Abstract
Objective
We aimed to evaluate all-cause and cause-specific mortality in patients with systemic lupus erythematosus (SLE) and neuropsychiatric (NP) symptoms in the Netherlands between 2007–2018.
Methods
Patients visiting the tertiary referral NPSLE clinic of the Leiden University Medical Center were included. NP symptoms were attributed to SLE requiring treatment (major NPSLE) or to other and mild causes (minor/non-NPSLE). Municipal registries were checked for current status (alive/deceased). Standardized mortality ratios (SMRs) and 95% confidence intervals (CI) were calculated using data from the Dutch population. Rate ratio (RR) and 95% CI were calculated using direct standardization to compare mortality between major NPSLE and minor/non-NPSLE.
Results
351 patients were included and 149 patients were classified as major NPSLE (42.5%). Compared with the general population, mortality was increased in major NPSLE (SMR 5.0 (95% CI: 2.6–8.5)) and minor/non-NPSLE patients (SMR 3.7 (95% CI: 2.2–6.0)). Compared with minor/non-NPSLE, mortality was similar in major NPSLE patients (RR: 1.0 (95% CI: 0.5–2.0)). Cause-specific mortality rates demonstrated an increased risk of death due to infections in both groups, whereas death due to cardiovascular disease was only increased in minor/non-NPSLE patients.
Conclusion
Mortality was increased in both major NPSLE and minor/non-NPSLE patients in comparison with the general population. There was no difference in mortality between major NPSLE and minor/non-NPSLE patients.
Keywords: Neuropsychiatric lupus, mortality, systemic lupus erythematosus
Introduction
Neuropsychiatric (NP) symptoms are a complex and heterogenous manifestation of systemic lupus erythematosus (SLE). Case definitions for nineteen NP syndromes have been described by the American College of Rheumatology (ACR) in 1999 and several attribution models have been developed, but attribution of NP symptoms to SLE remains difficult in clinical practice.1,2 Currently, a golden standard for the diagnosis of NPSLE is lacking and different approaches, such as multidisciplinary assessment, are used in clinical practice.3
Little is known about the outcome of NP involvement in SLE. However, in general, it is associated with a worse prognosis: previous research has indicated that NP involvement leads to decreased survival in patients with SLE, with up to a nine-fold increased mortality compared with the general population.4,5 As of yet, no study has compared mortality in patients with severe NPSLE requiring treatment with SLE patients with mild NP symptoms and alternative diagnoses, such as neuropsychiatric diseases not related to SLE, infections and medication related symptoms. Furthermore, it is known that main contributors to mortality in SLE are infections, cardiovascular disease (CVD) and renal involvement, but it is unknown what the factors influencing mortality in patients presenting with both SLE and NP symptoms are.6
This study aimed to evaluate all-cause and cause-specific mortality in patients with SLE and NP symptoms in a tertiary referral center in the Netherlands between 2007 and 2018. In addition, we investigated potential determinants of mortality in patients with SLE and NP symptoms.
Patients and methods
Study design
The NPSLE clinic of the Leiden University Medical Center (LUMC) is a tertiary referral center for patients with (suspicion of) SLE and neuropsychiatric symptoms. All patients are evaluated in a multidisciplinary setting, as described previously.7 All patients are evaluated in an outpatient clinic during one day by a rheumatologist, neurologist, clinical neuropsychologist, psychiatrist, vascular internal medicine expert and an advanced nurse practitioner. In addition, all patients undergo neuroimaging (MRI) and extensive laboratory investigation. Two weeks later, a multidisciplinary evaluation takes place. During this evaluation the following factors, as also described in SLICC decision rules and attribution model by Bortoluzzi et al., are taken into account: time between symptoms and SLE diagnosis, the presence of ‘non-SLE’ factors, the presence of minor symptoms, as described by Ainiala et al. and favoring factors such as general disease activity.8–10 During this consensus meeting, the diagnosis NPSLE was established. In this study, patients were divided in two subgroups: patients with NP symptoms attributed to SLE by multidisciplinary assessment and requiring treatment other than symptomatic treatment were classified as ‘major NPSLE’. If patients had NP symptoms not attributed to SLE, mild symptoms (e.g. mild cognitive dysfunction, mood disorder, headache) that did not require additional treatment or symptoms due to other causes, patients were classified as minor/non-NPSLE. If patients had both major NPSLE and minor/non-NPSLE symptoms, they were classified as major NPSLE. When patients were diagnosed with major NPSLE, the phenotype (inflammatory, ischemic or combined) and 1999 ACR NPSLE syndromes were assigned. If patients had clinical syndromes identified as NPSLE that were not part of the 1999 ACR NPSLE case definitions (such as cerebral vasculitis), they were categorized as ‘Other’. The diagnosis cerebral vasculitis was based on clinical and radiological parameters. Patients were seen for follow-up in the clinic if treatment of NPSLE was initiated, instead diagnostic difficulty arose and if patients were referred again by their treating physician because of (other) NP symptoms. Diagnosis at follow-up is considered the golden standard.
Electronical medical files of all patients who visited the NPSLE clinic between the 1st of September 2007 and the 31st of December 2018 were evaluated. All patients who visited the NPSLE clinic with the clinical diagnosis of SLE (based on rheumatologists’ assessment) that signed informed consent were included in the study. The study was approved by the local medical ethical committee.
Patient characteristics
Clinical characteristics including minor/non-NPSLE diagnoses were retrieved from electronical medical files. NPSLE phenotype was based on clinical multidisciplinary judgement and included laboratory and imaging results. NPSLE phenotype was only assigned when patients were classified as major NPSLE. Patients who started immunosuppressive treatment or had a dosage increase, either during or prior to the visit of the NPSLE clinic because of NP symptoms, were classified as inflammatory NPSLE. Patients who began anticoagulant therapy either during or prior to the visit of the NPSLE clinic because of NP symptoms due to SLE were classified as ischemic NPSLE. Patients who received a combination of both, were classified as combined. Patient who received anticoagulant therapy due to cerebrovascular disease with other important risk factors (smoking, hypertension, older age) were not classified as (ischemic) NPSLE. Patients with an uncertain diagnosis of NPSLE at baseline and follow-up visit were classified as undefined NPSLE and excluded from the main analyses. The presence of antiphospholipid syndrome (APS) was defined according to the revised classification criteria for APS.11 Years of education was used as marker for social economic status and divided in three categories: low (<6 years), medium (6–12 years), and high (>12 years).
Data on mortality, including date of death, were obtained using Chipsoft Hix in the LUMC. A link with the municipal registers enabled us to check vital status. Cause of death was obtained from the electronic health records in the hospital and classified according to the 10th revision of the International Classification of Diseases, Injuries, and Causes of Death-10 (ICD-10). When no information on cause of death was present, the general practitioner was contacted.
Laboratory assessment
The following autoantibodies were tested in all patients at baseline visit: anti-nuclear antibodies (ANA), anti-double stranded DNA (anti-dsDNA) and anti-extractable nuclear antigen antibodies (ENA). If anti-ENA was positive, amongst others, anti-Smith (anti-Sm) was tested as well. In addition, antiphospholipid antibodies including anticardiolipin (aCL), anti-Beta2 glycoprotein 1 antibodies (anti-β2GP1) and lupus anticoagulans (LAC) were tested. IgG anti-dsDNA antibodies were detected using the Crithidia luciliae indirect immune fluorescence technique (Immuno Concepts, Sacramento, CA, USA). IgG antibodies against Sm, IgG and IgM aCL and anti-β2GP1 were determined using a Phadia 250 EliA fluorescence enzyme immunoassay (Thermo Fisher Scientific, Freiburg, Germany). LAC was determined using STA-Rack and STA Evolution coagulation analyzers (Stago, Parsippany, NJ, USA). The following thresholds were used for the antiphospholipid antibodies, according to the manufactures’ guidelines: anti-β2GP1 IgM and IgG are considered positive >10 U/ml and aCL IgM and IgG are considered positive >40 GPL-U/ml and MPL-U/ml respectively. Levels of C3 and C4 in serum were measured using laser nephelometry. Based on the normal limits for our laboratory, C3 < 0.9 g/l and C4 < 95 mg/l were defined as low.
Disease activity
Disease activity was calculated using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K). When there was missing data to obtain the value of SLEDAI, the maximum possible value was calculated using the available data.
Disease damage
Disease damage was calculated using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (SDI).
Statistical analyses
All statistical analyses were performed using STATA version 14 (STATA Corp., Texas, USA).
All-cause mortality
Standardized mortality ratios (SMRs) were calculated by indirect standardization over the years 2007–2018 to estimate the risk of death among patients visiting our NPSLE clinic in comparison with the general Dutch population, adjusted for sex, age and calendar period. To calculate the SMR, death rates of the general population of 2007–2017, obtained from the Dutch Central Bureau of Statistics (CBS), were used.12 The 95% confidence intervals (CI) were calculated from a Poisson distribution for the observed number of deaths. When patients were diagnosed with major NPSLE prior to their baseline visit for which they received therapy, patients were counted in the major NPSLE group and phenotypes were attributed as described previously. In all cases, patient years were counted starting from baseline visit in the LUMC to avoid immortal time bias and ended at time of death or at the end of the follow-up time, the 30th of April, 2019. If a patient had a new event at follow-up, thereby switching from minor/non-NPSLE to major NPSLE or switching NPSLE phenotype, the number of patient years was counted in each respective group. We identified two types of new NP events in our clinic: patients with major NPSLE that had non/minor NPSLE at follow-up and patients with non/minor NPSLE that had new non/minor NPSLE at follow-up. Therefore, in the end no patients contributed patient years in both groups as no switch for minor/non-NPSLE to major NPSLE was present.
Comparison of mortality
Mortality in major NPSLE and minor/non-NPSLE patients in our cohort between 2007–2018 was compared using direct standardization methods. Crude mortality rates for 5-years age groups were calculated for both NPSLE and minor/non-NPSLE patients. The average amount of patient years of major NPSLE and minor/non-NPSLE patients was used as reference population. The incidence rate ratio and 95% confidence interval were calculated and reported.
Cause-specific mortality
Cause-specific SMRs were calculated by considering the specific cause of death as an endpoint and censoring patients with other causes of death. This analysis was performed for infections, cardiovascular disease and malignancies. To calculate cause-specific SMRs, cause-specific death rates of the general population of 2007–2017, obtained from the Central Bureau of Statistics (CBS), were used.
Determinants of mortality
Factors potentially influencing mortality in patients with NPSLE were analyzed by estimating hazard ratios (HR), using a Cox Proportional Hazard Model. The HR for NPSLE phenotype was corrected for SLE duration.
Sensitivity analyses
Patients who were classified as Undefined NPSLE were not included in the main analyses. To evaluate whether the classification of these patients in the major NPSLE vs. minor/non-NPSLE group altered the main results, a sensitivity analysis was performed. In addition, in our study all patients with the clinical diagnosis of SLE were included. A second sensitivity analysis was performed calculating all-cause mortality only for patients with ≥4 1997 ACR SLE classification criteria. Lastly, a sensitivity analyses excluding patients with NPSLE diagnosis before baseline visit at the LUMC NPSLE clinic was performed.
Results
Patient characteristics
In total, 351 patients fulfilled the inclusion criteria. NP symptoms were attributed to SLE (major NPSLE) in 149 patients (42.5%) and attributed to other causes (minor/non-NPSLE) in 202 patients (57.5%). In eleven patients, the diagnosis was unclear (undefined) and these patients were excluded. A flow-chart of patient inclusion is provided in Supplementary Figure 1.
Table 1 provides patient characteristics at first visit to the NPSLE clinic. The majority of major NPSLE and minor/non-NPSLE patients were female (87% and 89% respectively). Median age at inclusion was 42 years in major NPSLE patients and 45 years in minor/non-NPSLE patients. Median follow-up time was longer in major NPSLE patients than minor/non-NPSLE patients (6.1 vs. 4.9 years), but median SLE duration was similar (4.4 years). In patients with major NPSLE, the phenotype inflammatory NPSLE was observed in 38%, ischemic NPSLE in 32% and a combined phenotype in 30% of the patients, as shown in Table 2. 255 NPSLE symptoms were present in patients with major NPSLE, of which the majority was cerebrovascular disease (33%) and cognitive dysfunction (19%). 320 NP symptoms were present in minor/non-NPSLE patients. The majority of these NP symptoms were mild or not objectified cognitive complaints (24%), headache (23%) and depression, other mood disorders and coping disorder (18%). In addition, NP symptoms due to medication and alternative diagnoses were present, such as infections, tumours, neurodegenerative disorders and hernia nuclei pulposi.
Table 1.
Demographic and clinical characteristics of SLE patients visiting the neuropsychiatric systemic lupus erythematosus (NPSLE) clinic at first visit.
| Clinical characteristics | Major NPSLE (n = 149) | Minor/non-NPSLE (n = 202) |
|---|---|---|
| Gender (n (female, %)) | 130 (87) | 179 (89) |
| Age (years (median, range)) | 42.3 (13.6–72.2) | 44.9 (16.1–84.2) |
| Follow-up time (years (median, range)) | 6.1 (0.4–12.0) | 4.9 (0.3–11.6) |
| SLE duration (years (median, range)) | 4.4 (0.0–42.1) | 4.4 (0.0–38.9) |
| ACR 1997 criteria (n, %) | ||
| Malar rash | 58 (39) | 84 (42) |
| Discoid rash | 22 (15) | 41 (20) |
| Photosensitivity | 70 (47) | 107 (53) |
| Oral ulcers | 57 (38) | 91 (45) |
| Nonerosive arthritis | 98 (66) | 114 (56) |
| Pleuritis or pericarditis | 43 (29) | 50 (25) |
| Renal disorder | 43 (29) | 53 (26) |
| Neurologic disorder | 27 (18) | 16 (8) |
| Hematologic disorder | 81 (54) | 91 (45) |
| Immunologic disorder | 123 (83) | 146 (72) |
| Positive ANA | 146 (98) | 196 (97) |
| Antiphospholipid syndrome (n, %) | 39 (26) | 26 (13) |
| Hypertension (n, %) | 60 (40) | 63 (31) |
| SLEDAI-2K (median, range) | 6 (0–34) | 4 (0–34) |
| SDI (median, range) | 1 (0–11) | 0 (0–6) |
| Smoking status (n, %) | ||
| Present | 39 (26) | 60 (30) |
| Past | 27 (18) | 39 (19) |
| Never | 82 (55) | 101 (50) |
| Unknown | 1 (1) | 2 (1) |
| Education level (n, %) | ||
| Low | 10 (7) | 6 (3) |
| Middle | 94 (63) | 115 (57) |
| High | 39 (26) | 66 (33) |
| Unknown | 6 (4) | 15 (7) |
| Anti-inflammatory treatment (n, %)a | ||
| Corticosteroids | 117 (79) | 99 (49) |
| Methylprednisolone | 33 (22) | 2 (1) |
| Azathioprine | 59 (40) | 36 (18) |
| Hydroxychloroquine | 91 (61) | 141 (70) |
| Methotrexate | 8 (5) | 14 (7) |
| MMF | 25 (17) | 23 (11) |
| Tacrolimus | 0 (0) | 2 (1) |
| Cyclophosphamide | 44 (30) | 5 (3) |
| Biologicals (rituximab, belimumab) | 10 (7) | 0 (0) |
| Anti-coagulant treatment (n, %)a | ||
| Aspirin | 69 (46) | 33 (16) |
| Dipyridamole | 25 (17) | 9 (5) |
| P2Y12-inhibitor | 29 (20) | 7 (4) |
| Vitamin K antagonist | 38 (26) | 21 (10) |
| DOAC | 1 (1) | 2 (1) |
| LMWH | 6 (4) | 3 (2) |
| Statin | 49 (33) | 39 (19) |
aTreatment at baseline visit and initiated as treatment for major NPSLE.ANA: antinuclear antibodies; DOAC: direct oral anticoagulant; LMWH: low molecular weight heparin; MMF: mycophenolate mofetil; SDI: SLICC damage index; SLE: systemic lupus erythematosus; SLEDAI-2K: systemic lupus erythematosus disease activity index 2000.
Table 2.
Characteristics of major NPSLE patients visiting the clinic between 2007–2018.
| Clinical characteristics | Major NPSLE (n = 149) |
|---|---|
| NPSLE phenotype (n, %) | |
| Inflammatory | 57 (38) |
| Ischemic | 48 (32) |
| Combined | 44 (30) |
| NPSLE syndrome (n, %*) | |
| Aseptic meningitis | 5 (2) |
| Cerebrovascular disease | 85 (33) |
| Demyelinating syndrome | 0 (0) |
| Headache | 10 (4) |
| Movement disorder (chorea) | 5 (2) |
| Myelopathy | 13 (5) |
| Seizure disorders | 15 (6) |
| Acute confusional state | 7 (3) |
| Anxiety disorder | 1 (0) |
| Cognitive dysfunction | 49 (19) |
| Mood disorder | 14 (6) |
| Psychosis | 8 (3) |
| AIDPa | 0 (0) |
| Autonomic disorder | 1 (0) |
| Mononeuropathy | 1 (0) |
| Myasthenia gravis | 0 (0) |
| Neuropathy, cranial | 7 (3) |
| Plexopathy | 1 (0) |
| Polyneuropathy | 7 (3) |
| Otherb | 26 (10) |
*% of total neuropsychiatric symptoms (n = 255)
aAcute inflammatory demyelinating polyneuropathy.
bOther NPSLE symptoms: cerebral vasculitis (n = 10), organic brain syndrome (n = 4), pyramidal tract disorder (n = 3), visual disturbance other than optic neuritis (n = 2), apraxia (n = 2), walking disorder (n = 2), motor disorder left arm (n = 1), mononeuritis multiplex (n = 1), increased intracranial pressure (n = 1).
Table 3.
All-cause mortality in patients presenting with neuropsychiatric symptoms attributed to SLE (major NPSLE) or to other causes (minor/non-NPSLE).
| Parameters | Major NPSLE (n = 149) | Minor/non-NPSLE(n = 202) |
|---|---|---|
| Deaths (n, %) | 13 (9) | 17 (8) |
| Age at death (median, range) | 48.5 (32.0–79.4) | 59.2 (20.0–89.3) |
| Follow-up time (years) | 906 | 1047 |
| Crude mortality rate (per 1000 PY) | 14.3 | 16.2 |
| All-cause mortalitya | ||
| Female | 5.5 (2.8–9.6) | 3.4 (1.9–5.7) |
| Male | 2.3 (0.1–12.8) | 6.2 (1.3–18.2) |
| Combined | 5.0 (2.6–8.5) | 3.7 (2.2–6.0) |
aStandardized mortality ratio (SMR), ratio of the observed and expected number of deaths.
All-cause mortality
In total, 30 out of the 351 patients died during follow-up (9%), as shown in Table 3. In patients with major NPSLE, the median age at death was 48.5 years (range: 32.0–79.4) compared with 59.2 years (range 20.0–89.3) in patients with minor/non-NPSLE. Total follow-up time was 1,047 vs. 906 years respectively, which led to a crude mortality rate of 14.3 per 1,000 person-years in major NPSLE patients and 16.2 per 1,000 person-years in minor/non-NPSLE patients. The age-distribution of both groups is provided in Supplementary Figure 2. A Kaplan-Meier survival estimate is provided in Supplementary Figure 3.
Compared to the general population, mortality was five times higher in patients with major NPSLE (SMR: 5.0 (95% CI: 2.6–8.5). In patients with minor/non-NPSLE, mortality was nearly four times higher (SMR 3.7 (95% CI: 2.2–6.0)). Mortality in major NPSLE and minor/non-NPSLE was similar (rate ratio: 1.0, 95% CI: 0.5–2.0).
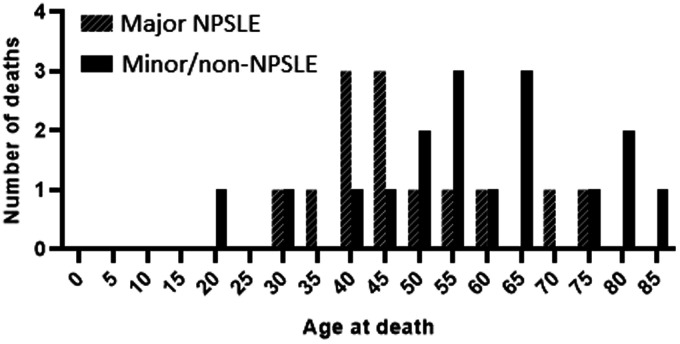
The age at death in both groups is demonstrated in Figure 1. The majority of patients died <50 years of age in major NPSLE patients, compared to >50 years in minor/non-NPSLE patients.
Figure 1.
Age at death of in patients with neuropsychiatric symptoms attributed to SLE requiring treatment (major NPSLE) and to mild and other causes (minor/non-NPSLE).
Cause-specific mortality
Information on cause-specific mortality in patients with major NPSLE and minor/non-NPSLE is provided in Table 4. In patients with major NPSLE, the main causes of death were infections, malignancies and SLE activity (all 23%). In particular, there was an increased risk of death due to infections compared to the general population (SMR 91.3 (95%CI: 18.8–266.9). In patients with minor/non-NPSLE, main causes of death were cardiovascular disease (29%) and malignancies (24%). Compared to the general population, infections and cardiovascular disease were more frequent causes of death (SMR 29.0 (95%CI: 3.5–104.9) and 6.2 (95% C1: 2.0–14.6) respectively). In both groups, malignancies were twice as common as in the general population.
Table 4.
Cause-specific mortality in patients presenting with neuropsychiatric symptoms attributed to SLE (major NPSLE) or to mild and other causes (minor/non-NPSLE).
|
Major NPSLE (n = 13) |
Minor/non-NPSLE (n = 17) |
|||||
|---|---|---|---|---|---|---|
| n a | % | SMR b | n a | % | SMR b | |
| Infections | 3 | 23 | 91.3 (18.8 – 266.9) | 2 | 12 | 29.0 (3.5 – 104.9) |
| Cardiovascular diseasec | 0 | 0 | 0.0 (0.0 – 13.7) | 5 | 29 | 6.2 (2.0 – 14.6) |
| Malignancies | 3 | 23 | 2.7 (0.6 – 7.8) | 4 | 24 | 2.3 (0.6 – 5.9) |
| SLE | 3 | 23 | – | 0 | 0 | – |
| NPSLE | 0 | 0 | – | – | – | – |
| Otherd | 2 | 15 | – | 3 | 18 | – |
| Unknown | 2 | 15 | – | 3 | 8 | – |
aNumber of deaths.
bStandardized mortality ratio, ratio of the observed and expected number of deaths and 95% confidence intervals.
cCardiac arrest (n = 4), stroke (n = 1).
dOther (major NPSLE): extra-abdominal hemorrhage (psoas hematoma) (n = 1), bowel perforation (n = 1). Other (minor/non-NPSLE): Alzheimer disease (n = 1), intra-abdominal hemorrhage (n = 1), suicide (n = 1).
Determinants of mortality in NPSLE patients
In both deceased and alive patients, the most common NPSLE syndromes were cerebrovascular disease (62% vs. 57%) and cognitive dysfunction (31% vs. 33%), as shown in Table 5.
Table 5.
Baseline characteristics of deceased and alive patients with neuropsychiatric symptoms attributed to SLE (major NPSLE).
| Parameters | Deceased patients (n = 13) | Surviving patients (n = 136) |
|---|---|---|
| Gender (n (female, %)) | 12 (92) | 118 (87) |
| Age (median, range) | 43.8 (24.3–72.2) | 42.0 (13.6–72.2) |
| Follow-up time (median, range) | 4.1 (0.5–8.6) | 6.2 (0.4–12.0) |
| SLE duration (median, range) | 6.3 (0.1–29.5) | 4.2 (0.0–42.1) |
| Antiphospholipid syndrome (n, %) | 6 (46) | 43 (32) |
| SLEDAI-2K (median, range) | 8 (2–24) | 5 (0–34) |
| SDI (median, range) | 1 (0–5) | 1 (0–11) |
| NPSLE phenotype (n, %) | ||
| Inflammatory | 4 (31) | 44 (32) |
| Ischemic | 5 (39) | 52 (38) |
| Combined | 4 (31) | 40 (29) |
| NPSLE syndrome (n, %) | ||
| Aseptic meningitis | 0 (0) | 5 (4) |
| Cerebrovascular disease | 8 (62) | 77 (57) |
| Demyelinating syndrome | 0 (0) | 0 (0) |
| Headache | 0 (0) | 10 (7) |
| Movement disorder (chorea) | 1 (8) | 4 (3) |
| Myelopathy | 0 (0) | 13 (10) |
| Seizure disorders | 0 (0) | 15 (11) |
| Acute confusional state | 0 (0) | 7 (5) |
| Anxiety disorder | 0 (0) | 1 (1) |
| Cognitive dysfunction | 4 (31) | 45 (33) |
| Mood disorder | 2 (15) | 12 (9) |
| Psychosis | 2 (15) | 6 (4) |
| AIDPa | 0 (0) | 0 (0) |
| Autonomic disorder | 0 (0) | 1 (1) |
| Mononeuropathy | 0 (0) | 1 (1) |
| Myasthenia gravis | 0 (0) | 0 (0) |
| Neuropathy, cranial | 1 (8) | 6 (4) |
| Plexopathy | 0 (0) | 1 (1) |
| Polyneuropathy | 1 (8) | 6 (4) |
| Other | 3 (23)a | 25 (18) |
aOther NP symptoms in deceased major NPSLE patients: cerebral vasculitis (n = 2), mononeuritis multiplex (n = 1).AIDP: acute inflammatory demyelinating polyneuropathy; NPSLE: neuropsychiatric systemic lupus erythematosus; SDI: SLICC damage index; SLE: systemic lupus erythematosus; SLEDAI-2K: systemic lupus erythematosus disease activity index 2000.
Hazard ratios were calculated for NPSLE phenotype, corrected for SLE duration. Compared to inflammatory NPSLE, mortality was not evidently higher in patients with ischemic NPSLE (HR 1.1, 95% CI 0.3–4.5) and combined NPSLE (HR 1.3, 95% CI: 0.3–5.5).
Sensitivity analyses
The sensitivity analyses regarding patients with Undefined NPSLE and patients with <4 ACR 1997 SLE classification criteria resulted in similar all-cause mortality, as shown in Supplementary Table 1. Of the patients with major NPSLE, 27 patients only had NPSLE prior to the visit to our clinic (18%). When these patients were excluded from the analyses, all-cause mortality was also similar.
Discussion
In the present study, we analysed mortality in patients with NP symptoms either attributed to SLE (major NPSLE) or to other causes (minor/non-NPSLE). We demonstrated that mortality in NPSLE patients was five times higher and mortality in minor/non-NPSLE patients was four times higher than in the general population. When comparing major NPSLE and minor/non-NPSLE patients directly, mortality appeared to be similar. Infections were an important cause of death in both groups.
Other research has as well shown an increased risk of mortality compared to the general population in SLE patients: a meta-analysis previously demonstrated a three-fold increased risk in mortality.6 A recent analysis in SLE patients in Canada also demonstrated an increased SMR especially in younger patients (19-45 years), which is similar to our findings in both major NPSLE and minor/non-NPSLE patients (see Supplementary Table 2).13 This indicates that the increased mortality compared to the general population is mainly due to deaths at a young age.
In our cohort, both crude mortality rate and rate ratio imply no clear difference in mortality between major NPSLE and minor/non-NPSLE patients. This is different than has been described in a previous study, which demonstrated a higher mortality in NPSLE compared to SLE patients.4 A possible explanation for this difference is that major NPSLE patients are now recognized earlier and treated on time, therefore normalizing their mortality risk to (minor/non-NP) SLE patients. In addition, the impact of other underlying NP disease such as Alzheimer’s or other major SLE organ involvement might contribute to this observation, but was not evaluated in our study. In our cohort, median age at death was ten years lower in major NPSLE patients than in minor/non-NPSLE patients, with eight patients with an age of death below 50 years (62% of total deaths). Of these eight patients, three died due to infections and three due to SLE activity. SLE activity as cause of death was due to renal failure (2x) and pulmonary hypertension. Although median age at death was lower in major NPSLE patients, SLE disease duration was similar in deceased patients of both groups (12 vs.14 years). This indicates that deceased patients with NPSLE were diagnosed with SLE at a younger age. It is unknown whether there is an actual association between SLE at young age, major NPSLE and mortality or that this is a coincidental finding in our cohort.
It is known that infections are an important cause of mortality in all patients with SLE, together with renal disease and cardiovascular disease.6 In our study, we also show that in both major NPSLE and minor/non-NPSLE patients’ infections are more prevalent as causes of death than in the general population. Causes of infectious death in our cohort were bacterial meningitis (2x), endocarditis, pneumonia and sepsis of unknown origin. Of the three major NPSLE patients that died due to infections, one patient received immunosuppressive therapy (cyclophosphamide) as treatment for NPSLE and died eighteen months after baseline visit. The increased risk of infections, especially in NPSLE patients, emphasizes the necessity to weigh potential treatment benefits against the risk of severe infection as a result of immunosuppressive treatment. In addition, other treatment related toxicity due to high-dose corticosteroids should be taken into account, such as an increased cardiovascular risk. In our cohort, however, the risk of death due to cardiovascular disease, including cerebrovascular disease, was only increased in minor/non-NPSLE patients. We expected to find more deaths due to cardiovascular disease in patients with major NPSLE, especially as APS is also more present in this population. It is possible that these patients are recognized earlier and receive appropriate treatment, preventing further cardiovascular damage. Of the five patients that died due to cardiovascular disease, only one patient had APS and two patients received anticoagulant treatment at baseline. However, most patients died long after this first visit (median 8 years, range 1–10 years) and it is unknown whether they (still) used anticoagulants at time of death. We also noticed that in our cohort a relatively large number of patients had malignancies (various types) as cause of death at a young age (median 55 years, range 46–79 years). Previous studies demonstrated a similar result, with a (nearly) two-fold increased risk of malignancies in SLE patients compared to the general population.14
In our hospital, mortality in major NPSLE was also studied previously, mainly in patients seen before the tertiary referral NPSLE clinic with multidisciplinary assessment was established (2007).5 Mortality was high in this cohort compared with the general population (SMR: 9). When comparing mortality with the current NPSLE cohort (2007–2018) as reference, mortality was higher in our center before 2007 (RR 1.9, 95% CI 1.0–3.7). Potential explanations for an improvement over time are earlier recognition and treatment of major NPSLE as well as a different patient selection with more severe cases before 2007 as a result of a different referral policy. Accordingly, before 2007, major NPSLE was the cause of death in five patients (16% of the total deaths), whereas no deaths due to NPSLE were reported in the current cohort. This is also in contrast with other studies, that showed NP involvement due to SLE, mainly cerebrovascular disease and acute confusional state, as frequent causes of death in NPSLE between 1990 and 2012.15,16 In our entire cohort, only two deaths were reported due to neurological disease, both in the minor/non-NPSLE group: one patient died of Alzheimer’s disease and another from an ischemic stroke at old age (90 years). It is noteworthy that despite the reported morbidity of major NPSLE, no deaths due to major NPSLE are present in our cohort, although two causes of death are missing for NPSLE patients.
We also aimed to study factors influencing mortality in patients with major NPSLE. We did not find any difference in mortality when comparing NPSLE phenotypes (inflammatory, ischemic, combined). This is also different than described previously: focal NPSLE has been associated with a strongly increased risk of mortality (HR: 7.8).4 Focal NPSLE manifestations include, amongst others, cerebrovascular disease and seizures, and is correlated with elevated antiphospholipid antibodies, thereby showing many similarities to ischemic NPSLE.
Our study has several strengths. In our cohort, major NPSLE and minor/non-NPSLE cases were well defined by multidisciplinary evaluation. As NPSLE lacks a golden standard, different approaches are used to define NPSLE in research, especially the SLICC decision rules and the attribution model by Bortoluzzi et al. are frequently used.8 Although similar factors are used in our multidisciplinary evaluation, our groups will not be entirely similar as when these models would have been used. We feel that our multidisciplinary assessment, however, provides a more accurate representation of severe NPSLE manifestations as seen in clinical practice by using ‘real life’ data. In clinical practice, it is after all often impossible to distinguish minor NPSLE from non-NPSLE. Therefore, we chose to combine these groups as both are approached with supportive/symptomatic therapy. On the other hand, major NPSLE, defined as requiring immunosuppressive or anticoagulant treatment is a clear and more severe subgroup and therefore especially important to study separately.
There are also several limitations to our study. As major NPSLE is a rare disease, the absolute number of patients remains small, even after more than ten years of follow-up. Larger cohorts are necessary to provide clear insight into factors influencing mortality. Furthermore, we performed this study in a tertiary referral center for patients with SLE and NP symptoms, which might make the results less generalizable to all SLE patients with NP symptoms as referral bias is present. This is for example reflected by the high amount of CVD and cerebral vasculitis in our cohort compared to other studies. However, The overall frequency of attribution of NP symptoms to SLE in our cohort is similar to that in other cohorts (approximately 1/3).3,17 Furthermore, five causes of death were missing in our study population (major NPSLE: 2, minor/non-NPSLE: 3), which might have influenced the cause-specific mortality.
In conclusion, we studied mortality in SLE patients with NP symptoms attributed to SLE requiring treatment (major NPSLE) and to mild NP symptoms and other causes (minor/non-NPSLE). In both major NPSLE and minor/non-NPSLE patients, mortality is increased compared to the general population, but no clear difference in mortality between major NPSLE and minor/non-NPSLE patients was present. The increased mortality is mainly due to deaths at a younger age. Death to infections was increased in both groups, but interestingly, no deaths due to cardiovascular or neuropsychiatric disease were reported in major NPSLE patients.
Supplemental Material
Supplemental material, sj-pdf-1-lup-10.1177_0961203320963815 for Mortality in patients with systemic lupus erythematosus and neuropsychiatric involvement: A retrospective analysis from a tertiary referral center in the Netherlands by Rory C Monahan, Rolf Fronczek, Jeroen Eikenboom, Huub AM Middelkoop, Liesbeth JJ Beaart-van de Voorde, Gisela M Terwindt, Nic JA van der Wee, Frits R Rosendaal, Tom WJ Huizinga, Margreet Kloppenburg and Gerda M Steup-Beekman in Lupus
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: RC Monahan https://orcid.org/0000-0003-2561-7085
Supplemental material: Supplemental material for this article is available online.
References
- 1.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed]
- 2.Bortoluzzi A, Scirè CA, Govoni M. Attribution of neuropsychiatric manifestations to systemic lupus erythematosus. Front Med (Lausanne) 2018; 5: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magro-Checa C, Zirkzee EJ, Beaart-van de Voorde LJJ, et al. Value of multidisciplinary reassessment in attribution of neuropsychiatric events to systemic lupus erythematosus: prospective data from the Leiden NPSLE cohort. Rheumatology (Oxford) 2017; 56: 1676–1683. [DOI] [PubMed] [Google Scholar]
- 4.Ahn GY, Kim D, Won S, et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus 2018; 27: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 5.Zirkzee EJ, Huizinga TW, Bollen EL, et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE). Lupus 2014; 23: 31–38. [DOI] [PubMed] [Google Scholar]
- 6.Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 2016; 25: 727–734. [DOI] [PubMed] [Google Scholar]
- 7.Zirkzee EJ, Steup-Beekman GM, van der Mast RC, et al. Prospective study of clinical phenotypes in neuropsychiatric systemic lupus erythematosus; multidisciplinary approach to diagnosis and therapy. J Rheumatol 2012; 39: 2118–2126. [DOI] [PubMed] [Google Scholar]
- 8.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum 2007; 56: 265–273. [DOI] [PubMed] [Google Scholar]
- 9.Bortoluzzi A, Scirè CA, Bombardieri S, et al. Development and validation of a new algorithm for attribution of neuropsychiatric events in systemic lupus erythematosus. Rheumatology (Oxford) 2015; 54: 891–898. [DOI] [PubMed] [Google Scholar]
- 10.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001; 57: 496–500. [DOI] [PubMed] [Google Scholar]
- 11.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 12.Central Bureau of Statistics. www.cbs.nl/nl/statline (accessed 10 August 2019).
- 13.Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971–2013). Ann Rheum Dis 2019; 78: 802–806. [DOI] [PubMed] [Google Scholar]
- 14.Björnådal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol 2004; 31: 713–719. [PubMed] [Google Scholar]
- 15.Feng M, Lv J, Fu S, et al. Clinical features and mortality in Chinese with lupus nephritis and neuropsychiatric lupus: a 124-patient study. J Res Med Sci 2014; 19: 414–419. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou HQ, Zhang FC, Tian XP, Leng XM, Lu JJ, Zhao Y. Clinical features and outcome of neuropsychiatric lupus in Chinese: analysis of 240 hospitalized patients. Lupus 2008; 17: 93–99. [DOI] [PubMed] [Google Scholar]
- 17.Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol 2014; 10: 338–347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-lup-10.1177_0961203320963815 for Mortality in patients with systemic lupus erythematosus and neuropsychiatric involvement: A retrospective analysis from a tertiary referral center in the Netherlands by Rory C Monahan, Rolf Fronczek, Jeroen Eikenboom, Huub AM Middelkoop, Liesbeth JJ Beaart-van de Voorde, Gisela M Terwindt, Nic JA van der Wee, Frits R Rosendaal, Tom WJ Huizinga, Margreet Kloppenburg and Gerda M Steup-Beekman in Lupus