, Kaiser J, Lachaux J-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Lewis DA, Curley AA, Glausier J, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, et al. Oscillations in schizophrenia: mechanisms and clinical significance. Brain Res. 2011;1413:98–114. doi: 10.1016/j.brainres.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 7.Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol. Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn, J. K. et al. EEG findings of reduced neural synchronization during visual integration in schizophrenia. PLoS ONE10, e0119849 (2015). [DOI] [PMC free article] [PubMed]
- 9.Spencer KM, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc. Natl Acad. Sci. USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grützner, C. et al. Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front. Hum. Neurosci. 7, 88 (2013). [DOI] [PMC free article] [PubMed]
- 11.Tikka SK, et al. Schneiderian first rank symptoms and gamma oscillatory activity in neuroleptic naïve first episode schizophrenia: a 192 channel EEG study. Psychiatry Investig. 2014;11:467–475. doi: 10.4306/pi.2014.11.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano Y, et al. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry. 2015;72:813–821. doi: 10.1001/jamapsychiatry.2014.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical γ synchrony and cognitive control in schizophrenia. Proc. Natl Acad. Sci. USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C-MA, et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka-Koshiyama, K. et al. Abnormal spontaneous gamma power is associated with underlying verbal learning and memory dysfunction in schizophrenia. Front. Psychiatry11, 832 (2020). [DOI] [PMC free article] [PubMed]
- 16.Light GA, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol. Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA. Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol. Psychiatry. 2012;71:873–880. doi: 10.1016/j.biopsych.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatard-Leitman VM, et al. Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biol. Psychiatry. 2015;77:556–568. doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thuné H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry. 2016;73:1145–1153. doi: 10.1001/jamapsychiatry.2016.2619. [DOI] [PubMed] [Google Scholar]
- 20.Kwon JS, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roach BJ, D’Souza DC, Ford JM, Mathalon DH. Test-retest reliability of time-frequency measures of auditory steady-state responses in patients with schizophrenia and healthy controls. Neuroimage Clin. 2019;23:101878. doi: 10.1016/j.nicl.2019.101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying J, Zhou D, Lin K, Gao X. Network analysis of functional brain connectivity driven by gamma-band auditory steady-state response in auditory hallucinations. J. Med Biol. Eng. 2015;35:45–51. doi: 10.1007/s40846-015-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmann I, Gutschalk A. Potential fMRI correlates of 40-Hz phase locking in primary auditory cortex, thalamus and midbrain. Neuroimage. 2011;54:495–504. doi: 10.1016/j.neuroimage.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 24.Herdman AT, et al. Intracerebral sources of human auditory steady-state responses. Brain Topogr. 2002;15:69–86. doi: 10.1023/A:1021470822922. [DOI] [PubMed] [Google Scholar]
- 25.Koshiyama, D. et al. A distributed frontotemporal network underlies gamma-band synchronization impairments in schizophrenia patients. Neuropsychopharmacology45, 2198–2206 (2020). [DOI] [PMC free article] [PubMed]
- 26.Zhou T-H, et al. Auditory steady state response deficits are associated with symptom severity and poor functioning in patients with psychotic disorder. Schizophr. Res. 2018;201:278–286. doi: 10.1016/j.schres.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshiyama D, et al. Auditory gamma oscillations predict global symptomatic outcome in the early stages of psychosis: a longitudinal investigation. Clin. Neurophysiol. 2018;129:2268–2275. doi: 10.1016/j.clinph.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Tada M, et al. Differential alterations of auditory gamma oscillatory responses between pre-onset high-risk individuals and first-episode schizophrenia. Cereb. Cortex. 2016;26:1027–1035. doi: 10.1093/cercor/bhu278. [DOI] [PubMed] [Google Scholar]
- 30.Koshiyama, D. et al. Hierarchical pathways from sensory processing to cognitive, clinical, and functional impairments in schizophrenia. Schizophr. Bull.10.1093/schbul/sbaa116. (2020). [DOI] [PMC free article] [PubMed]
- 31.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am. J. Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr. Bull. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas ML, et al. Targeted cognitive training improves auditory and verbal outcomes among treatment refractory schizophrenia patients mandated to residential care. Schizophr. Res. 2018;202:378–384. doi: 10.1016/j.schres.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher M, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr. Bull. 2015;41:250–258. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramaniam K, et al. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage. 2014;99:281–292. doi: 10.1016/j.neuroimage.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kambeitz-Ilankovic L, et al. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: Revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci. Biobehav. Rev. 2019;107:828–845. doi: 10.1016/j.neubiorev.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prikken M, Konings MJ, Lei WU, Begemann MJH, Sommer IEC. The efficacy of computerized cognitive drill and practice training for patients with a schizophrenia-spectrum disorder: a meta-analysis. Schizophr. Res. 2019;204:368–374. doi: 10.1016/j.schres.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Cella M, Preti A, Edwards C, Dow T, Wykes T. Cognitive remediation for negative symptoms of schizophrenia: a network meta-analysis. Clin. Psychol. Rev. 2017;52:43–51. doi: 10.1016/j.cpr.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow NR, Bhakta SG, Light GA. Room to move: plasticity in early auditory information processing and auditory learning in schizophrenia revealed by acute pharmacological challenge. Schizophr. Res. 2018;199:285–291. doi: 10.1016/j.schres.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarasenko M, et al. Measuring the capacity for auditory system plasticity: an examination of performance gains during initial exposure to auditory-targeted cognitive training in schizophrenia. Schizophr. Res. 2016;172:123–130. doi: 10.1016/j.schres.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez VB, et al. Mismatch negativity is a sensitive and predictive biomarker of perceptual learning during auditory cognitive training in schizophrenia. Neuropsychopharmacology. 2017;42:2206–2213. doi: 10.1038/npp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swerdlow NR, et al. Effects of amphetamine on sensorimotor gating and neurocognition in antipsychotic-medicated schizophrenia patients. Neuropsychopharmacology. 2018;43:708–717. doi: 10.1038/npp.2017.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Light GA, et al. Single-dose memantine improves cortical oscillatory response dynamics in patients with schizophrenia. Neuropsychopharmacology. 2017;42:2633–2639. doi: 10.1038/npp.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Light GA, Swerdlow NR. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Ann. N. Y. Acad. Sci. 2015;1344:105–119. doi: 10.1111/nyas.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Light GA, Swerdlow NR. Bending the curve on psychosis outcomes. Lancet Psychiatry. 2015;2:365–367. doi: 10.1016/S2215-0366(15)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hochberger WC, et al. Neurophysiologic measures of target engagement predict response to auditory-based cognitive training in treatment refractory schizophrenia. Neuropsychopharmacology. 2019;44:606–612. doi: 10.1038/s41386-018-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hochberger WC, et al. Oscillatory biomarkers of early auditory information processing predict cognitive gains following targeted cognitive training in schizophrenia patients. Schizophr. Res. 2020;215:97–104. doi: 10.1016/j.schres.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.First, M., Spitzer, R., Gibbon, M. & Williams, J. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/P) (2002).
- 49.Nuechterlein KH, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen, N. C. The Scale for the Assessment of Positive Symptoms. (University of Iowa, 1984).
- 51.Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS). (The University of Iowa, 1983).
- 52.van Erp TGM, et al. Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr. Res. 2014;152:289–294. doi: 10.1016/j.schres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauss GP, et al. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry. 2018;75:1271–1279. doi: 10.1001/jamapsychiatry.2018.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon SR, Sawilowsky SS. Impact of rank-based normalizing transformations on the accuracy of test scores. J. Mod. Appl. Stat. Methods. 2009;8:448–462. doi: 10.22237/jmasm/1257034080. [DOI] [Google Scholar]
- 55.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- 56.Bleuler, E. Dementia Praecox oder Gruppe der Schizophrenien. (F. Deuticke, 1911).
- 57.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 58.Thomas ML, et al. Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry. 2017;74:37–46. doi: 10.1001/jamapsychiatry.2016.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahshan C, Vinogradov S, Wynn JK, Hellemann G, Green MF. A randomized controlled trial comparing a ‘bottom-up’ and ‘top-down’ approach to cognitive training in schizophrenia. J. Psychiatr. Res. 2019;109:118–125. doi: 10.1016/j.jpsychires.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biagianti B, et al. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatr. Electrophysiol. 2017;3:2. doi: 10.1186/s40810-017-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Light GA, et al. Neurophysiological biomarkers for schizophrenia therapeutics. Biomark. Neuropsychiatry. 2020;2:100012. doi: 10.1016/j.bionps.2020.100012. [DOI] [Google Scholar]
- 62.Popova P, et al. The impact of cognitive training on spontaneous gamma oscillations in schizophrenia. Psychophysiology. 2018;55:e13083. doi: 10.1111/psyp.13083. [DOI] [PubMed] [Google Scholar]
- 63.Popov TG, et al. Targeted training modifies oscillatory brain activity in schizophrenia patients. Neuroimage Clin. 2015;7:807–814. doi: 10.1016/j.nicl.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramsay, I. S. et al. Increased global cognition correlates with increased thalamo-temporal connectivity in response to targeted cognitive training for recent onset schizophrenia. Schizophr. Res. 10.1016/j.schres.2020.01.020 (2020). [DOI] [PMC free article] [PubMed]
- 65.Dale CL, et al. Auditory cortical plasticity drives training-induced cognitive changes in schizophrenia. Schizophr. Bull. 2016;42:220–228. doi: 10.1093/schbul/sbv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina JL, et al. Memantine effects on electroencephalographic measures of putative excitatory/inhibitory balance in schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:562–568. doi: 10.1016/j.bpsc.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahshan C, et al. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol. Med. 2012;42:85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joshi YB, et al. Verbal learning deficits associated with increased anticholinergic burden are attenuated with targeted cognitive training in treatment refractory schizophrenia patients. Schizophr. Res. 2019;208:384–389. doi: 10.1016/j.schres.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lepock, J. R. et al. Relationships between cognitive event-related brain potential measures in patients at clinical high risk for psychosis. Schizophr. Res. 10.1016/j.schres.2019.01.014 (2019). [DOI] [PubMed]
- 70.Molina JL, et al. Prediction of Neurocognitive Deficits by Parkinsonian Motor Impairment in Schizophrenia: a study in neuroleptic-na‹ve subjects, unaffected first-degree relatives and healthy controls from an indigenous population. Schizophr. Bull. 2016;42:1486–1495. doi: 10.1093/schbul/sbw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Medalia A, Saperstein AM, Qian M, Javitt DC. Impact of baseline early auditory processing on response to cognitive remediation for schizophrenia. Schizophr. Res. 2019;208:397–405. doi: 10.1016/j.schres.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology. 2016;30:998–1008. doi: 10.1037/neu0000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.