Abstract
The treatment of pancreatic cancer with gemcitabine is hampered by its rapid metabolism in vivo, the dense stroma around the tumor site which prevents the drug from reaching the cancerous cells and drug resistance. To address these challenges, this study describes the preparation of a retinoid prodrug of gemcitabine, GemRA (gemcitabine conjugated to retinoic acid), and its formulation into a nanoparticulate system applicable for pancreatic cancer treatment. Retinoic acid targets stellate cells which are part of the stroma and can thus augment the delivery of gemcitabine. GemRA dissolved in dimethylsulfoxide presented efficacy towards PANC-1 (human) and mT4 (mouse) pancreatic cancer cell lines but its poor solubility in aqueous solution affects its applicability. Thus, the preparation of the nanoparticles was initially attempted through self-assembly of GemRA, which resulted in the formation of unstable aggregates that precipitated during preparation. As a result, encapsulation of the drug into micelles of polyethylene glycol-retinoic acid (PGRA) amphiphilic conjugates was accomplished and resulted in successful incorporation of GemRA into nanoparticlesof ca. 33 nm by dynamic light scattering and 25 nm by transmission electron microscopy. The nanoparticles had good stability in aqueous media and protected gemcitabine from the enzymatic action of cytidine deaminase, which converts gemcitabine to its inactive metabolite upon circulation. Cellular uptake of the nanoparticles by PANC-1 cells was confirmed by fluorescence spectroscopy and flow cytometry. Treatment of PANC-1 cells in vitro with the prodrug-loaded nanoparticles resulted in a significant reduction in cell viability (IC50 ca. 5 μM) compared to treatment with gemcitabine (IC50 >1000 μM). The ability of the GemRA-loaded nanoparticles to induce cellular apoptosis of treated PANC-1 cells was ascertained via a TUNEL assay suggesting these nanoparticles are effective in pancreatic cancer treatment.
Keywords: Pancreatic cancer, gemcitabine-retinoic acid prodrug, drug resistance, nanoparticle drug carrier, antitumor efficacy
Graphical abstract

1. Introduction
Pancreatic cancer remains one of the leading causes of cancer-related deaths despite the technological advancements in treatment and diagnosis of cancer over the years.[1, 2] The main treatment options, surgery and chemotherapy, are complicated by not all patients being suitable for surgery, the high propensity for metastases of the cancer and its resistance to chemotherapeutic drugs over time.[3–6] Furthermore, the drug of choice for the treatment of pancreatic cancer, gemcitabine, suffers from poor extravasation into pancreatic cancer tissues and rapid enzymatic deamination upon circulation which produces its inactive metabolite, 2′,2′-difluorodeoxyuridine (dFdU).[7, 8] Moreover, the presence of a desmoplastic stroma around the cancer site creates a barrier for the drug.[9] This results in high dosages of chemotherapy being required to attain an effect, which increases chances of side effects.[8, 10] Thus, significant research efforts have been made towards the design of drug delivery systems targeted at improving the therapeutic outcomes of chemotherapy with gemcitabine.
The main approaches that have been reported for improving the efficacy of gemcitabine in pancreatic cancer treatment include combination chemotherapy, formulation of gemcitabine into nanoparticles (polymeric/liposomal) and preparation/design of gemcitabine prodrugs that mitigate its deamination in vivo.[11–13] Effectiveness of combination chemotherapies often rely on harnessing the effect of two or more drugs to achieve an effect which in some cases is synergistic (e.g. nab-paclitaxel + gemcitabine and gemcitabine + cisplatin) and/or has reduced side effects.[14–17] Incorporation of gemcitabine into nanoparticles offers protection of the gemcitabine from the external environment during circulation thus curtailing deamination, and due to the size of the nanoparticles, the drug circulates longer with improved accumulation at the tumor site through the enhanced permeation and retention effect.[18–21] On the other hand, most prodrug approaches focus on the derivatization of gemcitabine through modification on its primary amine which prevents enzymatic attack by cytidine deaminase thus preventing its deactivation upon circulation.[22–24] The prodrugs can be self-assembled into nanoparticles through the nanoprecipitation method or encapsulated into polymeric or liposomal nanoparticles which also affords the advantages of nanoparticle formulations described above.[25, 26] Thus, an interesting and promising strategy to significantly enhance the therapeutic efficacy of gemcitabine would be to combine the prodrug, combination-chemotherapy and nanoparticle-formation approaches into a single drug delivery system.
In this work, we demonstrate the preparation of a gemcitabine nanoparticulate system embracing the three approaches above and determine its effectiveness towards gemcitabine-resistant human pancreatic cancer cells (PANC-1). A hydrophobic prodrug of gemcitabine was prepared by conjugating gemcitabine with retinoic acid (GemRA) which was encapsulated into amphiphilic polyethylene glycol-retinoic acid micelles. Retinoic acid shows some inhibitory effect on pancreatic cancer cells and has been shown to enhance the therapeutic index of chemotherapeutic drugs such as gemcitabine, cisplatin, doxorubicin, etoposide, and paclitaxel.[27–32] Moreover, retinoic acid can target pancreatic stellate cells which are part of the desmoplastic stroma that surrounds the cancer cells and acts as a barrier for chemotherapeutic drugs, thus generally promoting tumor growth and resistance.[33–36] Therefore, targeting the stellate cells is expected to lead to improved trafficking of the chemotherapeutic drug (gemcitabine) to the cancer cells. The application of retinoic acid is, however, hampered by its hydrophobicity, poor bioavailability, side effects upon systemic administration and instability towards light and oxidants which necessitates its incorporation into a nanoparticulate system for drug delivery purposes.[37, 38] Thus, the design of a nanoparticle platform capable of simultaneous delivery of gemcitabine and retinoic acid presents an intriguing strategy for pancreatic cancer treatment. For instance, Abu-Fayyad and Nazzal reported the conjugation of derivatives of vitamin E, γ-tocotrienol (γ-T3) and α-tocopherol (α-T, to gemcitabine which were then fabricated into nanoemulsions of ca.300 nm. These nanoemulsions showed better performance than gemcitabine against Bx-PC-3 and PANC-1 pancreatic cancer cell lines in vitro.[39] In work by Yalcin and co-workers, retinoic acid was conjugated to poly(amidoamine), PAMAM, dendrimer-coated iron oxide nanoparticles followed by loading of gemcitabine, and the drug-loaded nanoparticulate system was shown to be more effective at reducing viability of pancreatic cancer cells (PANC-1) in vitro compared to gemcitabine alone.[40] However, this system relied on the use of inorganic nanoparticles which raises toxicity concerns, and the payload release was most likely at different rates as retinoic drug was conjugated while the gemcitabine was physically encapsulated. Thus, when administered in vivo, the simultaneous delivery and entry of gemcitabine and retinoic acid into tumor cells is not guaranteed. Furthermore, the simultaneous loading and delivery of the two is not trivial due to the disparity in their polarity. Our prodrug approach ensures the formation of a single molecule that can be encapsulated in hydrophobic cores of core-shell micellar nanostructures that are easily accessible through block copolymer preparation and selfassembly. We demonstrate the nanoparticles obtained using this approach to be effective towards drug-resistant pancreatic cancer cells, and therefore anticipate their capability to improve chances of survival/prolong life in patients with pancreatic cancer.
2. Materials and methods
2.1. Materials
Gemcitabine hydrochloride was obtained from LC Laboratories (Woburn, MA). Retinoic acid, imidazole, tert-butyldimethylsilyl chloride (TBDMS-Cl), dimethyl sulfoxide, triethylamine, pyrene, N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCl), 4-Dimethylaminopyridine (DMAP), tetrabutylammonium fluoride solution 1.0 M in THF, cytidine deaminase, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), N,N′-Diisopropylcarbodiimide, N,N-Diisopropylethylamine (DIPEA) and Cathepsin B was purchased from Sigma (St. Louis, MO). Methoxy polyethyleneglycol 750 was obtained from Fisher Scientific (Santa Clara, CA). Methoxy-PEG2k-cholesterol (CholPEG) was purchased from Nano CS (Boston, MA). Anhydrous dimethylformamide was obtained from Acros Organics (Fair Law, NJ). The Cell Meter ™ TUNEL Apoptosis Assay kit was purchased from AAT Bioquest (Sunnyvale, CA).
2.2. Methods
2.2.1. Synthesis of TBDMS protected gemcitabine
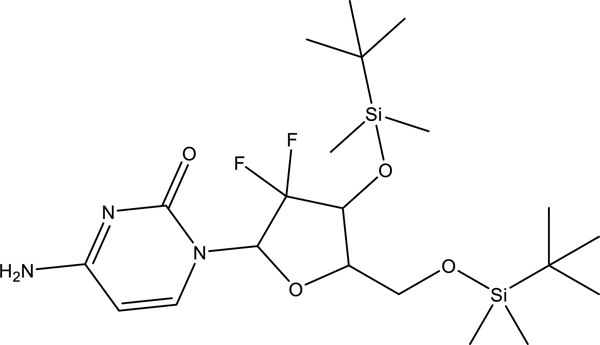
The compound was synthesized in accordance to the procedure previously reported by our group.[41] Briefly, to gemcitabine hydrochloride (0.5 g, 1.7 mmol) and imidazole (1.1574 g, 17.0 mmol) in anhydrous dimethylformamide (15 mL) was added tert-butyldimethylsilyl chloride (TBDMS-Cl, 2.5622 g, 10.0 mmol) under nitrogen. Triethylamine (0.4048 g, 560 μL, 3.4 mmol) followed by DMAP (0.0415 g, 0.34 mmol) were then added dropwise using gas tight syringes. The reaction was monitored by TLC using ethyl acetate. After 48 h, the reaction was stopped and concentrated in vacuo to a viscous oil. The residue was treated with aqueous sodium hydrogen carbonate (100 mL) and extracted with ethyl acetate (4 × 100 mL). The combined organic extract was washed with aqueous sodium hydrogen carbonate (1 × 50 mL), brine (1 × 50 mL) and dried over sodium sulfate. The sodium sulfate was removed via filtration and the solvent was removed under reduced pressure affording a pale-yellow oil. The crude product was dissolved in dichloromethane (200 mL) and washed with aqueous sodium hydrogen carbonate (1 × 50 mL) and brine (4 × 100 mL) after which the organic layer was dried over sodium sulfate. Removal of the solvent in vacuo gave an oil that solidified to a waxy off-white solid upon drying under high vacuum that was purified via column chromatography (SiO2), dichloromethane (100%) c.a. four column volumes then ethyl acetate (100%). Yield was 615 mg, 50% of the diprotected product.
**Increasing the amounts of TBDMS-Cl and imidazole plus adding DMAP (catalyst) was found to favor the formation of the disubstituted product.
2.2.2. Preparation of gemcitabine-retinoic acid conjugate
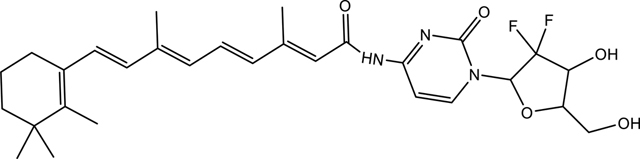
To two separate glass vials covered with aluminum foil was individually weighed out retinoic acid (100 mg, 0.33 mmol) and TBDMS-protected gemcitabine (178 mg, 0.36 mmol) under a blanket of nitrogen in a glove bag. The vials were immediately sealed with rubber stoppers followed by purging with a very gentle stream of nitrogen gas. The retinoic acid was dissolved in dry DMF (ca. 1 mL) which was transferred into the vial under nitrogen gas via a cannula. In a separate glass vial equipped with a stir bar, EDC.HCl (63 mg, 0.33 mmol) was dissolved in dry DMF (1 mL) before Oxyma Pure (47 mg, 0.33 mmol) was added and the vial was sealed with a rubber stopper then sparged with nitrogen gas. The EDC.HCl solution was cooled down to 4 °C before the retinoic acid solution was transferred to the EDC/Oxyma solution via a cannula followed by addition of DIPEA (86 μL, 0.50 mmol) using a gas tight syringe. TBDMS-protected gemcitabine was dissolved in dry DCM (ca. 1 mL) and transferred via a cannula to the vial containing EDC/Oxyma/retinoic acid solution at 4 °C. The resulting solution was left to stir at room temperature for 1 h then placed on a stirrer in a refrigerator at 4 °C and allowed to stir for 48 h. After the 48-hour period, TBAF (5 eq of TBDMS protected gemcitabine) was added to the solution via a syringe and the solution was left to stir for a further 48 h. Most of the solvent was removed using a stream of nitrogen gas to reduce the volume to ca. 0.5 mL. The crude product was diluted with a solution (2 mL) of acetonitrile /water/TFA (1/1/0.05) followed by purification via HPLC (protected from light, gradient 50–95% acetonitrile over 60 min, λ 325 nm). The fractions containing the product were combined and lyophilized to recover the product as a bright yellow solid which was stored at −20 °C under nitrogen (split into 2 mg per storage vial). Yield was 36 mg, 20%. 13C NMR, δ (ppm): 12.28, 14.23. 19.18, 21.73, 28.98, 33.09, 34.29, 39.65, 59.85, 95.34, 115.05, 129.21, 129.60, 130.37, 132.95, 143.22, 137.08, 137.58, 141.16, 157.21, 160.44, 165.43.
2.2.3. Self-assembly of GemRA (typical procedure)
GemRA (2 mg) was dissolved in 0.4 mL of nitrogen sparged acetone in an aluminum foil covered 4 mL rubber sealed glass vial that was equipped with a magnetic stir bar. The solution was left to stir for 5 min before filtered (0.2 μm membrane) degassed distilled water (2 mL) was added dropwise (0.04 mL/min) via a syringe pump. Acetone was initially removed slowly using the rotary evaporator (ca. 30 min, in the dark, no more than 30 °C) then using a stream of nitrogen which was blown over the remaining solution under continuous stirring for ca. 15 min. Precipitation was observed which was further ascertained via DLS analysis wherein the sizes were above 1 μm. No further characterization was conducted.
2.2.4. Conjugation of retinoic acid to PEG750 (PGRA)

Retinoic acid (100 mg, 0.33 mmol), methoxy polyethylene-glycol (248 mg, 0.33 mmol, Mn=750 g/mol) and Oxyma Pure (47 mg, 0.33 mmol) were weighed into a glass vial which was covered with aluminum foil and equipped with a magnetic stir bar under a nitrogen atmosphere in a glove bag. The vial was sealed with a rubber stopper and removed from the glove bag and the contents were dissolved in dry DMF (2 mL) which was added via a cannula. The vial was placed in an ice bath and sparged with nitrogen gas for 5 min followed by slow addition of DIC (77 μL, 0.5 mmol) via a gas tight syringe. The resulting solution was stirred at room temperature for 1 h at room temperature then at 4 °C for 48 h. After the reaction, the solution was diluted with degassed water (1 mL) and transferred into a dialysis bag (MWCO 3500 Da) followed by dialysis against nitrogen sparged water for 24 h at 4°C (in the dark). The contents of the dialysis bag were transferred into a Falcon tube and centrifuged at 4000 rpm for 10 min, and the supernatant was removed and transferred into a dialysis bag (MWCO 3500 Da) and dialysed against degassed water for 24 h at 4°C (in the dark). The solution in the dialysis bag was collected and lyophilized to recover the crude product which was further purified by HPLC (55–95% acetonitrile over 60 min, λ 325 nm). The isolated fractions were combined and lyophilized to recover the pure product (sticky yellow gel) which was dissolved in dichloromethane and stored as a stock solution at −20 °C. Yield was 125 mg, 38%. 13C NMR, δ (ppm):12.92, 13.88, 19.30, 21.73, 28.89, 33.10, 32.26, 39.61, 58.97, 62.81, 69.37, 70.43, 71.85, 118.19, 128.72, 129.39, 130.02, 131.14, 135.00, 137.19, 137.73, 139.75, 153.15, 166.96
2.2.5. Self-assembly of PGRA and CholPEG (Typical procedure)
2.2.5.1. PGRA
PGRA (2 mg) was dissolved in 0.4 mL of nitrogen sparged acetone in an aluminum foil covered 4 mL rubber sealed glass vial that was equipped with a magnetic stir bar. The solution was left to stir for 30 min before filtered distilled water (2 mL) was added dropwise (0.04 mL/min) via a syringe pump. Acetone was initially removed using the rotary evaporator (ca. 30 min, in the dark, no more than 30 °C) then using a stream of nitrogen which was blown over the remaining solution under continuous stirring for ca. 15 min. The micellar solution was characterized by DLS, TEM and HPLC. Micelle solutions were stored at 4 °C.
2.2.5.2. CholPEG
The procedure was similar to that employed for PGRA in 2.2.5.1 above.
2.2.6. Determination of critical micelle concentration (CMC)
The CMC was determined by fluorescence spectroscopy using pyrene as a probe as previously reported. Briefly, a solution of pyrene was prepared in methanol (0.025 mg/mL) and equal volumes (50 μL) were transferred into different vials from which the solvent was allowed to evaporate in the dark. To the pyrene containing vials were added different amounts of polymer-conjugate solution in water to obtain solutions of varying polymer concentrations in the range (0–0.5 mg/mL, total volume 3 mL, 30 μL PBS, pH 7.4 buffer added per vial). The solutions were left to equilibrate overnight at room temperature (in the dark/covered with aluminum foil) under gentle agitation on a rotary shaker. Fluorescence emission spectra of the solutions were obtained at 25 °C starting from 350 nm to 420 nm using an excitation wavelength of 334 nm. Intensities at 373 nm (I1) and 385 nm (I3) were recorded and the ratio I3/I1 was plotted against the logarithm of polymer-conjugate concentration. The concentration at the intersection of the tangents to the two linear portions of the plot was considered to be the CMC.
2.2.7. Encapsulation of GemRA in PGRA and CholPEG (Typical procedure)
2.2.7.1. PGRA
PGRA (0.7 mg) and GemRA (0.9 mg) (mixed) were dissolved in 0.2 mL of nitrogen sparged acetone in an aluminum foil covered 4 mL rubber sealed glass vial that was equipped with a magnetic stir bar. The solution was left to stir for 30 min before filtered distilled water (1 mL) was added dropwise (0.04 mL/min) via a syringe pump. Acetone was initially removed using the rotary evaporator (ca. 30 min, in the dark, no more than 30 °C) then using a stream of nitrogen which was blown over the remaining solution under continuous stirring for ca. 15 min. Unencapsulated drug sticks to the surfaces of the glass wall while the drug-encapsulating micelles form a clear solution. The micelle solutions were stored at 4 °C overnight and examined for precipitate formation. The solutions were clear with no signs of precipitation and centrifugation at 2000 rpm did not show any sedimentation either. Characterization was accomplished by DLS, TEM and HPLC. The amounts of GemRA and PGRA were determined by pipetting a known amount of the drug encapsulating micelle solution which was lyophilized, weighed and dissolved in acetone (100 μL) then injected into the HPLC. The amount of GemRA determined from the calibration curve based on peak area and the drug loading content (%) was calculated based on the equation below:
Drug loading content (LC) (%) = mass of GemRA in micelles / total mass of loaded micelles × 100%
2.2.7.2. CholPEG
The procedure was similar to that employed for PGRA in 2.2.7.1 above.
2.2.8. Nanoparticle stability
2.2.8.1. Stability on storage
Nanoparticles with and without encapsulated drug were prepared as detailed above and kept in water at 4 °C in the dark. Aliquots were taken and diluted to 0.1 mg/mL using deionized water containing PBS (pH 7.4) buffer (0.04×). The size and zeta potential were measured on different days using the Malvern Zetasizer over a 21-day period. Reported results are an average of at least 3 readings.
2.2.8.2. Stability in fetal bovine serum (FBS)
Nanoparticles with encapsulated drug were prepared as detailed above and aliquots were taken and diluted to 0.1 mg/mL using aqueous FBS. The size was measured every 10 min at 37 °C using the Malvern Zetasizer over a 20-hour period. One size reading was obtained per time point. The size for FBS without the drug-loaded nanoparticles was an average of 3 readings.
2.2.8.3. Metabolic stability
Solutions of gemcitabine (2 mL, 0.4 mM drug), PBS buffer pH 7.0 (adjusted from pH 7.4 (1×) using HCl, 10 N) and drug-encapsulating PGRA micelles (2 mL, 0.4 mM of drug conjugate, PBS buffer pH 7.0) were prepared in separate Eppendorf tubes to which Cathepsin B and cytidine deaminase were added to simulate in vivo conditions for cleavage of the gemcitabine from drug conjugate and deactivation of the freed gemcitabine to its inactive metabolite (dFdU). The solutions were placed in a water bath maintained at 37 °C and aliquots (100 μL) were withdrawn at predetermined intervals and treated with 10 μL of tetrahydrouridine (10 μg/mL) then 5 μL of glacial CH3COOH. The aliquots were diluted with acetonitrile to 500 μL and mixed thoroughly on a vortex mixer followed by centrifugation at 5000 rpm. The supernatants were withdrawn and analyzed by HPLC to determine the amounts of gemcitabine and dFdU.
2.2.9. In vitro drug release
Drug release was investigated at pH 7.4 (PBS, 0.5×), pH 5.5 (sodium acetate, 0.05 M) and at pH 5.5 (sodium acetate, 0.05 M) in the presence of Cathepsin B. Micellar solutions (2 mL) containing a known amount of drug (500 μg) were placed in separate dialysis bags (MWCO 2000 Da) containing 500 μL of buffer solution (pH 7.4 and pH 5.5) or Cathepsin B containing buffer solution and dialyzed against 30 mL of buffer at 37 °C. At predetermined intervals, 2 mL of the dialysate was withdrawn and replaced with fresh buffer solution of equal volume. The aliquots were analyzed via HPLC to determine the amount of released gemcitabine. For comparison, gemcitabine hydrochloride was also placed in a separate dialysis bag and dialyzed at pH 7.4 (37 °C) with the dialysate being analyzed by HPLC to determine the amount of drug released.
2.2.10. Nanoparticle uptake
2.2.10.1. Flow cytometry
Human PANC-1 pancreatic cancer cells were obtained from American Type Culture Collection (ATCC, CRL-1469). The murine mT4 pancreatic cancer cell line, derived from the genetically-engineered KPC (Kras+/LSLG12D ; Trp53+/LSL-R172H ; Pdx1-Cre) model, was a generous gift from Dr. David Tuveson (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). PANC-1 and mT4 cells were plated at 1.5 × 105 or 2 × 105 cells/well, respectively, in 24-well tissue culture plates 24 h prior to experiments. Three separate PGRA nanoparticle samples were prepared in which either (i) 3,3′-dioctadecyloxacarbocyanine perchlorate (dye 1) or (ii) 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (dye 2) encapsulation or (iii) an equimolar mixed dye (dyes 1 & 2) were encapsulated. Dye encapsulation was conducted in a similar manner to drug encapsulation detailed above. Non-encapsulated dye was removed via centrifugation and the samples were separately added to the plated mT4 and PANC-1 cells and incubated at 37 °C in complete media supplemented with 10% FBS and 1% Penicillin/Streptomycin (300 μL for 24-well plates) for 30 min, followed by removal of the nanoparticles and further incubation in nanoparticle-free complete media for another 2 h. In addition, samples of PGRA micelles with no encapsulated dye(s) and PBS (pH 7.4) only were also incubated with both mT4 and PANC-1 cells to serve as negative controls. For flow cytometry, cells were rinsed thrice with PBS and collected in 170 μL TrypLE Express dissociation buffer (ThermoFisher Scientific, Waltham MA). Samples were then analyzed on a BD FACScan flow cytometer with a 488 nm excitation wavelength and recording at least 10,000 events per sample. The emission signal in the 515–545 nm range was used for detection of dye 1 and in the 565–605 nm range for dye 2 detection. Results were analyzed using FlowJo v10 software.
2.2.10.2. Cellular internalization studies via microscopy
The internalization of nanoparticles was also confirmed using fluorescent microscopy. PANC-1 and mT4 cells were plated at 3 × 105 cells per dish in 35 mm tissue culture dishes 24 h prior to experiments. The cells were then continuously incubated with nanoparticles as detailed in (Section 2.2.10) above for 30 min followed by 2 h in nanoparticle-free media in an humidified 5% CO2 incubator at 37 °C. Images were acquired using a custom-built Mikron Instruments fluorescent microscope with a Carl Zeiss Achroplan 63X, NA 1.0 water immersion objective (Thornwood, NY), after staining of cells with LysoTracker® Blue DND-22 (5 μM final concentration in media, Invitrogen, Inc., Carlsbad, CA) as a means to image lysosomes.
2.2.11. Nanoparticle antitumor efficacy against PANC-1 (human) and mT4 (mouse) pancreatic cancer cell lines
Cell viability was assessed via a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. PANC-1 and mT4 cells (2 × 105) were separately plated into 96-well plates which were incubated for 24 h with complete cell media (Dulbecco’s Modified Eagle’s Medium (DMEM) with 4500 mg/L glucose (Gibco #11995) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin) at 37 °C in a 5% CO2 humidified incubator. Solutions of gemcitabine HCl, PGRA micelles, PGRA micelles loaded with drug conjugate, cholesterol PEG micelles, cholesterol PEG micelles loaded with drug conjugate (all containing 5% (w/v) dextrose, added after nanoparticle preparation) and 5% dextrose solution (control) were added to the plated cells yielding varying drug and polymer equivalent concentrations (1 μM to 500 μM) in duplicate. The plates were placed in an humidified incubator (37 °C, 5% CO2) and incubated for 72 h after which 10 uL/100 uL media of a 5 mg/mL solution of MTT in PBS without calcium/magnesium (PBS−/−) was added directly to the media and placed back into the incubator for an additional 3 h. The media was then removed and the resultant formazan crystals were solubilized in 100 μL DMSO. The absorbance was obtained using a TECAN M1000 microplate reader at 570 nm (reference at 670 nm). The cell viability was calculated by comparing the absorbances of the treated samples against the media+PBS only control.
2.2.12. TUNEL Apoptosis Assay
PANC-1 cells were plated at 5 × 105 cells/well in 96-well round bottom low adhesion plates (Corning #3879) 24 h prior to addition of treatments. Gem-nanoparticles or free gemcitabine at various concentrations were added to cells in 150 μL complete DMEM media supplemented with 10% FBS and 1% penicillin-streptomycin and incubated at 37°C for 24 h in a humidified CO2 incubator. After 24 h, DNA fragmentation in apoptotic cells was determined by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) using the Cell Meter ™ TUNEL Apoptosis Assay kit (AAT Bioquest) according to manufacturer’s instructions. Briefly cells were fixed with 4% paraformaldehyde for 30 min, followed by permeabilization in 0.2% Triton X-100 in PBS−/− for 10 min. 50 μL of TUNEL working solution was then added to cells for 60 min, followed by measurement of fluorescence intensity (ex/em 550nm/590nm) on a CytoFLEX flow cytometer (Beckman Coulter). Data were analyzed using FlowJo v10 software (TreeStar). Positive cell controls were prepared using DNAse I digestion of cells for 30 min at RT prior to addition of TUNEL working solution.
2.2.13. Annexin V-FITC/Propidium iodide apoptosis assay
An Annexin V Apoptosis experiment was performed to confirm the Gem-RA nanoparticle’s ability to induce apoptosis. An eBioscience™ Annexin V Apoptosis Detection Kit FITC (cat. 88–8005-72) purchased from Thermofisher Scientific was used to stain apoptotic cells with Annexin V conjugated to FITC. The propidium iodide (PI) solution was used to distinguish between early apoptotic cells (FITC+, PI−) and late apoptotic cells (FITC+, PI+). PANC-1 cells were plated into 35-mm dishes with 300,000 cells per dish and allowed to adhere for 24 h in a 37 °C humidified incubator with 5% CO2. Various concentrations of free gemcitabine or Gem-RA nanoparticles were added to the cells and incubated for an additional 24 h. Cells were then stained following the manufacturer’s protocol modified for microscopy. Media was removed and washed once with PBS−/− and once with 1X binding buffer. Next, 5 μL of fluorochrome-conjugated Annexin V was added and incubated for 15 min at room temperature, protected from light. Cells were then washed twice with 1X Binding Buffer and resuspended in 2 mL of 1X binding buffer. 5 μL of the provided PI solution was added to the cells. Cells were incubated for 10 min on ice and fixed with 1% PFA in PBS. FITC and PI fluorescence was captured by imaging the cell plates with an inverted fluorescence microscope (Mikron Instruments) using a 63x water-immersion objective and FITC/Texas Red filter set.
3. Results and discussion
3.1. Synthesis and characterization of GemRA
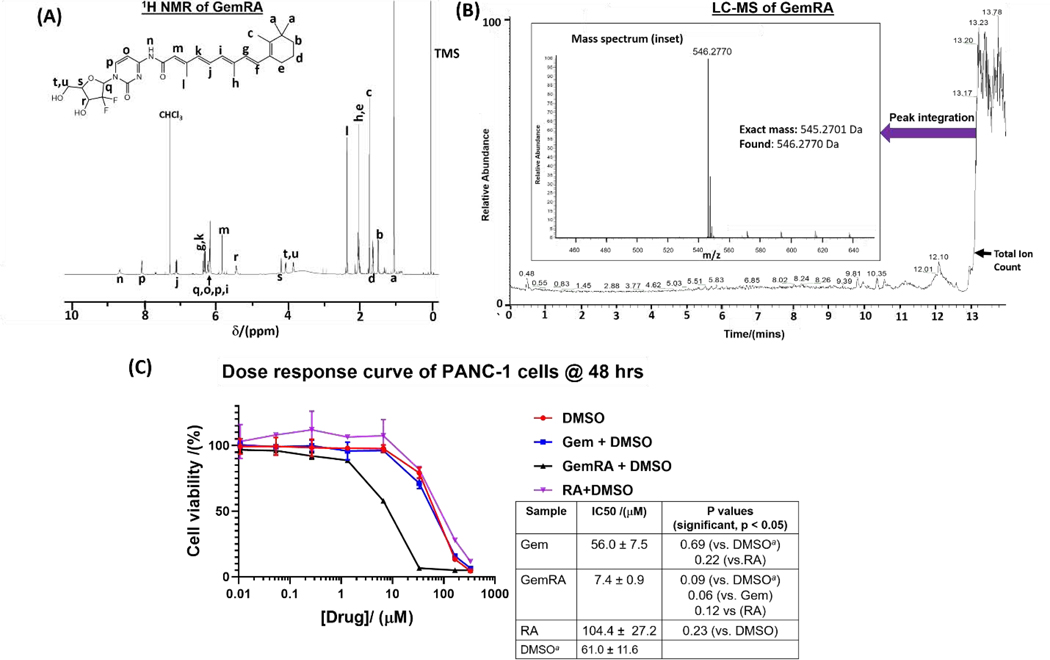
The synthetic approach used to obtain GemRA is shown in Fig. 1A. Initially, the hydroxyl groups of gemcitabine were protected using TBDMS to prevent esterification side reactions in the ensuing amide formation step with retinoic acid. In our previous work, we have found this approach to be effective at affording pure and higher amide conjugate product yields [41]. Coupling of the free amine of the protected gemcitabine (GemTBDMS) with retinoic acid was conducted under mild peptide bond formation conditions at low temperature to minimize possible RA and protected GemRA degradation. The intermediate product was not isolated but freed of the coupling agents and base by washing with water. Removal of the TBDMS protecting groups with TBAF as outlined in Fig. 1A gave the desired product which was isolated by HPLC and the structure was confirmed by 1H NMR (Fig. 2A) and 13C NMR. Furthermore, LC-MS revealed that the integration of the major peak in the total ion count chromatogram gave the expected mass of 546.2770 Da [M+H]+ which is in congruence with the structure of the product (Fig. 2B).
Fig. 1:
Synthetic and nanoparticle preparation approaches employed in this work. (A) Synthesis of gemcitabine-retinoic acid prodrug conjugate, GemRA, (B) synthesis of amphiphilic polymer, PGRA, used for encapsulation of GemRA and (C), strategies used to generate GemRA nanoparticles with the initial attempt being (i) direct self-assembly of GemRA which resulted in precipitation followed by (ii) encapsulation of GemRA through self-assembly in presence of amphiphilic PGRA which gave GemRA loaded nanoparticles dispersed in aqueous solution.
Fig. 2:
Characterization of the GemRA conjugate by (A) 1H NMR and (B) LC-MS demonstrating the purity of the conjugate and in (C) an assessment of the efficacy of the GemRA conjugate in vitro versus free gemcitabine (Gem), retinoic acid (RA) and DMSO alone (measurements in duplicate). Drug solubilization was done in DMSO and compared to DMSO alone due to poor solubility of GemRA in water. The p values are based on the Welch’s t-test (p ≤ 0.05 for significance). a The x-axis shows concentration of drugs not DMSO, the DMSO plot is based on matching the volume of DMSO used in solubilization of the drug that was added for each treatment.
3.2. In vitro efficacy of GemRA in the treatment of pancreatic cancer
The effectiveness of the GemRA prodrug as a pancreatic cancer treatment was evaluated through in vitro cell viability studies and compared against free gemcitabine alone. In vitro studies were conducted using mT4 (mouse) and PANC-1 (human) pancreatic cancer cell lines. From several in vitro experiments in our laboratory, we have found the mouse mT4 cell line to be sensitive to treatment with gemcitabine; however, the human PANC-1 cell line is resistant to gemcitabine. Indeed, we observed no difference in reduction of cell viability between GemRA and gemcitabine in the mT4 cell line (data not shown). Unlike the commercially-available gemcitabine hydrochloride, GemRA did not dissolve in aqueous media (water/PBS) and was thus dissolved in DMSO for the study. Consequently, for comparison purposes, gemcitabine hydrochloride was also solubilized in DMSO and DMSO alone at the same final concentration was used as a control. Gemcitabine and DMSO (control) had an IC50 of ca. 60 μM implying the reduction in viability was primarily from cell death caused by DMSO (Fig. 2C). However, the IC50 of GemRA (7.4 μM) was much lower than that of the gemcitabine and DMSO indicating the GemRA conjugate was more effective at inducing cell death in the drug-resistant PANC-1 cells. Moreover, a comparison of the IC50 of GemRA with that of gemcitabine shows a p value of 0.06 (p ≤0.05 for significance) which is strongly trending towards significance. However, for biological systems and clinical applications, administration of the drug in DMSO is not ideal. Thus, we turned to nanotechnology to develop a water-soluble nano-based delivery system that would be biologically/clinically relevant.[38] In addition, the delivery of drugs in nanoparticulate form has been shown to lead to improved circulation times leading to improved drug accumulation at tumor sites (hence improved therapeutic outcomes) through the enhanced permeation and retention (EPR) effect. [38],[21]
3.3. Preparation and characterization of nanoparticles (NPs)
3.3.1. NP preparation via direct self-assembly of GemRA
The structure of the GemRA is reminiscent of the structure of the gemcitabine-squalene (GemSq) prodrug pioneered by Couvreur et al.[7] GemSq has been reported to self-assemble into micelle-type nanostructures. Thus, self-assembly of GemRA into NPs was carried out using procedures similar to those reported for the formation of GemSq nanoassemblies but the material precipitated out of solution in the process. Since the direct self-assembly of GemRA was not possible, we proceeded to design a material that would self-assemble in aqueous solution in which the drug could be encapsulated (Fig. 1C).
3.3.2. NP preparation via encapsulation of GemRA in PGRA micelles (drug loaded NPs)
Retinoic acid was conjugated to PEG (Mn 750 Da) via esterification to obtain the amphiphilic PGRA as shown in Fig 1B. Formation of PGRA was confirmed by electrospray ionization mass spectrometry (ESI-MS) and 1H NMR. By ESI-MS, a distribution centered at ca 1000 Da (~750 (PEG) + 300 (RA) Da) (singly charged) with peak spacing of 44 Da, corresponding to the PEG repeating unit, was observed, which is in agreement with the structure of PGRA (Fig. 3A). The 1H NMR of the purified product also revealed peaks of both PEG and RA implying successful formation and isolation of the product (SI, Fig. S1). Self-assembly of PGRA in water gave NPs of about 8 nm as observed by dynamic light scattering (DLS) and ca. 7 nm by transmission electron microscopy (TEM). The difference arises from the DLS measuring the hydrated NPs while for TEM the measurements are on particles in dried state (Fig. 3B). The critical micelle formation concentration (CMC) for PGRA was also determined through fluorescence spectroscopy and found to be 0.0908 mg/mL (86 μM, Fig. 3C).[42] Such low CMC values are typical of polymer amphiphiles and they also have better stability than low molecular weight surfactants.[43] Low CMC values are desirable in drug delivery as it implies less material is required for micelle formation and the micelles are more resistant to dissociation on dilution such as that encountered upon injection of the micelles in vivo and thus can retain their cargo while circulating.
Fig. 3:
Characterization of the amphiphilic PGRA conjugate by (A) mass spectrometry and (B and C) an investigation of the self-assembly of PGRA into micelles; the NP hydrodynamic size as determined by dynamic light scattering (DLS) and the corresponding morphology is shown in the transmission electron microscopy image (inset) (B) while the critical micelle concentration (CMC) is shown in (C).
Drug loading was achieved by self-assembling the PGRA in the presence of the GemRA thus confining the GemRA to the cores of the micelles. Evidence of successful loading was first observed from size measurements by DLS (ca. 33 nm) wherein the sizes of the drug-loaded micelles were greater than those of PGRA-only micelles due to the presence of cargo in the micellar cores (Figs. 4A and 3B). By TEM, the morphology of the drug-loaded micelles was similar (spherical) to that of PGRA-micelles but the micelle diameters were greater (ca. 25 nm), in agreement with the observation made via DLS. Furthermore, following purification to remove any unencapsulated drug, an aliquot of the sample was dried and dissolved in DMSO and then analyzed by HPLC. This analysis confirmed the presence of the GemRA and enabled estimation of the drug loading (35% w/w) based on a calibration curve of GemRA. It is noteworthy that the loading is greater than that achieved for the loading of pure gemcitabine through encapsulation in micellar/liposomal NPs.
Fig. 4:
Size of drug (GemRA)-loaded PGRA micelles and the morphology determined via (A) dynamic light scattering (DLS) and transmission electron microscopy (TEM), size of the scale bar in TEM image = 50 nm; (B) stability of GemRA loaded micelles in aqueous solution (stored at 4 °C in between DLS and zeta potential measurements which were made at 25 °C (average of 5 measurements)); (C) stability of GemRA-loaded NPs in fetal bovine serum (FBS) at 37 °C (one measurement per time point); (D) drug retention characteristics of the GemRA-loaded NPs at pH 7.4, 37 °C in comparison to free gemcitabine (N=2); (E and F) comparison of the stability of free gemcitabine, GemRA and drug-loaded micelles towards cytidine deaminase deactivation to the inactive gemcitabine metabolite dFdU (2′,2′-difluorodeoxyuridine), (E) shows amount of drug left over time and (F) shows the amount of dFdU formed (N=2).
3.3.3. NP stability
Stability of the drug-loaded micelles is an important factor for their possible application as pancreatic cancer nanotherapeutics. The sizes, by DLS, of aqueous suspensions of drug-loaded micelles (ca. 1 mg/mL), were observed to increase slightly for samples measured soon after preparation and for the same samples upon incubation at 4 °C in the dark over a 7-day period (Fig. 4B). The slight increase in size suggests there was some minor NP aggregation that took place which can be attributed to low particle mobility and micelle corona shrinkage encountered during the storage period at low temperature (4 °C). Slow hydrolytic degradation of the PGRA’s ester linkage in aqueous solution may also have occurred reducing the stabilization effect of the corona thus promoting aggregation. However, the level of aggregation was not sufficient to cause formation of large particles or precipitation suggesting the drug-loaded micelles possessed good stability. The zeta potential was also observed to change slightly upon storage at 4 °C from ca. −3 mV to −9 mV. This change likely results from changes in solution ionic strength due to slow hydrolysis of PGRA and the diffusion/leakage of small amounts of drug from the micelles (Fig. 4B).
NP stability in biological milieu was also investigated by looking at the change in size of the drug-loaded NPs dispersed in fetal bovine serum (FBS) over time via DLS. The interaction of NPs with the biological environment such as those encountered in cell culture or in vivo can adversely alter the colloidal stability of the NPs. It is desirable that the drug-loaded NPs show good stability in such environments so that they can retain their cargo until internalized by cells in vitro or in circulation until uptake at the desired site of treatment in vivo. The GemRA-loaded NPs showed no dramatic size changes (ca. 55–75 nm) in FBS at 37 °C over a 17-hour incubation period indicating no aggregation or disaggregation took place and signifying good stability under those conditions (Fig. 4C). The observed size increase can be attributed to adsorption of serum components to the surface of the NPs thereby increasing their apparent hydrodynamic size.[44] Analysis of the serum alone by DLS gave a size of ca. 6–10 nm due to serum components (Fig. S2). Thus, assuming their adsorption onto the NPs surface leads to complete coverage, the expected diameter increase is ca. 20 nm which is in agreement with the observed size change. The result suggests that the GemRA-loaded NPs have the propensity to remain intact in cell culture media in vitro until uptake by cells, ensuring that the evaluation of efficacy is based on GemRA-loaded NP cell internalization events and not drug leakage into cell media (vide infra).
In addition to NP stability, the NPs are expected to have good drug retention characteristics. Drug retention was assessed via the dialysis method wherein solutions of free gemcitabine and drug-loaded micelles were separately dialyzed against PBS buffer pH 7.4 to simulate physiological conditions. Release from the free gemcitabine solution was rapid with most of the drug being released within 1 h (Fig. 4D). On the other hand, release from the GemRA-loaded NPs was minimal over ca. 3 days, with less than 5% of the drug being released. When the contents of the dialysis bag were collected at the end of the study, freeze dried and solubilized, the presence of the drug was ascertained by HPLC and mass spectrometry. The amount of GemRA recovered was ca. 90% (w/w) of the original amount indicating remarkable drug retention. Overall, the drug-loaded micelles exhibited stability and drug retention characteristics expected of an efficacious drug-delivery system.
Of concern when considering the delivery of gemcitabine in vivo is its deactivation by cytidine deaminase to the non-active metabolite 2’,2’-difluorodeoxyuridine (dFdU) thus reducing its efficacy.[45] Incorporation of gemcitabine into NPs or delivery in the form of prodrugs is a favorable route for providing protection against this adverse interaction. We thus investigated the susceptibility of free gemcitabine, free GemRA and GemRA-loaded NPs to the negative action of cytidine deaminase. When the aforementioned materials were incubated in the presence of cytidine deaminase and cathepsin B enzymes at 37 °C, calculation of the amount of drug left over time and dFdU formation by HPLC revealed that the amount of free gemcitabine was severely reduced within 30 mins and rapidly converted to dFdU (Fig. 4 E–F). However, about 40% (w/w) of the original drug amount remained and only a small amount of dFdU was formed for free GemRA, indicating better metabolic stability of the prodrug compared to gemcitabine alone. Deactivation of GemRA is slower since the prodrug needs to dissociate into RA and gemcitabine before cytidine deaminase can lead to the formation of dFdU (Fig. 4 E–F). The greatest stability towards the cytidine deaminase enzymatic deactivation was noticed for the drug-loaded NPs, demonstrating efficient protection of GemRA imparted by the PGRA shell. Moreover, the slow release of GemRA should occur from the loaded NPs followed by dissociation of the freed GemRA, leading to a very small amount of dFdU formation occurring. Thus, more than 90% (w/w) of the drug remained over a 2-hr period and less than 5% of dFdU was formed.
3.3.4. Cellular uptake of NPs
Uptake of the NPs by cells was studied by incubating the PGRA NPs loaded with fluorescence resonance energy transfer (FRET) paired dyes: dye 1 (3,3′-dioctadecyloxacarbocyanine perchlorate, donor, Ex/Em 488/501 nm) and dye 2 (1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, acceptor, Ex/Em 501/565 nm).[46] The dyes were separately encapsulated (two samples) and co-loaded/mixed (third sample) into PGRA micelles. Thus, energy transfer from the donor to acceptor molecules is expected if the molecules are closely packed together (encapsulated in the micelle). When excited at 488 nm, with set channel filters (channel 1, 530 ± 15 nm (pseudocolored red) and channel 2, 585 ± 20 nm (pseudocolored green)) to observe the fluorescence signals, only NPs with dye 1 show a signal in channel 1 (Fig. 5A) while in channel 2 (Fig. 5B) the mixed-dye NPs show a significantly greater fluorescence signal compared to the NPs loaded with either individual dye, signifying the dye molecules were close together, allowing energy transfer to occur. The signal from the individual dye-loaded NPs in channel 2 is likely due to background fluorescence from the PGRA micelles. Nevertheless, the signal from the PGRA micelles is significantly lower than that of the mixed-dye NPs, signifying dye encapsulation within the NPs when taken up by cells.
Fig. 5:
Cellular uptake of dye(s)-loaded PGRA micelles and efficacy of GemRA-loaded NPs towards PANC-1 cells. (A and B) PANC-1 cell uptake of PGRA-micelles loaded with FRET dyes: dye 1 (3,3′-dioctadecyloxacarbocyanine perchlorate) and dye 2 (1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) (separately loaded), and mixed dyes 1 and 2 (co-loaded) as assessed via flow cytometry (N=2), excitation was 488 nm and the detection channel filters, ((A) channel 1, 530 ± 15 nm (red) and (B) channel 2, 585 ± 20 nm (green)) were employed; (C) cell uptake of the mixed dye-loaded NPs by fluorescence microscopy, ((i) and (ii) show the bright field and blue channel (LysoTracker™ staining) images respectively, (iii) shows the green channel (co-loaded dyes) image while the merged image is shown in (iv), the white arrows show NP colocalization around the nuclei. Scale bars =20 μm. (D) Cell viability of PANC-1 cells following GemRA-loaded NP and gemcitabine incubation (***p<0.001, for significance, p≤0.05 based on one way ANOVA with a post Tukey test) (N=4), (E) a comparison of cell viability of PANC-1 cells following GemRA-loaded NP and drug-free NP incubation and (F) flow cytometry results showing GemRA-loaded NPs induce apoptosis (increased TUNEL-reagent fluorescence intensity) of PANC-1 cells (N=2) (*p<0.05, for significance, p≤0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The uptake of NPs with the mixed dyes could also be observed by fluorescence microscopy as shown in Fig. 5C. The fluorescence of dye-carrying NPs engulfed in endosomal and lysosomal compartments (small punctate green dots) could be observed (Fig. 5C (iii)). The merged image (Fig. 5C (iv)) of the NP fluorescence (Fig. 5C (iii)) and the LysoTracker™ staining (Fig. 5C (ii)) shows NP-lysosome colocalization with perinuclear NP accumulation (white arrows). Compared to images obtained wherein the mixed dyes were added to the mixture in free form, the results obtained with the NPs suggest the dye enters the cells while encapsulated in the NPs.
3.3.5. Antitumor efficacy against gemcitabine resilient PANC-1 cell line
Having established that the NPs can transport loaded cargo into cells, we determined the anticancer efficacy of GemRA-loaded NPs in vitro in the gemcitabine-resistant human PANC-1 cell line. Assessment of cell viability by MTT assay after 72 h showed that gemcitabine-treated cells were more than 50% viable at the highest drug concentration tested (1000 μM) (Fig. 5D). Conversely, the cell viability recorded for PGRA micelles loaded with GemRA was below 10% for a concentration above 30 μM resulting in an IC50 of 5±3 μM. This IC50 is significantly lower than that estimated for gemcitabine (p<0.05) suggesting that the delivery of GemRA results in more cytotoxicity than that realized from gemcitabine.
To further confirm the effectiveness of delivering GemRA, we encapsulated the drug in cholesterol-peg (Chol-PEG) nanoassemblies. Chol-PEG has building blocks that are generally considered to have negligible toxicity and good biocompatibility and is thus used in many lipid-based drug formulations.[47] The Chol-PEG micelles loaded with GemRA also showed significantly higher toxicity compared to that exhibited by free gemcitabine. To show that the effect observed in Fig. 5D resulted primarily from GemRA, we compared the cell viability of the materials alone (PGRA and Chol-PEG) against the drug-loaded micelles of each material at various concentrations as shown in Fig. 5E. It is noteworthy that the cell viability of the materials is based on the amount of material present at the given concentration of drug which is shown on the horizontal axis. A comparison of cell viabilities of PGRA (black) and PGRA-loaded with GemRA (purple) shows that the cell viability after PGRA incubation was significantly greater at all concentrations in comparison to the drug-loaded NPs indicating the effectiveness of GemRA. A similar trend can be observed for the comparison between Chol-PEG (red) and its drug-loaded NPs (blue). When we conducted a similar cell viability experiment using murine mT4 cells, no significant difference between the IC50 values were observed between the micelle-loaded NPs and gemcitabine alone due to the mT4 cells being more susceptible to gemcitabine antitumor activity (Fig. S3).
Finally, we proceeded to determine if the observed cell death associated with GemRA-loaded NPs was linked to DNA damage (due to inhibition of DNA synthesis and incorporation into DNA) which is the expected mode of action for gemcitabine.[4] Cellular apoptosis was determined via the terminal deoxynucleotidyl transferase dUTP nick end labeling assay (TUNEL) using flow cytometry for PANC-1 cells incubated with GemRA-loaded NPs (PGRA shell) for 72 h.[48] The three drug concentrations selected for analysis were 1) below the IC50 (0.01 μM GemRA), 2) at the IC50 (6.67 μM) and 3) above the IC50 (333.3 μM) as shown in Fig. 5F. All drug-particle incubations were compared to incubation with PBS alone at the same final volume in cell media as a control. At the drug concentration below the IC50, DNA damage was non-significant while at concentrations of 6.67 μM and 333.3 μM, apoptosis (hence DNA damage) was found to be significantly greater than that of the PBS control. The results indicate that GemRA effects apoptotic cell death on PANC-1 cells at concentrations equal to and above the IC50, highlighting its effectiveness against PANC-1 cells. Further confirmation of cell death was attained via an apoptosis experiment based on Annexin V-FITC/propidium iodide (PI) staining of PANC-1 cells previously incubated with free gemcitabine or GemRA+PGRA NPs. Analysis of the Annexin V-FITC/PI stained samples by fluorescence microscopy reveals the green colour of Annexin-FITC when there is early apoptosis, and red (PI) stains the nuclei of dead cells. Colocalization of the red and green stains is indicative of advanced apoptosis. Fluorescence microscopy images of PANC-1 cells treated with PBS buffer (no treatment control), free gemcitabine (333 μM) and PGRA+GemRA NPs (333 μM GemRA) then stained with Annexin V-FITC/PI are shown in Fig. 6. The greatest apoptotic signal was observed for the NP treated cells. A few cells in the gemcitabine treated group (white arrows) were positive for early apoptosis. No apoptotic signal was observed for the no treatment control. The results are in congruence with the TUNEL and cytotoxicity results suggesting that the PGRA+GemRA NPs are more effective at inducing apoptosis of PANC-1 cells than gemcitabine alone.
Fig. 6:
Determination of apoptosis by fluorescence microscopy using the Annexin V-FITC/propidium iodide staining kit. PANC-1 cells were initially incubated with gemcitabine or drug-loaded NPs (333 μM of drug) prior to treatment with Annexin V-FITC (green)/propidium iodide (PI) (red). Early apoptotic cells were considered to be Annexin FITC+ and PI− and late apoptotic cells were Annexin FITC+ and PI+. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusions
We prepared a gemcitabine-retinoic acid (GemRA) prodrug that showed significantly enhanced anticancer activity towards the gemcitabine-resistant PANC-1 pancreatic cancer cell line in vitro compared to gemcitabine. The conjugate was not directly soluble in water, so to make it suitable for drug delivery, formation of NPs through its self-assembly in aqueous solution was attempted but was unsuccessful due to precipitation. Successful formation of NPs was achieved by encapsulating the GemRA into an amphiphilic polymer conjugate, PEG-retinoic acid (PGRA) which yielded drug-loaded micelles of about 33 nm by DLS and 25 nm by TEM. The NPs showed good stability upon storage and against metabolic deactivation by cytidine deaminase action indicating the prodrug is shielded from the external environment by the polymer shell. Cellular uptake of the NPs by PANC-1 cells was confirmed by flow cytometry and confocal microscopy. In vitro cytotoxicity of the drug-loaded NPs in PANC-1 cells showed the NPs with the prodrug produced significantly lower cell viability after 72 h compared to the PGRA alone indicating the effectiveness of the prodrug in terminating PANC-1 cells. Moreover, the drug-loaded NPs exhibited a significantly lower IC50 (ca. 5μM) compared to gemcitabine (> 1000 μM) and caused apoptosis of PANC-1 cells based on the TUNEL assay. Our results suggest that the approach and system presented herein has potential application in pancreatic cancer therapy. Considering the prodrug was also encapsulated in a different encapsulating amphiphile and showed efficacy towards PANC-1 cells, the approach can thus be adapted to other polymeric materials to afford nanosized prodrug encapsulating systems with potential to overcome gemcitabine resistance in pancreatic cancer treatment.
Supplementary Material
Acknowledgements
This work was supported by funding from National Institute of Health grants R01CA112356, R01CA134659, R01CA199658, R01CA253316, R01CA210553 and R01CA211602.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hessmann E, Johnsen SA, Siveke JT and Ellenrieder V, Gut, (2017), 66, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amrutkar M. and Gladhaug IP, Cancers (Basel), (2017), 9, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jia Y, Gu D, Wan J, Yu B, Zhang X, Chiorean EG, Wang Y. and Xie J, Oncogene, (2019), 38, 1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Sousa Cavalcante L. and Monteiro G, Eur. J. Pharmacol, (2014), 741, 8–16. [DOI] [PubMed] [Google Scholar]
- [5].Adamska A, Domenichini A. and Falasca M, Int. J. Mol. Sci, (2017), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tucci ST, Kheirolomoom A, Ingham ES, Mahakian LM, Tam SM, Foiret J, Hubbard NE, Borowsky AD, Baikoghli M, Cheng RH and Ferrara KW, J. Control. Release, (2019), 309, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Couvreur P, Stella B, Reddy LH, Hillaireau H, Dubernet C, Desmaële D, Lepêtre-Mouelhi S, Rocco F, Dereuddre-Bosquet N, Clayette P, Rosilio V, Marsaud V, Renoir J-M and Cattel L, Nano Lett., (2006), 6, 2544–2548. [DOI] [PubMed] [Google Scholar]
- [8].Chitkara D, Mittal A, Behrman SW, Kumar N. and Mahato RI, Bioconjug. Chem, (2013), 24, 1161–1173. [DOI] [PubMed] [Google Scholar]
- [9].Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J. and Friess H, Nat. Rev. Gastroenterol. Hepatol, (2012), 9, 454. [DOI] [PubMed] [Google Scholar]
- [10].Joubert F, Martin L, Perrier S. and Pasparakis G, ACS Macro Lett, (2017), 6, 535–540. [DOI] [PubMed] [Google Scholar]
- [11].Nicolas J, Chem Mater, (2016), 28, 1591–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Federico C, Morittu VM, Britti D, Trapasso E. and Cosco D, Int. J. Nanomed, (2012), 7, 5423–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nair PR, Polymers, (2019), 11, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lan C. and Zhao S, J. Mater. Chem. B, (2018), 6, 6685–6704. [DOI] [PubMed] [Google Scholar]
- [15].Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J. and Renschler MF, N. Engl. J. Med, (2013), 369, 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoff DDV, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A. and Hidalgo M, J. Clin. Oncol, (2011), 29, 4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shi K, Xue B, Jia Y, Yuan L, Han R, Yang F, Peng J. and Qian Z, Nano Research, (2019), 12, 1389–1399. [Google Scholar]
- [18].Guégain E, Tran J, Deguettes Q. and Nicolas J, Chem. Sci, (2018), 9, 8291–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Probst CE, Zrazhevskiy P, Bagalkot V. and Gao X, Adv. Drug Del. Rev, (2013), 65, 703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Z, Ma R. and Shi L, Acc. Chem. Res, (2014), 47, 1426–1437. [DOI] [PubMed] [Google Scholar]
- [21].Fang J, Nakamura H. and Maeda H, Adv. Drug Del. Rev, (2011), 63, 136–151. [DOI] [PubMed] [Google Scholar]
- [22].Dasari M, Acharya AP, Kim D, Lee S, Lee S, Rhea J, Molinaro R. and Murthy N, Bioconjug. Chem, (2013), 24, 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Reddy LH and Patrick C, Curr. Pharm. Des, (2008), 14, 1124–1137. [DOI] [PubMed] [Google Scholar]
- [24].Moysan E, Bastiat G. and Benoit J-P, Mol. Pharm, (2013), 10, 430–444. [DOI] [PubMed] [Google Scholar]
- [25].Desmaële D, Gref R. and Couvreur P, J. Control. Release, (2012), 161, 609–618. [DOI] [PubMed] [Google Scholar]
- [26].Kattel K, Mondal G, Lin F, Kumar V. and Mahato RI, Mol. Pharm, (2017), 14, 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kuroda H, Tachikawa M, Uchida Y, Inoue K, Ohtsuka H, Ohtsuki S, Unno M. and Terasaki T, Eur. J. Pharm. Sci, (2017), 103, 116–121. [DOI] [PubMed] [Google Scholar]
- [28].Yao J, Zhang L, Zhou J, Liu H. and Zhang Q, Mol. Pharm, (2013), 10, 1080–1091. [DOI] [PubMed] [Google Scholar]
- [29].Pettersson F, Colston KW and Dalgleish AG, Pancreas, (2001), 23, 273–279. [DOI] [PubMed] [Google Scholar]
- [30].Pettersson F, Dalgleish AG, Bissonnette RP and Colston KW, Br. J. Cancer, (2002), 87, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang K, Baldwin GS, Nikfarjam M. and He H, Am J Physiol Gastrointest Liver Physiol, (2019), 316, G632–G640. [DOI] [PubMed] [Google Scholar]
- [32].Sun R, Liu Y, Li S-Y, Shen S, Du X-J, Xu C-F, Cao Z-T, Bao Y, Zhu Y-H, Li Y-P, Yang X-Z and Wang J, Biomaterials, (2015), 37, 405–414. [DOI] [PubMed] [Google Scholar]
- [33].Carapuça EF, Gemenetzidis E, Feig C, Bapiro TE, Williams MD, Wilson AS, Delvecchio FR, Arumugam P, Grose RP, Lemoine NR, Richards FM and Kocher HM, J. Pathol, (2016), 239, 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liang C, Shi S, Meng Q, Liang D, Ji S, Zhang B, Qin Y, Xu J, Ni Q. and Yu X, Cell. Mol. Life Sci, (2018), 75, 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han X, Li Y, Xu Y, Zhao X, Zhang Y, Yang X, Wang Y, Zhao R, Anderson GJ, Zhao Y. and Nie G, Nat. Commun, (2018), 9, 3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thomas D. and Radhakrishnan P, Mol. Cancer, (2019), 18, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cristiano MC, Cosco D, Celia C, Tudose A, Mare R, Paolino D. and Fresta M, Colloids Surf. B. Biointerfaces, (2017), 150, 408–416. [DOI] [PubMed] [Google Scholar]
- [38].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R. and Langer R, Nat. Nanotechnol, (2007), 2, 751. [DOI] [PubMed] [Google Scholar]
- [39].Abu-Fayyad A. and Nazzal S, Int. J. Pharm, (2017), 528, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yalçın S, Erkan M, Ünsoy G, Parsian M, Kleeff J. and Gündüz U, Biomed. Pharmacother, (2014), 68, 737–743. [DOI] [PubMed] [Google Scholar]
- [41].Tucci ST, Seo JW, Kakwere H, Kheirolomoom A, Ingham ES, Mahakian LM, Tam S, Tumbale S, Baikoghli M, Cheng RH and Ferrara KW, Nanotheranostics, (2018), 2, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilhelm M, Zhao CL, Wang Y, Xu R, Winnik MA, Mura JL, Riess G. and Croucher MD, Macromolecules, (1991), 24, 1033–1040. [Google Scholar]
- [43].Adams ML, Lavasanifar A. and Kwon GS, J. Pharm. Sci, (2003), 92, 1343–1355. [DOI] [PubMed] [Google Scholar]
- [44].Bhattacharjee S, Control J. Release, (2016), 235, 337–351. [DOI] [PubMed] [Google Scholar]
- [45].Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W. and Raber MN, J. Clin. Oncol, (1991), 9, 491–498. [DOI] [PubMed] [Google Scholar]
- [46].Wang Y, Fan W, Dai X, Katragadda U, McKinley D, Teng Q. and Tan C, Mol. Pharm, (2014), 11, 1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].He Z-Y, Chu B-Y, Wei X-W, Li J, Edwards CK, Song X-R, He G, Xie Y-M, Wei Y-Q and Qian Z-Y, Int. J. Pharm, (2014), 469, 168–178. [DOI] [PubMed] [Google Scholar]
- [48].Kyrylkova K, Kyryachenko S, Leid M. and Kioussi C, Detection of Apoptosis by TUNEL Assay. In Odontogenesis: Methods and Protocols, Kioussi C, Ed. Press Humana: Totowa, NJ, 2012; pp 41–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.