Abstract
Blueberry is an important agricultural crop with high nutritional, health, and economic value. Despite the well‐studied blueberry cultivation methods and soil requirements, little is known about how beneficial bacteria function in organic blueberry cultivation systems and their effects on acidic soils. In this study, a single bacteria Bacillus amyloliquefaciens JC65 and three biocontrol bacteria consortiums containing JC65 were applied to organic system. The effect of bacteria to blueberry growth, yield, fruit quality, and soil quality was investigated. A consortium of three mixed Bacillus (B. amyloliquefaciens JC65, B. licheniforims HS10 and B. subtilis 7ze3) showed the highest growth improvement efficiency. The bacterial inoculation increased blueberry leaf chlorophyll content, net photosynthetic rate by 21.50%, 13.21% at 30 days, and increased average plant height by 2.72% at 69 days. Compared with the control, the inoculated plants showed an increased yield of 14.56%. Interestingly, blueberry fruit quality was also improved with supplement of the bacterial consortium. Fruit anthocyanin, soluble sugar, vitamin C, soluble solids, and soluble protein content were increased by 5.99%, 4.21%, 17.31%, 2.41%, and 21.65%, respectively. Besides, beneficial bacterial consortium also enables sustainable agriculture by improving soil ammonium nitrogen and organic matter by 3.77% and 2.96% after blueberry planting. In conclusion, the combination of beneficial bacteria showed a synergistic activity in organic system to promote the blueberry yield, fruit quality, and soil nutrient preservation.
Keywords: beneficial bacteria consortium, blueberry, organic system, soil quality, yield
We conclude that addition of beneficial microbe in organic blueberry production significantly promotes plant growth and improves blueberry fruit quality. Interestingly, by combination of different beneficial microorganism, the promotion effect on blueberry production was enhanced significantly, indicating synergistic activity among specific mixture of bacteria. The soil retention ability of the beneficial microbe is also shown, especially for increase of soil organic matter.

1. INTRODUCTION
Blueberry is attracting keen interest all over the world for its high nutritional value, rich flavors, and health properties. As a result of consumer's well receiving of healthy food, world blueberry production has increased rapidly over the recent decades. With the development of the economy and the awareness of the people's health care, China's blueberry production has rapidly prospered from a small industry to a leader in the Asia‐Pacific region in just a few years. In 2016, China's blueberry planting acreage accounts for more than 20% of the world's total, and produce around 20 thousand tons of fresh blueberry (Brazelton & Young, 2014). Even so, the growth of blueberry production still lags behind the consumers’ demand (Villata, 2012). Therefore, optimizing blueberry production conditions and increasing blueberry yield are of great significance to society and economy.
Most consumers have a positive attitude toward organic foods, and the market prospects of healthy organic foods are generously optimistic (Kihlberg & Risvik, 2007). Specifically, consumers who are interested in purchasing blueberry as a health‐friendly fruit are often willing to pay for additive value of organic blueberry as a “reduced‐risk” product (Drummond, Smagula, Annis, & Yarborough, 2009). Besides benefits on food safety, organic farming system enables formation of a benign cycle based on a healthy ecological environment by improving biodiversity, soil environment, and protecting the ecological environment. Broad‐spectrum insecticides, for instance, are effective in controlling agricultural pests. However, they also threaten the living and multiply of natural enemies and seriously endanger the agricultural ecological environment. On the contrary, products approved for use in organic agriculture are compatible with beneficial insects, which in turn support natural biological pest management (Roubos, Rodriguez‐Saona, Holdcraft, Mason, & Isaacs, 2014). Moreover, compared with other crops, blueberry has unique advantages in embracing organic cultivation because of its relatively high vigor and resistance to pests due to a short domestication history of <100 years (Sciarappa et al., 2008).
Although organic blueberry production has become an important part of the blueberry industry worldwide, in most areas, organic blueberry production still faces many challenges, such as increased production costs or inputs (especially in fertilization and weed management in an organic way), limited choice of disease or pest control methods, and low production of organic blueberries (Pretty, 1997). Beneficial microbe, often known as plant growth‐promoting rhizobacteria (PGPR), is considered to be one of the solutions to those problems. PGPR participate in multiple physiological processes of plants including establishment of plant morphology, plant growth, cycling of nutrient, and disease defense (Wu, Cao, Li, Cheung, & Wong, 2005). Some of the PGPR have been reported to induce plant systemic resistance against both pathogen and insect by stimulating SA and JA/ET pathway (Niu et al., 2011). In another study, application of two Bacillus strains, OSU‐142 and M3, has shown positive effect on raspberry growth and yield (Orhan, Esitken, Ercisli, Turan, & Sahin, 2006). Besides beneficial effect on plant, PGPR also have the potential to alter soil properties in the long term. Secretions of B. cereus AR156 are able to influence plant root secrets (Zhou et al., 2016). The change of root secrets has effects on plant growth; moreover, it provides more organic matter, such as lactic acid and caproic acid, for the soil (Wang et al., 2019). It has also been shown that PGPR are able to promote soil water retention (Zheng et al., 2018). Nevertheless, information about the effect of PGPR on blueberry growth, yield, and soil nutrients in organic planting system is still scarce.
In this study, we evaluated the effect of single and complex beneficial microbe on blueberry growth, yield, fruit quality as well as soil content after one season of planting in an organic cultivation system by analyzing the major corresponding parameters.
2. MATERIALS AND METHODS
2.1. Preparation of bacteria consortium
Bacillus amyloliquefaciens JC65, B. subtilis 7ze3, B. subtilis JC03, and B. licheniforims HS10 were grown at 28°C in LB medium for 24 hr. The four bacterial cultures were then adjusted to ~5 × 107 cfu/ml with water. The diluted bacteria culture was mixed by the ratio of 1:1:1 in M1 (JC65 + 7ze3 + JC03), M2 (JC65 + 7ze3 + HS10), and M3 (JC65 + JC03 + HS10).
2.2. Field experiment
The field trial began in December 2016 and last for 2 years. The experiment site is located at Lanmei agricultural ecological park, Kuizhang village, Nanjing city (E 118° 22′ 51.67′′, N 31° 56′ 12.14′′). The blueberry variety used in this trial is “Lanmei No.1”, which was selected by Zhejiang Lanmei Agricultural Co., Ltd. The blueberry seedlings were transplanted in 2016.
Five treatments were carried out in this study, including a control treatment C, for an organic system with no addition of beneficial microbe; treatment S, for an organic system supplemented with single beneficial bacteria B. amyloliquefaciens JC65; treatment M1, M2, and M3, for organic systems supplemented with mixed beneficial bacteria of JC65 + 7ze3 + JC03, JC65 + 7ze3 + HS10, and JC65 + JC03 + HS10, respectively. We used JC65 as the core strain in the formula because, firstly, JC65 had previously been reported to have significant promotion effect on the watermelon's resistance to Acidovorax avenae subsp. citrulli, as well as on plant growth (Jiang et al., 2015). The purpose of this experiment design was to screen for more effective consortiums in promoting organic blueberry growth by using the beneficial microorganism JC65 as the core strain with two other alternate Bacillus environmental isolates. The feasibility of the three‐strain mixtures was analyzed by testing the difference between the effects of single bacteria and different consortiums on blueberry production. Moreover, we also wanted to summarize the preliminary rules of bacteria mixture to guide the development of other bacteria consortiums by comparing the effects of different three‐strain consortiums.
In 2016, well‐ventilate field with loose soil, high organic content, and no water accumulation was chosen for experiment. One tone per hectare of sulfur and organic compost made from peat, wood chips, rotten, and pine crust were applied into the selected field. Soil was covered with black film to maintain soil temperature and humidity. Dry straw was placed in the furrows as cover crop to help control the grass. Blueberries were planted on mounds with a space of 1.5 m, and plant spacing of each blueberry was 1.2 m. Approximately 3,750 seedlings were planted per hectare. One mound of the field with 30 seedlings was set as one treatment. Each treatment was repeated 3 times on the mounds randomly distributed in the whole field. In February 2017 and February 2018, organic fertilizer, which was mainly composed of sheep manure, was applied into the field with an amount of 7,500 kg/ha. Drip irrigation was employed in each system to control soil moisture and prevent the disease‐leading excessive humidity. Manual weeding was equally carried out in all tested organic systems. Sweet and sour liquid (sugar: vinegar: wine: juice: clear water = 1.5:1:1:1:5) was applied near the orchard to trap adult flies and chafers during mature and harvesting period (from late May to July 2018). Diluted 0.6% matrine water solution was sprayed 600 ml/ha to control pests such as flies. Blueberry fruit was picked by hand. In order to maintain strong shoots and optimize fruit quality rather than maximizing yield, trees were pruned during winter.
2.3. Determination of growth index
In each treatment, 24 plants from the three repeat mounds were randomly selected. Plant height was measured with a tape measure. The leaf chlorophyll content was determined by chlorophyll meter (TYS‐A) from Zhejiang Top Yunnong Technology Co., Ltd. The net photosynthetic rate of the leaves of 9 randomly selected blueberry trees for each treatment was determined with LI‐COR Li‐6400 photosynthetic apparatus.
2.4. Soil sampling and determination
Soil samples were taken before treatment (December 2016) and after harvest (August 2018). Soil samples were collected according to the five‐point sampling method at the same sampling depth of about 10–15 cm below the ground. Plant roots and small rocks were removed and the soil was air‐dried. After grinding, the ammonium nitrogen (AN), available phosphorus (AP), and available potassium (AK) were determined with a soil tester (TPY‐6A type), and the pH value of the soil was measured with a pH meter (Ray‐Magnetic PHS‐3C type). Soil EC values were determined using a conductivity meter (Bante 950). Soil organic matter (OM) percentage was determined with oil bath heated potassium dichromate oxidation‐reduction titration (Schollenberger, 1931).
2.5. Fruit quality determination
For each treatment, 15 blueberry fruits with similar size and hardness were selected and squeezed. The juice was mixed thoroughly and sampled 3 times to determine soluble sugar with a saccharimeter LB32T and soluble solids with a refractometer ATAGO PAL‐1. One gram of fruit flesh was sampled and ground from five fruits with similar size and hardness in each treatment. Vitamin C was determined with ultraviolet spectrophotometry (Santos, Lima, Março, & Valderrama, 2016). The Coomassie Brilliant Blue G‐250 staining method was used to determine the soluble protein content (Jones, Hare, & Compton, 1989). The titratable acid content was determined by phenolphthalein titration (Alamo, Maquieira, Puchades, & Sagrado, 1993). Fourteen fruits with similar maturity were lyophilized and ground in liquid nitrogen. Two grams of ground material was collected to determine total anthocyanin content (Solomakhin & Blanke, 2010). Assays were repeated three times.
2.6. Statistical analysis
Data were analyzed using Statistical Analysis Software (SAS Institute). One‐way analysis of variance (ANOVA) was used. Means were separated using the least‐significant difference test (LSD).
3. RESULTS
3.1. PGPR effects on blueberry growth and yield
Thirty days after treatment, the growth index of blueberry, chlorophyll content, and net photosynthetic rate of blueberry leaves were measured. Compared with the organic cultivation control C, the composite bacteria treatment (M1, M2, M3) increased the chlorophyll content of the blueberry leaves by 15.33%, 21.50%, and 9.47%, respectively. There was no significant difference on chlorophyll content between the single bacterial treatment S and the control C. Moreover, M1, M2, and M3 treatments increased the average net photosynthetic rate of blueberry leaves by 11.79%, 13.21%, and 9.65%, respectively, compared with the control C. However, due to a substantial in‐treatment variation of leaves, no significant difference on net photosynthetic rate was found among the treatments (Table 1). Sixty‐nine days after treatment, blueberry growth index and leaves chlorophyll content were also recorded. Compared with the control, the composite bacteria treatment (M1, M2, M3) increased the chlorophyll content of the blueberry leaves by 1.47%, 3.03%, and 2.49%, respectively. In addition, M1, M2, and M3 increased the average height of blueberry than control C by 2.40%, 2.72%, and 2.62%, respectively, but without significant difference between treatments (Table 1).
Table 1.
Growth indexes of blueberries at different time periods after treatment
| Treatment | 0 day aftertreatment | 30 days after treatment | 69 days after treatment | |||
|---|---|---|---|---|---|---|
| Plant height (cm) | Plant height (cm) | Chlorophyll (SPAD) | Net photosynthetic rate (μmol CO2 m−2 s−1) | Plant height (cm) | Chlorophyll (SPAD) | |
| C | 92.00 ± 0.75A | 93.25 ± 0.96A | 34.32 ± 0.33D | 6.300 ± 0.466A | 93.83 ± 0.48A | 57.05 ± 0.16BC |
| S | 92.00 ± 0.71A | 93.75 ± 1.15A | 35.20 ± 0.51D | 6.225 ± 0.326A | 94.58 ± 0.77A | 57.02 ± 0.70C |
| M1 | 92.00 ± 0.81A | 94.13 ± 0.52A | 39.58 ± 0.62B | 7.043 ± 0.644A | 96.08 ± 1.16A | 57.89 ± 0.32ABC |
| M2 | 92.17 ± 0.95A | 93.79 ± 0.82A | 41.70 ± 0.27A | 7.132 ± 0.558A | 96.38 ± 1.68A | 58.78 ± 0.27A |
| M3 | 92.00 ± 0.73A | 93.88 ± 0.65A | 37.57 ± 0.57C | 6.908 ± 0.523A | 96.29 ± 1.17A | 58.47 ± 0.20AB |
Data are showed as mean ± standard deviation. The values with different uppercases are significantly different among different treatments at p < .01. C: blank control; S: JC65; M1: JC65 + 7ze3 + JC03; M2: JC65 + HS10 + 7ze3; M3: JC65 + JC03 + HS10.
The total yield of blueberry was also calculated after harvest. M1, M2, and M3 improved blueberry yield by 3.45%, 14.56%, and 6.78% compared with the control C, respectively. The yield of blueberry treated with M2 is significantly higher than that of other treatments. However, single bacterial treatment S showed no significant yield improvement than the control. No significant difference on single fruit weight was shown among all treatments (Figure 2).
FIGURE 2.
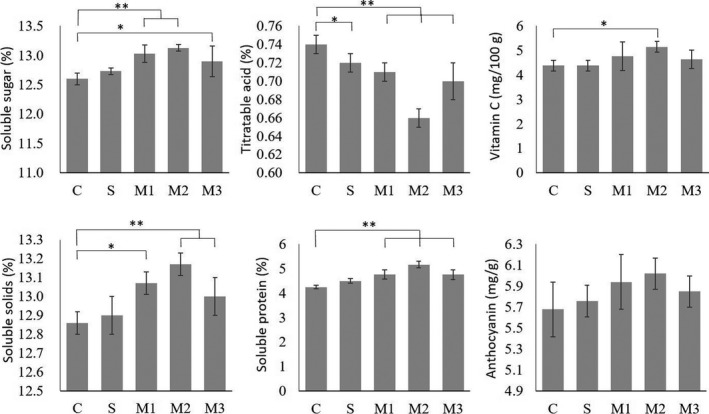
Soluble sugar content (%), titratable acid content (%), vitamin C content (mg/100g), soluble solids content (%), soluble protein content (%), and anthocyanin content (mg/g) of blueberry under different treatments. C: blank control; S: JC65; M1: JC65 + 7ze3 + JC03; M2: JC65 + HS10 + 7ze3; M3: JC65 + JC03 + HS10. Significant differences are marked as: “*” for p < .05, “**” for p < .01
3.2. PGPR effects on blueberry fruit quality
The quality of blueberry fruit, including soluble sugar, titratable acid, vitamin C, soluble solid, soluble protein, and anthocyanin content, was also determined. Results showed that compared with the control C, the compound bacteria treatment M1, M2, and M3 significantly improve the fruit quality of blueberries. Among all, the M2 treatment showed the best effect on fruit quality with an increase on soluble sugar, vitamin C, soluble solids, and soluble protein content by 4.21%, 17.31%, 2.41%, and 21.65% (Figure 2), respectively. Moreover, the titratable acid was reduced by 10.81% in M2 treatment (Figure 2). The average anthocyanin content of M2 treatment was also 5.99% higher than control. The single‐strain treatment S also increased the quality of blueberry fruit by 1.03% on average soluble sugar content and 5.88% on average soluble protein (Figure 2). However, this increase was not statistically significant.
3.3. PGPR effects on soil quality
We collected soil samples before and after harvest and determined the basic physical and chemical properties of the soil. The soil properties changed after one season of planting in organic system, which was used as control in this study. The content of ammonium nitrogen in the soil decreased 20.64% in the control, while an increase of 3.77% was detected in M2 treatment (Figure 3). Soil organic matter decreased 6.17% in the control organic system by the harvest time; on the contrary, organic matter content in soil increased significantly in M1, M2, and M3 treatment after planting by 2.07%, 2.79%, and 2.04%, respectively (Figure 3). For available potassium and phosphorus content, although decreased in all the treatments, the decrease rate in M2 treatment was significantly lower than other treatments. In the single bacterial treatment S, the decline rate of soil organic matter content was significantly lower than that of control treatment; however, no other significant difference on soil physical and chemical properties in this treatment was detected compared with the control C treatment (Table 2). Soil EC value decreased at harvest in C treatment, while in composite bacteria treatment, especially in M2 and M3, the drop was more significant.
FIGURE 3.
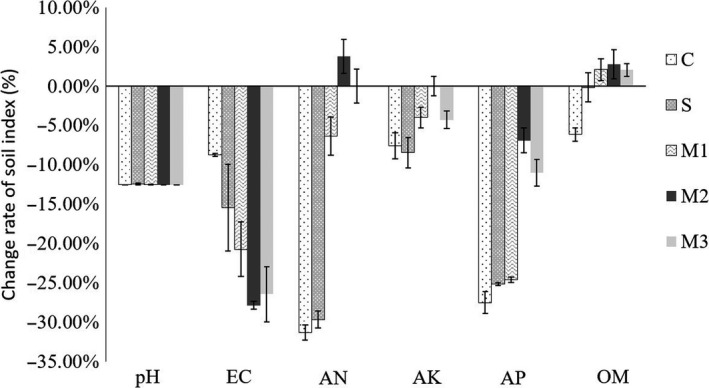
Change rate of soil indexes in different treatments after one season of organic cultivation of blueberry. C: blank control; S: JC65; M1: JC65 + 7ze3 + JC03; M2: JC65 + HS10 + 7ze3; M3: JC65 + JC03 + HS10. AN, ammonium nitrogen; AK, available potassium; AP, available phosphorus; OM, organic matter
Table 2.
Soil indexes before and after treatment
| Treatment | pH | EC (µS cm−1) | AN (‰) | AK (‰) | AP (‰) | OM (%) | |
|---|---|---|---|---|---|---|---|
| C | Before treatment | 6.38 ± 0.01a | 355.00 ± 14.11a | 26.61 ± 0.59ab | 140.40 ± 1.40ab | 39.97 ± 0.19a | 2.14 ± 0.02a |
| After treatment | 5.58 ± 0.01b | 324.00 ± 14.11b | 18.27 ± 0.37e | 129.73 ± 2.91d | 28.97 ± 0.82d | 2.01 ± 0.02c | |
| S | Before treatment | 6.38 ± 0.01a | 373.33 ± 29.74a | 25.84 ± 0.53b | 141.50 ± 2.31a | 40.03 ± 0.09a | 1.99 ± 0.07c |
| After treatment | 5.58 ± 0.02b | 315.67 ± 10.69b | 20.50 ± 0.40d | 129.50 ± 6.84d | 34.97 ± 0.19c | 1.99 ± 0.02c | |
| M1 | Before treatment | 6.38 ± 0.02a | 358.00 ± 16.70a | 26.27 ± 0.87b | 141.23 ± 2.71a | 39.95 ± 0.43a | 2.00 ± 0.06c |
| After treatment | 5.58 ± 0.02b | 283.67 ± 8.50c | 24.61 ± 0.79c | 135.57 ± 3.39bc | 35.12 ± 0.14c | 2.05 ± 0.02bc | |
| M2 | Before treatment | 6.38 ± 0.01a | 365.67 ± 19.14a | 26.55 ± 0.52ab | 140.23 ± 0.96ab | 40.06 ± 0.16a | 2.03 ± 0.05bc |
| After treatment | 5.58 ± 0.01b | 263.67 ± 10.60c | 27.55 ± 0.96a | 140.23 ± 2.80ab | 37.29 ± 1.07b | 2.09 ± 0.02ab | |
| M3 | Before treatment | 6.37 ± 0.01a | 369.00 ± 27.22a | 26.54 ± 0.56ab | 139.23 ± 1.21ab | 39.96 ± 0.45a | 2.03 ± 0.04bc |
| After treatment | 5.57 ± 0.01b | 271.33 ± 14.98c | 26.54 ± 0.44ab | 133.23 ± 3.83cd | 35.56 ± 0.77c | 2.07 ± 0.01b | |
Data are showed as mean ± standard deviation. The values with different lowercases are significantly different among different treatments at p < .05. C: blank control; S: JC65; M1: JC65 + 7ze3 + JC03; M2: JC65 + HS10 + 7ze3; M3: JC65 + JC03 + HS10.
Abbreviations: AK, available potassium; AN, ammonium nitrogen; AP, available phosphorus; OM, organic matter.
4. DISCUSSION
Successful symbioses of plant and microbe, such as leguminous plants and rhizobia, have long been recognized to benefit plant health and growth on various aspects. For highbush blueberry (Vaccinium corymbosum L.), ericoid mycorrhizae spontaneously colonize blueberry roots and form mutually beneficial symbiotic relationship (Smith & Read, 2008). Ericoid mycorrhizal promote the plant growth by degrading the soil organic nutrient and delivering it to mycorrhizal plant (Read, Leake, & Langdale, 1989), for instance, producing acid extracellular protease to break down soil proteins into blueberry available N sources (Bajwa & Read, 1985). However, little is known about the effect of PGPRs on blueberry planting. PGPRs have been shown to have beneficial effect on plant by controlling plant disease by either indirectly repressing pathogens or inducing plant systemic resistance, helping to improve soil quality or directly motivating plant growth by activating changes on expression profiles of plant growth‐related genes (Bhattacharyya & Jha, 2012). There have been reports that PGPRs could be applicable agents against plant disease in blueberry production. With a targeted delivery by honeybee, B. subtilis effectively control mummy berry disease by suppressing growth of the causal agent Monilinia vaccinii‐corymbosi (Dedej, Delaplane, & Scherm, 2004; Scherm, Ngugi, Savelle, & Edwards, 2004). In our study, we compared blueberry growth and production in organic agriculture system with or without different bacteria treatments. We found that addition of beneficial bacteria into organic blueberry cultivation system promotes blueberry plant growth and significantly increases blueberry yield, especially in M2 (7ze3 + JC65 + HS10) treatment, in which a 14.56% higher blueberry yield was achieved (Figure 1). Jha and Subramanian (2018) have reported that the accumulation of soluble sugars in plants negatively regulates plant photosynthesis and sugar biosynthesis, while inoculation with beneficial microorganisms increases the accumulation of soluble sugar in plants by enhancing photosynthesis. Similarly, we found that leaf chlorophyll content in M2 treatment was significantly higher than the control treatment (Table 1). Additionally, mean net photosynthetic rate in M2 treatment was also 13.21% higher than the control (Table 1). These results suggest that beneficial bacteria, especially bacterial consortium M2, have the ability to induce photosynthesis and consequently result in higher growth indexes and yield. The improved growth and yield are in consistent with former report that B. amyloliquefaciens JC65 (former name 54) was able to promote growth of watermelon (Jiang et al., 2015). However, the growth promotion effect of JC65 in this study is 2.72% which is much lower than the total protein promotion rate of 110% reported on watermelon. This may be due to the limited experimental period compared with growth cycle of perennial woody plant. Alternatively, the acidic soil condition required for satisfactory growth of blueberry (Harmer, 1945) may also influence the growth and the plant growth‐promoting effect of bacteria. In the future, screening for PGPRs that are compatible with acidic soil will be a potential direction to improve the efficacy of PGPRs utilization on blueberry production.
FIGURE 1.
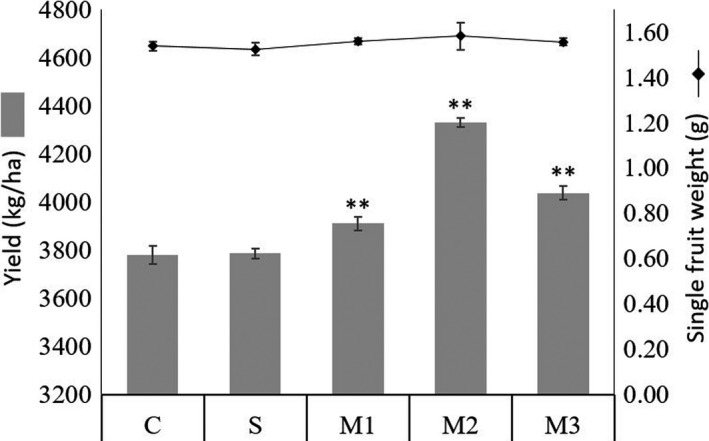
Blueberry yield (gray columns) and single fruit weight (connected scatter plots) under different treatments. C: blank control; S: JC65; M1: JC65 + 7ze3 + JC03; M2: JC65 + HS10 + 7ze3; M3: JC65 + JC03 + HS10. Significant differences are marked as: “**” for p < .01
Blueberry fruit is not only a delicious fruit, but also a health food. Its rich variety of substances, such as polyphenolic compounds, especially anthocyanins, polysaccharides, and triterpenoids, has been proven to have health benefits in many aspects. Anthocyanins, as strong antioxidants and anti‐inflammatory agents for mammalian cells (Bornsek et al., 2012; Krikorian et al., 2010), are shown to be capable of reversing the course of neuronal and behavioral aging (Joseph et al., 1999), inhibiting cancer cell proliferation and invasion (Faria et al., 2010), preventing obesity, other metabolic syndrome disorders (Norberto et al., 2013), etc. Our results show that addition of Bacillus promote average anthocyanin content of blueberry fruit with a highest increase of 5.99% in the M2 treatment (Figure 2). Besides the differences among blueberry varieties, the anthocyanin content of blueberry is affected by many factors including light, pH, moisture, fertilization, geographic location, and sampling time during the planting process (Akerström, Forsum, Rumpunen, & Jäderlund, 2009; Akerström, Jaakola, Bång, & Jäderlund, 2010; Rieger, Müller, Guttenberger, & Bucar, 2008). Interestingly, it has been reported that application of plant defense regulator, such as methyl jasmonate, positively affects anthocyanins content in blueberry, however, at a cost of yield loss (Percival & MacKenzie, 2007). Ample research indicates that some bacteria in Bacillus sp. are capable to induce plant systemic resistance (Niu et al., 2011) by releasing resistance‐inducing compounds such as extracellular polysaccharides (Jiang, Fan, Xie, & Guo, 2016) and lipopeptides (Ongena et al., 2007). Above reports offer a feasible explanation that the promotion of anthocyanin content achieved in organic planting system supplemented with single or mixed Bacillus could be at least partially caused by beneficial bacterial induced plant defense.
Fruit‐related index is also important for quality of blueberry. The additional bacteria in M2 treatment increased the soluble sugar content of blueberry fruit by 4.21%, the content of vitamin C by 17.31%, the content of soluble solids by 2.41%, and the content of soluble protein by 21.65%, while titratable acid in fruit was reduced by 10.81% (Figure 2). These results are consistent with the previously report, in which the application of the microbial consortium improved the fruit quality of pepper (Yu et al., 2019). It has also been reported that the beneficial microorganisms are able to increase the accumulation of soluble sugar in plants by enhancing photosynthesis (Jha & Subramanian, 2018). It is noteworthy that BBP3‐1, a blueberry polysaccharide, is shown to inhibit tumor progression and has the potential to be an immunomodulatory (Sun, Liu, Wu, Feng, & Meng, 2015). Therefore, the improvement of soluble sugar not only promote fruit flavor, but may also promote blueberry health function.
Soil quality has a long‐term impact on crop growth. Plant, grown in organic farms, often suffers from limited soil nutrient supply, as addition of chemical fertilizers is restricted in organic systems (Kitchen, McDonald, Shepherd, Lorimer, & Graham, 2003). Understanding on the soil quality dynamics is important to reveal crop nutrient condition and assess sustainability of individual cropping systems in agricultural practices. Nitrogen, phosphorus, and potassium are commonly recognized as the three most important nutrients in plants. NPK fertilization was also found to have positive influence on blueberry yield (Starast, Karp, Vool, Paal, & Albert, 2007). In this study, we compared soil nutrient changes after one season of planting in organic planting system motivated by single bacterium JC65 (S), microbial consortium JC65 + 7ze3 + JC03 (M1), JC65 + 7ze3 + HS10 (M2), and JC65 + JC03 + HS10 (M3) with control organic planting system (C) (Table 2). The reduction in soil nutrient after one season of planting was significantly less in beneficial microbe motivated organic planting system compared with the control organic planting system (Figure 3), indicating the soil nutrient preservation effect of the beneficial microbe. These results are supported by the reported ability of soil‐dwelling Bacillus to increase soil available nitrogen, phosphorus, and potassium nutrition. Wang, Liu, and Li (2014) have shown that, in acidic soil condition, Bacillus increases the content of available phosphorus by converting insoluble phosphorus into soluble ions. Similarly, as reported by Sheng and He (2006), the dissolution of potassium‐containing minerals increased after the inoculation with Bacillus, resulting in an improvement in the plant accessibility of soil potassium. Moreover, soil nitrogen fixed by Bacillus has also been reported (Lucas García, Probanza, Ramos, Colón Flores, & Gutiérrez, 2004). Although the combination of Bacillus in this study affected soil nutrient indicators, it did not change soil pH (Table 2). This feature is conducive to the application of the bacteria consortium in organic blueberry cultivation with special requirements on soil pH (Harmer, 1945). Few studies have shown the effects of Bacillus on plants under acidic conditions. The results of this study suggest that both the single B. amyloliquefaciens JC65 and the Bacillus consortiums in this study are able to function in acidic soils and deliver beneficial effects to plants and soil.
Another important indicator of soil fertility is the organic matter content. Haynes increased the yield of blueberries by applying soil conditioners such as peat and pine bark, which significantly increase soil organic matter content (Haynes & Swift, 1986). Farooque, Zaman, Schumann, Madani, and Percival (2012) also showed that, when planted in soil with significant spatial variability, blueberry yield positively correlated to soil organic matter of the specific site. Our results showed that addition of Bacillus positively contributes to the increase of soil organic matter (Figure 3). The increased soil organic matter content in the beneficial microbe motivated organic planting systems (S/M1/M2/M3) can thus explain the increase of blueberry yield. Specifically, Wang et al. (2019) reported that B. cereus AR156 can induce the secretion of organic acids, including lactic acid and caproic acid, from tomato roots, thereby promoting the growth and metabolism of root‐dwelling microorganisms. It suggested an important method that bacteria employ to improve their viability in barren soil, while consequently promoting soil organic matter. Moreover, the salinization of the soil, often caused by overuse of chemical fertilizers, leads to the risk of salt damage when the soil salinity exceeds the salt tolerance threshold of the plant. The soil EC value was reduced after bacteria treatment, indicating an effective effect of microbial agent on preventing soil salinization (Table 2). PGPRs have been developed as one of the strategies to decrease the toxic effect caused by high salinity. Besides inducing plant systemic tolerance to the salt stress, PGPRs are able to promote plant growth by facilitating plant nutrient uptake (Kohler, Caravaca, Carrasco, & Roldan, 2006), which can possibly result in the decrease of soil EC value in this study.
Compared with the single bacteria treatment, the organic planting system supplemented with three‐bacteria complex JC03 + JC65 + HS10 showed higher efficiency in improving blueberry yield (Figure 1), fruit quality nutrients (Figure 2), and maintaining soil nutrients (Figure 3). The superiority of the composite microbes could be simply due to an additive effect, a synergistic activity, or both. In order to make better use of environmental resources and gain advantage in competition, microbes have differentiated into unique material decomposition systems and acted differently in disease control (Yu et al., 2017). Thus, an additive effect is theoretically rational to achieve by mixing bacteria with different functions. Furthermore, bacteria extensively interact with each other in comprehensive community such as soil environment. Quorum sensing system by a cycle of recognizing, responding to endogenous and exogenous extracellular signals as well as secreting its own signals is a universal mechanism employed by bacteria and thereby creating interactions and regulations on different cell fates and activities (Cloud‐Hansen et al., 2006). For instance, it has been proved that biofilm formation is critical for the successful colonization and biological control efficiency of B. subtilis (Chen et al., 2013). Biofilm formation of B. subtilis can be stimulated by lactose, which is governed by quorum sensing system (Duanis‐Assaf, Steinberg, Chai, & Shemesh, 2016). Additionally, microbial peptidoglycan and cell wall fragments can act as an important signaling molecule to benefit other bacteria (Cloud‐Hansen et al., 2006). B. cereus and its purified peptidoglycan can promote the growth of other bacterial population by being used as a carbon source for growth (Peterson, Dunn, Klimowicz, & Handelsman, 2006). Besides the universal interaction mechanism, Bacillus owns its unique interaction within the genus. It has been shown that, among the tested strains, most of bacteria with the ability to promote biofilm formation of B. subtilis through interspecies interaction are members of Bacillus own genus (Shank et al., 2011). Together, the composite microbial agents in the M1/M2/M3 treatments in this study may not only act in an additive matter; instead, the combination of Bacillus may show a synergistic effect through comprehensive interspecies interactions. It seems promising that the compounding of microbe is a potential means for efficient growth promoting, disease prevention, and soil improvement in organic agriculture practice.
5. CONCLUSIONS
Based on the results, we conclude that addition of beneficial microbe in organic blueberry production significantly promotes plant growth and improves blueberry fruit quality. Interestingly, by combination of different beneficial microorganism, the promotion effect on blueberry production was enhanced significantly, indicating synergistic activity among specific mixture of bacteria. The soil retention ability of the beneficial microbe is also shown, especially for increase of soil organic matter. The change of soil nutrients may be one of the reasons that addition of bacteria affects the growth and quality of blueberry. Further studies should be focused on screening for more effective bacterial consortium and studying the interactions among the mixed species causing this synergistic activity.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ETHICAL APPROVAL
No human subjects or vertebrate animals were used in this study.
ACKNOWLEDGMENTS
This work was funded by Nanjing Science and Technology Project (201716066), China Postdoctoral Science Foundation (2019M651863), Key Technologies Innovation of Modern Agricultural Industry in Jiangsu Province (CX(19)2008), and National Natural Science Foundation of China (31672075).
Yu Y‐Y, Xu J‐D, Huang T‐X, et al. Combination of beneficial bacteria improves blueberry production and soil quality. Food Sci Nutr. 2020;8:5776–5784. 10.1002/fsn3.1772
Contributor Information
Jing‐Ping Qiu, Email: 781104935@qq.com.
Jian‐Hua Guo, Email: jhguo@njau.edu.cn.
REFERENCES
- Akerström, A. , Forsum, A. , Rumpunen, K. , & Jäderlund, A. (2009). Effects of sampling time and nitrogen fertilization on anthocyanidin levels in Vaccinium myrtillus fruits. Journal of Agricultural and Food Chemistry, 57, 3340–3345. 10.1021/jf8037743 [DOI] [PubMed] [Google Scholar]
- Akerström, A. , Jaakola, L. , Bång, U. , & Jäderlund, A. (2010). Effects of latitude‐related factors and geographical origin on anthocyanidin concentrations in fruits of Vaccinium myrtillus L. (Bilberries). Journal of Agricultural and Food Chemistry, 58, 11939–11945. 10.1021/jf102407n [DOI] [PubMed] [Google Scholar]
- Alamo, J. M. , Maquieira, A. , Puchades, R. , & Sagrado, S. (1993). Determination of titratable acidity and ascorbic acid in fruit juices in continuous‐flow systems. Fresenius Journal of Analytical Chemistry, 347, 293–298. 10.1007/BF00323975 [DOI] [Google Scholar]
- Bajwa, R. , & Read, D. J. (1985). The biology of mycorrhiza in the ricaceae. New Phytologist, 101, 459–467. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, P. , & Jha, D. (2012). Plant growth‐promoting rhizobacteria (PGPR): emergence in agriculture. World Journal of Microbiology & Biotechnology, 28(4), 1327–1350. 10.1007/s11274-011-0979-9 [DOI] [PubMed] [Google Scholar]
- Bornsek, S. M. , Ziberna, L. , Polak, T. , Vanzo, A. , Ulrih, N. P. , Abram, V. , … Passamonti, S. (2012). Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chemistry, 134, 1878–1884. 10.1016/j.foodchem.2012.03.092 [DOI] [PubMed] [Google Scholar]
- Brazelton, C. , & Young, K. (2014). World blueberry statistics and global market analysis. Folsom, CA: US Highbush Blueberry Council. [Google Scholar]
- Chen, Y. , Yan, F. , Chai, Y. , Liu, H. , Kolter, R. , Losick, R. , & Guo, J. H. (2013). Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environmental Microbiology, 15, 848–864. 10.1111/j.1462-2920.2012.02860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud‐Hansen, K. A. , Peterson, S. B. , Stabb, E. V. , Goldman, W. E. , McFall‐Ngai, M. J. , & Handelsman, J. (2006). Breaching the great wall: Peptidoglycan and microbial interactions. Nature Reviews Microbiology, 4, 710 10.1038/nrmicro1486 [DOI] [PubMed] [Google Scholar]
- Dedej, S. , Delaplane, K. S. , & Scherm, H. (2004). Effectiveness of honey bees in delivering the biocontrol agent Bacillus subtilis to blueberry flowers to suppress mummy berry disease. Biological Control, 31, 422–427. 10.1016/j.biocontrol.2004.07.010 [DOI] [Google Scholar]
- Drummond, F. , Smagula, J. , Annis, S. , & Yarborough, D. (2009). B852: Organic wild blueberry production. [Google Scholar]
- Duanis‐Assaf, D. , Steinberg, D. , Chai, Y. , & Shemesh, M. (2016). The LuxS based quorum sensing governs lactose induced biofilm formation by Bacillus subtilis . Frontiers Microbiology, 6, 1517 10.3389/fmicb.2015.01517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria, A. , Pestana, D. , Teixeira, D. , De Freitas, V. , Mateus, N. , & Calhau, C. (2010). Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytotherapy Research, 24, 1862–1869. 10.1002/ptr.3213 [DOI] [PubMed] [Google Scholar]
- Farooque, A. A. , Zaman, Q. U. , Schumann, A. W. , Madani, A. , & Percival, D. C. (2012). Response of wild blueberry yield to spatial variability of soil properties. Soil Science, 177, 56–68. 10.1097/ss.0b013e3182376ed6 [DOI] [Google Scholar]
- Harmer, P. M. (1945). The effect of varying the reaction of organic soil on the growth and production of the domesticated blueberry 1. Soil Science Society of America Journal, 9, 133–141. 10.2136/sssaj1945.036159950009000C0021x [DOI] [Google Scholar]
- Haynes, R. J. , & Swift, R. S. (1986). Effect of soil amendments and sawdust mulching on growth, yield and leaf nutrient content of highbush blueberry plants. Scientia Horticulturae, 29, 229–238. 10.1016/0304-4238(86)90066-X [DOI] [Google Scholar]
- Jha, Y. , & Subramanian, R. B. (2018). Effect of Root‐Associated Bacteria on Soluble Sugar Metabolism in Plant Under Environmental Stress In Plant Metabolites and Regulation Under Environmental Stress, Copyright © 2018 Elsevier Inc. 10.1016/B978-0-12-812689-9.00012-1 [DOI] [Google Scholar]
- Jiang, C. H. , Fan, Z. H. , Xie, P. , & Guo, J. H. (2016). Bacillus cereus AR156 extracellular polysaccharides served as a novel micro‐associated molecular pattern to induced systemic immunity to Pst DC3000 in Arabidopsis. Frontiers in Microbiology, 7, 664 10.3389/fmicb.2016.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, C. H. , Wu, F. , Yu, Z. Y. , Xie, P. , Ke, H. J. , Li, H. W. , … Guo, J. H. (2015). Study on screening and antagonistic mechanisms of Bacillus amyloliquefaciens 54 against bacterial fruit blotch (BFB) caused by Acidovorax avenae subsp. citrulli . Microbiological Research, 170, 95–104. 10.1016/j.micres.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Jones, C. G. , Hare, J. D. , & Compton, S. J. (1989). Measuring plant protein with the Bradford assay. Journal of Chemical Ecology, 15, 979–992. 10.1007/BF01015193 [DOI] [PubMed] [Google Scholar]
- Joseph, J. A. , Shukitt‐Hale, B. , Denisova, N. A. , Bielinski, D. , Martin, A. , McEwen, J. J. , & Bickford, P. C. (1999). Reversals of age‐related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. Journal of Neuroscience, 19, 8114–8121. 10.1016/0304-4165(84)90046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlberg, I. , & Risvik, E. (2007). Consumers of organic foods–value segments and liking of bread. Food Quality and Preference, 18, 471–481. 10.1016/j.foodqual.2006.03.023 [DOI] [Google Scholar]
- Kitchen, J. L. , McDonald, G. K. , Shepherd, K. W. , Lorimer, M. F. , & Graham, R. D. (2003). Comparing wheat grown in South Australian organic and conventional farming systems. 1. Growth and grain yield. Australian Journal of Agricultural Research, 54, 889–901. 10.1071/AR03039 [DOI] [Google Scholar]
- Kohler, J. , Caravaca, F. , Carrasco, L. , & Roldan, A. (2006). Contribution of Pseudomonas mendocina and Glomus intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions. Soil Use and Management, 22(3), 298–304. 10.1111/j.1475-2743.2006.00041.x [DOI] [Google Scholar]
- Krikorian, R. , Shidler, M. D. , Nash, T. A. , Kalt, W. , Vinqvist‐Tymchuk, M. R. , Shukitt‐Hale, B. , & Joseph, J. A. (2010). Blueberry supplementation improves memory in older adults. Journal of Agricultural and Food Chemistry, 58, 3996–4000. 10.1021/jf9029332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas García, J. A. , Probanza, A. , Ramos, B. , Colón Flores, J. J. , & Gutiérrez, M. F. J. (2004). Effects of plant growth promoting rhizobacteria (PGPRs) on the biological nitrogen fixation, nodulation, and growth of Lupinus albusl. cv. Multolupa. Engineering in Life Sciences, 4, 71–77. 10.1002/elsc.200400013 [DOI] [Google Scholar]
- Niu, D. D. , Liu, H. X. , Jiang, C. H. , Wang, Y. P. , Wang, Q. Y. , Jin, H. L. , & Guo, J. H. (2011). The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate‐and jasmonate/ethylene‐dependent signaling pathways. Molecular Plant‐Microbe Interactions, 24, 533–542. 10.1094/MPMI-09-10-0213 [DOI] [PubMed] [Google Scholar]
- Norberto, S. , Silva, S. , Meireles, M. , Faria, A. , Pintado, M. , & Calhau, C. (2013). Blueberry anthocyanins in health promotion: A metabolic overview. Journal of Functional Foods, 5, 1518–1528. 10.1016/j.jff.2013.08.015 [DOI] [Google Scholar]
- Ongena, M. , Jourdan, E. , Adam, A. , Paquot, M. , Brans, A. , Joris, B. , … Thonart, P. (2007). Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environmental Microbiology, 9, 1084–1090. 10.1111/j.1462-2920.2006.01202.x [DOI] [PubMed] [Google Scholar]
- Orhan, E. , Esitken, A. , Ercisli, S. , Turan, M. , & Sahin, F. (2006). Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient contents in organically growing raspberry. Scientia Horticulturae, 111, 38–43. 10.1016/j.scienta.2006.09.002 [DOI] [Google Scholar]
- Percival, D. , & MacKenzie, J. L. (2007). Use of plant growth regulators to increase polyphenolic compounds in the wild blueberry. Canadian Journal of Plant Science, 87, 333–336. 10.4141/p06-120 [DOI] [Google Scholar]
- Peterson, S. B. , Dunn, A. K. , Klimowicz, A. K. , & Handelsman, J. (2006). Peptidoglycan from Bacillus cereus mediates commensalism with rhizosphere bacteria from the cytophaga‐flavobacterium group. Applied and Environmental Microbiology, 72, 5421–5427. 10.1128/AEM.02928-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretty, J. N. (1997). The sustainable intensification of agriculture. Natural Resources Forum, 21(4), 247–256. 10.1111/j.1477-8947.1997.tb00699.x [DOI] [Google Scholar]
- Read, D. J. , Leake, J. R. , & Langdale, A. R. (1989). The nitrogen nutrition of mycorrhizal fungi and their host plants, in Nitrogen, phosphorus and sulphur utilization by fungi, (pp. 181–204). [Google Scholar]
- Rieger, G. , Müller, M. , Guttenberger, H. , & Bucar, F. (2008). Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus . Journal of Agricultural and Food Chemistry, 56, 9080–9086. 10.1021/jf801104e [DOI] [PubMed] [Google Scholar]
- Roubos, C. R. , Rodriguez‐Saona, C. , Holdcraft, R. , Mason, K. S. , & Isaacs, R. (2014). Relative toxicity and residual activity of insecticides used in blueberry pest management: Mortality of natural enemies. Journal of Economic Entomology, 107, 277–285. 10.1603/EC13191 [DOI] [PubMed] [Google Scholar]
- Santos, D. A. , Lima, K. P. , Março, P. H. , & Valderrama, P. (2016). Vitamin C determination by ultraviolet spectroscopy and multiproduct calibration. Journal of the Brazilian Chemical Society, 27, 1912–1917. 10.5935/0103-5053.20160071 [DOI] [Google Scholar]
- Scherm, H. , Ngugi, H. K. , Savelle, A. T. , & Edwards, J. R. (2004). Biological control of infection of blueberry flowers caused by Monilinia vaccinii‐corymbosi . Biological Control, 29, 199–206. 10.1016/s1049-9644(03)00154-3 [DOI] [Google Scholar]
- Schollenberger, C. J. (1931). Determination of soil organic matter. Soil Science, 31, 483–486. [Google Scholar]
- Sciarappa, W. , Polavarapu, S. , Barry, J. , Oudemans, P. , Ehlenfeldt, M. , Pavlis, G. , … Holdcraft, R. (2008). Developing an organic production system for highbush blueberry. HortScience, 43, 51–57. 10.21273/HORTSCI.43.1.51 [DOI] [Google Scholar]
- Shank, E. A. , Klepac‐Ceraj, V. , Collado‐Torres, L. , Powers, G. E. , Losick, R. , & Kolter, R. (2011). Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proceedings of the National Academy of Sciences of the United States of America, 108, 1236–1243. 10.1073/pnas.1103630108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, X. F. , & He, L. Y. (2006). Solubilization of potassium‐bearing minerals by a wild‐type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Canadian Journal of Microbiology, 52, 66–72. 10.1139/w05-117 [DOI] [PubMed] [Google Scholar]
- Smith, S. E. , & Read, D. J. (2008). Mycorrhizal symbiosis, (3)rd Edition. San Diego: Academic Press. [Google Scholar]
- Solomakhin, A. , & Blanke, M. M. (2010). Can coloured hailnets improve taste (sugar, sugar: Acid ratio), consumer appeal (colouration) and nutritional value (anthocyanin, vitamin C) of apple fruit? LWT‐ Food Science and Technology, 43, 1277–1284. 10.1016/j.lwt.2010.02.020 [DOI] [Google Scholar]
- Starast, M. , Karp, K. , Vool, E. , Paal, T. , & Albert, T. (2007). Effect of NPK fertilization and elemental sulphur on growth and yield of lowbush blueberry. Agricultural and Food Science, 16, 34–45. 10.2137/145960607781635859 [DOI] [Google Scholar]
- Sun, X. , Liu, N. , Wu, Z. , Feng, Y. , & Meng, X. (2015). Anti‐tumor activity of a polysaccharide from blueberry. Molecules, 20, 3841–3853. 10.3390/molecules20033841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villata, M. (2012). Trends in world blueberry production. Amer. Fruit Growers, 132, 30. [Google Scholar]
- Wang, N. , Wang, L. , Zhu, K. , Hou, S. , Chen, L. , Mi, D. , … Guo, J.‐H. (2019). Plant root exudates are involved in Bacillus cereus AR156 mediated biocontrol against Ralstonia solanacearum . Frontiers in Microbiology, 10, 98 10.3389/fmicb.2019.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. , Liu, M. Q. , & Li, H. X. (2014). Inoculation of phosphate‐solubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agriculturae Scandinavica Seceion B‐Soil and Plant Science, 64, 8 10.1080/09064710.2014.905624 [DOI] [Google Scholar]
- Wu, S. C. , Cao, Z. H. , Li, Z. G. , Cheung, K. C. , & Wong, M. H. (2005). Effects of biofertilizer containing N‐fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma, 125, 155–166. 10.1016/j.geoderma.2004.07.003 [DOI] [Google Scholar]
- Yu, Y. Y. , Jiang, C. H. , Wang, C. , Chen, L. J. , Li, H. Y. , Xu, Q. , & Guo, J. H. (2017). An improved strategy for stable biocontrol agents selecting to control rice sheath blight caused by Rhizoctonia solani . Microbiological Research, 203, 1–9. 10.1016/j.micres.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Yu, Y. Y. , Li, S. M. , Qiu, J. P. , Li, J. G. , Luo, Y. M. , & Guo, J. H. (2019). Combination of agricultural waste compost and biofertilizer improves yield and enhances the sustainability of a pepper field. Journal of Plant Nutrition and Soil Science, 182, 560–569. 10.1002/jpln.201800223 [DOI] [Google Scholar]
- Zheng, W. , Zeng, S. , Bais, H. , LaManna, J. M. , Hussey, D. S. , Jacobson, D. L. , & Jin, Y. (2018). Plant growth‐promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resources Research, 54, 3673–3687. 10.1029/2018WR022656 [DOI] [Google Scholar]
- Zhou, D. , Huang, X. F. , Chaparro, J. M. , Badri, D. V. , Manter, D. K. , Vivanco, J. M. , & Guo, J. (2016). Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant and Soil, 401, 259–272. 10.1007/s11104-015-2743-7 [DOI] [Google Scholar]


