Abstract
Background and Aims
The temporal dimensions of floral adaptation to pollinators are not yet well understood, partly because we lack accurate information on the diel rhythms of flower visitation for many pollinators. We investigated whether diel patterns of pollinator visitation to flowers of the African woodland orchid Bonatea polypodantha are synchronized with rhythms of floral anthesis, scent emission and nectar availability.
Methods
Direct observations and motion-activated cameras were used to identify pollinators of B. polypodantha and to document their activity periods. The timing of pollinaria removal from flowers, emission of scent and availability of nectar was also measured.
Results
We found that B. polypodantha is pollinated exclusively by short-tongued hawkmoths. Pollinaria of the orchid are affixed between the labial palps of the moths and brush over the protruding stigmatic arms. The flowers also receive visits by long-tongued hawkmoths, but these act as nectar thieves. Tracking of pollinaria removal from flowers confirmed that pollination occurs only at night. Camera footage revealed a striking crepuscular pattern of foraging by short-tongued hawkmoths with peaks of activity during the twilight periods at dusk and at dawn. In contrast, long-tongued hawkmoths were found to visit flowers throughout the night. Flowers of B. polypodantha exhibit unimodal peaks of anthesis, scent emission (dominated by nitrogenous aromatics) and nectar availability before or around dusk.
Conclusions
Flowers of B. polypodantha are pollinated exclusively by short-tongued hawkmoths, which show crepuscular foraging activity at dusk and dawn. Floral phenophases of the orchid are closely synchronized with the peak of pollinator activity at dusk.
Keywords: Breeding system, Bonatea polypodantha, floral advertising, floral rewards, motion-activated cameras, nocturnal pollination, pollen transfer efficiency, pollination syndrome
INTRODUCTION
Plant–pollinator interactions are thought to have played an important role in shaping the immense floral diversity exhibited in angiosperms (Fenster et al., 2004; Harder and Johnson, 2009). A key factor in this process is floral specialization for pollination by various animal groups (Armbruster, 2017). Distantly related angiosperm species that become specialized for pollination by the same animal group tend to exhibit convergent suites of traits known as floral or pollination syndromes (Vogel, 1954; Faegri and van der Pijl, 1979; Grant, 1983; Schiestl and Johnson, 2013). The traits that are most often used to characterize these syndromes include flower size and shape, colour and nectar chemistry (Faegri and van der Pijl, 1979). However, floral adaptation to pollinators also includes temporal aspects such as diel rhythms of anthesis, nectar production and scent emission (Hoballah et al., 2005; Jürgens et al., 2014; Van der Niet et al., 2014). Understanding these diel floral rhythms requires combined knowledge of plant and pollinator rhythms. However, accurate information on diel activity rhythms of insect pollinators, particularly nocturnal species, is scarce (Kawahara et al., 2018) and this reflects the practical difficulties of making unbiased observations throughout 24-h periods.
Hawkmoths are widespread and important floral visitors of many specialized angiosperm flowers, particularly in the tropics and subtropics (Faegri and van der Pijl, 1979; Proctor et al., 1996; Agosta and Janzen, 2005; Johnson et al., 2017). Hawkmoths can be light-trapped throughout the night (Nilsson et al., 1985; Amorim et al., 2009, 2014), but this does not necessarily reveal their feeding patterns. Direct observations have indicated that nectar-feeding activity of hawkmoths is often most intense at dusk, although feeding has also been reported to occur throughout the night and even at dawn (Murcia, 1990; Martins and Johnson, 2013). The dawn foraging observed previously by early-rising biologists has been an enigma as it has not been clear if this constitutes a distinct peak of hawkmoth activity or the tail end of foraging through the night. Studying activity patterns of pollinators using direct observations can be challenging due to various factors, such as low visitation rates, problems associated with the presence of human observers as a disturbing factor, and inaccessibility of plants in time and space. These challenges are exacerbated for studying nocturnally active animals, due to the required stamina to observe all night, and the potentially disturbing effects of using illumination required for detailed behavioural observations. Remote motion-activated cameras have addressed some of these challenges and results derived have provided many new insights into plant–pollinator interactions of vertebrate pollinators (Letten and Midgley, 2009; Micheneau et al., 2009; Wester et al., 2009; Hobbhahn and Johnson, 2015; Melidonis and Peter, 2015; Van der Niet et al., 2015; Cozien et al., 2019, Johnson and Van der Niet, 2019; Amorim et al., 2020). Recently, motion-activated cameras with close-up lenses and infrared illumination have been successfully used to document nocturnally active insect pollinators (Johnson and Raguso, 2016; Peter and Venter, 2017; Johnson et al., 2019, 2020). These cameras not only reveal activity times, but can also provide additional information on pollinator identity and behaviour. Recent results from a meta-analysis of camera studies of six African hawkmoth-pollinated plant species showed that short-tongued hawkmoths feed mostly at dusk, while long-tongued hawkmoths feed throughout the night with a peak around midnight (Johnson et al., 2019).
Hawkmoth-pollinated plants are generally strongly scented in the early evening, coinciding with the assumed peak of hawkmoth activity (Luyt and Johnson, 2001; Raguso et al., 2003; Hoballah et al., 2005; Peter et al., 2009; Van der Niet et al., 2014; Chapurlat et al., 2018; Balducci et al., 2019a; Steen et al., 2019). However, most analyses of the timing of scent emission in hawkmoth-pollinated plants do not involve quantification of scent production at different times of the night and also do not include objective measurements of the activity cycles of the pollinators. It is therefore still unclear whether there is synchronization between diel patterns of pollinator activity and floral phenophases such as anthesis, scent emission and nectar production in hawkmoth-pollinated plants.
The present study is focused on Bonatea polypodantha, an African terrestrial orchid that we hypothesized to be hawkmoth-pollinated based on its long floral spurs and strong nocturnal scent, as assessed subjectively by the human nose. The pollen of this orchid is packaged in large distinctive pollinaria, which allows easy assessment of the timing of pollination and identity of pollinators based on rates of pollinarium removal and identification of pollen loads on moths in video footage. The aim of this study was to establish whether B. polypodantha is pollinated by hawkmoths and to test whether diel rhythms of floral anthesis, advertising and reward availability are synchronized with pollinator activity patterns. Specific objectives were (1) to test whether B. polypodantha has a pollinator-dependent breeding system, (2) to test whether the species is pollinated nocturnally by hawkmoths, (3) to establish the timing of hawkmoth activity on flowers using motion-activated cameras, and (4) to quantify the diel patterns of floral anthesis, scent emission and nectar availability.
MATERIALS AND METHODS
Study species and study site
Bonatea polypodantha (Fig. 1A) is a terrestrial orchid endemic to South Africa that grows in woodland vegetation (Ponsie et al., 2007a). Bonatea is a member of the subtribe Habenariinae and phylogenetic data suggest that the genus may be nested inside the larger genus Habenaria (Ponsie et al., 2007b; Batista et al., 2013). Plants produce a single terminal racemose inflorescence bearing 2–12 pale-green to white flowers that open acropetally, with a relatively long (~44 mm) nectar-producing spur (Fig. 1B, C). The dorsal sepal is pale green (forming a hood around the anther sacs) whereas the stigmatic arms, lateral sepals, anterior petal and lip lobes are white (Ponsie et al., 2007a). A characteristic feature of Bonatea is that the central rostellum lobe forms a tooth-like structure around the opening of the spur (Fig. 1C). There are two pollinaria and each consists of a sticky viscidium which attaches to the body of the pollinator, a long elastic caudicle and a pollinium comprising several hundred tightly packaged pollen units known as massulae (Johnson and Edwards, 2000). Bonatea species have long rostellum lobes which serve to present the viscidia in a position for precise attachment to the body of the pollinator. Once attached, massulae from a single pollinium can be deposited on the stigmas of numerous conspecific flowers (Johnson et al., 2005). Flowering of B. polypodantha occurs mainly from the late austral summer to early winter (March–June) (Ponsie et al., 2007a). The pollination biology of B. polypodantha has not been investigated previously.
Fig. 1.
Floral morphology and pollination of Bonatea polypodantha. (A) Plant in situ. Scale bar = 50 mm. (B, C) Close-up (B) and (C) side view of B. polypodantha flowers. Abbreviations: ds, dorsal sepal; upl, upper petal lobe; a, anther sacs; r, rostellum hood; v, viscidium; lpl, lower petal lobe; ls, lateral sepal; st, stigma; l, lip lobe; sp, spur. Scale bar = 8 mm. (D, E) An individual Nephele comma approaching (D) and feeding (E) on the flowers of B. polypodantha. Scale bar = 20 mm. (F) Close-up of N. comma with the median sepal accommodating the head of the hawkmoth. Note the large number of pollinaria attached between the palpi of the hawkmoth. Scale bar = 5 mm. Photographs by Herbert Stärker (A) and Steven D. Johnson (B–F).
Research was conducted during two flowering seasons (2016 and 2017) in a large population of ~1000 plants located 30 km north-west of Rustenburg in the North West Province, South Africa. The population is largely restricted to a ~300 × 50-m area in full shade in forest habitat on steeply sloping terrain, with a few other scattered patches found within a 5-km radius. The exact coordinates of the study site are withheld here to protect the population. A voucher specimen is deposited in the Bews Herbarium (NU) at the University of KwaZulu-Natal, Pietermaritzburg (Balducci NU0068129).
Breeding system assessment
To test the degree of self-compatibility and whether B. polypodantha is dependent on pollinators for fruit set, a breeding system experiment was carried out in 2017. Pollinators were excluded by placing fine mesh nylon bags over developing inflorescences (n = 48). After anthesis, three flowers on each inflorescence were marked and assigned to one of the following treatments: (1) self-pollination (with pollinia from another flower on the same inflorescence); (2) cross-pollination (with pollinia from plants growing at least 10 m away); and (3) left unmanipulated to test for autonomous self-pollination. Flowers were pollinated just before dusk and plants were then rebagged. We also assessed fruit and seed set in a further 32 naturally pollinated plants.
After 5 weeks, fruit formation was assessed and mature intact capsules were harvested and weighed. Seed viability was quantified based on a random sample of 300–400 seeds from each capsule using a staining procedure described by Vujanovic et al. (2000). Seeds were stained with 1 % triphenyl tetrazolium chloride (TTC) solution for 24 h at 40 °C under dark conditions and viewed under a compound microscope. Seeds with plump embryos that stained red were considered viable, whereas shrivelled unstained seeds were considered inviable (Vujanovic et al., 2000).
Differences in fruit set and seed viability among treatments were analysed using generalized linear models (GLMs) with a binomial error structure and logit link function. To account for lack of independence among flowers on the same plant, we used generalized estimating equations (GEEs) with an exchangeable correlation matrix and plant as a subject variable using SPSS 25 (IBM). Significance was tested using Wald statistics. Because GLMs require each level in the analysis to have variance, we substituted a single zero value with a value of one in the data for the unmanipulated treatment. This procedure makes the test slightly more statistically conservative. Capsule seed weight was analysed using a GEE with a Gaussian distribution and identity link function. The Dunn–Šidák procedure was used for post hoc comparisons among treatments.
Pollinator observations and camera trapping
Observations of flower visitation took place in March 2016 and 2017 and comprised ~112 h of observations over 14 d in 2016 and 14 d in 2017. Daytime observations were carried out between 0900 and 1500 h on five warm sunny days to quantify diurnal visitation to flowers of B. polypodantha. Evening observations typically started before dusk at 1700 h and lasted until ~2030 h. A dimmed flashlight with a red filter (Zartek, rechargeable LED spotlight) was used to detect the presence of pollinators once ambient light levels were too low for human vision. To directly assess pre-dawn foraging, three observers were stationed at different parts of the population between 0500 and 0600 h on 16 and 17 March 2017. The pollen loads and behaviour of flower visitors were recorded from direct observations, photographs, netted specimens, and in some cases from videos (see below). The proboscis length of netted moths was measured and pollinaria loads were recorded (the pollinaria of B. polypodantha are highly distinctive on account of the relatively long caudicle). A subset of caught moths were pinned for identification and voucher specimens deposited in the insect collection at the Centre for Functional Biodiversity at the University of KwaZulu-Natal, Pietermaritzburg.
To further identify and quantify activity rhythms of diurnal and nocturnal floral visitors to B. polypodantha, seven Bushnell Natureview motion-activated cameras [equipped with passive infrared sensors and close-up lenses (460 mm)] were directed at patches consisting of five to ten plants in 2016 and 2017. Camera traps were configured with the highest sensitivity setting to record a 60-s video when triggered and were left in the field for the duration of the flowering season. Information obtained from video footage included (1) functional group (short-tongued hawkmoths with tongues <8 cm versus long-tongued hawkmoths with tongues >8 cm) (Johnson and Raguso, 2016); (2) time of commencement of foraging activity by each hawkmoth (calculated as decimal hours and minutes in a 24-h period starting at midday); (3) the number of flowers probed per plant; (4) time spent foraging on nectar on each flower (i.e. feeding time); and (5) whether or not pollinaria were affixed to the foraging moth.
To assess whether the distributions of the times of commencement of foraging activity by short-tongued and long-tongued hawkmoths were significantly multimodal, we used Hartigan’s dip test under the null hypothesis that the distributions were unimodal (Hartigan and Hartigan, 1985). Hartigan’s dip test was implemented using the dip test package for R software.
Comparisons of number of flowers probed per plant (n = 112 visits to plants made by 35 hawkmoth individuals) and the mean probing time per flower per plant among hawkmoth species were performed using GEEs either incorporating a Poisson distribution and log link for counts of flowers probed or a Gaussian distribution and identity link function for log-transformed probing time values. Hawkmoth individual was treated as the subject (to control for repeated measures) and the correlation matrix was exchangeable. In these analyses, time (treated as a continuous covariate of decimal hours and minutes after midday) and the interaction between time and moth type were incorporated as predictors.
Pollination success
As a measure of the timing of pollinator visits, we recorded whether pollinaria were removed from flowers during the day or evening. We marked the bracts with small dots applied with a permanent marker pen on a total of 60 fresh intact flowers on 20 plants distributed throughout the population and inspected these flowers just before dusk and again at the beginning of the next day to record the number of pollinaria removed (Van der Niet et al., 2014). This procedure was repeated for 6 d until all pollinaria were removed from these inflorescences. The percentage of pollinaria removed during the day versus night was analysed using GEEs that incorporated a binomial distribution and logit link function with an autoregressive correlation matrix. Plant was treated as the subject variable and period as the within-subject variable.
To determine the overall level of pollination success and the efficiency of pollen transfer in B. polypodantha, the number of pollinaria removed (mr) and number of massulae (the subunits of sectile pollinia) deposited on the stigma (ms) was recorded for 118 flowers from 59 different plants using a ×10 hand lens. The mean number of massulae per pollinium (m) was determined from pollinia sampled from 20 different plants using a dissecting microscope. Pollen transfer efficiency (PTE; expressed as a percentage) was determined from the equation PTE = total ms/(mean m × total mr) (Johnson et al., 2005).
Plant trait analysis
To quantify plant size and floral morphology, plant height, number of basal leaves, number of flowers (including buds) per inflorescence and floral spur length were measured for 70 plants. Spur length was measured using digital callipers and considered to be the distance from the opening of the spur to the tip of the spur. Floral longevity was measured by monitoring 21 flowers on different plants from the opening stage until signs of floral senescence (loss of perianth turgidity). Flowers were checked every day for a maximum of 10 d to determine how long an individual flower remained open.
To measure rates of floral anthesis, we selected 20 plants in early stages of flowering and counted the number of flowers at 1400, 1700 and 2000 h over a 4-d period from 17 to 20 March 2016. We calculated the number of flowers that opened during each time period on each day and then analysed these data using a GEE with a negative binomial distribution, an auto-regressive correlation matrix, and plant as the subject. To obtain values as a rate of flowers opening per plant per hour, we included the natural log of the hours in each time period as an offset in the model.
In the 2017 flowering season diel patterns of nectar availability over time were determined by measuring the standing crop nectar volume and sugar concentration of 15 flowers (from different plants), at each of the following times: 1330, 1700, 1900, 0600 and 0830 h. The volume was measured using calibrated 5-µL micropipettes (Fisherbrand) and the sugar concentration (w/w) was determined with a Bellingham and Stanley 0–50 % hand-refractometer. As the spur is too narrow to accommodate micropipettes, nectar was extracted by capillary action after pinching off the tip of the spur. Since this procedure destroys the spur, different flowers were used at each sampling time in order to measure nectar volume accurately. Differences in mean volume and concentration values among the sampling times were assessed using GLMs with a Gaussian distribution and identity link function. Post hoc comparisons among time periods were done using the Dunn–Šidák procedure. To establish the chemical composition of nectar, samples from one flower from each of five different plants were stored on Whatman no 0.1 filter paper and kept at −20°C in the laboratory until further analysis. Sugar composition ratio of fructose:glucose:sucrose was quantified using high-pressure liquid chromatography (HPLC) according to the methods described by Van der Niet et al. (2010).
Floral scent
To characterize the chemical composition and timing of emission of floral scent of B. polypodantha in the field, floral volatiles were collected using dynamic headspace extraction methods. Inflorescences with four to six flowers were enclosed in polyacetate bags (Kalle Bratschlauch, Wiesbaden, Germany). Air from the bag was drawn through small glass cartridges filled with 1 mg of Tenax® TA 60/80 and 1 mg of Carbotrap® B 20/40 mesh activated charcoal for 30 min per sample, using a realized flow rate of 50 mL min−1. Air flow during sampling was facilitated by making a small incision on the opposite side of the polyacetate bag. To control for any volatiles present in ambient air, samples were taken simultaneously from empty polyacetate bags for the same duration. Scent samples were collected from seven plants at 1930 h during the 2016 season. In 2017 we sampled scent from three inflorescences over a 24-h period with samples taken at 1200, 1700, 1930, 0530 and 0830 h using the same sampling techniques as previously described. Samples taken at 1930 h were very similar across different years (Supplementary Data Appendix S1) and were thus combined for analysis.
Headspace samples were analysed using coupled gas chromatography and mass spectrometry (GC–MS) on a Varian CP-3800 gas chromatograph (Varian, Palo Alto, CA, USA) with a 30 × 0.25-mm internal diameter (film thickness 0.25 µm) Alltech EC-WAX column coupled to a Varian 1200 quadrupole mass spectrometer in electron-impact ionization mode. Cartridges were placed in a Varian 1079 injector equipped with a Chromatoprobe thermal desorption device (Gordin and Amirav, 2000; Dötterl et al., 2005). The flow of helium carrier gas was 1 mL min−1. The injector was held at 40 °C for 2 min with a 20:1 split and then increased to 200 °C at 200 °C min−1 in splitless mode for thermal desorption. After a 3-min hold at 40 °C, the temperature of the GC oven was ramped up to 240 °C at 10 °C min−1 and held there for 12 min. Compounds were tentatively identified using Varian Workstation software with the NIST 2011 mass spectral library (NIST/EPA/NIH Mass Spectral Library, data version NIST 2011; MS search software version 2.0 d) and verified, where possible, using retention times of synthetic standards or published Kovats retention indices. For unknown compounds the ten largest mass fragments were recorded. Compounds present in similar abundance in the control samples were excluded from further analyses. For quantification of emission rates per hour, the peak surface area of a known amount of methyl benzoate was used. One nanolitre of methyl benzoate was injected into thermodesorption cartridges and thermally desorbed under conditions identical to those used for the original samples. This procedure was repeated three times and peak area calculated for each subsequent run. A grand average was calculated to arrive at the average total ion count per nanogram of methyl benzoate. The emission rate per flower per hour was calculated by dividing the emission rate per inflorescence by the number of open flowers. For the comparison of mean absolute emission rates and the mean number of scent compounds emitted at the different time intervals, we used a GEE with plant as the subject variable for repeated measures and an exchangeable correlation matrix. These models incorporated a Gaussian distribution with an identity link function and Poisson distribution with a log link function, respectively. Time of sampling was the predictor variable and model significance was assessed using Wald χ2 statistics. The Dunn–Šidák procedure was used for post hoc pairwise comparisons among time intervals.
To visualize and quantify variation in the proportion of scent compounds between different time periods, we implemented multivariate methods using Bray–Curtis similarity of square-root-transformed proportions of scent compounds. Visualization was done using non-metric multidimensional scaling. To determine whether day versus night samples differed in scent composition we implemented a one-way analysis of similarity (ANOSIM). The null distribution was generated based on 9999 permutations of the scent matrix. To determine which compounds characterize evening and day samples we implemented a similarity percentage (SIMPER) analysis. All multivariate analyses were performed in PAST 3.13 (Hammer et al., 2001).
RESULTS
Breeding system assessment
Unmanipulated bagged flowers did not set fruit, while >80 % of those that were self-, cross- and naturally pollinated set fruit (χ2 = 5.63, P = 0.0059; Table 1). There was no significant difference between fruit set of self- and cross-pollinated flowers (Table 1). However, seed pod weight and percentage of filled embryos differed significantly among treatments (seed pod weight, χ2 = 18.1, P < 0.0001; percentage filled embryos, χ2 = 25.6, P < 0.0001) and were lower for fruits from self-pollination than for fruits from cross-pollination (Table 1). The percentage of filled embryos in fruits from natural pollination was intermediate between fruits from self-pollination and those from cross-pollination (Table 1).
Table 1.
Results of controlled pollination treatments and naturally pollinated Bonatea polypodantha flowers. Means ± s.e. that share letters do not differ significantly (see text for details of statistical analysis)
| Bagged and unmanipulated | Self-pollinated | Cross-pollinated | Naturally pollinated | |
|---|---|---|---|---|
| Fruit set (%) | 0a | 56.3 ± 0.13b | 87.5 ± 1.74b | 87.5 ± 2.09b |
| Capsule mass (mg) | – | 220 ± 8.9a | 320 ± 12.0b | 262 ± 11.0a |
| Seeds with embryos (%) | – | 56.3 ± 6.02a | 94.9 ± 3.79b | 65.4 ± 10.6a |
Pollinator observations and camera trapping
Flowering plants of B. polypodantha were visited exclusively by hawkmoths (Sphingidae). We directly observed 80 hawkmoth visits to plants and from camera trapping documented a further 133 visits to plants involving 35 hawkmoth individuals The majority of short-tongued hawkmoths (76 %) carried pollinaria of the orchid (Table 2), which were accumulated in large masses as they probed each flower for nectar, clearly visible through direct observations, photographic evidence and video footage and on netted individuals (Fig. 1D, E, Table 2, Supplementary Data Appendix S2). Pollinaria are affixed to the ventral region between the palps (Fig. 1D–F; Table 2) when the proboscis is fully inserted and one of the two rostellum arms that bear the viscidia is inserted between the palps. Pollination occurs when pollinaria dangling from the hawkmoths brush over the protruding stigmatic lobes (Fig. 1F).
Table 2.
Records of hawkmoths visiting inflorescences of Bonatea polypodantha
| Taxon (Sphingidae) | Individuals directly observed (with pollinaria) | Individuals observed on videos (with pollinaria) | Number of individuals captured (with pollinaria) | Mean number of pollinaria (range) | Mean ± s.e. proboscis length (mm) |
|---|---|---|---|---|---|
| Nephele accentifera | 9 (4) | – | 1 (1) | 20 | 43 |
| Nephele comma | 34 (23) | 23 (16) | 9 (7) | 16 (2–29) | 37.05 ± 0.97 |
| Hippotion celerio | 18 (8) | 5 (2) | 3 (1) | 16 | 32 |
| Hippotion eson | 7 (0) | – | 0 (0) | – | – |
| Agrius convolvuli | 12 (0) | 8 (0) | 1 (0) | – | 81 |
The short-tongued hawkmoth Nephele comma was the most frequently observed pollinator (Table 2). The proboscis of this moth species is ~37 mm in length and thus closely matches the ~44-mm long orchid spur. Other short-tongued hawkmoths observed carrying pollinaria included Nephele accentifera and Hippotion celerio (Table 2). The only other floral visitor observed was the long-tongued hawkmoth, Agrius convolvuli (n = 12 observed directly and n = 7 recorded on video). None of the individuals of A. convolvuli carried pollinaria and video footage revealed that they were non-pollinating nectar thieves (Table 2, Supplementary Data Appendix S2).
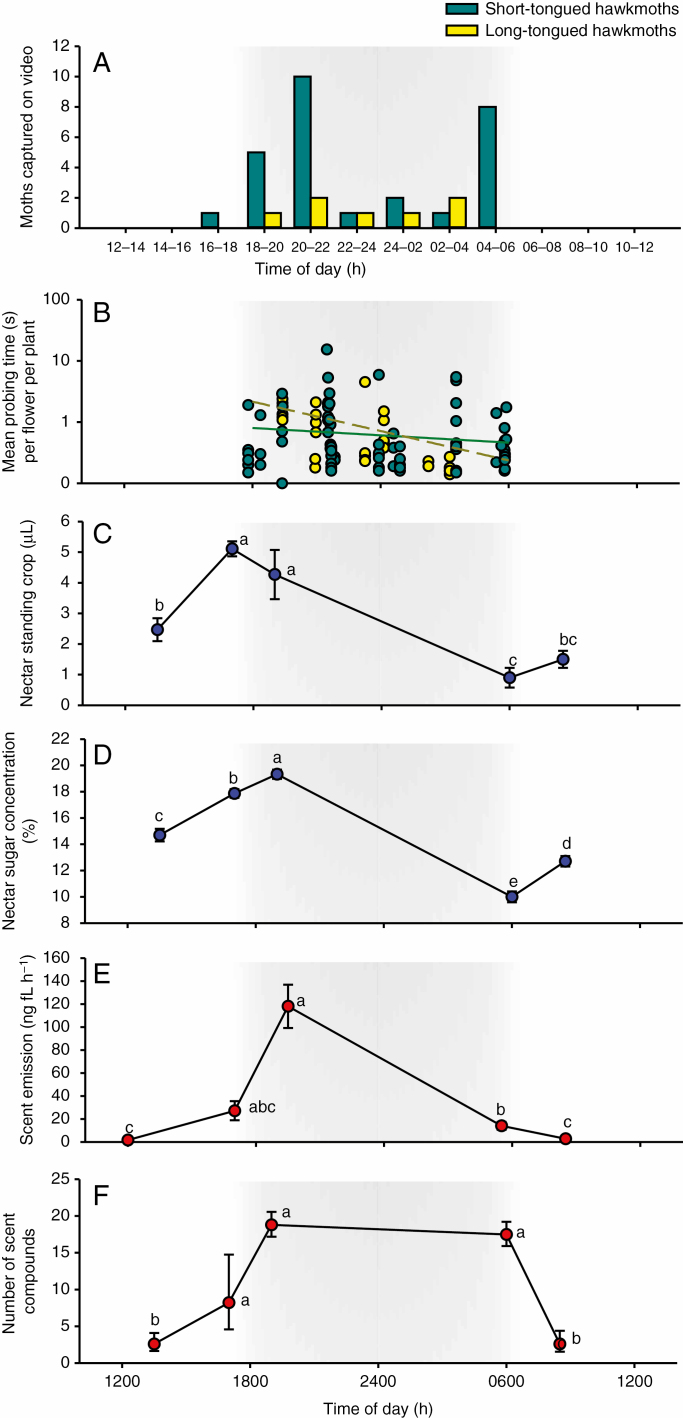
Short-tongued hawkmoths were the first to arrive at the population, with the earliest visit observed at 1730 h. There was a peak in activity of these moths during the first 3 h after dusk (1900–2200 h) and a second peak in pollinator activity before sunrise (0400–0600 h) (Fig. 2A). Hartigan’s dip test revealed that timing of visits by short-tongued hawkmoths is bimodal (dip test statistic = 0.032, P = 0.008, n = 24) with peaks after dusk and before dawn, while this was not the case for long-tongued hawkmoths (dip test statistic = 0.054, P = 0.98, n = 7) (Fig. 2A).
Fig. 2.
Timing of hawkmoth behaviour in relation to rhythms of floral reward availability and scent emission. (A) Distribution of foraging activity by hawkmoths based on video records. Bars represent the number of individual hawkmoths recorded in each 2-h time interval. (B) Mean probing time per flower per plant (log scale) for hawkmoths. Regression lines were fitted by mixed-effects modelling (see Results section). (C) Diel variation in nectar standing crop. (D) Diel variation in nectar sugar concentration (g/100 g). (E) Diel variation in scent emission. (F) Diel variation in the number of compounds detected in the scent. Values in (C–F) represent marginal means ± s.e. Means that share letters are not significantly different. The shaded area in the graphs represent the time between dusk and dawn.
Hawkmoths visited an overall mean ± s.e. of 2.87 ± 0.13 flowers per inflorescence (n = 115). The number of flowers visited per inflorescence differed significantly between short- and long-tongued hawkmoths (short, 2.39 ± 0.22 s; long, 3.50 ± 0.02 s, χ2 = 17.48, P < 0.001), but did not vary according to time of day (χ2 = 0.024, P = 0.87). The mean flower-probing time by long-tongued hawkmoths was marginally significantly higher than that by short-tongued hawkmoths (χ2 = 3.84, P = 0.049). Probing time ranged from a few milliseconds to just under 7 s, with an overall mean ± s.e. of 0.91 ± 0.16 s (n = 110). Overall flower probing times decreased through the night (β = −0.02, χ2 = 19.9, P < 0.001) and this decrease occurred mainly among long-tongued hawkmoths (hawkmoth type × time interaction, χ2 = 7.95, P = 0.004; Fig. 2B)
Pollination success
Pollinarium removal from flowers occurred only at night (day, 0 %; night, 96 %; χ2 = 34.50, P < 0.0001). Overall, 28 % of flowers surveyed had just one pollinarium removed and 56.8 % of flowers had both pollinaria removed. Massulae were deposited on at least one stigmatic lobe of 81 % of the flowers, while 61 % showed evidence of massula deposition on both stigmatic lobes. The mean ± s.e. number of massulae per pollinium in the population was 239.6 ± 16.21 while the mean ± s.e. number of massulae deposited on stigmas was 36.4 ± 2.74. Pollen transfer efficiency in the population was thus 10.8 %.
Plant trait analysis
The mean ± s.e. height of B. polypodantha plants at the study site was 21.3 ± 4.3 cm. The mean ± s.e. number of flowers per inflorescence was 4.77 ± 0.17 and the mean ± s.e. spur length was 43.9 ± 0.85 mm (range 36.8–49.9 mm).
Anthesis occurred in 56 flowers on the 20 plants that we monitored for 4 d and 21 of these flowers opened in the 3-h period between 1400 and 1700 h. Anthesis varied significantly among the three time periods (χ2 = 16.1, P < 0.0001). The mean ± s.e. rates of anthesis in terms of number of flowers opening per plant per hour was 0.08 ± 0.02 between 1400 and 1700 h, which was significantly greater than the values of 0.03 ± 0.01 between 1700 and 2000 h and 0.02 between 0800 and 1400 h. Individual flowers remained open for 7.86 ± 0.51 d (n = 21 plants).
The volume of nectar standing crop varied significantly among time periods (χ2 = 68.1, P < 0.0001). It increased throughout the day, and peaked at ~5 µL just before the onset of pollinator activity (Fig. 2C). Nectar sugar concentration similarly differed among sampling times (χ2 = 413.9, P < 0.0001) and peaked at ~20 % around the onset of pollinator activity (Fig. 2D). Sugar composition was 9.97 ± 0.33 % fructose, 8.75 ± 0.47 % glucose and 81.27 ± 0.91 % sucrose.
Floral scent
A total of 40 compounds of various classes were identified in the scent profile of B. polypodantha flowers (Supplementary Data Appendix S1). Benzyl nitrile, (E)-ocimene and indole occurred in relatively high amounts and were consistently abundant across samples in 2016 and 2017 (Supplementary Data Appendix S1). Scent emission differed significantly between samples taken over the 24-h time period (χ2 = 57.1.1, P < 0.0001), and was lowest at 0830 and 1200 h, but increased in the late afternoon and peaked at 1930 h, with some traces of scent still present at 0530 h (Fig. 2E, Supplementary Data Appendix S1). Furthermore, the mean number of scent compounds varied significantly among different time intervals and was lowest at 1200 h and highest at 1930 h (χ2 = 34.4, P < 0.0001; Fig. 2F).
We found strong clustering in multivariate space of samples taken in the evening, regardless of the year of sampling (Supplementary Data Appendix S3) and these samples differed significantly in composition from samples taken during the day (ANOSIM, R = 0.478, P = 0.0002). The compounds that characterized the evening samples were benzylnitrile, (E)-ocimene and indole, whereas the day samples were mostly characterized by octanal and decanal (Supplementary Data Appendix S4).
DISCUSSION
The results of this study show that B. polypodantha depends on pollinators for fruit set and is pollinated exclusively by short-tongued hawkmoths, which exhibit a bimodal pattern of foraging with a major peak after dusk followed by a distinct second peak of activity before dawn (Fig. 2). This is the first study to show a clearly bimodal pattern of flower foraging by hawkmoths based on consistent sampling through the night. Interestingly, we found no evidence for bimodal activity by long-tongued hawkmoths and this corroborates other studies which suggest that long-tongued hawkmoths tend to be fully nocturnal, while short-tongued hawkmoths tend to be crepuscular (Baum, 1995; Gregory, 1963; Broadhead et al., 2017; Johnson et al., 2019).
Diel floral rhythms
Diel rhythms of anthesis, nectar availability and scent emission in B. polypodantha coincide with the peak in activity by short-tongued hawkmoths at dusk, but not the second peak of activity before dawn. These diel floral rhythms are consistent with other studies showing that scent and nectar availabilities in hawkmoth-pollinated plants tend to peak around dusk and that anthesis occurs in the afternoon, thus maximizing exposure of newly opened flowers to hawkmoth activity (Luyt and Johnson, 2001; Dötterl et al., 2012; Chapurlat et al., 2018; Balducci et al., 2019b).
Nectar volume and concentration in flowers of B. polypodantha are similar to those reported for other hawkmoth-pollinated plants in South Africa (Johnson, 1995; Johnson and Liltved, 1997; Luyt and Johnson, 2001; Peter et al., 2009). It is clearly evident that nectar is secreted in the afternoon so that the standing crop peaks shortly before hawkmoths begin foraging (Fig. 2B). It is also possible that nectar is produced continuously through the night, but decreases in availability after dusk due to consumption, particularly by large long-tongued nectar-robbing hawkmoths. This, together with a corresponding decrease in nectar sugar concentration, may explain the decrease in probing time throughout the night. The sucrose-dominated sugar composition is similar to that reported more generally for hawkmoth-pollinated plants (Baker and Baker, 1983). Pollination of B. polypodantha flowers does not trigger nectar reabsorption (M. Balducci, unpubl. data). Unlike orchids with solid pollinaria, some of which are known to reabsorb nectar after pollination (Luyt and Johnson, 2002 and references therein), Bonatea flowers may require several visits to saturate female and male function and this may select against nectar reabsorption after the first pollination event.
The mean rate of scent emission during the evening is consistent with that in other hawkmoth-pollinated plants (Van der Niet et al., 2014 and references therein). The functionality of floral scent compounds emitted by B. polypodantha remains to be tested, but indole, benzyl nitrile and (E)-ocimene, which dominate the scent profile, have been shown to trigger electrophysiological responses and behavioural responses in other moth species (e.g. Knudsen and Tollsten, 1993; Jürgens et al., 2003; Sun et al., 2012). The scent of B. polypodantha is consistent with the basic framework for moth-pollinated flowers, which has been described as a sweet ‘white flower smell’ characterized by acyclic terpenes and simple aromatic alcohols (Kaiser, 1993). Absolute scent emission rate, number of compounds and overall chemical composition differed markedly between evening and day samples, as observed in other moth-pollinated plant species (Raguso et al., 2003; Peter et al., 2009; Dötterl et al., 2012; Van der Niet et al., 2014). The presence of some scent during the second smaller activity peak at dawn suggests that olfactory cues may still play an important role in hawkmoth attraction through the night. Hawkmoths tended to visit flowers in quick succession at dawn and may thus use mainly visual stimuli to locate flowers during this time when ambient light levels are increasing.
Pollen transfer among flowers
The pollination mechanism of B. polypodantha is highly selective and dictates that only hawkmoths with tongues that are shorter than the ~44-mm floral spur can serve as pollinators. In contrast, long-tongued hawkmoths visit the flowers, but function as nectar thieves as their tongues are never fully inserted into the flower. Pollinaria are placed in an unusual scale-free location between the palps (Fig. 1) and this requires that the tongue is fully inserted and that the hawkmoth presses firmly against the rostellum. Hawkmoths probing the flowers are deflected slightly to the left or right by a tooth at the mouth of the spur and as a consequence only one of the two rostellum arms is inserted between the palps (Fig. 1D) and a single pollinarium is thus removed during each visit. This also accounts for the high frequency of flowers with only one pollinarium removed and massulae deposited on one stigmatic lobe. A similar pollination mechanism was recently reported in a butterfly-pollinated Bonatea species in South Africa (Balducci et al., 2019b). Placement of pollinaria between the palps was documented previously in a South American Habenaria species (Pedron et al., 2012), but in that case the tips of the rostellum arms are abutting and both pollinaria are removed simultaneously. Based on rostellum structure, we suspect that placement of pollinaria between palps is a common pollination mechanism in Habenaria and allied genera such as Bonatea.
Our results show that B. polypodantha is self-compatible, but entirely dependent on pollinator visits for seed production (Table 1). Selfing significantly decreased fruit capsule weight and the percentage of seeds with embryos, most likely reflecting early-acting inbreeding depression (Tremblay et al., 2004; Jersáková et al., 2006; Micheneau et al., 2009; Tao et al., 2018). The percentage of seeds with embryos in naturally pollinated fruits was lower than in cross-pollinated fruits, which could reflect that natural pollination involves a fraction of self-pollen, as shown for other orchids that lack mechanisms to prevent geitonogamy (Johnson, 1995; Peter and Johnson, 2009). Reduced seed set in some amaryllids has also been attributed to a process of ovule discounting resulting from the self fraction in natural pollen loads on stigmas (Vaughton et al., 2010). However, we cannot exclude the alternative possibility that naturally pollinated stigmas of B. polypodantha did not receive sufficient pollen to fertilize all ovules, even though this seems unlikely given the large number of massulae we recorded on stigmas of naturally pollinated flowers. The overall pollen transfer efficiency of 10.8 % in the B. polypodantha population is comparable to that in other rewarding orchid species with sectile pollinia (4.6–17.6 %; Harder and Johnson, 2008).
Conclusions
Our results shed new light on the diel rhythms of pollination in hawkmoth-pollinated plants. In particular, the evidence from motion-activated cameras revealed a bimodal pattern of foraging by hawkmoths with peaks shortly after dusk and before dawn. This study highlights the utility of using motion-activated cameras to explore aspects of pollinator behaviour, such as diel activity patterns. We were able to identify insect species, presence of pollinaria, feeding behaviour, activity patterns and frequencies of visitation from the video footage obtained from these cameras. These cameras can thus accelerate the rate of discovery of new pollination systems, particularly in remote localities and for plants with flowers that are visited during the night. Interestingly, diel rhythms of flower-opening and nectar and scent production of B. polypodantha were synchronized mainly with the shortly-after-dusk peak of hawkmoth activity, rather than the pre-dawn peak. This suggests that plants are adapted mainly to dusk foraging by hawkmoths. Whether or not pre-dawn foraging by hawkmoths contributes significantly to plant pollination and plays a role in selection on floral traits remains to be determined. The benefits to a plant of maintaining floral advertising and rewards until dawn may ultimately not outweigh the physiological costs and risks of nocturnal florivory (Ramos and Schiestl, 2019).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix S1: mean ± s.d. percentage of floral scent compounds emitted by Bonatea polypodantha flowers sampled at different periods, including mean ± s.d. emission rates and number of compounds. Appendix S2: sample video footage obtained from motion-activated cameras, showing short- and long-tongued hawkmoths visiting flowers of B. polypodantha. Appendix S3: non-metric multidimensional scaling of B. polypodantha scent based on Bray–Curtis similarity of square-root-transformed proportions of scent compounds. Appendix S4: compounds that characterize dissimilarity between evening and day samples of B. polypodantha based on a similarity percentage analysis in descending order.
FUNDING
This study was supported by the National Research Foundation of South Africa (Grant 46372 to S.D.J.).
ACKNOWLEDGEMENTS
We thank Peter and Belinda De Wet for allowing access to their farm for fieldwork. We gratefully acknowledge Paul Minnaar for his assistance with fieldwork and logistical support. We would like to thank Ruth Cozien for help with analysis of nectar sugar composition in the laboratory. Herbert Stärker kindly provided locality information.
LITERATURE CITED
- Agosta SJ, Janzen DH. 2005. Body size distributions of large Costa Rican dry forest moths and the underlying relationship between plant and pollinator morphology. Oikos 108: 183–193. [Google Scholar]
- Amorim FW, DeÁvila RS Jr, De Camargo AJ, Vieira AL, Oliveira PE. 2009. A hawkmoth crossroads? Species richness, seasonality and biogeographical affinities of Sphingidae in a Brazilian Cerrado. Journal of Biogeography 36: 662–674. [Google Scholar]
- Amorim FW, Wyatt GE, Sazima M. 2014. Low abundance of long-tongued pollinators leads to pollen limitation in four specialized hawkmoth-pollinated plants in the Atlantic rain forest, Brazil. Naturwissenschaften 101: 893–905. [DOI] [PubMed] [Google Scholar]
- Amorim FW, Ballarin CS, Mariano G, et al. 2020. Good heavens what animal can pollinate it? A fungus-like holoparasitic plant potentially pollinated by opossums. Ecology 101: e03001. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. 2017. The specialization continuum in pollination systems: diversity of concepts and implications for ecology, evolution and conservation. Functional Ecology 31: 88–100. [Google Scholar]
- Baker HG, Baker I. 1983. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold, 117–141. [Google Scholar]
- Balducci MG, Martins DJ, Johnson SD. 2019a Pollination of the long-spurred African terrestrial orchid Bonatea steudneri by long-tongued hawkmoths, notably Xanthopan morganii. Plant Systematics and Evolution 305: 765–775. [Google Scholar]
- Balducci MG, Van der Niet T, Johnson SD. 2019b Butterfly pollination of Bonatea cassidea (Orchidaceae): solving a puzzle from the Darwin era. South African Journal of Botany 123: 308–316. [Google Scholar]
- Batista JA, Borges KS, de Faria MW, et al. 2013. Molecular phylogenetics of the species-rich genus Habenaria (Orchidaceae) in the New World based on nuclear and plastid DNA sequences. Molecular Phylogenetics and Evolution 67: 95–109. [DOI] [PubMed] [Google Scholar]
- Baum DA. 1995. The comparative pollination and floral biology of baobabs (Adansonia – Bombacaceae). Annals of the Missouri Botanical Garden 82: 322–348. [Google Scholar]
- Broadhead GT, Basu T, von Arx M, Raguso RA. 2017. Diel rhythms and sex differences in the locomotor activity of hawkmoths. Journal of Experimental Biology 220: 1472–1480. [DOI] [PubMed] [Google Scholar]
- Chapurlat E, Anderson J, Ågren J, Friberg M, Sletvold N. 2018. Diel pattern of floral scent emission matches the relative importance of diurnal and nocturnal pollinators in populations of Gymnadenia conopsea. Annals of Botany 121: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozien RJ, van der Niet T, Johnson SD, Steenhuisen SL. 2019. Saurian surprise: lizards pollinate South Africa’s enigmatic hidden flower. Ecology 100: e02670. [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jahreiß K, Jhumur US, Juergens A. 2012. Temporal variation of flower scent in Silene otites (Caryophyllaceae): a species with a mixed pollination system. Botanical Journal of the Linnean Society 169: 447–460. [Google Scholar]
- Dötterl S, Wolfe LM, Jürgens A. 2005. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 66: 203–213. [DOI] [PubMed] [Google Scholar]
- Faegri K, van der Pijl L. 1979. Principles of pollination ecology. Oxford: Pergamon. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology and Systematics 35: 375–403. [Google Scholar]
- Gordin A, Amirav A. 2000. SnifProbe: new method and device for vapor and gas sampling. Journal of Chromatography 903: 155–172. [DOI] [PubMed] [Google Scholar]
- Grant V. 1983. The systematic and geographical distribution of hawkmoth flowers in the temperate North American flora. Botanical Gazette 144: 439–449. [Google Scholar]
- Gregory DP. 1963. Hawkmoth pollination in the genus Oenothera. Aliso 5: 357–419. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software: package for education and data analysis. Palaeontologia Electronica 4 (1): 1–9. [Google Scholar]
- Harder LD, Johnson SD. 2008. Function and evolution of aggregated pollen in angiosperms. International Journal of Plant Sciences 169: 59–78. [Google Scholar]
- Harder LD, Johnson SD. 2009. Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist 183: 530–545. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Hartigan PM. 1985. The dip test of unimodality. Annals of Statistics 13: 70–84. [Google Scholar]
- Hoballah ME, Stuurman J, Turlings TC, Guerin PM, Connétable S, Kuhlemeier C. 2005. The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta 222: 141–150. [DOI] [PubMed] [Google Scholar]
- Hobbhahn N, Johnson SD. 2015. Sunbird pollination of the dioecious root parasite Cytinus sanguineus (Cytinaceae). South African Journal of Botany 99: 138–143. [Google Scholar]
- Jersáková J, Johnson SD, Kindlmann P. 2006. Mechanisms and evolution of deceptive pollination in orchids. Biological Reviews of the Cambridge Philosophical Society 81: 219–235. [DOI] [PubMed] [Google Scholar]
- Johnson SD. 1995. Observations of hawkmoth pollination in the South African orchid Disa cooperi. Nordic Journal of Botany 15: 121–125. [Google Scholar]
- Johnson SD, Edwards TJ. 2000. The structure and function of orchid pollinaria. Plant Systematics and Evolution 222: 243–269. [Google Scholar]
- Johnson SD, Liltved WR. 1997. Hawkmoth pollination of Bonatea speciosa (Orchidaceae) in a South African coastal forest. Nordic Journal of Botany 17: 5–10. [Google Scholar]
- Johnson SD, Raguso RA. 2016. The long-tongued hawkmoth pollinator niche for native and invasive plants in Africa. Annals of Botany 117: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Van der Niet T. 2019. Bird pollination in an African Satyrium (Orchidaceae) confirmed by camera traps and selective exclusion experiments. Plant Systematics and Evolution 305: 477–484. [Google Scholar]
- Johnson SD, Neal PR, Harder LD. 2005. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnean Society 86: 175–190. [Google Scholar]
- Johnson SD, Moré M, Amorim FW, et al. 2017. The long and the short of it: a global analysis of hawkmoth pollination niches and interaction networks. Functional Ecology 31: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Balducci MG, Bijl A, et al. 2019. From dusk till dawn: camera traps reveal the diel patterns of flower feeding by hawkmoths. Ecological Entomology 10.1111/een.12827. [Google Scholar]
- Johnson SD, Balducci MG, Shuttleworth A. 2020. Hawkmoth pollination of the orchid Habenaria clavata: mechanical wing guides, floral scent and electroantennography. Biological Journal of the Linnean Society 129: 213–226. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. 2003. Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochemical Systematics and Ecology 31: 345–357. [Google Scholar]
- Jürgens A, Glück U, Aas G, Dötterl S. 2014. Diel fragrance pattern correlates with olfactory preferences of diurnal and nocturnal flower visitors in Salix caprea (Salicaceae). Botanical Journal of the Linnean Society 175: 624–640. [Google Scholar]
- Kaiser R. 1993. The scent of orchids: olfactory and chemical investigations. Amsterdam: Elsevier. [Google Scholar]
- Kawahara AY, Plotkin D, Hamilton CA, et al. 2018. Diel behavior in moths and butterflies: a synthesis of data illuminates the evolution of temporal activity. Organisms Diversity & Evolution 18: 13–27. [Google Scholar]
- Knudsen JT, Tollsten L. 1993. Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Botanical Journal of the Linnean Society 113: 263–284. [Google Scholar]
- Letten AD, Midgley JJ. 2009. Rodent pollination in the Cape legume Liparia parva. Austral Ecology 34: 233–236. [Google Scholar]
- Luyt R, Johnson SD. 2001. Hawkmoth pollination of the African epiphytic orchid Mystacidium venosum, with special reference to flower and pollen longevity. Plant Systematics and Evolution 228: 49–62. [Google Scholar]
- Luyt R, Johnson SD. 2002. Postpollination nectar reabsorption and its implications for fruit quality in an epiphytic orchid. Biotropica 34: 442–446. [Google Scholar]
- Martins DJ, Johnson SD. 2013. Interactions between hawkmoths and flowering plants in East Africa: polyphagy and evolutionary specialization in an ecological context. Biological Journal of the Linnean Society 110: 199–213. [Google Scholar]
- Melidonis CA, Peter CI. 2015. Diurnal pollination, primarily by a single species of rodent, documented in Protea foliosa using modified camera traps. South African Journal of Botany 97: 9–15. [Google Scholar]
- Micheneau C, Johnson SD, Fay MF. 2009. Orchid pollination: from Darwin to the present day. Botanical Journal of the Linnean Society 161: 1–19. [Google Scholar]
- Murcia C. 1990. Effect of floral morphology and temperature on pollen receipt and removal in Ipomoea trichocarpa. Ecology 71: 1098–1109. [Google Scholar]
- Van der Niet T, Jürgens A, Johnson SD. 2010. Pollinators, floral morphology and scent chemistry in the southern African orchid genus Schizochilus. South African Journal of Botany 76: 726–738. [Google Scholar]
- Van der Niet T, Jürgens A, Johnson SD. 2014. Is the timing of scent emission correlated with insect visitor activity and pollination in long-spurred Satyrium species? Plant Biology 17: 226–237. [DOI] [PubMed] [Google Scholar]
- Van der Niet T, Cozien RJ, Johnson SD. 2015. Experimental evidence for specialized bird pollination in the endangered South African orchid Satyrium rhodanthum and analysis of associated floral traits. Botanical journal of the Linnean Society. 177: 141–150. [Google Scholar]
- Nilsson LA, Jonsson L, Rason L, Randrianjohany E. 1985. Monophily and pollination mechanisms in Angraecum arachnites Schltr. (Orchidaceae) in a guild of long-tongued hawk-moths (Sphingidae) in Madagascar. Biological Journal of the Linnean Society 26: 1–19. [Google Scholar]
- Pedron M, Buzatto CR, Singer RB, Batista JA, Moser A. 2012. Pollination biology of four sympatric species of Habenaria (Orchidaceae: Orchidinae) from southern Brazil. Botanical Journal of the Linnean Society 170: 141–156. [Google Scholar]
- Peter CI, Johnson SD. 2009. Reproductive biology of Acrolophia cochlearis (Orchidaceae): estimating rates of cross-pollination in epidendroid orchids. Annals of Botany 104: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CI, Venter N. 2017. Generalist, settling moth pollination in the endemic South African twig epiphyte, Mystacidium pusillum Harv. (Orchidaceae). Flora 232: 16–21. [Google Scholar]
- Peter CI, Coombs G, Huchzermeyer CF, et al. 2009. Confirmation of hawkmoth pollination in Habenaria epipactidea: leg placement of pollinaria and crepuscular scent emission. South African Journal of Botany 75: 744–750. [Google Scholar]
- Ponsie ME, Edwards TJ, Johnson SD. 2007a A taxonomic revision of Bonatea Willd. (Orchidaceae: Orchidoideae: Habenariinae). South African Journal of Botany 73: 1–21. [Google Scholar]
- Ponsie ME, Mitchell A, Edwards TJ, Johnson SD. 2007b Phylogeny of Bonatea (Orchidaceae: Habenariinae) based on molecular and morphological data. Plant Systematics and Evolution 263: 253–268. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OR: Timber Press. [Google Scholar]
- Raguso RA, Levin RA, Foose SE, Holmberg MW, McDade LA. 2003. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry 63: 265–284. [DOI] [PubMed] [Google Scholar]
- Ramos SE, Schiestl FP. 2019. Rapid plant evolution driven by the interaction of pollination and herbivory. Science 364: 193–196. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends in Ecology & Evolution 28: 307–315. [DOI] [PubMed] [Google Scholar]
- Steen R, Norli HR, Thöming G. 2019. Volatiles composition and timing of emissions in a moth-pollinated orchid in relation to hawkmoth (Lepidoptera: Sphingidae) activity. Arthropod-Plant Interactions 13: 581–592. [Google Scholar]
- Sun J-G, Huang L-Q, Wang C-Z. 2012. Electrophysiological and behavioral responses of Helicoverpa assulta (Lepidoptera: Noctuidae) to tobacco volatiles. Arthropod-Plant Interactions 6: 375–384. [Google Scholar]
- Tao Z-B, Ren Z-X, Bernhardt P, et al. 2018. Nocturnal hawkmoth and noctuid moth pollination of Habenaria limprichtii (Orchidaceae) in sub-alpine meadows of the Yulong Snow Mountain (Yunnan, China). Botanical Journal of the Linnean Society 187: 483–498. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2004. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society 84: 1–54. [Google Scholar]
- Vaughton G, Ramsey M, Johnson SD. 2010. Pollination and late-acting self-incompatibility in Cyrtanthus breviflorus (Amaryllidaceae): implications for seed production. Annals of Botany 106: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. 1954. Floral-biological syndromes as elements of diversity within tribes in the flora of South Africa. Aachen: Shaker. [Google Scholar]
- Vujanovic V, St-Arnaud M, Barabé D, Thibeault G. 2000. Viability testing of orchid seed and the promotion of colouration and germination. Annals of Botany 86: 79–86. [Google Scholar]
- Wester P, Stanway R, Pauw A. 2009. Mice pollinate the pagoda lily, Whiteheadia bifolia (Hyacinthaceae) – first field observations with photographic documentation of rodent pollination in South Africa. South African Journal of Botany 75: 713–719. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.