Abstract
Mammalian glycosylated rhesus (Rh) proteins include the erythroid RhAG and the non-erythroid RhBG and RhCG. RhBG and RhCG are expressed in multiple tissues, including hepatocytes and the collecting duct (CD) of the kidney. Here we expressed human RhAG, RhBG, and RhCG in Xenopus oocytes (vs. H2O-injected control oocytes) and used microelectrodes to monitor the maximum transient change in surface pH (ΔpHS) caused by exposing the same oocyte to 5% CO2/33 mM HCO3– (an increase) or 0.5 mM NH3/NH4+ (a decrease). Subtracting the respective values for day-matched, H2O-injected control oocytes yielded channel-specific values (*). (ΔpHS*)CO2 and (−ΔpHS*)NH3 were each significantly > 0 for all channels, indicating that RhBG and RhCG—like RhAG—can carry CO2 and NH3. We also investigated the role of a conserved aspartate residue, which was reported to inhibit NH3 transport. However, surface biotinylation experiments indicate the mutants RhBGD178N and RhCGD177N have at most a very low abundance in the oocyte plasma membrane. We demonstrate for the first time that RhBG and RhCG—like RhAG—have significant CO2 permeability, and we confirm that RhAG, RhBG, and RhCG all have significant NH3 permeability. However, as evidenced by (ΔpHS*)CO2/(−ΔpHS*)NH3 values, we could not distinguish among the CO2/NH3 permeability ratios for RhAG, RhBG, and RhCG. Finally, we propose a mechanism whereby RhBG and RhCG contribute to acid secretion in the CD by enhancing not only the transport of NH3 but also the transport of CO2 across the membranes of CD cells.
Keywords: gas channels, Rhesus protein, biotinylation, surface pH, collecting duct
INTRODUCTION
The regulation of blood pH within the normal range (7.35–7.45) is one of the most important physiological processes because the structure and function of virtually all proteins are directly influenced by pH.
With a person on a typical Western diet, cellular metabolism produces ~40 mmol of net H+/day (Giebisch and Windhager 2009). In addition, the transfer of dietary H+ from the gastrointestinal tract to the extracellular fluid, as well as the obligatory loss of alkali in stool, represents a net gain of an additional 30 mmol of H+/day (Giebisch and Windhager 2009). The total daily H+ load of ~70 mmol titrates ~70 mmol of HCO3– from body fluids to produce CO2 (which the lungs excrete) and H2O. If the HCO3– consumed in this buffer reaction were not constantly replenished, a catastrophic metabolic acidosis would ensue. To maintain plasma [HCO3–] and thus pH, the kidney—operating in a steady state—must perform three related tasks. [1] Reabsorb the ~4000 mmol/day of HCO3– filtered in the glomeruli; this operation merely prevents the loss of HCO3– into the urine. [2] Transfer to the blood plasma an additional ~70 mmol/day of “new” HCO3– to replenish the HCO3– lost to H+ buffering. And [3] secrete into the tubule lumen ~70 mmol/day of H+ that is produced during the process of generating the “new” HCO3–. Virtually all of the secreted H+ titrates buffers that increase the H+-carrying capacity of the tubule fluid.
The most important of these urinary buffers is NH3/NH4+, nearly all of which is synthesized de novo by the proximal tubule (PT). Deamidation of glutamine to glutamate and then α-ketoglutarate in the PT mitochondria yields two molecules of NH4+. Metabolism of α-ketoglutarate generates two HCO3– ions, which exit the cell across the basolateral membrane via the Na/HCO3– cotransporter (NBCe1-A; (Boron and Boulpaep 1983; Romero et al. 1997) for entry into the blood. The newly formed NH4+ dissociates in the PT cell to form NH3 and H+. The NH3 exits across the apical membrane of the cell—probably at least in part via AQP1 (Nakhoul et al. 2001; Musa-Aziz et al. 2009a)—entering the tubule lumen. There the NH3 reacts with H+—secreted mainly via the Na-H exchanger NHE3 (Nagami 1988)—to reform NH4+. The medullary thick ascending limb (mTAL) reabsorbs much of the NH4+, which—as considered in the Discussion—eventually enters the lumen of the collecting duct in a complex series of events.
From the above discussion, it is clear that the net movement of NH3/NH4+ from the TAL lumen to the CD lumen is critical. Four lines of evidence suggest that the Rh glycoproteins RhBG and RhCG make a substantial contribution to the movement of NH3 across the membranes of the CD cells:
In heterologous expression systems RhBG and RhCG both transport NH3 (Bakouh et al. 2006; Mak et al. 2006; Weiner and Verlander 2010).
In the kidney, RhBG and RhCG are expressed mainly in the CD α-intercalated cells (Eladari et al. 2002; Quentin et al. 2003; Verlander et al. 2003; Seshadri et al. 2006; Biver et al. 2008; Brown et al. 2009), far more so than in principal cells (Seshadri et al. 2006). RhBG is expressed only in the basolateral membrane (Quentin et al. 2003; Verlander et al. 2003; Han et al. 2006; Kim et al. 2009), whereas RhCG is expressed both in the basolateral (Han et al. 2006; Seshadri et al. 2006; Brown et al. 2009; Kim et al. 2009) and apical membranes (Eladari et al. 2002; Quentin et al. 2003; Verlander et al. 2003; Han et al. 2006; Seshadri et al. 2006; Biver et al. 2008; Brown et al. 2009; Kim et al. 2009).
In response to chronic metabolic acidosis, wild-type (WT) mice increase RhBG (Bishop et al. 2010) and RhCG (Seshadri et al. 2006) protein abundance, consistent with a role in support of acid excretion.
When subjected to chronic metabolic acidosis, mice with intercalated-cell–specific knockouts of RhBG (Bishop et al. 2010) or RhCG (Lee et al. 2010) transiently excrete less urinary NH3/NH4+ than WT mice.
Previous work shows that the Rh glycoprotein related to RhBG and RhCG—namely, RhAG—transports not only NH3 (Ripoche et al. 2006; Musa-Aziz et al. 2009a) but also CO2 (Endeward et al. 2008; Musa-Aziz et al. 2009a). Moreover, when compared to the bacterial Rh protein AmtB and various mammalian aquaporins, RhAG exhibits a characteristic selectivity for NH3 vs. CO2 (Musa-Aziz et al. 2009a; Geyer et al. 2013b). Thus, important questions are whether RhBG and RhCG also conduct CO2 and, if so, how the CO2/NH3 selectivities of RhBG and RhCG compare to those of AQPs and other Rh proteins. In the present study we express RhBG, RhCG, or—as a control—RhAG in Xenopus oocytes and use microelectrodes to monitor the transient changes in cell-surface pH (pHS) as we expose cells to CO2 or NH3. The maximal excursions of pHS (ΔpHS) are semiquantitative indices of CO2 and NH3 permeability. We find that both RhBG and RhCG conduct CO2 and NH3. However, the CO2/NH3 permeability ratios for RhAG, RhBG, and RhCG were indistinguishable from one another. Based on our results, we propose a model whereby basolateral RhBG and RhCG, not only enhance the uptake of NH3 (Mak et al. 2006; Kim et al. 2009; Wagner et al. 2009; Gruswitz et al. 2010) but also of CO2 across the basolateral membranes of the CD cells. This CO2 uptake would be anticipated to promote basolateral Cl-HCO3– exchange and thus help drive H+ secretion into the CD lumen.
MATERIALS AND METHODS
Expression in Xenopus oocytes
cDNA clones.
RhAG and the Xenopus expression vector BSXG have been described previously (Bruce et al. 2009). BSXG-RhBG and RhCG were generated from pGFPC1-RhBG and pGFPC1-RhCG constructs (Brown et al. 2009) by double restriction digest, using Bg1II and XmaI and ligated into BglII and XmaI precut BSXG4 vector. The site-directed mutants RhBGD178N and RhCGD177N were generated using the QuikChange Site-Directed Mutagenesis Kit (Catalog # 200518, Stratagene, Cedar Creek, TX) according to the manufacturer’s protocol. The sequence of all clones was confirmed by DNA sequencing (Eurofin MWG Operon, UK and Keck DNA Sequencing Facility, New Haven, CT).
cRNA synthesis.
The restriction enzyme XhoI was used to linearize the pBSXG plasmid containing human RhBG, RhBGD178N, RhCG, or RhCGD177N cDNAs. The linearized cDNAs were then purified using the QIAquick PCR purification kit (Qiagen Inc., Valencia, CA). Transcribed, capped cRNA was generated using the T7 mMessage mMachine kit (Ambion, Austin, TX) and these cRNAs were purified and concentrated using the RNeasy MinElute RNA Cleanup Kit (Qiagen).
Xenopus oocyte isolation.
Oocytes were isolated from female Xenopus laevis frogs according to methods described previously (Musa-Aziz et al. 2010). Briefly, we surgically removed ovaries from frogs anesthetized in 0.2% MS-222 (Ethyl 3-aminobenzoate methanesulfonate, Sigma-Aldrich, St. Louis, MO). The ovarian lobes were dissected into small pieces and washed in 0-Ca solution (in mM: 98 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.5, osmolality 195 mOsm/kg) prior to enzymatic defolliculation with 2 mg/mL type IA collagenase (Sigma-Aldrich) in 0-Ca. Stage V–VI oocytes were selected and stored at 18°C in filter-sterilized OR3 medium that contained (per 2 liters) one pack of powdered Leibovitz L-15 media (13,7 g/pack) with l-glutamine (GIBCO-BRL), 100 mL of 10,000 U/mL penicillin, 10,000 U/mL streptomycin solution (Sigma-Aldrich), and 5 mM HEPES titrated to pH 7.5, osmolality ~195 mOsm/kg H2O until use.
Microinjection of cRNAs.
One day after isolation, oocytes were injected with either 25 ng of cRNA encoding human RhBG, RhBGD178N, RhCG, or RhCGD177N cRNA (delivered as 25 nL of a 1 ng/nL cRNA solution) or 25 nL of sterile water (Ambion) for control H2O-injected oocytes. After injection, we stored oocytes at 18°C in OR3 medium for 4–5 days before using them in experiments.
Protein expression measurements
Biotinylation.
Biotinylation of plasma-membrane resident proteins was performed using the EZ-Link Sulfo-NHS-Biotinylation Kit (part # 21425, Thermo Fisher Scientific, Rockford, IL) according to the manufacturers recommendations, with some previously described modifications (Geyer et al. 2013a; Geyer et al. 2013b). Briefly, groups of 30 oocytes were incubated with Sulfo-NHS-Biotin biotinylation reagent at 4°C × 1 h. The biotinylation reaction was terminated by adding the supplied quenching buffer and washing the cells in TBS. Cells were lysed by trituration in Lysis buffer (TBS that contained 1% Triton X100 and a cOmplete EDTA-free protease inhibitor tablet; part # 11873580001; Roche, Indianapolis, IN). The insoluble fraction was pelleted by centrifugation and a sample of the supernatant containing solubilized protein (“total protein” fraction) was set aside for western-blot analysis. Biotinylated protein was isolated from the remainder of the solubilized fraction by an incubation at RT × 1 h with immobilized NeutrAvidin gel. Non-biotinylated protein was rinsed from the gel by repeated washing with lysis buffer. Biotinylated protein was subsequently eluted from the gel using 300 μl of 1 × SDS sample buffer (Invitrogen, Carlsbad, CA) containing 50 mM DTT (“biotinylated protein” fraction).
Western-blot analysis.
Total and biotinylated protein samples were separated by SDS-PAGE on 12% Tris-Glycine gels (Invitrogen). The samples were transferred to PVDF membranes using the iBlot apparatus (Invitrogen) × 8 min. The membranes were rinsed with TBST (Tris-buffered saline/Tween, in mM: 50 Tris-Base, 150 NaCl, pH 7.4, 0.1% Tween 20 [#P7949, Sigma-Aldrich]) and then transferred to TBST plus 5% powdered milk. The membranes were probed with one of a number of rabbit primary C-terminal polyclonal antibodies raised against human RhAG (Toye et al. 2008), RhBG or RhCG (Brown et al. 2009), followed by a goat anti-rabbit secondary monoclonal antibody (# AP132P; Millipore, Billerica, MA), and detected using ECL plus Western Blotting Detection Reagents (GE Healthcare Life Sciences, Pittsburgh, PA).
Electrophysiological measurements
Chamber.
Oocytes were placed in plastic perfusion chamber, with a channel 3 mm wide × 30 mm long; saline constantly flowed down this channel at a rate of 4 mL/min. Perfusing solutions were delivered using syringe pumps (Harvard Apparatus, South Natick, MA). Switching between solutions was performed by pneumatically operated valves (Clippard Instrument Laboratory, Cincinnati, OH). All experiments were performed at room temperature (~22°C).
Transport Assay Solutions.
The ND96 solution contained in mM: 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES, pH 7.50, osmolality 195 mOsm. The CO2/HCO3– solution was identical to ND96 except that 33 mM NaHCO3 replaced 33 mM NaCl, and the solution was bubbled with 5% CO2/balanced O2. The 0.5 mM NH3/NH4+ solution was made by first replacing 5 mM NaCl with 5 mM NH3/NH4+ and then diluted the solution 1:10 with standard ND96 solution.
Measurement of surface pH.
Our approach for monitoring surface pH (pHS) of an oocyte has been described in detail elsewhere (Musa-Aziz et al. 2009a; Geyer et al. 2013b). We measured pHS using a pH-electrode with a tip diameter of ~15 μm, which was filled with H+ ionophore mixture B (# 95293 Fluka Chemical Corp., Ronkonkoma, NY), and amplified by a FD223 electrometer (World Precision Instruments, Inc., Sarasota, FL). The external reference electrode for the pHS measurements was a calomel half-cell (connected to a model 750 electrometer, World Precision Instruments) contacting a 3M-KCl–filled micropipette, which contacted the fluid in the chamber. We also recorded pHi and Vm in each experiment, as described previously (Musa-Aziz et al. 2009a; Musa-Aziz et al. 2009b), but do not report these data. The analog subtraction of the calomel-electrode signal from the pHS-electrode signal produced the signal due to pHS. We used an ultra-fine micromanipulator (model MPC-200 system, Sutter Instrument Company, Novato, CA) to position the pHS-electrode tip at the surface of the oocyte, and then to advance it ~40 μm further, forming a slight dimple in the membrane. For routine recalibration of the electrode, we periodically withdrew the electrode from the surface of the oocyte and positioned it in the bulk extracellular fluid (BECF, pH 7.50). The tip of the pHS microelectrode, with respect to the flow of solution, was positioned near the oocyte’s equator, in the “shadow” of the oocyte.
Analysis of pHS data
We used an approach described previously (Endeward et al. 2006; Musa-Aziz et al. 2009a; Musa-Aziz et al. 2009b) to compute the maximum magnitude (i.e., “spike height” or ΔpHS) of the pHS transient elicited by applying a solution containing either extracellular 5% CO2/HCO3– or 0.5 mM NH3/NH4+. In brief, we determined the initial pHS—before the application of CO2/HCO3– or NH3/NH4+—by comparing the pHS-electrode voltage signal when the electrode tip was at the oocyte surface with the voltage signal obtained when the tip was in BECF lacking CO2/HCO3– and NH3/NH4+ (7.50). We determined the maximum pHS during exposure to CO2/HCO3–, or the minimum pHS during exposure to NH3/NH4+ by comparing the voltage signal (at a time corresponding to the extreme pHS value) when the electrode tip was at the oocyte surface with the voltage signal obtained a few minutes later, when the tip was in the BECF containing CO2/HCO3– or NH3/NH4+ (7.50). ΔpHS is the algebraic difference between the extreme and initial pH values. All oocytes used in this study had initial Vm values at least as negative as −40 mV.
In vitro assay of carbonic-anhydrase activity
To determine carbonic anhydrase (CA) activity, we used a colorimetric assay—conducted at 0°C—to monitors a fall in pH (Brion et al. 1988; Musa-Aziz et al. 2009a). The sample mixture consisted of 10 μl of protein (20 μg) from a membrane preparation of oocytes injected with cRNA (25 ng) encoding human CA IV, RhAG, RhBG or RhCG, plus 185 μl H2O and 5 μl of 1-octanol, for a total volume of 200 μl. In some experiments, we reduced the amount of injected hCA IV to 0.25 μg. We bubbled the sample mixture with 100% CO2, and then added 200 μl of buffer/indicator mix (5.0 mM Tris·HCl, 20 mM imidazole, and 0.4 mM para-nitrophenol, pH 8.00), thereby reducing [CO2] by half. The color of this CO2-rich solution was initially yellow, indicating a relatively alkaline solution. A yellow-to-clear color change—due to the reaction CO2 + H2O → HCO3– + H+—indicates the reaction endpoint. We used a stopwatch to measure the time to achieve the endpoint. Protein concentrations of samples were determined using an assay from Pierce (Pierce® BCA Protein Assay Kit, Thermo Scientific).
Statistics
Data are presented as mean ± SEM. To compare the difference between two means, we performed Student’s t tests (two tails). To compare more than two means, we performed a 1-way ANOVA followed by a Student–Newman–Keuls posthoc analysis, using KaleidaGraph (Version 4, Synergy Software). P < 0.05 was considered significant.
RESULTS
Our goal was to use pHS measurements to determine whether—like RhAG—RhBG and RhCG are permeable both to CO2 and NH3. We examined not only the WT proteins but also constructs in which we mutated a highly conserved Asp residue to Asn (Figure 1).
Figure 1.

Multiple sequence alignment of RhAG, RhBG, and RhCG. Using CLUSTALW, a sequence alignment was generated to illustrate the conserved aspartate group in AmtB, RhAG, RhBG, and RhCG. The residue D160 in AmtB (Javelle et al. 2004) and the homologous D177 in RhCG (Marini et al. 2006) have been reported to be critical for NH3 transport. This residue is also conserved in RhAG and RhBG. The WT sequence used for AmtB was Swissprot P69681. GenBank accession numbers for the other proteins were AF031548 (RhAG), AF193807 (RhBG), and AF193809 (RhCG).
pHS transients induced by CO2 entry
Oocytes injected with H2O.
When we add CO2/HCO3– to bulk extracellular fluid, then—if the influx of CO2 dominates over the entry of HCO3– in terms of pHS changes—the entry of CO2 into the cell creates a deficit of CO2 near the outer surface of the membrane (Figure 2a: left side). CO2 diffusion from the BECF partially replenishes the deficit. However, additional replenishment occurs via the following reactions at the outer surface of the cell: HCO3– + H+ → H2CO3 → H2O + CO2. The result is the consumption of protons and thus a rapid rise—or ‘spike’—in pHS that decays exponentially from its peak towards pHBulk as the CO2 influx gradually slows. The light gray record on the left side of Figure 2b shows the pHS trajectory for a H2O-inected oocyte (replicated in Figure 2c–d). The maximal pHS spike height (ΔpHS) is a semi-quantitative index of the rate of CO2 entry. Thus, other things being equal, ΔpHS is an index of the permeability to CO2, although the relationship between ΔpHS and permeability is not expected to be linear (Somersalo et al. 2012).
Figure 2.
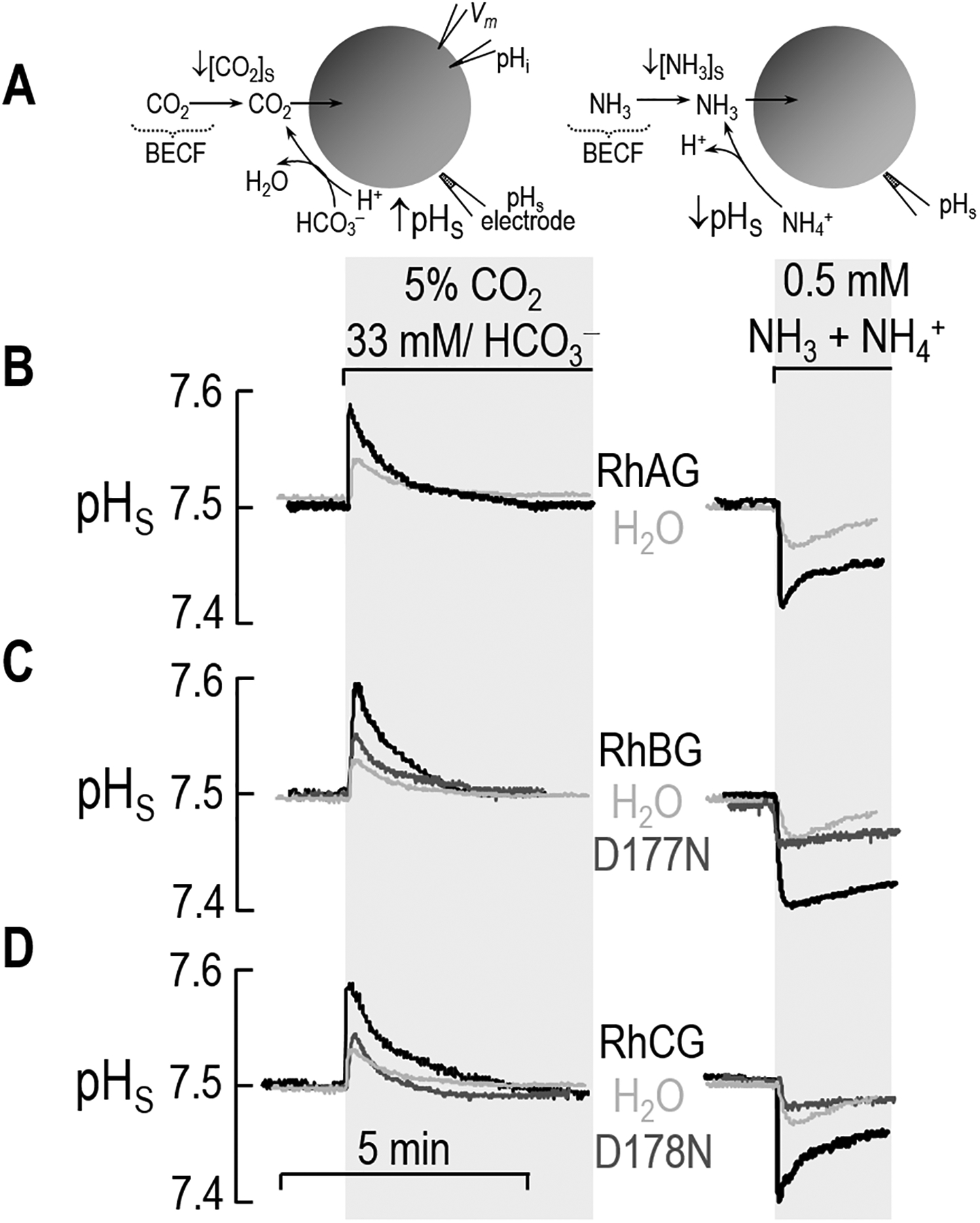
Surface pH (pHS) measurements in oocytes exposed to CO2/HCO3– or NH3/NH4+. a Cell models. b RhAG and H2O. c RhBG, RhBGD178N, and H2O. d RhCG, RhCGD177N, and H2O. In each experiment, the same oocyte is sequentially exposed to ND96, the 5% CO2/33 mM HCO3– solution, ND96 again, and then finally the 0.5 mM NH3/NH4+ solution. During the exposure, pHS measurements are recorded throughout the course of the experiment. We expose the oocyte to CO2/HCO3– for a period of time long enough for the pHS to rise and then decay to a stable value. Then following the washout of CO2/HCO3– (~15 min, long enough for pHi to stabilize), the same oocyte is then exposed to NH3/NH4+. Routinely, we move the electrode away from the surface of the oocyte to calibrate it in the bath solution.
Oocytes expressing RhAG.
The left side of Figure 2b also shows the effect of expressing RhAG on the pHS trajectory elicited by an exposure to CO2 (black record). As was the case with the H2O-injected oocyte, the exposure to CO2/HCO3– causes a rapid rise in pHS, followed by an exponential decay. However, the expression of RhAG increases the magnitude of ΔpHS compared to day-matched H2O-injected control oocyte. Thus, RhAG increases the CO2 permeability of the oocyte, consistent with the earlier work of Musa Aziz et al. (Musa-Aziz et al. 2009a).
Oocytes expressing RhBG.
The left side of Figure 2c (black record) shows that expressing RhBG produces a CO2-induced pHS trajectory that is similar to that observed above with RhAG. On the other hand, with an oocyte expressing RhBGD178N (Figure 2c: gray record), the magnitude of ΔpHS is only slightly greater than that of the day-matched H2O-injected oocyte.
Oocytes expressing RhCG.
The left side of Figure 2d (black record) shows that an oocyte expressing RhCG, likewise, exhibits a much greater ΔpHS than the day-matched H2O-injected control. On other hand, the oocyte expressing RhCGD177N (gray record)—like the one expressing RhBGD178N—produces a pHS trajectory that is indistinguishable from that of the H2O-injected control oocyte.
pHS transients induced by NH3 entry
Oocytes injected with H2O.
When we add NH3/NH4+ to the BECF, then—if the entry of NH3 dominates over the entry of NH4+ in terms of pHS changes—the influx of NH3 creates a deficit in NH3 at the outer surface of the cell membrane (Figure 2a: right side). Diffusion of NH3 from the BECF partially replenishes the deficit. However, the NH3 deficit is also replenished by the following reaction at the outer surface of the cell: NH4+ → NH3 + H+. This results in the production of protons and thus a rapid fall in pHS that decays towards pHBulk as the NH3 influx gradually slows. The light gray record on the right side of Figure 2b (replicated in Figure 2c–d) illustrates the pHS trajectory for a H2O-injected oocyte. The maximum pHS spike depth ΔpHS is a semi-quantitative index of the rate of NH3 entry. Thus, (−ΔpHS) reflects the permeability to NH3.
Oocytes expressing RhAG.
The right side of Figure 2b (black record) shows the effect of expressing RhAG on the ΔpHS changes induced by exposing the cell to NH3. As we saw with the H2O-injected oocyte above, the NH3/NH4+ exposure causes a rapid pHS decrease and a slower decay. However, the magnitude of ΔpHS is substantially greater for the RhAG-expressing oocyte as compared to the H2O-injected oocyte. These results confirm the results of others (Ripoche et al. 2004; Ripoche et al. 2006) and of Musa-Aziz et al. (2009a) that RhAG is an NH3 channel.
Oocytes expressing RhBG.
Previous studies, using methodologies different from those presented in the present work, have demonstrated that RhBG transports NH3 (Ludewig 2004; Zidi-Yahiaoui et al. 2005; Mak et al. 2006). The right side of Figure 2c (black record) shows that an oocyte expressing RhBG responds to NH3 in much the same way as an oocyte expressing RhAG, with a large (−ΔpHS) indicative of NH3 permeability. As we saw with CO2, the oocyte expressing RhBGD178N has a ΔpHS response that is very similar to that of a H2O-injected oocyte when exposed to NH3/NH4+ (Figure 2c: gray record).
Oocytes expressing RhCG.
As is the case for RhBG, the NH3 permeability of RhCG has been reported (Ludewig 2004; Zidi-Yahiaoui et al. 2005; Bakouh et al. 2006; Mak et al. 2006), though using pHi monitoring. The right side of Figure 2d (black record) confirms that RhCG is an effective NH3 channel. However, in the oocyte expressing RhCGD177N, an exposure to NH3/NH4+ elicits a pHS response that is very similar to that of the day-matched H2O-injected oocyte (Figure 2d: gray record).
Although not shown, our recordings of Vm reveal no evidence of an electrogenic flux of NH4+.
Taken together, our data indicate that the WT proteins RhAG, RhBG, and RhCG all transport both CO2 and NH3.
Analysis of surface expression
It has been proposed by Gruswitz et al. (2010) that these conserved Asp residues—D178N in RhBG and D177N in RhCG—serves to stabilize these Rh proteins. If the D-to-N mutations destabilize RhBG and RhCG, then it is possible that the oocyte synthesizes but rapidly degrades the mutants, fails to traffic the mutant to the plasma membrane at a normal rate, or retrieves the mutant from the plasma membrane at an excessive rate. In any case, the surface abundance of the mutant proteins would be low. To investigate the extent to which the low functional expression of RhBGD178N and RhCGD177N reflects a low plasma-membrane abundance, we biotinylated oocytes expressing RhAG, RhBG, RhBGD178N, RhCG, and RhCGD177N, and performed western blotting of both the total protein fraction and the biotinylated (i.e., plasma-membrane–resident) protein fractions using previously described polyclonal C-terminal RhAG, RhBG and RhCG antibodies.
Figure 3a is a western blot that shows the total and surface expression of RhAG. The band at ~38 kDa represents the unglycosylated or core-glycosylated protein, whereas the immunoreactive higher molecular weight pattern centered around 50 kDa is consistent with mature N-linked glycosylated protein. Based on such blots, we estimate that a substantial proportion of the total RhAG expression is resident in the plasma-membrane fraction (44 ± 3%, n = 4), and that most of the surface protein has a mature glycosylation (70 ± 9%, n = 4).
Figure 3.
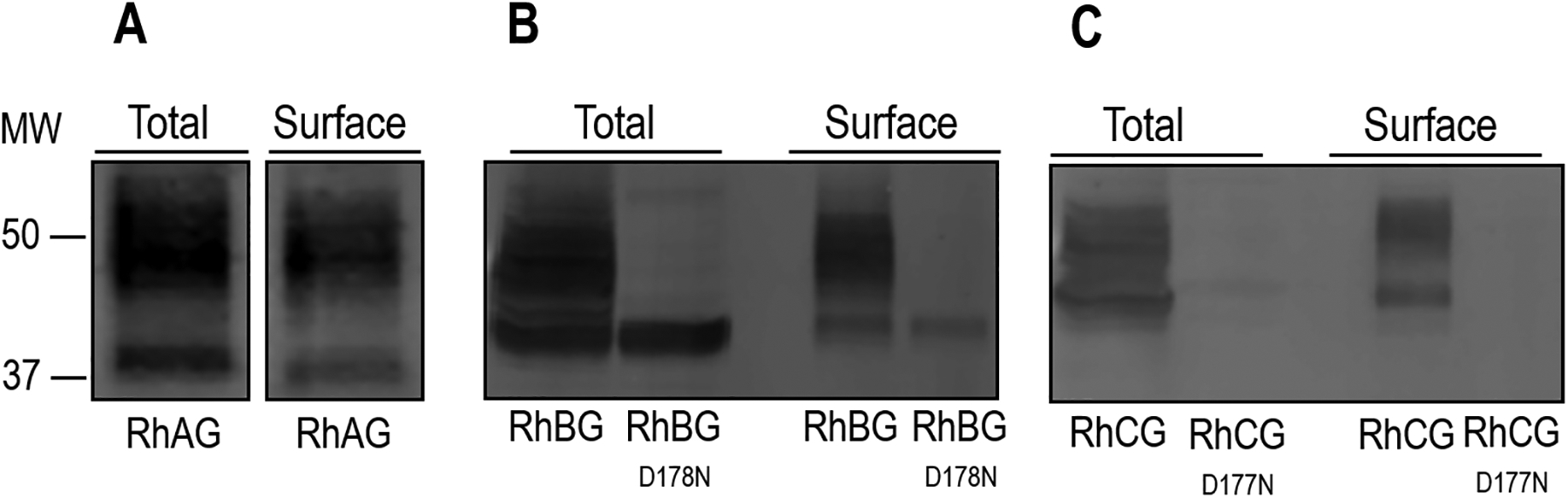
Surface expression of RhAG, RhBG, RhBGD178N, RhCG, and RhCGD177N. We assessed the total and surface expression of RhAG, RhBG, RhBG, RhBGD177N, RhCG, and RhCGD178N by biotinylating 30 intact oocytes injected with cRNA for each protein channel, and used anti-RhAG, anti-RhBG, or anti-RhCG to detect protein abundance. a RhAG. We detect the protein in both the total and surface fractions. There is also a characteristic high molecular weight pattern consistent with mature N-linked glycosylation in both samples. b RhBG. We also detect glycosylated WT RhBG in both total and surface fractions. However, the abundance of RhBGD178N in the total fraction is greatly reduced and lacks any detectable glycoslyation. The abundance of the mutant protein is also greatly reduced at the cell surface. c RhCG. We detect WT protein in the total and surface fractions, but we are unable to detect appreciable amounts of RhCGD177N in either fraction. Molecular weight (MW) markers are displayed to the left.
Figure 3b is similar to Figure 3a except that it focuses on WT RhBG and RhBGD178N. The introduction of the D178N mutation substantially reduces total expression (in our experiments we estimate an average 68 ± 3 % reduction compared to wild-type, n=4). Both WT and RhBGD178N are expressed to the plasma membrane: 43 ± 6 % (n=4) of total in the case of WT and 22 ± 7 % (n=4) for the mutant. An interesting observation is that virtually all of the RhBGD178N protein—both total and surface—is at a molecular weight consistent with unglycosylated or core-glycosylated protein.
Figure 3c is similar to Figure 3b except that it focuses on RhCG and RhCGD177N. We estimate that 30 ± 3 % (n=4) of total WT RhCG protein is resident in the plasma-membrane fraction. On the other hand, RhCGD177N expression is barely detectable in either the total or plasma-membrane fractions. Thus, although injecting oocytes with cRNA encoding WT RhBG or RhCG results in the robust accumulation of the cognate protein in the plasma membrane, the injection of cRNA encoding the mutants RhBGD178N or RhCGD177N results in little protein in the plasma membrane.
Summary of ΔpHS data
Figure 4 summarizes the ΔpHS data for a larger number of experiments like those in Figure 2b–d. Here we ignore the two mutants, which were not appreciably present at the plasma membrane. We pair each oocyte expressing a WT channel with its day-matched, H2O-injected control. Figure 4a shows that the application of CO2/HCO3– yields to a mean ΔpHS for RhAG, RhBG, or RhCG that is significantly greater than that for day-matched H2O-injected controls.
Figure 4.
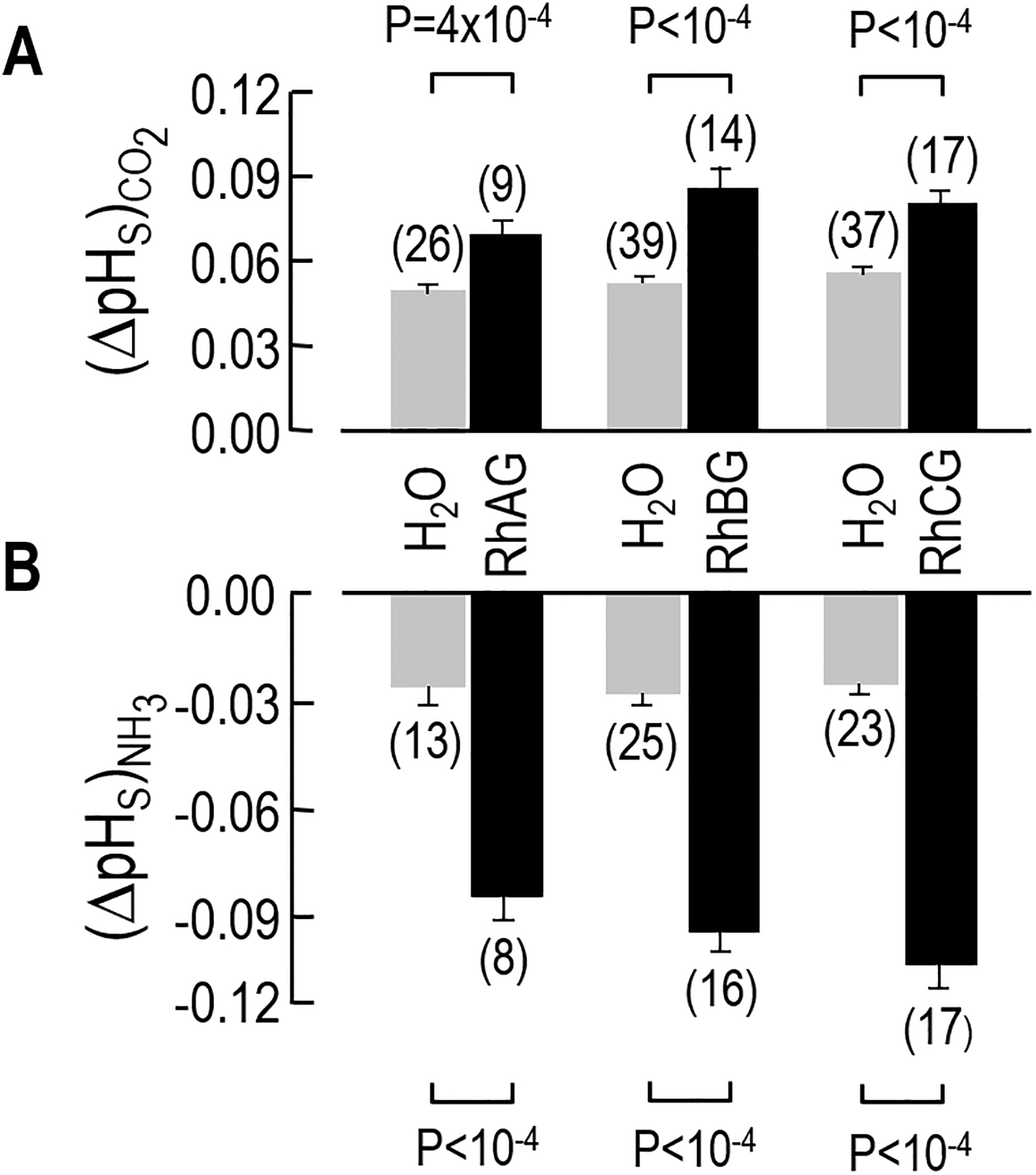
Data summary for the ΔpHS measurements. The bars summarize the results of a larger number of experiments, like those shown in Figure 2. a Maximum pHS excursions evoked by CO2 exposure. Upon exposure to a flowing solution of 5% CO2/33 mM HCO3–, H2O-injected control oocytes become more alkaline. However, in the oocytes expressing RhAG, RhBG, or RhCG, the alkalinization—as shown by a larger (ΔpHS)CO2 value—is greater than that of H2O injected oocytes. b Maximum pHS excursions evoked by NH3 exposure. When the same oocyte is exposed to 0.5 mM NH3/NH4+, the magnitude of the acidification (ΔpHS)NH3 in the RhAG-, RhBG-, or RhCG-expressing oocytes is also greater than in H2O-injected control oocytes. For RhBGD178N and the RhCGD177N the (ΔpHS)CO2 and (ΔpHS)NH3 values (not shown) are not statistically different from the H2O injected controls. We performed Student’s t-test (two tails) for statistical comparisons.
Figure 4b shows that the mean ΔpHS produced by the application of NH3/NH4+ for RhAG, RhBG, or RhCG is significantly greater than that for day-matched H2O-injected controls. These observations demonstrate that RhBG and RhCG—like RhAG—function not only as NH3 channels but also as CO2 channels.
Channel-dependent gas transport
The portion of the CO2-induced ΔpHS signal that we can ascribe to a particular channel is the difference between the ΔpHS of each channel-expressing oocyte (e.g., black bars in Figure 4a) and the mean ΔpHS of the day-matched H2O-injected controls (e.g., light gray bars in Figure 4a). Figure 5a summarizes these differences, computed oocyte by oocyte, for the CO2 data—the channel-dependent signal (ΔpHS*)CO2. Similarly, Figure 5b summarizes the analogous differences for the NH3 data—the channel-specific signal (ΔpHS*)NH3. The six mean values—semiquantitative indices of channel-dependent gas permeability—are all significantly greater than zero. Note that the values in Figure 5 are not true permeabilities, but rather indices of relative CO2 or NH3 permeabilities, as determined by the product of intrinsic (or per-channel) gas conductance and the number of channels proteins in the plasma membrane.
Figure 5.
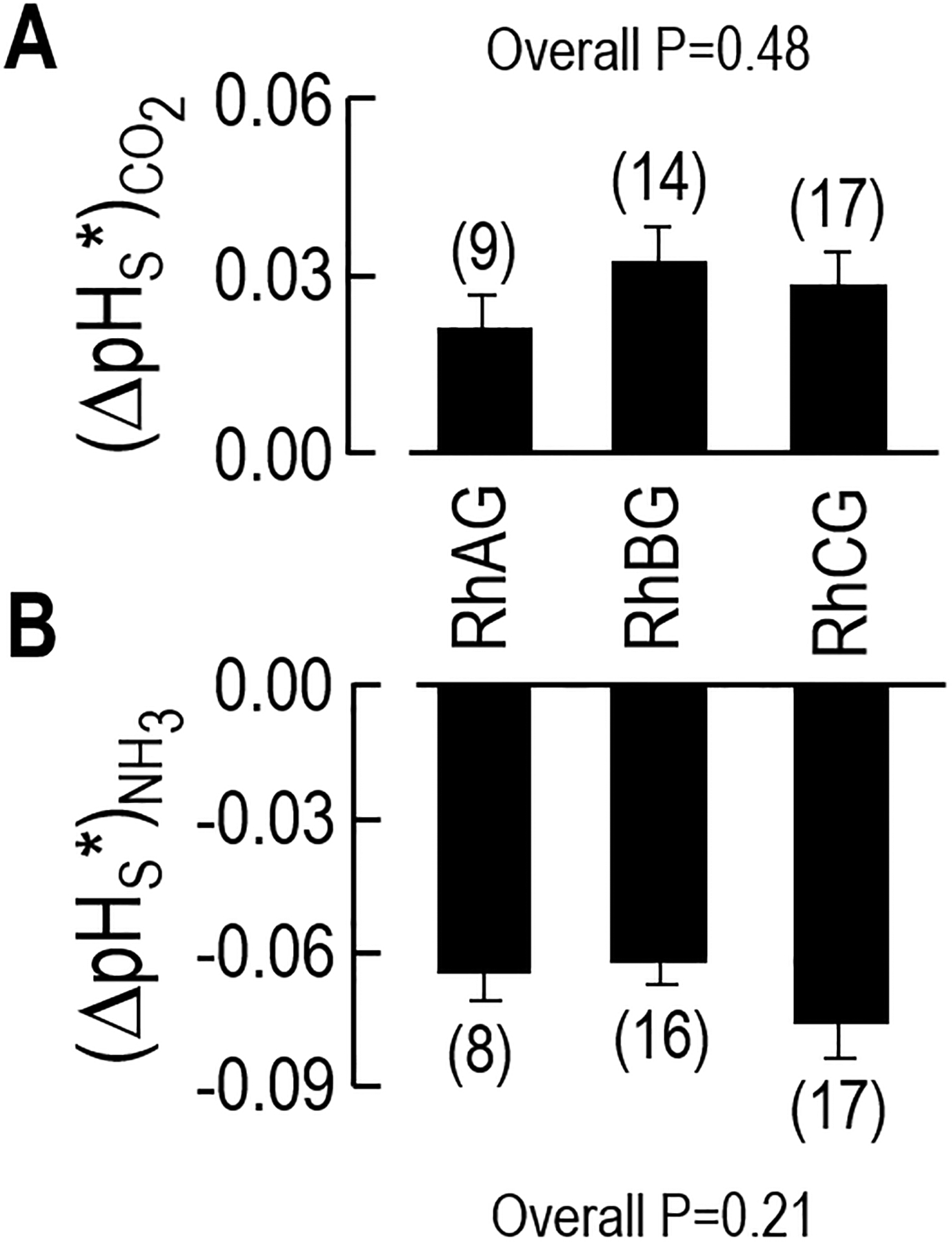
Index of channel-dependent permeability to CO2 or NH3. a Channel-dependent ΔpHS for CO2. b Channel-dependent ΔpHS for NH3. By subtracting the (ΔpHS)CO2 or (ΔpHS)NH3 for the H2O-injected control oocytes (see Figure 4) from the (ΔpHS)CO2 or (ΔpHS)NH3 of RhAG-, RhBG-, or RhCG-expressing oocytes (see Figure 4), we obtain (ΔpHS*)CO2, a semiquantitative index of channel-dependent CO2 permeability, or (−ΔpHS*)NH3, a semiquantitative index of channel-dependent NH3 permeability. In all cases, the ΔpHS values are significantly different from zero. However, the (ΔpHS*)CO2 values are similar for RhAG, RhBG, and RhCG; the same is true for the (−ΔpHS*)NH3 values. We did not compute these values for RhBGD178N and RhCGD177N, inasmuch as these proteins are not expressed at the oocyte membrane surface. We performed a 1-way ANOVA to assess statistical significance.
Ratios of indices of permeability—Gas Selectivity
In three previous studies, we have examined the relative CO2/NH3 selectivities of the aquaporins 0–9 (Musa-Aziz et al. 2009a; Geyer et al. 2013b), RhAG (Musa-Aziz et al. 2009a), the bacterial Rh homolog AmtB (Musa-Aziz et al. 2009a), and the urea transporter UT-B (Geyer et al. 2013a). We found that each channel has a characteristic ratio (ΔpHS*)CO2/(ΔpHS*)NH3, which is a relative index of the actual CO2/NH3 permeability ratio. From the data that contribute to Figure 5, we can obtain similar information about RhBG and RhCG by dividing, oocyte by oocyte, (ΔpHS*)CO2 by the (ΔpHS*)NH3—or conversely, dividing (ΔpHS*)NH3 by (ΔpHS*)CO2. The numerical values in Figure 6 are not ratios of true permeabilities, but relative indices of CO2/NH3 or NH3/CO2 permeability ratios that we can compare from channel to channel if we obtain the data under identical experimental conditions. Our 1-way ANOVA indicates no statistically significant difference among the ratios in Figure 6.
Figure 6.
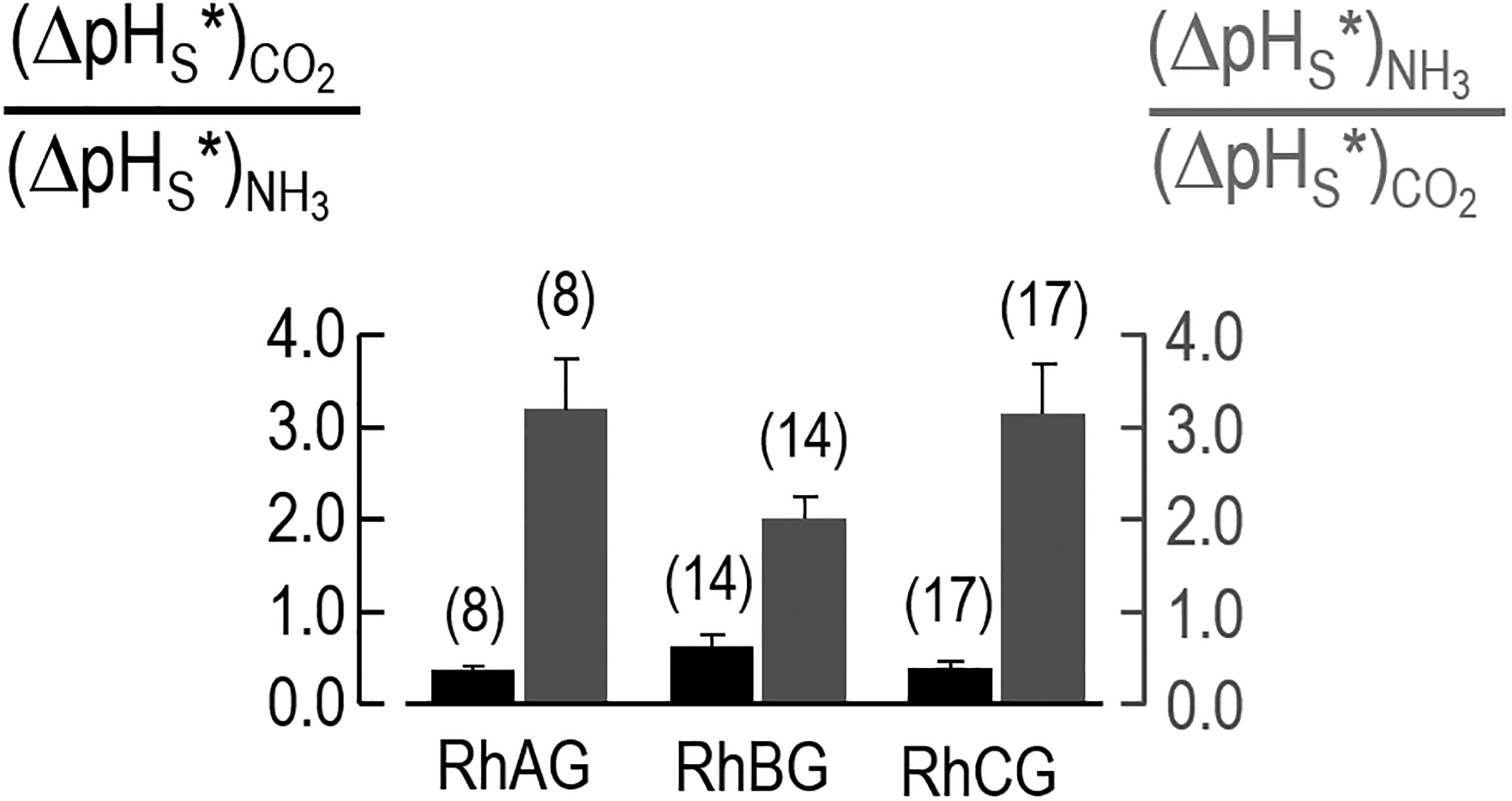
Gas selectivity of RhAG, RhBG, and RhCG. Using the data underlying Figure 5, we calculated an index of relative CO2/NH3 permeability ratio by dividing, oocyte by oocyte, (ΔpHS*)CO2 by (−ΔpHS*)NH3 or (−ΔpHS*)NH3 by (ΔpHS*)CO2.
Carbonic Anhydrase Activity
In principle, the enhanced pHS spike produced by exposing Rh-expressing oocytes to CO2 (see left side of Figure 2b–d, and data summarized in Figure 4a and Figure 5a) could have been caused, not by CO2 conduction through the Rh protein, but by carbonic anhydrase (CA) activity in the Rh protein itself or an oocyte protein expressed in response to the Rh protein. To test the CA hypothesis, we injected oocytes with H2O or with cRNA encoding CA IV (in which the catalytic domain is coupled via a GPI linkage to the outer surface of the membrane), RhAG, RhBG or RhCG. Previous work has shown that graded increases in the amount of injected cRNA encoding CA IV causes a graded increase in ΔpHS (see supplemental Fig 2 in (Musa-Aziz et al. 2009a)). Figure 7a shows that membrane preparations of oocytes injected with 12 ng CA IV cRNA/oocyte (we obtained similar results with 0.25 ng/oocyte; not shown)—compared to H2O oocytes—require a much shorter time to achieve the pH endpoint in a colorimetric CA assay. However, membrane preparations of RhAG, RhBG or RhCG oocytes are indistinguishable from those of H2O. Figure 7b–c show that oocytes from this preparation, when exposed to CO2 or NH3, exhibited pHS changes similar to those in Figure 5. Thus, we can rule out the hypothesis that the expression of RhAG, RhBG, or RhCG increase the size of CO2-induced pHS changes by engendering CA activity in either the cytosol or on the surface of the oocyte.
Figure 7.
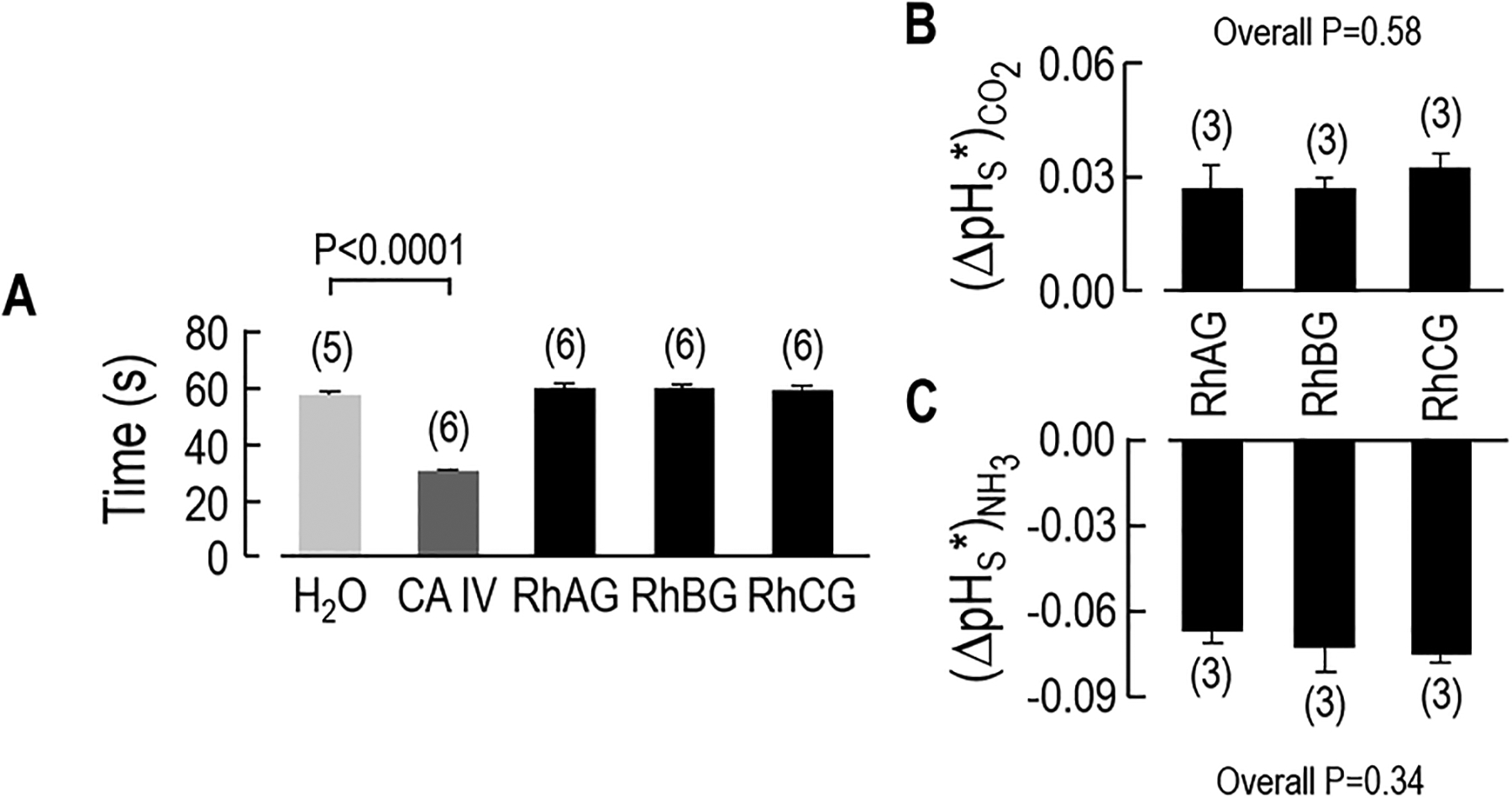
Assessing the carbonic-anhydrase activity of oocytes expressing Rh proteins. a Colorimetric assays of carbonic-anhydrase activity of membrane preparations created from oocytes injected with H2O (negative control) or 12 ng/oocyte of cRNA encoding CA IV (positive control) or 25 ng/oocyte of cRNA encoding RhAG, RhBG, or RhCG. The value on the y axis indicates the time necessary for the color to change. We injected 100 oocytes of each type, made a membrane preparation of each group, and then repeated the colorimetric assay the indicated number of times. Other experiments (not shown) revealed that the time to the color change (~30 s) was the same after injecting either 25 ng cRNA/oocyte or 0.25 ng/oocyte. b Channel-dependent ΔpHS for CO2 addition in 3 oocytes from the same batch of oocytes used in panel ‘a’. c Channel-dependent ΔpHS for NH3 addition for the same 3 oocytes as in panel ‘b’. Values are means ± SE, with nos. of oocytes in parentheses. For panel a, we performed Student’s t-test (two tails) for statistical comparisons and for panel b and c, we performed one-way ANOVA, followed by Student-Newman-Keuls analyses.
DISCUSSION
Overview
In the present study, we have made two main observations. First, we show for the first time that RhBG and RhCG transport not only NH3 but also CO2 (Figure 2, Figure 4). Bakouh et al performed one preliminary experiment on a H2O-injected oocyte and one on a RhCG-expressing oocyte in which they monitored intracellular pH (pHi) while exposing the cells to a solution containing CO2 (Bakouh et al. 2006). Their data are consistent with the hypothesis that RhCG increase the rate of CO2-induced fall in pHi. We are aware of no reports concerning the CO2 permeability of RhBG. Han et al point out that RhBG and RhCG, although present in the bronchial epithelium of the lung, are absent from alveoli and thus are not in a position to contribute to CO2 transport (Han et al. 2009). However, they did not examine the CO2 permeabilities of the two Rh proteins.
Second, we find that the mutation of the conserved aspartate residues in RhBG (D178N) or RhCG (D177N) substantially reduces the abundance of the channel protein in the oocyte plasma membrane (Figure 3). Consistent with this interpretation, we find that the mutation of RhBG results in the near-total loss of the high-MW product that presumably represents mature, glycosylated RhBG. Others had proposed that these residues play a role in the deprotonation of NH4+ (Javelle et al. 2004; Marini et al. 2006). Our data do not allow us to address the deprotonation hypothesis, inasmuch as the surface abundance of the mutant RhBG and RhCG is so low as to preclude the detection of channel-mediated transport. Our data do support the proposal of Gruswitz et al. (2010) that the mutation of the residues would cause a structural disruption.
The RhBG cDNA used in the present study is the original human full-length clone (Lopez et al. 2005). Recently, Han et al. (2013) described a new variant of human RhBG, caused by a deletion of a single cytosine base, resulting in a frame shift in which residues 425–441 (17 aa) are replaced by new residues 425–459 (35 aa). Because the N terminus and the entire transmembrane spanning domains are unaffected, we think it is likely that the permeability properties of the original and new forms of RhBG are identical. The implications of the new variant for trafficking and regulation remain to be explored.
Gas channels
The dogma had been that all gases freely diffuse through all membranes simply by dissolving into and diffusing through the lipid phase of the membrane. However, numerous publications have challenged this view. The first evidence challenging the diffusion of gases across membranes came from the observation that apical membranes of gastric-gland cells are impermeable to CO2 and NH3 (Waisbren et al. 1994), and apical membranes of colonic crypts are impermeable to NH3 (Singh et al. 1995). The second piece of evidence challenging the dogma was the identification of the first family of gas channels, with the demonstration that AQP1, heterologously expressed in Xenopus oocytes, can conduct CO2 (Nakhoul et al. 1998; Cooper and Boron 1998). In these experiments, the authors used as an index of CO2 permeability the initial rate at which pHi declines (dpHi/dt). Because this dpHi/dt approach is somewhat insensitive, Nakhoul et al enhanced CO2 influx by injecting CA II protein into the oocytes, whereas Cooper and Boron dissected away the vitelline membrane. Neither of these auxiliary maneuvers is necessary with the pHS approach used in the present study. Co-expression of CA II presumably would make the increase ΔpHS to an extent in the present study, limited by the overall dynamic range of our system (solution changes, chamber, pHS electrode).
Later work showed that AQP1 also conducts NH3 (Nakhoul et al. 2001) and nitric oxide (Herrera et al. 2006; Herrera and Garvin 2007). The rhesus proteins became the second known family of gas channels with the demonstration that they can conduct NH3 (Ripoche et al. 2004). Work with red blood cells (RBCs) demonstrated that the Rh complex contributes CO2 permeability (Endeward et al. 2008). We recently described a third family of gas channels, exemplified by the urea transporter UT-B, which conducts NH3 (Geyer et al. 2013a). Moreover, work on AQPs and rhesus proteins expressed in Xenopus oocytes indicates that each channel has a characteristic selectivity for CO2 vs. NH3 (Musa-Aziz et al. 2009a; Geyer et al. 2013b).
The CO2 permeability of plant aquaporins is important for providing CO2 for photosynthesis (Uehlein et al. 2003; Kaldenhoff and Fischer 2006; Kaldenhoff 2012; Uehlein et al. 2012), and the CO2 permeability of AQP1 (Endeward et al. 2006) is responsible for about half of the CO2 permeability of RBCs.
Preliminary work shows that either the injection of CA II or the expression of CA IV increases both the maximal rate of pHi descent and ΔpHS spike caused by CO2 influx (Musa-Aziz, Occhipinti & Boron, unpublished). The CA assays summarized in Figure 7a rule out the possibility that the enhanced CO2-induced pHS changes produced by RhAG, RhBG, and RhCG are due to the CA activity of the rhesus proteins per se, or of endogenous oocyte proteins. Previous work (Musa-Aziz et al. 2009a) led to a similar conclusion for AQP1. Thus, we can conclude that—like several AQPs—the three rhesus proteins examined in the present study act as channels for CO2 and NH3.
Possible physiological roles of RhBG and RhCG
The ability of RhBG and RhCG to conduct both CO2 and NH3 is reminiscent of the gas-transport properties of the related erythroid rhesus protein, RhAG (Musa-Aziz et al. 2009a). In RBCs, the CO2 permeability of RhAG, could enhance the uptake of CO2 in systemic tissues, for delivery to the lung. Similarly, the NH3 permeability of RhAG could promote the uptake the NH3 from systemic tissues, for delivery to the liver for detoxification.
What roles do RhBG and RhCG play in the CD in acid-base homeostasis? Others have proposed that the NH3 permeabilities of RhBG and RhCG are critical for NH3/NH4+ secretion during the defense against metabolic acidosis (Biver et al. 2008; Lee et al. 2009; Bishop et al. 2010; Gruswitz et al. 2010; Lee et al. 2010; Wagner et al. 2011; Weiner and Verlander 2011). Figure 8 summarizes the handling of NH3/NH4+ by the mTAL and CD. The mTAL reabsorbs NH4+ via apical Na/K/2Cl cotransporters (with NH4+ replacing K+), and K+ channels (Attmane-Elakeb et al. 2001). Inside the TAL cell, NH4+ dissociates into H+ and NH3. Via unknown mechanisms, the NH3 exits across the TAL basolateral membrane and enters the interstitial fluid of the renal medulla. A portion of this NH3 recycles back to the late PT and thin descending limb, some NH3 enters the blood stream for detoxification to urea in the liver, and the remaining NH3 passes through the basolateral and apical membranes of the collecting duct (CD) cell and enters the lumen, where it is trapped as NH4+ and excreted in the urine. To the extent that NH3/NH4+ moves from the TAL lumen to the CD lumen, it bypasses the cortical segments of the distal nephron, where—due to the permeability of the cortical nephron segments to NH3/NH4+ and the greater blood flow of the cortex vs. the medulla—toxic quantities of NH3/NH4+ could otherwise escape into the blood. Moreover, any NH4+ that escapes into the blood represents a net loss of urinary NH3/NH4+ that would compromise acid-base balance.
Figure 8.
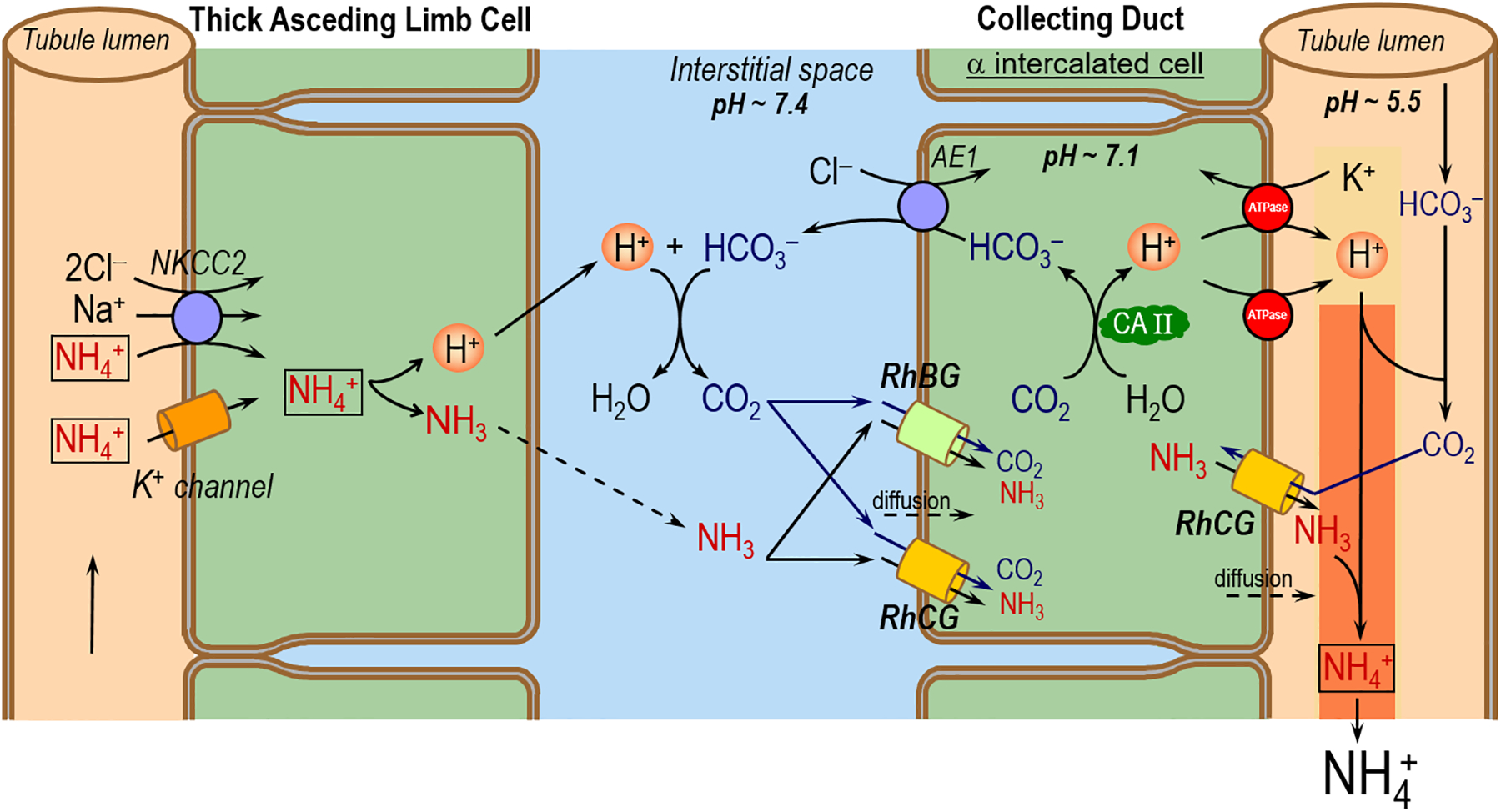
Proposed novel model for CO2 and NH3 transport across the basolateral and apical membranes of αIC cell in the CD. NKCC2 (Na-K-2Cl cotransporter). AE1 (chloride bicarbonate exchanger). CA II (carbonic anhydrase II). This mechanism, particularly the aspects related to CO2 movements, is an extension to the models proposed by others (Gruswitz et al. 2010). The dashed arrows represent the possible diffusion of CO2 or NH3 across plasma membranes.
The sustained formation of NH4+ in the CD lumen requires not only NH3 secretion across the apical membrane, but also H+ secretion to titrate the secreted NH3 to NH4+. This secreted H+ also titrates other luminal buffers, including some HCO3–. Regardless of what the secreted H+ titrates, an equivalent amount of HCO3– must exit the cell across the basolateral membrane of the CD cell via the renal form of AE1 (Romero 2005). The source of this cytosolic HCO3– is CO2. The aforementioned models neither implicitly assume that CO2 can freely enter the collecting-duct cell across the basolateral membrane by an unspecified mechanism, or explicitly state that the CO2 enters by dissolving in the lipid phase of the plasma membrane. We propose that the bifunctional RhBG and RhCG channels in the CD are important not only for mediating NH3 uptake across the basolateral membrane but also for mediating CO2 uptake. Moreover, to the extent that the H+ secreted into the CD lumen titrates a small amount of luminal HCO3– to form CO2, this CO2 could enter the αIC via apical RhCG. Thus, the RhBG and RhCG would enhance CO2 uptake into the αIC, thereby speeding luminal H+ secretion.
Conclusions
Our results confirm the observation that the human Rhesus family of transporters—namely RhAG, RhBG, and RhCG—exhibit significant permeability to NH3 and show for the first time that RhBG and RhCG can conduct CO2. We could not assess the effect of specific Asp to Asn mutations (equivalent to D160 in AmtB) on the CO2 and NH3 permeability of RhBG and RhCG because these mutants have an extremely low abundance in the oocyte plasma membrane. Finally, we could not distinguish the CO2/NH3 permeability ratios of RhAG, RhBG, and RhCG in this study.
ACKNOWLEDGEMENTS
We thank Dale Huffman for computer support, Dr Alice Brown (University of Bristol) for plasmid cloning, and Dr. Nancy Amaral Rebouças (University of São Paulo) and Dr. Seong-Ki Lee for helpful discussions. RRG was supported by postdoctoral fellowship N00014-09-1-0246 from the Office of Naval Research. RMA was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP # 08/128663). This work was supported by Office of Naval Research grant N00014-11-1-0889 and NIH grant DK81567 to WFB. AMT received funding from Kidney Research UK and NHS Blood and Transplant R&D.
REFERENCES
- Attmane-Elakeb A, Amlal H, Bichara M (2001) Ammonium carriers in medullary thick ascending limb. Am J Physiol Renal Physiol 280:F1–9. [DOI] [PubMed] [Google Scholar]
- Bakouh N, Benjelloun F, Cherif-Zahar B, Planelles G (2006) The challenge of understanding ammonium homeostasis and the role of the Rh glycoproteins. Transfus Clin Biol 13:139–146. doi: 10.1016/j.tracli.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Bishop JM, Verlander JW, Lee H-W, et al. (2010) Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299:F1065–1077. doi: 10.1152/ajprenal.00277.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biver S, Belge H, Bourgeois S, et al. (2008) A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456:339–343. doi: 10.1038/nature07518 [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL (1983) Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol 81:53–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion LP, Schwartz JH, Zavilowitz BJ, Schwartz GJ (1988) Micro-method for the measurement of carbonic anhydrase activity in cellular homogenates. Anal Biochem 175:289–297. [DOI] [PubMed] [Google Scholar]
- Brown ACN, Hallouane D, Mawby WJ, et al. (2009) RhCG is the major putative ammonia transporter expressed in the human kidney, and RhBG is not expressed at detectable levels. Am J Physiol Renal Physiol 296:F1279–1290. doi: 10.1152/ajprenal.00013.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LJ, Guizouarn H, Burton NM, et al. (2009) The monovalent cation leak in overhydrated stomatocytic red blood cells results from amino acid substitutions in the Rh-associated glycoprotein. Blood 113:1350–1357. doi: 10.1182/blood-2008-07-171140 [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Boron WF (1998) Effect of pCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol 275:C1481–1486. [DOI] [PubMed] [Google Scholar]
- Eladari D, Cheval L, Quentin F, et al. (2002) Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13:1999–2008. [DOI] [PubMed] [Google Scholar]
- Endeward V, Cartron J-P, Ripoche P, Gros G (2008) RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22:64–73. doi: 10.1096/fj.07-9097com [DOI] [PubMed] [Google Scholar]
- Endeward V, Musa-Aziz R, Cooper GJ, et al. (2006) Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20:1974–1981. doi: 10.1096/fj.04-3300com [DOI] [PubMed] [Google Scholar]
- Geyer RR, Musa-Aziz R, Enkavi G, et al. (2013a) Movement of NH3 through the Human Urea Transporter B (UT-B): A New Gas Channel. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00609.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer RR, Musa-Aziz R, Qin X, Boron WF (2013b) Relative CO2/NH3 selectivities of mammalian Aquaporins 0–9. Am J Physiol, Cell Physiol. doi: 10.1152/ajpcell.00033.2013 [DOI] [PubMed] [Google Scholar]
- Giebisch G, Windhager EE (2009) Urine Concentration and Dilution In: Boron WF, Boulpaep EL (eds) Medical Physiology. A Cellular and Molecular Approach, 2nd ed. Elsevier Saunders, Philadelphia, PA, pp 835–850 [Google Scholar]
- Gruswitz F, Chaudhary S, Ho JD, et al. (2010) Function of human Rh based on structure of RhCG at 2.1 Å. Proc Natl Acad Sci USA 107:9638–9643. doi: 10.1073/pnas.1003587107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-H, Croker BP, Clapp WL, et al. (2006) Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17:2670–2679. doi: 10.1681/ASN.2006020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-H, Lee H-W, Handlogten ME, et al. (2013) Expression of the ammonia transporter family member, Rh B Glycoprotein, in the human kidney. Am J Physiol Renal Physiol 304:F972–981. doi: 10.1152/ajprenal.00550.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-H, Mekala K, Babida V, et al. (2009) Expression of the gas-transporting proteins, Rh B glycoprotein and Rh C glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol 297:L153–163. doi: 10.1152/ajplung.90524.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera M, Garvin JL (2007) Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol 292:F1443–1451. doi: 10.1152/ajprenal.00353.2006 [DOI] [PubMed] [Google Scholar]
- Herrera M, Hong NJ, Garvin JL (2006) Aquaporin-1 transports NO across cell membranes. Hypertension 48:157–164. doi: 10.1161/01.HYP.0000223652.29338.77 [DOI] [PubMed] [Google Scholar]
- Javelle A, Severi E, Thornton J, Merrick M (2004) Ammonium sensing in Escherichia coli. Role of the ammonium transporter AmtB and AmtB-GlnK complex formation. J Biol Chem 279:8530–8538. doi: 10.1074/jbc.M312399200 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R (2012) Mechanisms underlying CO2 diffusion in leaves. Curr Opin Plant Biol 15:276–281. doi: 10.1016/j.pbi.2012.01.011 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Fischer M (2006) Aquaporins in plants. Acta Physiol (Oxf) 187:169–176. doi: 10.1111/j.1748-1716.2006.01563.x [DOI] [PubMed] [Google Scholar]
- Kim H-Y, Verlander JW, Bishop JM, et al. (2009) Basolateral expression of the ammonia transporter family member Rh C glycoprotein in the mouse kidney. Am J Physiol Renal Physiol 296:F543–555. doi: 10.1152/ajprenal.90637.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-W, Verlander JW, Bishop JM, et al. (2010) Effect of intercalated cell-specific Rh C glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299:F369–379. doi: 10.1152/ajprenal.00120.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-W, Verlander JW, Bishop JM, et al. (2009) Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296:F1364–1375. doi: 10.1152/ajprenal.90667.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Métral S, Eladari D, et al. (2005) The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J Biol Chem 280:8221–8228. doi: 10.1074/jbc.M413351200 [DOI] [PubMed] [Google Scholar]
- Ludewig U (2004) Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J Physiol (Lond) 559:751–759. doi: 10.1113/jphysiol.2004.067728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D-OD, Dang B, Weiner ID, et al. (2006) Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol 290:F297–305. doi: 10.1152/ajprenal.00147.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Boeckstaens M, Benjelloun F, et al. (2006) Structural involvement in substrate recognition of an essential aspartate residue conserved in Mep/Amt and Rh-type ammonium transporters. Curr Genet 49:364–374. doi: 10.1007/s00294-006-0062-5 [DOI] [PubMed] [Google Scholar]
- Musa-Aziz R, Boron WF, Parker MD (2010) Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes. Methods 51:134–145. doi: 10.1016/j.ymeth.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Chen L-M, Pelletier MF, Boron WF (2009a) Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106:5406–5411. doi: 10.1073/pnas.0813231106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa-Aziz R, Grichtchenko II, Boron WF (2005) Evidence from surface-pH transients that CA IV & CA II enhances CO2 influx into Xenopus oocytes. J Am Soc Nephrol 16:TH–PO016. [Google Scholar]
- Musa-Aziz R, Jiang L, Chen L-M, et al. (2009b) Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4+. J Membr Biol 228:15–31. doi: 10.1007/s00232-009-9155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagami GT (1988) Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest 81:159–164. doi: 10.1172/JCI113287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF (1998) Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol 274:C543–548. [DOI] [PubMed] [Google Scholar]
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL (2001) Transport of NH3/NH4+ in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281:F255–263. [DOI] [PubMed] [Google Scholar]
- Occhipinti R, Musa-Aziz R, Boron WF (2012) Mathematical modeling of the role of carbonic anhydrase II and IV on the influx of CO2 in a Xenopus oocyte. FASEB J 26:882.9.22075646 [Google Scholar]
- Quentin F, Eladari D, Cheval L, et al. (2003) RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14:545–554. [DOI] [PubMed] [Google Scholar]
- Ripoche P, Bertrand O, Gane P, et al. (2004) Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc Natl Acad Sci USA 101:17222–17227. doi: 10.1073/pnas.0403704101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche P, Goossens D, Devuyst O, et al. (2006) Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: a stopped-flow analysis. Transfus Clin Biol 13:117–122. doi: 10.1016/j.tracli.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Romero MF (2005) Molecular pathophysiology of SLC4 bicarbonate transporters. Curr Opin Nephrol Hypertens 14:495–501. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF (1997) Expression cloning and characterization of a renal electrogenic Na+CO3- cotransporter. Nature 387:409–413. doi: 10.1038/387409a0 [DOI] [PubMed] [Google Scholar]
- Seshadri RM, Klein JD, Kozlowski S, et al. (2006) Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290:F397–408. doi: 10.1152/ajprenal.00162.2005 [DOI] [PubMed] [Google Scholar]
- Singh SK, Binder HJ, Geibel JP, Boron WF (1995) An apical permeability barrier to NH3/NH4+ in isolated, perfused colonic crypts. Proc Natl Acad Sci USA 92:11573–11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somersalo E, Occhipinti R, Boron WF, Calvetti D (2012) A reaction-diffusion model of CO2 influx into an oocyte. J Theor Biol 309:185–203. doi: 10.1016/j.jtbi.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye AM, Williamson RC, Khanfar M, et al. (2008) Band 3 Courcouronnes (Ser667Phe): a trafficking mutant differentially rescued by wild-type band 3 and glycophorin A. Blood 111:5380–5389. doi: 10.1182/blood-2007-07-099473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425:734–737. doi: 10.1038/nature02027 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R (2012) The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ 35:1077–1083. doi: 10.1111/j.1365-3040.2011.02473.x [DOI] [PubMed] [Google Scholar]
- Verlander JW, Miller RT, Frank AE, et al. (2003) Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol 284:F323–337. doi: 10.1152/ajprenal.00050.2002 [DOI] [PubMed] [Google Scholar]
- Wagner CA, Devuyst O, Belge H, et al. (2011) The rhesus protein RhCG: a new perspective in ammonium transport and distal urinary acidification. Kidney Int 79:154–161. doi: 10.1038/ki.2010.386 [DOI] [PubMed] [Google Scholar]
- Wagner CA, Devuyst O, Bourgeois S, Mohebbi N (2009) Regulated acid-base transport in the collecting duct. Pflugers Arch 458:137–156. doi: 10.1007/s00424-009-0657-z [DOI] [PubMed] [Google Scholar]
- Waisbren SJ, Geibel JP, Modlin IM, Boron WF (1994) Unusual permeability properties of gastric gland cells. Nature 368:332–335. doi: 10.1038/368332a0 [DOI] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW (2010) Molecular physiology of the Rh ammonia transport proteins. Curr Opin Nephrol Hypertens 19:471–477. doi: 10.1097/MNH.0b013e32833bfa4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ID, Verlander JW (2011) Role of NH3/NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300:F11–23. doi: 10.1152/ajprenal.00554.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidi-Yahiaoui N, Mouro-Chanteloup I, D’Ambrosio A-M, et al. (2005) Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391:33–40. doi: 10.1042/BJ20050657 [DOI] [PMC free article] [PubMed] [Google Scholar]


