We read the article published recently in Critical Care by Tan et al. with great interest. We appreciate their effort to evaluate high flow nasal cannula (HFNC) usage in post-extubated chronic obstructive pulmonary disease (COPD) patients with respiratory failure [1]. A non-inferiority study is a reasonable approach given NIV has shown benefit in post-extubation studies [2–4]. Unlike superiority trials, non-inferiority studies establish non-inferiority by rejecting a null hypothesis that the tested treatment is worse than the comparator by a pre-established minimum difference (non-inferiority cutoff or delta) based on results from prior studies [5]. However, several issues in this study prevent us from reaching this conclusion.
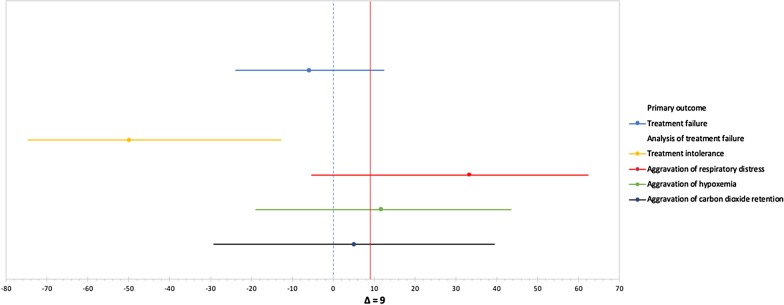
First, Tan et al. anticipated an NIV failure rate of 22% based on prior trials. The authors determined a delta of 9% for non-inferiority cutoff. They found that the experimental group (HFNC) had failure rates less than the control group (NIV), 22.7% vs. 28.6%, respectively. The absolute risk difference was − 5.8% (CI 95%; − 23.8 to 12.5). Since the CI range extends beyond the predetermined non-inferiority cut-off, inferiority is still a possibility and the study should be considered inconclusive [5]. For further clarification, we created a forest plot to visualize the primary outcomes CIs in relation to the delta point (Fig. 1).
Fig. 1.
Forest plot of confidence intervals (CI) for primary outcome and the causes of treatment failure
Second, the suggested sample size of 44 subjects per group seems insufficient. We don’t have a description of the calculations used by Tan et al., but using their assumptions we calculated that at least 216 patients per arm would be required to prove non-inferiority with a difference of less than 9%, an alpha of 0.05, and a beta of 0.2 (it is worth noting that in the paper it is stated an alpha of 0.5 instead of 0.05, which we think is a typo since such a large error margin is not considered acceptable). A trial with a similar design referenced by Tan et al. [4] calculated a sample size of 300 patients per arm using similar assumptions.
Third, the failure rate in the NIV arm is higher than expected (28.6% vs 22%, OR = 1.3), and is higher than other previous studies [2–4]. This may create bias in favor of non-inferiority and should have been discussed further in the paper.
In summary, we conclude that the results of Tan et al.’s study can’t prove non-inferiority of HFNC compared to NIV, although it doesn’t exclude it either. Besides, further clarification regarding the sample size is required.
Acknowledgements
None.
Authors’ contributions
RVC, BAK, and SA contributed to interpretation of results. RVC, BAK, and SA contributed to manuscript preparation. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tan D, Walline JH, Ling B, Xu Y, Sun J, Wang B, Shan X, Wang Y, Cao P, Zhu Q, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. 2020;24(1):489. doi: 10.1186/s13054-020-03214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns KE, Adhikari NK, Keenan SP, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010;2010(8):CD004127. doi: 10.1002/14651858.CD004127.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguia C, Gonzalez M, Epstein SK, Hill NS, Nava S, Soares MA, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350(24):2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez G, Vaquero C, Colinas L, Cuena R, Gonzalez P, Canabal A, Sanchez S, Rodriguez ML, Villasclaras A, Fernandez R. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 5.Mauri L, D'Agostino RB., Sr Challenges in the design and interpretation of noninferiority trials. N Engl J Med. 2017;377(14):1357–1367. doi: 10.1056/NEJMra1510063. [DOI] [PubMed] [Google Scholar]
- 6.Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, Cosserant B, Flicoteaux G, Imbert A, Pilorge C, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 7.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 8.Doshi P, Whittle JS, Bublewicz M, Kearney J, Ashe T, Graham R, Salazar S, Ellis TW, Jr, Maynard D, Dennis R, et al. High-velocity nasal insufflation in the treatment of respiratory failure: a randomized clinical trial. Ann Emerg Med. 2018;72(1):73–83.e5. doi: 10.1016/j.annemergmed.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, Cleary JD, Rubinstein E, Church LWP, Brown JM, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–1442. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]