Abstract
Cancer genome sequencing studies have revealed a number of variants in coding regions of several genes. Some of these coding variants play an important role in activating specific pathways that drive proliferation. Coding variants present on cancer cell surfaces by the major histocompatibility complex serve as neo-antigens and result in immune activation. The success of immune therapy in patients is attributed to neo-antigen load on cancer cell surfaces. However, which coding variants are expressed at the protein level can’t be predicted based on genomic data. Complementing genomic data with proteomic data can potentially reveal coding variants that are expressed at the protein level. However, identification of variant peptides using mass spectrometry data is still a challenging task due to the lack of an appropriate tool that integrates genomic and proteomic data analysis pipelines. To overcome this problem, and for the ease of the biologists, we have developed a graphical user interface (GUI)-based tool called CusVarDB. We integrated variant calling pipeline to generate sample-specific variant protein database from next-generation sequencing datasets. We validated the tool with triple negative breast cancer cell line datasets and identified 423, 408, 386 and 361 variant peptides from BT474, MDMAB157, MFM223 and HCC38 datasets, respectively.
Keywords: Next-generation sequencing, Variant protein database, NGS-pipeline
Introduction
Cancer genome sequencing projects have revealed thousands of genomic variations in cancers ( Forbes et al., 2010; Tomczak et al., 2015; Tate et al., 2019; Zhang et al., 2011). It is reasonable to presume that genomic variations in cancers might result in several new proteoforms. Genomic variations in coding regions may encode mutant proteins in cancer cells. These mutant proteins are known to drive proliferation in various cancers. For example, amino acid substitution of arginine to leucine at position 858 (L858R) in the epidermal growth factor receptor (EGFR) gene has been observed in 17% of pulmonary adenocarcinoma patients ( Morgensztern et al., 2015). In addition, a mutation in gene BRAF V600E is known to result in increased possibility of metastatic melanoma ( Chapman et al., 2011). Some of these mutant proteins are proteolytically processed in cancer cells, resulting in major histocompatibility complex (MHC) presentation of mutant peptides. These mutant peptides serve as neo-antigens that recruit T cells and result in immune activation ( Kreiter et al., 2015). Therefore, it is important to identify mutant proteins expressed by cancer cells. However, there are no easy-to-use pipelines for biologists to identify such coding variants, which alter the protein sequences and may play an important role in tumorigenesis. Detection of cancer-specific proteoforms has been studied by several research teams using proteogenomics methods. This approach integrates proteomics data with genome sequences to identify novel protein-coding regions ( Menschaert & Fenyö, 2017; Nesvizhskii, 2016; Ruggles & Fenyö, 2016), which can be easily adopted in the field of cancer biology for the detection of genomic aberrations and coding single nucleotide polymorphisms (SNPs). Cancer is a heterogeneous disease with a large number of genomic variations. However, detection of mutations and SNPs at the proteomic level will aid in further investigations on altered molecular networks and functional ramifications and may provide candidate molecules for novel therapeutic interventions ( Subbannayya et al., 2016) for various types of cancers.
Several qualitative and quantitative proteomics studies have been reported, which identify altered expression of proteins in cancers ( Subbannayya et al., 2015). Often, general workflows were used in such investigations, wherein a reference protein database was used to search the experimentally derived tandem mass spectrometry data for the identification and quantification of the proteins ( Kelkar et al., 2014). However, such a reference database is usually deprived of sample-specific amino acid variations brought about by genomic aberrations and coding SNPs reported for the various cancers. The publicly available databases such as dbSNP ( Sherry et al., 2001), COSMIC ( Forbes et al., 2015) and UniProt ( Apweiler et al., 2004) can be used to identify the variant peptides ( Alfaro et al., 2017) but millions of protein-variants from these databases might increase the probability of identifying false positives. Therefore, there is a need for an improved specific method to characterize germline and somatic variations at the proteomic level ( Alfaro et al., 2017) specific to the cancer type. Hence, we developed CusVarDB with an in-built pipeline for genomics suite in deriving variants and creating custom variant protein databases.
Methods
Implementation
CusVarDB is available at https://sourceforge.net/projects/cusvardb/ and http://bioinfo-tools.com/Downloads/CusVarDB/V1.0.0/. Our tool supports graphical user interface (GUI) for the easy execution of the next-generation sequencing (NGS) pipelines. The GUI was developed using Microsoft Visual Studio Community edition 2017. Linux commands were executed through the Python program (terminal.py) developed by Linwei available on GitHub. A portable version of Perl was used to execute the script for downloading the annotation database and executing the variant annotation. A python script was used to generate the custom variant database; the scripts were made portable using PyInstaller. The dry-run concept is implemented in the tool to customize the commands according to user need and run in batches.
CusVarDB inherits different NGS pipelines for genomics, RNA-Seq and exome-seq datasets. The tool performs the following steps: i) alignment of genomic data to a reference genome; ii) variant calling; iii) variant annotation; and iv) generate variant protein database ( Figure 1). Burrows-Wheeler Aligner (BWA) is executed for alignment of genome and exome ( Li & Durbin, 2009) data, while HISAT2 is used for RNA-Seq data ( Kim et al., 2015). Subsequent steps involving sample labelling, variant calling and annotation are performed using Picard, GATK ( McKenna et al., 2010) and ANNOVAR ( Wang et al., 2010), respectively. CusVarDB substitutes the amino acid variations from the previous steps to generate a custom variant protein database.
Figure 1.

Schematic representation of the workflow.
Operation
CusVarDB runs on Windows 10 system. It requires Linux Bash Shell and ANNOVAR tool to be enabled and installed by the user. Minimum system requirements include Intel i5 or i7, having at least four cores with 8 GB of RAM and 1 TB hard drive. High performance processors such as Intel i9 or Xeon and large quantity of RAM can enable faster execution of tasks.
The tool requires FASTA, FASTQ and dbSNP database as input files. FASTA file will be loaded at configuration panel and indexed using bwa index or hisat2-build command. The quality control panel loads the FASTQ file and produces a quality check report using the FastQC tool. The third panel asks the user to load the FASTQ file(s), set the number of threads and RAM to perform the alignment and variant calling steps. This step produces the raw VCF file, which will be used for annotation in annotation tab. The annotation panel annotates the variants and incorporates those variants to the protein database to create a custom variant protein database. Detailed information of tool installation and execution of a test dataset are available in the manual (Manual.pdf) available to download here and on Zenodo ( Kasaragod et al., 2020b).
Use cases methods
The genomics datasets downloaded from the SRA repository were subjected to a quality check using the FastQC tool. Poor quality reads with Phred score less than twenty were trimmed using fastx_trimmer. Trimmed reads were mapped to human reference genome version 19 (hg19). AddOrReplaceReadGroup and Markduplicates operations from GATK were performed to add label information and to remove polymerase chain reaction (PCR) artefacts. Further, post alignment processing steps such as IndelRealigner and BaseRecalibrator were performed to correct the mapping errors made by alignment tool to increase variant calling accuracy. Variant calling was performed using HaplotypeCaller. Finally, raw variants were annotated using ANNOVAR and the annotated variants were incorporated to the protein database to create custom variant protein database.
Proteomic searches were carried out using Proteome Discoverer 2.3 (Thermo Scientific, Bremen, Germany). Searches could also be carried out using freely available open source alternatives such as MaxQuant, MsFragger, MS-GF+, MyriMatch or OMSSA. Mass spectrometry raw files were obtained from the PRIDE archive ( PXD008222) and searched against the customized variant protein database using the SEQUEST-HT search algorithm. Trypsin and LysC were set as proteolytic enzymes with a maximum missed cleavage of one. Carbamidomethylation of cysteine was set as a fixed modification, and acetylation of protein N-terminus and oxidation of methionine were set as variable modifications with a minimum peptide length of seven amino acids. The precursor mass tolerance was fixed as 10 ppm, and 0.05 Da for fragment ions. The mass spectrometry data were searched against the decoy database with a 1% false discovery rate cut-off at the peptide level.
We have provided a dataset for users to test the tool. The test dataset was taken from the study SRR7418758 archived on the NCBI Sequence Read Repository (SRA) and aligned using human reference genome version 19 (hg19). Using samtools view command, reads mapped to chromosome 22 were extracted and converted to FASTQ files (paired-end). We have also provided chromosome 22 nucleotide sequences from hg19 and corresponding variant information from dbSNP database.
Use cases
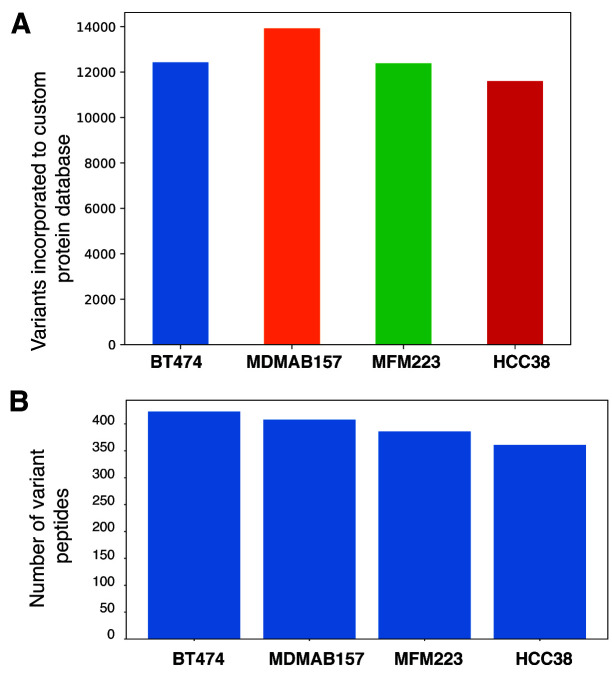
To determine the functionality of our tool, we have taken exome ( Daemen et al., 2013) and proteome datasets ( Lawrence et al., 2015). We incorporated 12,429; 13,923; 12,386 and 11,600 non-synonymous SNPs from BT474 (accession number SRR925752), MDMAB157 (accession number SRR925788), MFM223 (accession number SRR925796) and HCC38 (accession number SRR925778) cells, respectively, to the protein database ( Figure 2A). These non-synonymous SNPs were incorporated to the reference protein database (Human RefSeq release 93) to create the customized variant protein database. The mass spectrometry-based raw files were searched against their respective custom variant protein database, which resulted in the identification of 423, 408, 386 and 361 variant peptides from BT474, MDMAB157, MFM223 and HCC38, cell line datasets ( Figure 2B). The resultant variant peptide lists are available as Underlying data ( Kasaragod et al., 2020a). The data generated from the tool demonstrates the usefulness and the ease of detection of variant peptides in an integrated omics analysis.
Figure 2.
A) Number of nonsynonymous variants incorporated to protein database. B) Mutant peptides identified across four cell lines (BT474, MDMAB157, MFM223 and HCC38) where the mass spectrometry raw files were searched against custom mutant protein database.
Conclusions
CusVarDB generates customized variant protein database from NGS datasets. To our knowledge, this is the first tool which uses the Linux Bash Shell to execute the NGS tools on a Windows OS. The tool provides the additional feature of dry-run, making the commands highly customizable. The variant protein database generated by this tool is highly compatible for use as a reference protein database for analysis of variants at the proteomic level.
Data availability
Source data
Whole exome data from Daemen et al. (2013) on BioProject, Accession number PRJNA210427
Mass spectrometry proteome data from Lawrence et al. (2015) on PRIDE, Accession number PXD008222: https://identifiers.org/pride.project:PXD008222
Test dataset on SRA, Accession number SRR7418758: https://identifiers.org/insdc.sra:SRR7418758
Underlying data
Zenodo: CusVarDB: A tool for building customized sample-specific variant protein database from Next-generation sequencing datasets. http://doi.org/10.5281/zenodo.3747108 ( Kasaragod et al., 2020a)
This project contains the following underlying data:
-
-
Gowda_CusVarDB_Supplementary_table1.xlsx (This table contains the resultant variant peptides along with the wild-type peptides from BT474, MDMAB157, MFM223, and HCC38 datasets. Along with mutant peptides, this section also provides additional information such as peptide-spectrum match [PSM], Protein accession, cross-correlation value from the search [Xcorr] and retention time [RT])
-
-
Gowda_CusVarDB_Supplementary_table2.xlsx (This table provides the complete details of the resultant peptides. Here the mutant and corresponding wild-type peptides are mentioned in different sheets. For a given mutant peptide its wild-type peptide and corresponding information can be mapped using the VLOOKUP function in Excel by keeping column A [Sl.No] as lookup parameter.)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Software availability
Software available from: https://sourceforge.net/projects/cusvardb/ and http://bioinfo-tools.com/Downloads/CusVarDB/V1.0.0/
Source code available from: https://github.com/sandeepkasaragod/CusVarDB
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3780645 ( Kasaragod et al., 2020b)
License: Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
We thank Mr. Hitesh Ugaram Kore, Ph.D. student, Cancer Precision Medicine Group, QIMR Berghofer Medical Research Institute, Australia for his assistance with testing the tool and fixing the bugs which has greatly improved the performance of the tool.
Funding Statement
SK is a recipient of the Indian Council of Medical Research (ICMR) Senior Research Fellow (SRF) application number [ISRM/11(27)/2017]. VM is a recipient of Women Scientist-A award from the Department of Science and Technology (DST). SKB is a recipient of DBT-BINC Junior Research Fellow. SMP is a recipient of INSPIRE Faculty Award from the Department of Science and Technology (DST), Government of India. We thank Karnataka Biotechnology and Information Technology Services (KBITS), Government of Karnataka, for the support to the Center for Systems Biology and Molecular Medicine at Yenepoya (Deemed to be University) under the Biotechnology Skill Enhancement Programme in Multiomics Technology (BiSEP GO ITD 02 MDA 2017).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved with reservations]
References
- Alfaro JA, Ignatchenko A, Ignatchenko V, et al. : Detecting protein variants by mass spectrometry: a comprehensive study in cancer cell-lines. Genome Med. 2017;9(1):62. 10.1186/s13073-017-0454-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apweiler R, Bairoch A, Wu CH, et al. : UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32(Database issue):D115–9. 10.1093/nar/gkh131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, et al. : Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen A, Griffith OL, Heiser LM, et al. : Modeling precision treatment of breast cancer. Genome Biol. 2013;14(10):R110. 10.1186/gb-2013-14-10-r110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Tang G, Bindal N, et al. : COSMIC (the Catalogue of Somatic Mutations in Cancer): a resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010;38(Database issue):D652–D657. 10.1093/nar/gkp995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Beare D, Gunasekaran P, et al. : COSMIC: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(Database issue):D805–11. 10.1093/nar/gku1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaragod S, Mohanty V, Tyagi A, et al. : CusVarDB: A tool for building customized sample-specific variant protein database from Next-generation sequencing datasets [Data set]. Zenodo. 2020a. 10.5281/zenodo.3747108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaragod S, Mohanty V, Tyagi A, et al. : CusVarDB: A tool for building customized sample-specific variant protein database from Next-generation sequencing datasets: First release (Version 1.0.0). Zenodo. 2020b. 10.5281/zenodo.3780645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar DS, Provost E, Chaerkady R, et al. : Annotation of the zebrafish genome through an integrated transcriptomic and proteomic analysis. Mol Cell Proteomics. 2014;13(11):3184–98. 10.1074/mcp.M114.038299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL, et al. : HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter S, Vormehr M, van de Roemer N, et al. : Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–6. 10.1038/nature14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RT, Perez EM, Hernández D, et al. : The proteomic landscape of triple-negative breast cancer. Cell Rep. 2015;11(4):630–44. 10.1016/j.celrep.2015.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R: Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, et al. : The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menschaert G, Fenyö D: Proteogenomics from a bioinformatics angle: A growing field. Mass Spectrom Rev. 2017;36(5):584–599. 10.1002/mas.21483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgensztern D, Politi K, Herbst RS: EGFR Mutations in Non-Small-Cell Lung Cancer: Find, Divide, and Conquer. JAMA Oncol. 2015;1(2):146–8. 10.1001/jamaoncol.2014.278 [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI: Proteogenomics: concepts, applications and computational strategies. Nat Methods. 2016;11(11):1114–25. 10.1038/nmeth.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggles KV, Fenyö D: Next Generation Sequencing Data and Proteogenomics. Adv Exp Med Biol. 2016;926:11–19. 10.1007/978-3-319-42316-6_2 [DOI] [PubMed] [Google Scholar]
- Sherry ST, Ward MH, Kholodov M, et al. : dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. 10.1093/nar/29.1.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya Y, Mir SA, Renuse S, et al. : Identification of differentially expressed serum proteins in gastric adenocarcinoma. J Proteomics. 2015;127(Pt A):80–8. 10.1016/j.jprot.2015.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya Y, Pinto SM, Gowda H, et al. : Proteogenomics for understanding oncology: recent advances and future prospects. Expert Rev Proteomics. 2016;13(3):297–308. 10.1586/14789450.2016.1136217 [DOI] [PubMed] [Google Scholar]
- Tate JG, Bamford S, Jubb HC, et al. : COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019;47(D1):D941–D947. 10.1093/nar/gky1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak K, Czerwińska P, Wiznerowicz M, et al. : The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol (Pozn). 2015;19(1A):A68–77. 10.5114/wo.2014.47136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H, et al. : ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Baran J, Cros A, et al. : International Cancer Genome Consortium Data Portal--a one-stop shop for cancer genomics data. Database (Oxford). 2011;2011:bar026. 10.1093/database/bar026 [DOI] [PMC free article] [PubMed] [Google Scholar]