Abstract
BACKGROUND
(-)-Fenchone is a bicyclic monoterpene present in essential oils of plant species, such as Foeniculum vulgare and Peumus boldus, used to treatment of gastrointestinal diseases. Pharmacological studies report its anti-inflammatory, antioxidant, and antinociceptive activity.
AIM
To investigate antidiarrheal activity related to gastrointestinal motility, intestinal secretion and antimicrobial activity.
METHODS
A castor oil-induced diarrhea model was used to evaluate antidiarrheal activity. Intestinal transit and gastric emptying protocols were used to assess a possible antimotility effect. Muscarinic receptors, presynaptic α2-adrenergic and tissue adrenergic receptors, KATP channels, nitric oxide were investigated to uncover antimotility mechanisms of action and castor oil-induced enteropooling to elucidate antisecretory mechanisms. The antimicrobial activity was evaluated in the minimum inhibitory concentration model, the fractional inhibitory concentration index using the (-)-fenchone association method with standard antifungal agents.
RESULTS
(-)-Fenchone (75, 150 and 300 mg/kg) showed antidiarrheal activity, with a significant decrease in the evacuation index. This activity is possibly related to a percentage of reduced intestinal transit (75, 150 and 300 mg/kg). The antimotility effect of (-)-fenchone decreased in the presence of pilocarpine, yohimbine, propranolol, L-NG-nitroarginine methyl ester or glibenclamide. In the enteropooling model, no reduction in intestinal fluid weight was observed. (-)- Fenchone did not show antibacterial activity; on the other hand, inhibits the growth of strains of fungi with a minimum fungicide concentration of 32 μg/mL. However, when it was associated with amphotericin B, no synergism was observed.
CONCLUSION
The antidiarrheal effect of (-)-fenchone in this study involves antimotility effect and not involve antisecretory mechanisms. (-)-Fenchone presents antifungal activity; however, it did not show antibacterial activity.
Keywords: (-)-Fenchone, Monoterpenes, Diarrhea, Motility, Antifungal
Core Tip: (-)-Fenchone is a bicyclic monoterpene present in essential oils of plant species, such as Foeniculum vulgare and Peumus boldus, used to treatment of gastrointestinal diseases. Diarrhea is a pathological condition characterized by an increase in three or more defecations in 24 h, being of multiple origins, whether infectious or not. In the search for new therapeutic alternatives, natural products, and medicinal plants are of great relevance. Many species of plants and their isolated compounds, including terpenes, have shown promising antidiarrheal and motility, based on this result, the monoterpene (-)-fenchone was selected for this study.
INTRODUCTION
Diarrhea is one of the most prevalent conditions affecting the gastrointestinal tract worldwide, contributing significantly to morbidity and mortality of the population[1]. The prevalence of chronic diarrhea is estimated at 1%-5% of the adult population, and in developed countries[2]. This condition can be defined as water and electrolyte loss caused by an increase in defecation frequency and abnormality in stool consistency throughout three or more times over 24 h[3-5].
The etiology of diarrheal disorders is multifactorial, attributed to factors such as infectious agents[6], food allergies, disturbances in intestinal function[7], alcohol intake[8], malabsorption of bile salts[9] and use of some medications, e.g., antimicrobials, antineoplastic, antiretrovirals, oral hypoglycemic agents, β-blockers, nonsteroidal anti-inflammatory drugs and proton pump inhibitors[10].
In adults, the most common causes of diarrhea include irritable bowel syndrome, inflammatory bowel disease, celiac disease, malabsorption of syndromes and microscopic colitis. The assessment and management of this condition can be a challenge, since the diagnosis is quite broad, especially about the differentiation between organic or functional causes that may be involved in its etiology[2-11].
Treatment of this disorder aims to reduce the dehydration and discomfort caused by frequent bowel movements through pharmacological and non-pharmacological actions[12,13]. Oral rehydration therapy is the primary non-pharmacological approach used for fluid and electrolyte replacement through the use of an oral rehydration solution, which may be associated with zinc supplementation[14]. Pharmacological therapy is nonspecific and indicated for the reduction of persistent and clinically significant symptoms. Among the main classes of drugs used are antisecretory and motility suppressing agents, probiotics, enkephalinase inhibitors, bismuth compounds and α2-adrenergic receptor agonists [12,15,16].
However, current therapy is limited due to side effects, e.g., dry mouth, distension and abdominal cramps, severe constipation, nausea and vomit[17]. Besides, respiratory depression and paralytic ileus are among the most dangerous side effects of loperamide in children[18].
In the search for new therapeutic alternatives, natural products and medicinal plants are of great relevance. Plant extracts, their semi-synthetic derivatives and synthetic compounds inspired by natural products make up the majority of medicines in use currently[19]. Thus, natural products are good starting points for the development of new drugs used in the treatment of gastrointestinal disorders such as diarrhea[20,21].
In fact, in recent years many species of plants and their isolated compounds, including terpenes, have shown promising antidiarrheal and motility effects, such as the friedelan-3β-ol, friedelin, volvalerenol A triterpenes[22,23] and the monoterpene carvone[24] and α-terpineol[25].
Based on this criterion, the monoterpene (-)-fenchone (1,3,3-trimethylbicyclo[2.2.1] heptane-2-one) was selected for this study. This monoterpene is made up of two enantiomers, (+)-fenchone and (-)-fenchone, obtained from fennel oil (Foeniculum vulgares) and thuya oil (Thuja occidentalis L.), respectively[26,27]. Research shows that the (-)-fenchone isomer has antinociceptive activity[28], antimicrobial[29], anti-inflammatory and antioxidant[30,31].
From this perspective, the present study aimed to evaluate the antidiarrheal and antimicrobial activity of (-)-fenchone using experimental models.
MATERIALS AND METHODS
Animals
Swiss adult male (Mus musculus), weighing between 25-35 g, were obtained from the Animal Production Unit of the Institute for Research on Drugs and Medicines of Federal University of Paraiba (IPeFarM/UFPB), protocol No. 035/2017 and No. 4996090518/2018. They were housed under 23 ± 1 °C, with a 12/12 h light/dark cycle, fed with Purina® chow and water ad libitum for two weeks before experimentation. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection, following internationally accepted principles for the use of laboratory animals.
Substances
The following drugs were used: Carboxymethylcellulose (Formula Brasil®, Brazil); castor oil (Tayuyna Lab Ltda®, Brazil); loperamide hydrochloride (2 mg; Janssen Cilag Farmacêutica Ltda®, Brazil); activated charcoal (Proquímios®, Brazil); glibenclamide, L-NG-nitroarginine methyl ester (L-NAME), propranolol and yohimbine (Sigma Aldrich®, Brazil).
Study material
(-)-Fenchone was purchased from SIGMA-ALDRICH Brasil Ltda (product reference: 196436) 98% purity, density: 0.948 g/cm3 at 25 °C, CAS: 7787-20-4, molecular mass 152.23, boiling point: 192-194 °C and melting point 5-6 °C.
Culture mediums
The culture media used were Brain Heart Infusion (BHI), Sabouraud Dextrose Agar purchased from Difco Laboratories Ltd, United States, France, RPMI 1640 with L-glutamine, and bicarbonate (Difco Laboratories Ltd, United States, France, and INLAB, San Paulo, Brazil). All media were prepared as per the manufacturer’s descriptions.
Microorganisms
For the biological activity assays of the test products, strains of Staphylococcus aureus ATCC-25923 and LM-177, Pseudomonas aeruginosa ATCC-25853 and LM-297, Escherichia coli ATCC-18739 and LM-39, Candida albicans ATCC-76645 and LM-05; Candida tropicalis ATCC-13803 and LM-20; Candida Krusei ATCC-6258 and LM-13; obtained from the Laboratory of Mycology, Department of Pharmaceutical Sciences (DCF), Health Sciences Center (CCS) of the Federal University of Paraíba (UFPB). For inoculum preparation, the colonies of microorganisms were suspended in 0.85% sterile 0.9% NaCl solution and adjusted according to the 0.5 scale of McFarland standard to obtain an inoculum of 1 × 106-5 × 106 colony-forming units per milliliter (CFU/L) for fungi and 1 × 108-2 × 108 CFU/mL for bacteria[32].
Pharmacological assays
Effects of (-)-fenchone on castor oil-induced diarrhea: For the evaluation of (-)-fenchone antidiarrheal activity, the protocol described by Awouters et al[33] was used. Male Swiss mice (n = 7 per group), fasting 12 h, were treated orally with vehicle (5% tween 80, 10 mL/kg), loperamide (5 mg/kg) or (-)-fenchone (37.5, 75, 150 or 300 mg/kg). After 1 h, 10 mL/kg of castor oil was administered orally. The animals were placed separately in boxes with a paper-lined floor for analysis of fecal cakes. The severity of diarrhea was observed during 4 h, analyzing the following parameters: Evacuation index (formed or solid, semi-solid or pasty and liquid), percentage of liquid stools, and percentage of diarrhea inhibition. Evacuation index: ∑ (Solid stool × 1) + (Liquid stool × 2) + (Liquid stool × 3) % ID = (Tween group average - Treated group average) / Tween group average × 100.
Effect of (-)-fenchone on gastric emptying: The effect on gastric emptying was evaluated according to experiments conducted by Scarpignato et al[34]. Male Swiss mice (n = 7 per group), fasted for 24 h, orally received the vehicle (5% tween 80; 10 mL/kg), loperamide (5 mg/kg) or (-)-fenchone (37.5, 75, 150, 300 mg/kg). After 1 h the animals received the 10 mL/kg phenol red-colored marker (0.05% phenol red-colored in 1.5% carboxymethylcellulose-thickening agent). To the non-treated control group (the zero-time control group), the phenol red-colored marker was administrated and the mice were immediately euthanized. The treated groups received the same marker and were euthanized 30 min after administration. The abdominal cavity was opened; the pylorus and distal esophagus were clamped. The stomach was removed, opened, and washed with 7 mL of distilled water. Gastric contents were collected and centrifuged at 3000 rpm for 15 min. Then, 1 mL of the supernatant was collected, and 1 mL of 1 mol/L NaOH (pH = 12) was added. The results were obtained by spectrophotometry with a wavelength reading of 560 nm and expressed as a percentage of gastric emptying, calculated by the formula: % Gastric emptying = (100 - mean absorbance of sample) / mean absorbance of zero-time control group × 100.
Effect of (-)-fenchone on castor oil-induced intestinal transit: The effect on intestinal transit was evaluated according to the methodology described by Stickney et al[35]. Male Swiss mice (n = 7 per group) were fasted for 24 h and orally received the vehicle (5% tween 80; 10 mL/kg), loperamide (5 mg/kg) or (-)-fenchone (37.5, 75, 150, 300 mg/kg). After 1 h the oral administration of castor oil (1 mL/100 g) was performed. After 30 min, 10 mL/kg of activated charcoal marker [activated charcoal (10%) in gum arabic (5% thickening agent)] was orally administered to the animals. After 30 min, the animals were euthanized, the intestine removed from the pylorus to the ileocecal junction and using a ruler the total length of the intestinal segment and the distance covered by the activated charcoal were measured to calculate the percentage of intestinal transit: % Intestinal transit = (distance traveled by phenol red)/total intestine length × 100.
(-)-Fenchone antimotility mechanisms of action
Evaluation of the participation of muscarinic, adrenergic, nitrergic pathway and ATP-dependent potassium channels (KATP) in (-)-fenchone antimotility mechanisms in the intestinal transit model: To investigate the antimotility mechanisms involved in the antidiarrheal activity fenchone were evaluated according to the model described by Santos et al[36]. The role of muscarinic receptors, alpha and beta-adrenergic receptors, nitric oxide (NO) and KATP were evaluated. The mice were fasted for 24 h and distributed in different groups (n = 7 per group). Two groups received intraperitoneal NaCl solution 0.9% (10 mL/kg), the other two received pilocarpine (non-selective muscarinic receptor agonist, 1 mg/kg), yohimbine (α2-adrenergic presynaptic receptor antagonist, 1 mg/kg), propranolol (non-selective β-adrenergic receptor antagonist), L-NAME (NO synthase activity inhibitor, 25 mg/kg) or glibenclamide (KATP channel blocker, 1 mg/kg). These drugs were dissolved in NaCl 0.9% and given intraperitoneally. After 30 min, the animals were treated orally with 5% tween 80 (control group), or fenchone 150 mg/kg (most effective dose). After 60 min, 10 mL/kg (p.o.) of the black marker (10% activated charcoal suspension in 5% arabic gum) was administered and 30 min later, the animals were euthanized for removal of the small intestine to calculate the percentage of intestinal transit.
Evaluation of (-)-fenchone antisecretory mechanism in castor oil-induced intraluminal fluid accumulation (enteropooling) model: The effects on intestinal enteropooling were evaluated according to protocols described by Ezeja et al[37] adapted. Mice (n = 7 per group), after 24 h fasting, were orally pretreated with 5% tween 80 (vehicle 10 mL/kg), loperamide (5 mg/kg) or (-)-fenchone (150 mg/kg). After 60 min, the animals received orally 10 mL/kg of castor oil to induce diarrhea. After 1 h, the mice were euthanized, their abdomens were opened, and their intestines removed (pylorus ileocecal junction) and weighed with the intestinal contents (full intestine). The intestinal contents were removed and weighed again (empty intestine). Difference between the first and second weight is considered as the weight of the intestinal content of each animal.
Evaluation of antibacterial and antifungal activity
Minimum inhibitory concentration: Antimicrobial activity assays were performed according to the protocols of Cleeland et al[38], Eloff[39], and CLSI[32]. Minimum inhibitory concentration (MIC) determination of the (-)-fenchone on bacterial and fungal strains was performed by the microdilution technique in 96 well plates. Initially, 100 μL of double concentrated RPMI/BHI broth was distributed to the wells of the microdilution plates. Then, 100 μL of (-)-fenchone was dispensed into the wells of the first line of the plate and by serial dilution at a ratio of two concentrations of 1024 μg/mL to 16 μg/mL were obtained. Finally, 10 μL of bacterial and fungal inoculum (strains of Staphylococcus aureus ATCC-25923 and LM-177, Pseudomonas aeruginosa ATCC-25853 and LM-297, Escherichia coli ATCC-18739 and LM-39, Candida albicans ATCC-76645 and LM-05, Candida tropicalis ATCC-13803 and LM-20, Candida Krusei ATCC-6258 and LM-13) were added to the wells. Controls performed: Microorganisms and culture medium to check the viability of the strains and the sterility of the medium and control with Gentamicin (64 μg/mL) and amphotericin B (32 μg/mL). The prepared plates were sealed aseptically and incubated at 35 ± 2 °C for 24-48 h. The antimicrobial activity of the products was interpreted and considered as active or inactive according to the following criteria: Up to 600 μg/mL = strong activity; 600-1500 μg/mL = moderate activity; > 1500 μg/mL = weak activity or inactive product[40-42].
Minimum fungicide concentration: After MIC, 10 μL aliquots of the supernatant from the wells in which complete fungal growth inhibition (MIC, MIC × 2, MIC × 4, and MIC × 8) was observed on the microdilution plates were added to 100 μL RPMI broth contained in new culture plates. Plates were incubated for 24-48 h at 35 ± 2 °C. Minimum fungicide concentration (MFC) was considered as the lowest concentration of the product that was able to inhibit the growth of microorganisms.
Association test: The effect of the association of (-)-fenchone with amphotericin B was determined from the checkerboard method for derivation of the fractional inhibitory concentration index (FICI) against C. albicans strains ATCC-76645 and LM-05. Solutions of the tested products were used at concentrations determined from their MICs. Initially, 100 μL RPMI broth was added to the 96 well plates. Then, 50 μL of each product tested at several levels (1/8 MIC, 1/4 MIC, 1/2 MIC, MIC, MIC × 2 and MIC × 4) were added vertically (amphotericin B) and horizontally (-)-fenchone. Finally, 20 μL of the fungal suspension was added after the incubation period of the microplates at 35 ± 2 °C for 24-48 h. The fractional inhibitory concentration (FIC) was calculated by summing the FICA + FICB (FICA = FIC of the test product; FICB = FIC of the standard antifungal). The FICA, in turn, is calculated using the combined MICA / MICA isolated, while FICB = combined MICB / isolated MICB. This index is interpreted as follows: Synergism (FICI ≤ 0.5), antagonism (FICI > 4.0) and indifference (0.5 < FICI ≤ 4).
Statistical analyses
Data were expressed as mean ± standard deviation from the mean for parametric data and median (minimum-maximum values) for nonparametric data. These values were statistically analyzed by one-way analysis of variance (parametric data) or Kruskal-Wallis (nonparametric data), followed by Dunnet or Dunn’s posttests, respectively. Results were considered significant when P < 0.05. GraphPad Software© 5.0 (United States) was used for data processing. The statistical methods applied in this study were reviewed by Gessenildo P. Rodrigues, PhD, Padronize - Academic Consulting Firm.
RESULTS
Effect of (-)-fenchone in diarrhea induced by castor oil
According to the observed results, a negative control group treated with the vehicle (5% tween 80, 10 mL/kg) presented diarrhea, with evacuation index 23 (20-24) and 81% of liquid feces. Pre-treatment with (-)-fenchone (75, 150 and 300 mg/kg, p.o.) reduced the evacuation by 7 (6-7) with 51% inhibition of diarrhea (P < 0.05), 4 (3-6) and 81% (P < 0.01), 3 (1-4) and 88% (P < 0.001), respectively, when compared to the negative control group. A standard antidiarrheal drug, loperamide (5 mg/kg, p.o.), produced an 87% inhibition of diarrhea (Table 1).
Table 1.
Effect of oral (-)-fenchone and loperamide administration on castor oil-induced diarrhea in mice
| Tratament (p.o.) | Dose (mg/kg) | EI | Liquid stools (%) | Diarrheal inhibition (%) |
| Tween 80 5% | 23 (20-24) | 81 | ||
| Loperamide | 5 | 7 (4-7)e | 8 | 87 |
| (-)-Fenchone | 37, 5 | 16 (14-17) | 52 | 28 |
| (-)-Fenchone | 75 | 7 (6-7)a | 19 | 51 |
| (-)-Fenchone | 150 | 4 (3-6)b | 4 | 81 |
| (-)-Fenchone | 300 | 3 (3-4)e | 4 | 88 |
Data expressed as median (minimum-maximum values) and analyzed by Kruskal-Wallis test followed by Dunn’s test (
P < 0.05,
P < 0.01,
P < 0.001, compared to the 5% tween 80 group) (n = 7/per group). EI: Evacuation index.
Effect of (-)-fenchone on gastric emptying
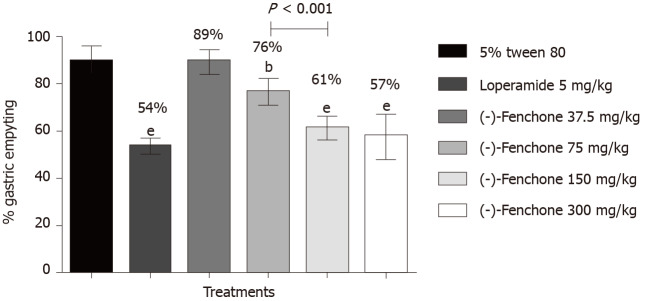
Results of this model showed that the group treated with the vehicle (5% tween 80, 10 mL/kg) presented 89% gastric emptying. The groups treated with loperamide (5 mg/kg, p.o.) and (-)-fenchone at doses of 75, 150 and 300 mg/kg reduced gastric emptying to 54% (P < 0.01), 76% (P < 0.01), 61% (P < 0.001) and 57% (P < 0.001), respectively, when compared to the negative control group (Figure 1).
Figure 1.
Effect of oral administration of (-)-fenchone and loperamide on gastric emptying in male Swiss mice. Data expressed as mean ± standard deviation and analyzed by ANOVA, followed by Dunnet and Tukey's multiple comparison tests (bP < 0.01 and eP < 0.001, compared to the 5% tween 80 group) (n = 7/per group).
Effect of (-)-fenchone on intestinal transit
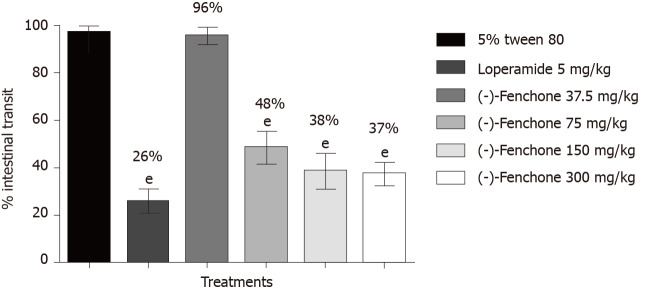
Results of this study showed that the distance travelled by a marker (activated charcoal) in terms of percentage of the total length of the intestine was 97% in the negative control group. Treatment with loperamide (5 mg/kg, p.o.) and (-)-fenchone at doses of 75, 150 and 300 significantly reduced (P < 0.001) the percentage of intestinal transit to 26%, 48%, 38% and 37%, respectively, when compared to the control group (Figure 2).
Figure 2.
Effect of oral administration of (-)-fenchone and loperamide in intestinal transit in male Swiss mice. Data expressed as mean ± standard deviation and analyzed by ANOVA, followed by Dunnet and Tukey’s multiple comparison tests (eP < 0.001, compared to the 5% tween 80 group) (n = 7/per group).
Mechanisms of antimotility action of (-)-fenchone
Treatment with (-)-fenchone at its most effective dose (150 mg/kg, p.o.) significantly reduced the percentage of intestinal transit to 37% when compared to the negative control group (85%). In the group pretreated with pilocarpine (non-selective muscarinic receptor agonist, 1 mg/kg, i.p.), fenchone significantly reduced muscarinic action in intestinal transit to 75% (Figure 3).
Figure 3.
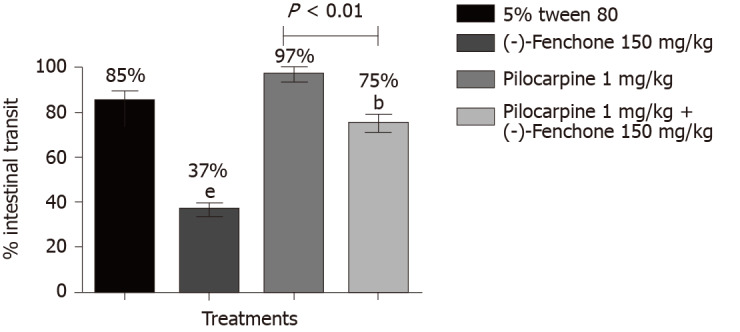
Effect of oral administration of (-)-fenchone after treatment with pilocarpine and yohimbine, and on intestinal transit of mice. Data expressed as mean ± standard deviation and analyzed by ANOVA, followed by Dunnet and Tukey's tests (bP < 0.01 and eP < 0.001, compared whit tween 5% tween 80) (P < 0.01 compared to the pilocarpine whit pilocarpine + (-)-fenchone group (n = 7/per group).
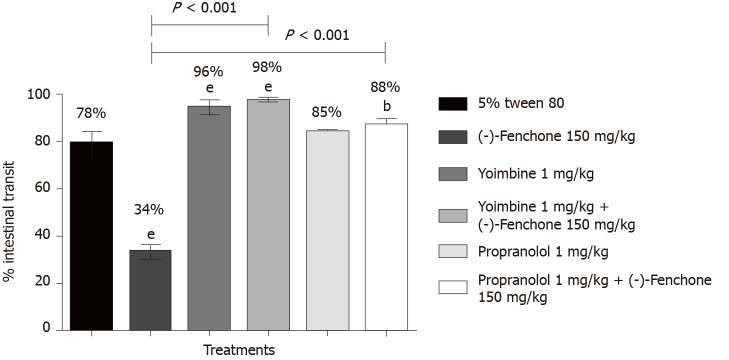
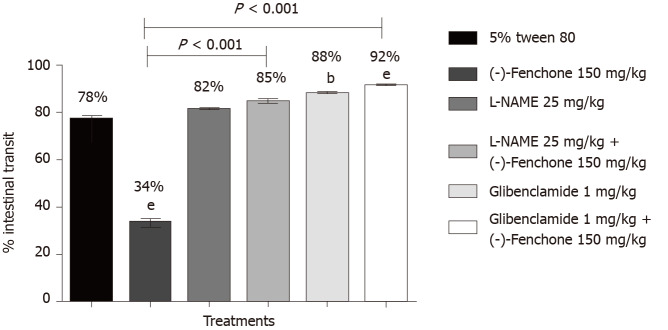
(-)-Fenchone (150 mg/kg, orally), reduced the percentage of intestinal transit to 34% compared to the control group (78%). In the presence of yohimbine (α2-adrenergic antagonist 1 mg/kg, i.p.) or propranolol (non-selective β-adrenergic receptor antagonist, 1 mg/kg, i.p.) the inhibitory effect was significantly reduced to 98% and 88%, respectively, when compared to the group (-)-fenchone (unlocked, Figure 4). Pre-treatment with L-NAME (inhibitor of NO synthase activity, 25 mg/kg, i.p.) or glibenclamide (KATP channel blocker, 1 mg/kg, i.p.) reversed the inhibitory effect of this monoterpene on intestinal transit to 85% and 92%, respectively, when compared to the unblocked group (-)-fenchone (Figure 5).
Figure 4.
Effect of oral administration of (-)-fenchone after treatment with propranolol on intestinal transit of mice. Data expressed as mean ± standard deviation and analyzed by ANOVA, followed by Dunnet and Tukey's tests (bP < 0.01 and eP < 0.001, compared whit tween 5% tween 80) (P < 0.001 compared to the unblocked (-)-fenchone group) (n = 7/per group).
Figure 5.
Effect of oral administration of (-)-fenchone after treatment with L-NG-nitroarginine methyl ester, and glibenclamide on intestinal transit of mice. Data expressed as mean ± standard deviation and analyzed by ANOVA, followed by Dunnet and Tukey's tests (bP < 0.01 and eP < 0.001, compared whit tween 5% tween 80 groups) (P < 0.001 compared to the unblocked (-)-fenchone group) (n = 7/per group). Statistical analysis: ANOVA followed by the Tukey test.
Mechanisms of anti-secretion of (-)-fenchone (enteropooling)
Treatment with (-)-fenchone (150 mg/kg, p.o.) showed no significant reduction in intestinal fluid accumulation (0.53 ± 0.04) when compared to the control group (0.51 g ± 0.01), treatment with loperamide (5 mg/kg, p.o) reduced the weight of intestinal fluid (0.28 ± 0.01 g) significantly when compared to the control group (Figure 6).
Figure 6.
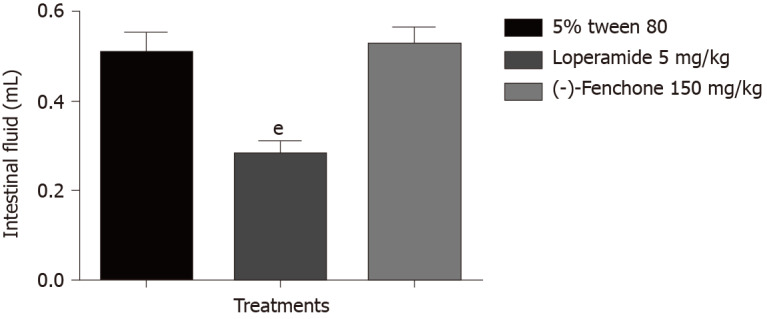
Effect of oral administration of (-)-fenchone and loperamide on castor oil-induced enteropooling in mice. Data expressed as mean ± standard deviation and analyzed by ANOVA, followed by Dunnet and Tukey's tests (eP < 0.001, compared whit tween 5% tween 80 groups) (n = 7/per group).
Antibacterial and antifungal activity
(-)-Fenchone did not show inhibitory activity against any of the bacterial strains in the concentration range used. Thus, for bacterial strains, it was not possible to determine the MIC and minimum bactericidal concentration. The results of the antifungal activity (Table 2) shown that (-)-fenchone inhibited the growth of 4 (66%) of the six fungal strains studied up to a concentration of 32 μg/mL, with only two strains being inhibited to a concentration of 64 μg/mL. Therefore, the MIC50 (level capable of inhibiting the growth of up to 50% of the species) was considered to be 32 μg/mL MFC of the product against fungal strains varied between 32 μg/mL and 256 μg/mL, with the concentration of 32 μg/mL fungicidal for 3 (50%) of the strains tested. In this way, the MFC50 was established as 32 μg/mL.
Table 2.
Effect of the evaluation of the minimum inhibitory concentration and minimum fungicide concentration (μg/mL) of (-)-fenchone against fungal strains
| Species | Strains |
(-)-Fenchone |
|
| MIC (µg/mL) | MFC (µg/mL) | ||
| Candida albicans | ATCC-76645 | 32 | 32 |
| Candida albicans | LM-05 | 32 | 32 |
| Candida tropicalis | ATCC-13803 | 32 | 32 |
| Candida tropicalis | LM-20 | 32 | 256 |
| Candida kruzei | ATCC-6258 | 64 | 256 |
| Candida krusei | LM-13 | 64 | 256 |
MIC: Minimum inhibitory concentration; MFC: Minimum fungicide concentration.
Association assay
Tests demonstrated that (-)-fenchone maintained the MIC of 32 μg/mL and amphotericin B the MIC of 0.2 μg/mL for the strains tested, maintaining the MIC at equal values both individually and in an association. Since the FIC, that is, the ratio of the combined inhibitory concentrations between the isolated inhibitory concentrations, for each substance was equivalent to 1, the FICI was equal to 2 (Table 3).
Table 3.
Determination of the fractional inhibitory concentration index of the association between (-)-fenchone and amphotericin B on Candida albicans strains
| Strains | FICA, (-)-fenchone | FICB, Amphotericin B | FICI | Interaction type |
| Candida albicans (ATCC-76645) | 1 | 1 | 2 | Indifferent |
| Candida albicans (LM-05) | 1 | 1 | 2 | Indifferent |
FICA: Fractional inhibitory concentration of the test product; FICB: Fractional inhibitory concentration of the standard antifungal; FICI: Fractional inhibitory concentration index.
DISCUSSION
To evaluate the antidiarrheal activity of (-)-fenchone the castor oil-induced diarrhea model was used in mice. Castor oil is a natural product obtained from the seeds of Ricinus communis, that when ingested is metabolized by intestinal lipases, releasing ricinoleic acid in the intestinal lumen, creating extensive contractions in the transverse and distal colon[43]. This substance causes a reduction in Na+, K-ATPase activity, inhibition of sodium, chloride, and water absorption, increased contractions of smooth intestine muscles, produces a cytotoxic effect on enterocytes, resulting in abundant watery diarrhea with an accumulation of intestinal fluid[44].
(-)-Fenchone (75, 150, and 300 mg/kg, p.o.) presented antidiarrheal activity, decreasing (P > 0.05) the evacuation index (evacuation index, percentage of liquid stools, and percentage of diarrhea inhibition). These results corroborate a study by Dos Santos Negreiros et al[25] with the monoterpene α-terpineol showed a reduction in the total number of stools and diarrheal stools in the castor oil-induced diarrhea model. Study with essential oil of Mentha longifolia L., whose main components are monoterpenes pulegone and 1.8-cineol showed antidiarrheal activity these same model[45].
To evaluate if (-)-fenchone influenced gastrointestinal motility, the evaluation of gastric emptying and intestinal transit protocols were assessed. The findings suggested an antimotility activity mediated by (-)-fenchone since it was efficient in decreasing gastric emptying and intestinal transit. Similar results were found for monoterpene 1.8-cineol showed a reduction in gastric emptying[46]. (-)-Fenchone declined the propulsion of the marker (activated charcoal suspension) through the intestine. It suggests that (-)-fenchone influenced peristaltic movements of the intestine, characterizing an antimotility activity. Silva et al[24] shown that the monoterpene carvone also reduced the percentage of intestinal transit in this model.
An investigation was then carried out to determine whether fenchone can act through cholinergic mechanisms. Acetylcholine (ACh) exerts an excitatory effect on the gastrointestinal smooth muscle by activating muscarinic M3 receptors (coupled to Gq/11), there is an increase in the cytosolic concentration of Ca2+, which results in contraction of the smooth muscle and increased intestinal transit[47]. Therefore, a reduction in the release of this neurotransmitter, as well as the inhibition of its action on its respective receptors, can delay intestinal transit.
Pilocarpine, a cholinergic agonist, was used to induce intestinal motility. It can be seen that fenchone significantly reduced the stimulating effects promoted by pilocarpine resulting in a decrease of intestinal transit. From this result, we can infer that (-)-fenchone may be competing for GIT M3 receptors, acting as a partial antagonist of muscarinic receptors and that its antimotility involves the cholinergic pathway. This result corroborates the study by Dos Santos Negreiros et al[25], with the monoterpene α-terpineol, which presented the same behavior when associated with bethanechol (muscarinic agonist), suggesting the participation of the muscarinic pathway in its antimotility effect.
Sympathetic innervation (via noradrenaline) acts as inhibitory feedback modulating the release of ACh in the myenteric plexus (via presynaptic α2-adrenergic receptors coupled to Gi/Go), and also by its action on receptors present in the intestinal smooth muscle (via post receptorsβ2-adrenergic synaptic coupled to Gs)[48]. Both actions result in inhibition of peristaltic activity and decreased tone of intestinal smooth muscle, leading to reduced intestinal motility[49]. Therefore, a blockade of pre or postsynaptic receptors can increase intestinal transit.
The presynaptic α2-adrenergic receptor antagonist yohimbine or postsynaptic β-adrenergic receptor antagonist propranolol were used to induce intestinal motility. It can be observed that in the presence of blockers the antimotility effect of (-)-fenchone was significantly reduced, with this, it can be inferred that the adrenergic pathway is related to the antimotility effect of fenchone.
NO is a neurotransmitter released by inhibitory enteric neurons and causes post-junction hyperpolarization responses resulting in smooth muscle relaxation[50]. The KATP channels are expressed in large quantities in the intestinal smooth muscle modulating gastrointestinal motility in physiological and pathophysiological states[51]. NO activates soluble guanylyl cyclase, leading to the production of cyclic guanosine monophosphate (cGMP) that enables its dependent protein kinase, which activates potassium channels[50]. Activation of KATP promotes hyperpolarization of the cell membrane, reduction of Ca2+ influx and inhibition of cellular excitability, thus generating smooth muscle relaxation[51].
L-NAME an inhibitor of NO synthase or glibenclamide a KATP channel blocker was used as blockers of this pathway. From the results, it was observed that in the presence of the blockers the antimotility effect of fenchone was significantly reduced; thus, it can be suggested that the antimotility effect to fenchone may involve the NO-cGMP-KATP pathway.
These results corroborate with the study by Formiga et al[52] with the ethanolic extract of Maytenus erythroxylon Reissek, which has triterpenes in its composition, the administration of the extract associated with propranolol, L-NAME or glibenclamide reduced its antimotility effect, suggesting the participation of adrenergic and nitrergic pathways and KATP channels in their effect.
To evaluate the antisecretory effect of (-)-fenchone was used the intestinal fluid accumulation model (enteropooling) induced by castor oil. The results show that (-)-fenchone did not reverse the accumulation of fluid caused by the inducing agent. It can suppose that the antidiarrheal effect of this monoterpene does not involve antisecretory and pro-absorptive mechanisms. This result differs the Sisay et al[53], in which the extract of Verbena officiinalis rich in terpenoids, had an inhibitory effect on enteropooling induced by castor oil.
According to the results obtained in the tests, it was found that (-)-fenchone at concentrations of 1024 to 16 μg/mL does not show antibacterial activity against any of the tested bacterial strains. A study conducted by Balogun et al[54] demonstrated that the essential oil of Moringa oleifera at a concentration of 25 mg/mL. With monoterpenes as the most abundant class of compounds, exhibited relatively weak antibacterial activity against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa.
Several studies have reported a variety of funges can cause diarrhea and opportunistic infections, such as genus Candida (C. albicans, C. tropicalis, and C. krusei)[55,56]. Many essential oils already have known antifungal activity[57]. The results obtained in this study showed that monoterpene (-)-fenchone in concentrations of 32 μg/mL and 64 μg/mL, inhibited the growth of all tested strains (Candida albicans ATCC-76645 and LM-05; Candida tropicalis ATCC-13803 and LM-20; Candida Krusei ATCC-6258 and LM-13).
The results obtained corroborate the study by Dias et al[58] the antifungal activity of monoterpene linalool in clinical strains of C. albicans, C. krusei, and C. tropicalis was evaluated. The results showed that this monoterpene inhibited the growth of all strains of fungi at the concentrations evaluated.
Several studies have demonstrated the antifungal potential of products of natural origin used alone or in combination with medications already used. It was evaluated the association between (-)-fenchone and amphotericin B, an antifungal agent standard, utilizing the checkerboard method[59]. Candida albicans is still the most prevalent species in infections caused by fungi, bringing an even more worrying scenario due to the high rates of resistance to antifungals[60]. Amphotericin B is an antifungal of the polyenes class, and its mechanism of action involves interaction with ergosterol. It leads to the formation of pores in the membrane, loss of integrity, rapid extravasation of potassium and other ions causing cell death[61].
When tested in combination (-)-fenchone and amphotericin B, they did not show any synergism or antagonism effect, indicating that the combination between these two substances does not present significant interaction, that is, indifferent. The antimicrobial action of essential oils and their constituents such as terpenes is being associated with their high lipophilicity, which facilitates access to the lipid layer of the bacterial cell membrane and fungal mitochondria. It acts to increase the permeability of these structures, resulting in extravasation cellular contents and ions, leading to cell lysis[62-64]. The lack of synergism may be related to the fact that (-)-fenchone and amphotericin B possibly act by the same mechanism of action, competing for the same binding site, which limits an effect potentiation.
Another association study was carried which the monoterpene geraniol, showed varying degrees of interaction with antifungal patterns fluconazole and amphotericin B, demonstrating synergism for some strains of C. albicans and indifference for others, showing that there is no pattern of the synergistic effect of monoterpenes[65].
CONCLUSION
In brief, it can be observed that (-)-fenchone presents antidiarrheal activity related to an antimotility effect. This antimotility effect involves anticholinergic mechanisms, which can be partially reversed in the presence of a muscarinic agonist. It can be blocked by α2- and β-adrenergic receptor antagonists, suggesting the participation of the adrenergic pathway. It can also be blocked by L-NAME and glibenclamide, indicating a possible involvement of NO and KATP channels. Not related to antisecretory or pro-absorption activities. In the evaluation of antimicrobial activity, monoterpene inhibited the growth of fungal strains, being considered a product with intense antifungal activity, but without antibacterial activity. Besides, it has no synergistic or antagonistic effect in combination with the standard antifungal.
ARTICLE HIGHLIGHTS
Research background
Pharmacological therapy for diarrhea is associated with contraindications and side effects. In the search for new therapeutic alternatives, natural products and medicinal plants are of great relevance, plant extracts, their semi-synthetic derivatives and synthetic compounds inspired by natural products make up the majority of drugs in use today. Many plant species and their isolated compounds, including terpenes, showed promising effects in the context of diarrhea, based on this criterion, the monoterpene (-)-fenchone was selected for this study.
Research motivation
(-)-Fenchone is a bicyclic monoterpene present in essential oils of plant species, such as Foeniculum vulgare and Peumus boldus, used in the treatment of gastrointestinal diseases. It has relevant pharmacological activities described in the literature. Many species of plants and their isolated compounds, including terpenes, have shown promising antidiarrheal and motility, based on this result, the monoterpene (-)-fenchone was selected for this study.
Research objectives
The main objective of our study was to evaluate the antidiarrheal activity related to gastrointestinal motility, intestinal secretion and antimicrobial and antifungal activity of (-)-fenchone.
Research methods
In this study, antidiarrheal activity was evaluated in vivo, using male Swiss mice. The effects of (-)-fenchone in the castor oil-induced diarrhea model. Intestinal transit and gastric emptying protocols were used to evaluate a possible antimotility impact. Muscarinic receptors, presynaptic α2-adrenergic and tissue adrenergic receptors, KATP channels, nitric oxide were investigated to uncover antimotility mechanisms of action and castor oil-induced enteropooling to elucidate antisecretory mechanisms. The antimicrobial activity was evaluated in the minimum inhibitory concentration model, the fractional inhibitory concentration index using the (-)-fenchone association method with standard antimicrobial agents.
Research results
(-)-Fenchone at doses (75, 150 and 300 mg/kg) has antidiarrheal activity, with a significant decrease in the evacuation index. This activity is possibly related to a percentage of reduced intestinal transit (75, 150 and 300 mg/kg). The antimotility effect of (-)-fenchone decreased in the presence of pilocarpine, yohimbine, propranolol, L-NG-nitroarginine methyl ester or glibenclamide. In the enteropooling model, no reduction in intestinal fluid weight was observed. (-)-Fenchone did not show antibacterial activity, inhibits the growth of strains of fungi with a minimum fungicidal concentration of 32 μg/mL. As for the association between (-)-fenchone and amphotericin B in strains of Candida albicans, it was observed that the association was indifferent.
Research conclusions
The antidiarrheal effect of (-)-fenchone found in this study involves antimotility and not involve antisecretory mechanisms. (-)-Fenchone has antifungal activity; however, it did not show antibacterial activity.
Research perspectives
The main limitations of our study include strains of tested bacteria that are not the most prevalent in infectious diarrhea, as well as other in vivo models of diarrhea and post-exposure treatment. The prospects are to perform other models of diarrhea in vivo that can help to reinforce these data, as well as other analyzes of molecular markers to characterize mechanisms.
ACKNOWLEDGEMENTS
We are grateful to Parentoni RN, Duarte JC, and all LFTGI members. We would like to extend our gratitude to the Federal University of Paraíba.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Commission for Ethics in Animal Experimentation (CEUA) of the Research Ethics Committee of the Federal University of Paraíba.
Institutional animal care and use committee statement: All animal experiments conformed to the internationally accepted principles for the care and use of laboratory animals (approved by the Institutional Commission in Animal Use from Federal University of Paraíba (CEUA/UFPB) under No. 035/2017 and No. 4996090518/2018).
Conflict-of-interest statement: Authors declare that no conflict of interest exists.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: May 20, 2020
First decision: July 29, 2020
Article in press: October 1, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdulnour-Nakhoul SM, Medeiros PHQS, Pop TL, Rakhshan V, Xu XY S-Editor: Huang P L-Editor: A P-Editor: Liu JH
Contributor Information
Michelle Liz de Souza Pessoa, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Leiliane Macena Oliveira Silva, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Maria Elaine Cristina Araruna, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Catarina Alves de Lima Serafim, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Edvaldo Balbino Alves Júnior, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Alessa Oliveira Silva, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Matheus Marley Bezerra Pessoa, Health Sciences Center, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Hermes Diniz Neto, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Edeltrudes de Oliveira Lima, Department of Pharmaceutical Sciences, IPeFarM, Federal University of Paraíba, João Pessoa 58051-970, Paraíba, Brazil.
Leônia Maria Batista, Postgraduate Program in Natural and Synthetic Bioactive Products, Universidade Federal da Paraiba, João Pessoa 58051-900, Brazil. leoniab@uol.com.br.
Data sharing statement
No additional data are available.
References
- 1.GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrasco-Labra A, Lytvyn L, Falck-Ytter Y, Surawicz CM, Chey WD. AGA Technical Review on the Evaluation of Functional Diarrhea and Diarrhea-Predominant Irritable Bowel Syndrome in Adults (IBS-D) Gastroenterology. 2019;157:859–880. doi: 10.1053/j.gastro.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Tadesse WT, Hailu AE, Gurmu AE, Mechesso AF. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complement Altern Med. 2014;14:460. doi: 10.1186/1472-6882-14-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Diarrhoeal disease. 2 May 2017 [cited 10 March 2020]. Available from: http://www.who.int/mediacentre/factsheets/fs330/en/
- 5.UNICEF , WHO , World Bank Group, United Nations. Levels and Trends in Child Mortality Report 2017. [cited: 20 April 2020]. Available from: https://www.unicef.org/publications/index_101071.html .
- 6.Tribble DR. Resistant pathogens as causes of traveller's diarrhea globally and impact(s) on treatment failure and recommendations. J Travel Med. 2017;24:S6–S12. doi: 10.1093/jtm/taw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anbazhagan AN, Priyamvada S, Alrefai WA, Dudeja PK. Pathophysiology of IBD associated diarrhea. Tissue Barriers. 2018;6:e1463897. doi: 10.1080/21688370.2018.1463897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller LR. Definitions, pathophysiology, and evaluation of chronic diarrhoea. Best Pract Res Clin Gastroenterol. 2012;26:551–562. doi: 10.1016/j.bpg.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Can J Gastroenterol. 2013;27:653–659. doi: 10.1155/2013/485631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philpott HL, Nandurkar S, Lubel J, Gibson PR. Drug-induced gastrointestinal disorders. Postgrad Med J. 2014;90:411–419. doi: 10.1136/postgradmedj-2013-100316rep. [DOI] [PubMed] [Google Scholar]
- 11.Burgers K, Lindberg B, Bevis ZJ. Chronic Diarrhea in Adults: Evaluation and Differential Diagnosis. Am Fam Physician. 2020;101:472–480. [PubMed] [Google Scholar]
- 12.Schiller LR. Antidiarrheal Drug Therapy. Curr Gastroenterol Rep. 2017;19:18. doi: 10.1007/s11894-017-0557-x. [DOI] [PubMed] [Google Scholar]
- 13.Nalin DR, Cash RA. 50 years of oral rehydration therapy: the solution is still simple. Lancet. 2018;392:536–538. doi: 10.1016/S0140-6736(18)31488-0. [DOI] [PubMed] [Google Scholar]
- 14.Greenland K, Chipungu J, Chilengi R, Curtis V. Theory-based formative research on oral rehydration salts and zinc use in Lusaka, Zambia. BMC Public Health. 2016;16:312. doi: 10.1186/s12889-016-2984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng SX. Calcium-sensing receptor: A new target for therapy of diarrhea. World J Gastroenterol. 2016;22:2711–2724. doi: 10.3748/wjg.v22.i9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szymaszkiewicz A, Storr M, Fichna J, Zielinska M. Enkephalinase inhibitors, potential therapeutics for the future treatment of diarrhea predominant functional gastrointestinal disorders. Neurogastroenterol Motil. 2019;31:e13526. doi: 10.1111/nmo.13526. [DOI] [PubMed] [Google Scholar]
- 17.Wu PE, Juurlink DN. Clinical Review: Loperamide Toxicity. Ann Emerg Med. 2017;70:245–252. doi: 10.1016/j.annemergmed.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Zarghami M, Rezapour M. Loperamide Dependency: A Case Report. Addict Health. 2017;9:59–63. [PMC free article] [PubMed] [Google Scholar]
- 19.Wright GD. Unlocking the potential of natural products in drug discovery. Microb Biotechnol. 2019;12:55–57. doi: 10.1111/1751-7915.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen B. A New Golden Age of Natural Products Drug Discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017;50:111–116. doi: 10.5483/BMBRep.2017.50.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Queiroga CL, Silva GF, Dias PC, Possenti A, de Carvalho JE. Evaluation of the antiulcerogenic activity of friedelan-3beta-ol and friedelin isolated from Maytenus ilicifolia (Celastraceae) J Ethnopharmacol. 2000;72:465–468. doi: 10.1016/s0378-8741(00)00237-3. [DOI] [PubMed] [Google Scholar]
- 23.Bashir S, Memon R, Gilani AH. Antispasmodic and Antidiarrheal Activities of Valeriana hardwickii Wall. Rhizome Are Putatively Mediated through Calcium Channel Blockade. Evid Based Complement Alternat Med. 2011;2011:304960. doi: 10.1155/2011/304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva CMS, Wanderley CWS, Lima-Junior FJB, de Sousa DP, Lima JT, Magalhães PJC, Santos AA, Palheta-Juniot RC. Carvone (R)-(-) and (S)-(+) enantiomers inhibits upper gastrointestinal motility in mice. Flavour Fragr J. 2015;30:439–444. [Google Scholar]
- 25.Dos Santos Negreiros P, da Costa DS, da Silva VG, de Carvalho Lima IB, Nunes DB, de Melo Sousa FB, de Souza Lopes Araújo T, Medeiros JVR, Dos Santos RF, de Cássia Meneses Oliveira R. Antidiarrheal activity of α-terpineol in mice. Biomed Pharmacother. 2019;110:631–640. doi: 10.1016/j.biopha.2018.11.131. [DOI] [PubMed] [Google Scholar]
- 26.Miyazawa M, Miyamoto , Y Biotransformation of (1R)-(+)- and (1S)-(−)-camphor by the larvae of common cutworm (Spodoptera litura) J Mol Catal B-Enzym. 2005;32:123–130. [Google Scholar]
- 27.NIH National Library of Medicine. PubChem. Available from: https://pubchem.ncbi.nlm.nih.gov .
- 28.Him A, Özbek H, Turel I, Öner AC. Antinociceptive activity of alpha-pinene and fenchone. Pharmacologyonline. 2008;3:363–369. [Google Scholar]
- 29.Slavchev I, Dobrikov GM, Valcheva V, Ugrinova I, Pasheva E, Dimitrov V. Antimycobacterial activity generated by the amide coupling of (-)-fenchone derived aminoalcohol with cinnamic acids and analogues. Bioorg Med Chem Lett. 2014;24:5030–5033. doi: 10.1016/j.bmcl.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Küpeli Akkol E, İlhan M, Ayşe Demirel M, Keleş H, Tümen I, Süntar İ. Thuja occidentalis L. and its active compound, α-thujone: Promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J Ethnopharmacol. 2015;168:25–30. doi: 10.1016/j.jep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Algieri F, Rodriguez-Nogales A, Vezza T, Garrido-Mesa J, Garrido-Mesa N, Utrilla MP, González-Tejero MR, Casares-Porcel M, Molero-Mesa J, Del Mar Contreras M, Segura-Carretero A, Pérez-Palacio J, Diaz C, Vergara N, Vicente F, Rodriguez-Cabezas ME, Galvez J. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J Ethnopharmacol. 2016;190:142–158. doi: 10.1016/j.jep.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard- Ninth Edition. CLSI document M07-A9. 2012; 32: Available from: https://www.researchgate.net/file.PostFileLoader.html?id=564ceedf5e9d97daf08b45a2&assetKey=AS%3A297254750572544%401447882463055 .
- 33.Awouters F, Niemegeers CJ, Lenaerts FM, Janssen PA. Delay of castor oil diarrhoea in rats: a new way to evaluate inhibitors of prostaglandin biosynthesis. J Pharm Pharmacol. 1978;30:41–45. doi: 10.1111/j.2042-7158.1978.tb13150.x. [DOI] [PubMed] [Google Scholar]
- 34.Scarpignato C, Capovilla T, Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980;246:286–294. [PubMed] [Google Scholar]
- 35.Stickney JC, Northup DW. Effect of gastric emptying upon propulsive motility of small intestine of rats. Proc Soc Exp Biol Med. 1959;101:582–583. doi: 10.3181/00379727-101-25024. [DOI] [PubMed] [Google Scholar]
- 36.Santos FA, Rao VS. Quinine-induced inhibition of gastrointestinal transit in mice: possible involvement of endogenous opioids. Eur J Pharmacol. 1999;364:193–197. doi: 10.1016/s0014-2999(98)00842-5. [DOI] [PubMed] [Google Scholar]
- 37.Ezeja MI, Anaga AO. Anti-diarrheal activities of the methanolic root bark extract of Cochlospermum planchonii (Hook f) Intl J Toxicol Pharmacol Res. 2010;2:40–45. [Google Scholar]
- 38.Cleeland R, Squires E. Evaluation of new antimicrobials in vitro and experimental animal infections. In: Lorian VMD. Antibiotics in Laboratory Medicine, Baltimore: Williams & Wilkins. 1991; 739-787. [Google Scholar]
- 39.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 40.Holetz FB, Pessini GL, Sanches NR, Cortez DA, Nakamura CV, Filho BP. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz. 2002;97:1027–1031. doi: 10.1590/s0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 41.Sartoratto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT, Rehder VLG. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol . 2004;35:275–280. [Google Scholar]
- 42.Houghton PJ, Howes MJ, Lee CC, Steventon G. Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant. J Ethnopharmacol. 2007;110:391–400. doi: 10.1016/j.jep.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Aleem A, Janbaz KH. Dual mechanisms of anti-muscarinic and Ca++ antagonistic activities to validate the folkloric uses of Cyperus niveus Retz. as antispasmodic and antidiarrheal. J Ethnopharmacol. 2018;213:138–148. doi: 10.1016/j.jep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Shahed-Al-Mahmud M, Shawon MJA, Islam T, Rahman MM, Rahman MR. In Vivo Anti-diarrheal Activity of Methanolic Extract of Streblus asper Leaves Stimulating the Na+/K+-ATPase in Swiss Albino Rats. Indian J Clin Biochem. 2020;35:72–79. doi: 10.1007/s12291-018-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalilzadeh-Amin G, Maham M. Antidiarrheal activity and acute oral toxicity of Mentha longifolia L. essential oil. Avicenna J Phytomed. 2015;5:128–137. [PMC free article] [PubMed] [Google Scholar]
- 46.Rocha Caldas GF, Oliveira AR, Araújo AV, Lafayette SS, Albuquerque GS, Silva-Neto Jda C, Costa-Silva JH, Ferreira F, Costa JG, Wanderley AG. Gastroprotective Mechanisms of the Monoterpene 1,8-Cineole (Eucalyptol) PLoS One. 2015;10:e0134558. doi: 10.1371/journal.pone.0134558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Semenov I, Brenner R. Voltage effects on muscarinic acetylcholine receptor-mediated contractions of airway smooth muscle. Physiol Rep. 2018;6:e13856. doi: 10.14814/phy2.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. 48 Murray D, Abell T. Neuromodulation (Second Edition), Chapter 114Neural Control of the Gastrointestinal System. 2018; 1373-1378 . [Google Scholar]
- 49.Browning KN, Travagli RA. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes. 2019;26:11–16. doi: 10.1097/MED.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Groneberg D, Voussen B, Friebe A. Integrative Control of Gastrointestinal Motility by Nitric Oxide. Curr Med Chem. 2016;23:2715–2735. doi: 10.2174/0929867323666160812150907. [DOI] [PubMed] [Google Scholar]
- 51. Radulovic M, Anand P, Korsten MA, Gong B. Targeting Ion Channels: An Important Therapeutic Implication in Gastrointestinal Dysmotility in Patients With Spinal Cord Injury. J Neurogastroenterol Motil. 2015;21:494–502. doi: 10.5056/jnm15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Formiga RO, Quirino ZGM, Diniz MFFM, Marinho AF, Tavares JF, Batista LM. Maytenus erythroxylon Reissek (Celastraceae) ethanol extract presents antidiarrheal activity via antimotility and antisecretory mechanisms. World J Gastroenterol. 2017;23:4381–4389. doi: 10.3748/wjg.v23.i24.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sisay M, Bussa N, Gashaw T. Evaluation of the Antispasmodic and Antisecretory Activities of the 80% Methanol Extracts of Verbena officinalis L: Evidence From In Vivo Antidiarrheal Study. J Evid Based Integr Med. 2019;24:2515690X19853264. doi: 10.1177/2515690X19853264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Balogun OS, Fadare RY, Fadare AO, Akinpelu DA, Obafemi CA. Chemical Composition and In-vitro Antibacterial Activity of the Essential Oil of Nigerian Moringa oleifera Lam. Flowers. Eur J Med Plants. 2017;18:1–9. [Google Scholar]
- 55. Kaur R, Dhakad MS, Goyal R, Kumar R. Emergence of non-albicans species and antifungal resistance in intensive care unit patients. Asian Pac J Trop Biomed . 2016;6:455–460. [Google Scholar]
- 56. Nigar L, Tarafder S, Khan RR, Ahmed SMA, Saleh AA. Species identification of candida isolated from clinical specimens in a tertiary care hospital. BSMMU J . 2016;9 [Google Scholar]
- 57. Nazzaro F, Fratianni F, Coppola R, Feo V. Essential Oils and Antifungal Activity. Pharmaceuticals (Basel) 2017;10 doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dias IJ, Trajano ERIS, Castro RD, Ferreira GLS, Medeiros HCM, Gomes DQC. Antifungal activity of linalool in cases of Candida spp. isolated from individuals with oral candidiasis. Braz J Biol. 2018;78:368–374. doi: 10.1590/1519-6984.171054. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell G, Lafrance M, Boulanger S, Séguin DL, Guay I, Gattuso M, Marsault E, Bouarab K, Malouin F. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J Antimicrob Chemother. 2012;67:559–568. doi: 10.1093/jac/dkr510. [DOI] [PubMed] [Google Scholar]
- 60. Meirelles GC, Pippi B, Hatwig C, de Barros FMC, de Oliveira LFS, von Poser GL, Fuentefria AM. Synergistic antifungal activity of the lipophilic fraction of Hypericum carinatum and fluconazole. Rev Bras Farmacogn. 2017;27:118–123. [Google Scholar]
- 61. Chudzik B, Bonio K, Dabrowski W, Pietrzak D, Niewiadomy A, Olender A, Malodobry K, Gagoś M. Synergistic antifungal interactions of amphotericin B with 4-(5-methyl-1,3,4-thiadiazole-2-yl) benzene-1,3-diol. Sci Rep. 2019;9:12945. doi: 10.1038/s41598-019-49425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vergis J, Gokulakrishnan P, Agarwal RK, Kumar A. Essential oils as natural food antimicrobial agents: a review. Crit Rev Food Sci Nutr. 2015;55:1320–1323. doi: 10.1080/10408398.2012.692127. [DOI] [PubMed] [Google Scholar]
- 63. Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crop Prod . 2014;62:250–264. [Google Scholar]
- 64. Dagli N, Dagli R, Mahmoud RS, Baroudi K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J Int Soc Prev Community Dent. 2015;5:335–340. doi: 10.4103/2231-0762.165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khan MS, Malik A, Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med Mycol. 2012;50:33–42. doi: 10.3109/13693786.2011.582890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.