Abstract
Introduction
Lower urinary tract reconstruction in paediatric urology represents a physiologically stressful event that is associated with high complication rates, including readmissions and emergency room visits. Enhanced recovery after surgery (ERAS) protocol is a set of multidisciplinary, perioperative strategies designed to expedite surgical recovery without adversely impacting readmission or reoperation rates. Early paediatric urology data demonstrated ERAS reduced complications in this population.
Methods and analysis
In 2016, a working group of paediatric urologists and anaesthesiologists convened to develop an ERAS protocol suitable for patients undergoing lower urinary tract reconstruction and define study process measures, patient-reported outcomes and clinically relevant outcomes in paediatric and adolescent/young adult patients. A multicentre, prospective, propensity-matched, case–control study design was chosen. Each centre will enrol five pilot patients to verify implementation. Subsequent enrolled patients will be propensity matched to historical controls. Eligible patients must be aged 4–25 years and undergoing planned operations (bladder augmentation, continent ileovesicostomy or appendicovesicostomy, or urinary diversion). 64 ERAS patients and 128 controls will be needed to detect a decrease in mean length of stay by 2 days. Pilot phase outcomes include attainment of ≥70% mean protocol adherence per patient and reasons for protocol deviations. Exploratory phase primary outcome is ERAS protocol adherence, with secondary outcomes including length of stay, readmissions, reoperations, emergency room visits, 90-day complications, pain scores, opioid usage and differences in Quality of Recovery 9 scores.
Ethics and dissemination
This study has been registered with authors’ respective institution review boards and will be published in peer-reviewed journals. It will provide robust insight into the feasibility of ERAS in paediatric urology, determine patient outcomes and allow for iteration of ERAS implementations as new best practices and evidence for paediatric surgical care arise. We anticipate this study will take 4 years to fully accrue with completed follow-up.
Trial registration number
Keywords: enhanced recovery after surgery, bladder augmentation, Mitrofanoff, urinary diversion, protocol
Strengths and limitations of this study.
This protocol outlines a multicentre, prospective, propensity score-matched cohort study of an enhanced recovery after surgery (ERAS) protocol applied to paediatric and adolescent/young adult patients undergoing lower urinary tract reconstructive surgery.
Each participating free-standing paediatric centre will take part in a pilot phase to understand barriers to implementation, protocol compliance, and protocol uptake and an exploratory phase to demonstrate clinical outcomes related to the ERAS care pathway as compared with propensity-matched recent historical controls.
Primary and secondary outcomes of interest are relevant to the underlying quality improvement initiative to implement a standardised care pathway (ERAS), reflect clinical outcomes and include patient-reported outcome measures to understand the patient and family perspective.
The comparator group will be recent historical patients who have undergone the same operations. Propensity score matching based on a priori identified covariates will be used to reduce confounding based on non-random assignment of perioperative care (eg, routine care vs ERAS protocol). Time-series analysis will provide insight into any ongoing changes occurring over time with regard to either process, clinical or balancing measures.
Introduction
Background
Lower urinary tract reconstruction represents some of the most challenging surgical operations performed by paediatric urologists. These operations can be long, complex and often involve a bowel resection and anastomosis. Patients undergoing these operations are at high risk for postoperative complications, including nausea and vomiting, ileus, surgical site infection, urinary tract infection and pyelonephritis.1 To date, the optimal perioperative care for these patients has not been well defined, with practices varying widely from institution to institution.2 3
Relevance
Since its initial description in the late 1990s, enhanced recovery after surgery (ERAS) has emerged as an innovative tool in the care of adult surgical patients. ERAS represents a multidisciplinary protocol with a strong implementation framework that targets all phases of care for the surgical patient.4 ERAS has been shown in various adult surgical populations to maintain adequate pain control and facilitate earlier return to baseline function without adverse impact on complication or readmission rate through evidence-based care.5–7 A large multicentre ERAS study of adult colorectal resection and hip fracture repairs demonstrated clinically significant reductions in length of stay (LOS) (0.4–0.9 days), postoperative major complications (rate ratio 0.28 compared with pre-ERAS controls (95% CI 0.12 to 0.68)) and decreased opioid use (by 31%–42%).7 Data audits function as an essential part of an ERAS protocol, allowing teams to review compliance and use continuous quality improvement methodology to iterate and target areas requiring amelioration, further improving clinical outcomes.8 Standardising perioperative care of complex paediatric urology patients using an approach like ERAS has potential to reduce undesirable variations in care, optimise recovery and lead to improvements in surgical outcomes.9
To date, experience with ERAS in paediatric patients has been limited. Published studies have methodological limitations including retrospective nature, lack of specified inclusion and exclusion criteria, poorly defined ERAS protocol elements, small sample size, lack of audits and/or limited follow-up.10 11 A single-centre, prospective pilot trial of 13 paediatric urology patients undergoing procedures with ERAS, compared with 26 historical controls, demonstrated fewer complications and reduced LOS from 8 to 5.7 days.12 Another group studying a similar population retrospectively reported even greater improvements in LOS.13 These small experiences reflect that some paediatric operations occur with far less frequency than common adult operations, increasing variability in postoperative care and overall experience from centre to centre and surgeon to surgeon. The evidence base for perioperative practices is not necessarily well developed or even valid in a paediatric population. Additionally, parents, guardians and families are integral to the care of paediatric surgical patients and their involvement in a pathway should be considered.11
Anticipated impact
To address these issues, a collaborative multicentre effort was initiated by the study authors with goals of defining and implementing an ERAS protocol adapted for paediatric urology patients and studying both implementation and outcomes prospectively. Centres will gain valuable experience in implementation, which requires stakeholder engagement and multidisciplinary participation, while the study group seeks to understand system, provider and patient-level barriers to protocolised surgical care. Furthermore, study of ERAS in a paediatric and emerging young adult population undergoing metabolically stressful operations has the potential to demonstrate the value of standardised care similar to gains seen in adult counterparts.
Objectives
Pediatric Urology Recovery After Surgery Endeavor (PURSUE) study has two primary objectives: (1) to determine if an ERAS protocol can decrease variation in care for complex paediatric patients while simultaneously improving recovery time from surgery without any change in balancing measures, and (2) to broaden exposure of the paediatric urology community to ERAS by engaging study centres in ERAS protocol implementation at geographically diverse medical centres. In this report, we describe study design considerations, ERAS protocol definitions and rationale for the PURSUE study.
Methods and analysis
ERAS protocol development
The study group first met to discuss the proposal for a multicentre study at the Societies for Pediatric Urology Fall Congress in September 2016 in Dallas, Texas, USA. Follow-up phone conferences were held several times over the following year. The participants at the initial meeting included attendings, fellows and residents from paediatric urology and paediatric anaesthesiology. Six institutions participated in the original discussions and committed to the study (represented by the authors).
Prior to this work, the study group was only aware of a single paediatric urology ERAS protocol that was adapted for use in patients predominantly with neurogenic bowel and bladder (eg, myelomeningocele).12 This was used as a starting point and was similar to existing adult urology ERAS protocols for radical cystectomy.14 Modification and addition to this protocol were arrived at by literature review and group consensus. Highlights of the original protocol include omission of formal preoperative bowel preparation, multimodal analgesia with regional blocks for all, no nasogastric tube postoperatively, early feeding (clear liquids in the evening after leaving the operating room, regular diet the following day) and early discontinuation of intravenous fluids by postoperative day 2. Table 1 lists all 20 ERAS protocol elements defined for the purpose of this study.
Table 1.
Comprehensive list of preoperative, intraoperative and postoperative ERAS protocol items targeted by the care pathway, customised for paediatric urology patients. The definitions for these items were arrived at through multidisciplinary consensus of the study group
| Preoperative | Intraoperative | Postoperative |
| Counsel about ERAS | Regional anaesthesia (catheter-based block) | Nausea/vomiting prevention |
| Clear-liquid carbohydrate load (10 mL/kg up to 350 mL) | Avoiding excess drains (intraperitoneal or subcutaneous) | Early feeding (clears POD 0, regular POD 1) |
| Avoid prolonged fasting (eat regular diet and avoid prolonged clears-only diet day prior to surgery) | Euvolaemia (4–7 mL/kg/hour crystalloid) | Early mobilisation (out-of-bed POD 1) |
| No bowel preparation (continue bowel regimen if on one) | Normothermia (36°C–38°C during skin-to-skin time) | Adjunctive pain medication (acetaminophen and NSAID) |
| Antibiotic prophylaxis per American Urological Association guidelines | Minimising opioids (<0.15 mg/kg intravenous morphine equivalents) | Early stoppage of intravenous fluids (either discontinue or lower rate to keep vein open (TKO) by POD 2) |
| DVT prophylaxis (age ≥14 or risk factors) | Minimally invasive assistance (at surgeon discretion) | Early removal of extra drains/catheters (non-urinary drain removal by POD 4) |
| No nasogastric tube on leaving OR | Minimising opioids (<0.30 mg/kg/day intravenous morphine equivalents) |
DVT, deep vein thrombosis; ERAS, enhanced recovery after surgery; NSAID, non-steroidal anti-inflammatory drug; OR, operating room; POD, postoperative day.
The authors identified several important components to add to the original pilot protocol, including ensuring a preoperative clear liquid complex carbohydrate load up to 2 hours prior to surgery and encouraging patients to eat a regular diet the night before surgery. Minimising nothing by mouth duration is crucial to limit metabolic stress, minimise catabolic response from surgery, reduce risk of short-term atrophy of the gastrointestinal villi and protect patients against developing insulin resistance, which has been associated with postoperative complications.5 Specifically, the study group sought to avoid situations where patients drink only clear liquids for several days prior to the operation, nullifying the intentions of ERAS precepts. Bowel preparation remains an open debate.3 Given the lack of supporting data for this specific patient population, the study group chose to omit formal bowel preparation in the ERAS protocol but aims to study this question secondarily.
Venous thromboembolism (VTE) prophylaxis was added with the caveat that it should apply only to those patients with certain risk factors (age ≥14 years, body mass index ≥30 kg/m2, history of VTE, history of malignancy, history of coagulation disorder). The primary recommendation was for sequential compression devices to be placed on the patient prior to induction. No recommendation was made for pharmacological prophylaxis on the basis that the risk/benefit profile may not make sense in children.15 16 Since creating this clinical pathway, there have been new reports regarding clarification of paediatric risk factors for VTE.16 17 Future revisions of the protocol will require adjustment to match newer evidence. Normothermia was also added and defined as a core body temperature between 36°C and 38°C from incision to close time. Any value outside this range nullifies the measure. Maintaining normal temperatures may minimise risk of wound infection in adults.18 19 Notably, normothermia promotes normal metabolic demands on the body (including pharmacokinetics of anaesthetics) and minimises stress from hypothermic or hyperthermic conditions.20 This was a Surgical Care Improvement Project core measure (SCIP-INF-10).21 The evidence underlying this measure is not level I, but the study group included it for its importance from a physiological perspective and to match existing published ERAS principles.
Minimising surgical drains is another ERAS goal. To adapt this to lower urinary tract reconstruction, it was defined as avoiding placement of intraperitoneal or subcutaneous drains. This measure does not include urinary drains, as the group felt these to be important to protect and maximise drainage of the urinary tract postoperatively as a matter of urological principle. To account for those patients in whom a clinical decision has been made to leave an intraperitoneal or subcutaneous drain, a postoperative measure was added that any such drains should be removed on or by postoperative day 4. This day was proposed on the basis of pilot data showing that many patients are ready to go home and that these drains are rarely helpful.
The remainder of the measures from the pilot study were adopted and definitions updated to be internally consistent and account for most foreseeable scenarios. Refer to online supplemental table 1 for complete definitions of all 20 ERAS protocol measures. Importantly, the ERAS protocol as defined was to be adopted by all participating centres as standard of care for treatment of patients undergoing urological reconstructive surgery.
bmjopen-2020-039035supp003.pdf (97.8KB, pdf)
Study design
Several study designs were debated and discussed, including randomised controlled trials (RCT) of various permutations as well as prospective observational studies. Important characteristics discussed included ensuring both implementation and study design were feasible with minimal overhead, robust data collection through the use of a shared Research Electronic Data Capture (REDCap) database, a priori defined ERAS protocol definitions and outcomes, inclusion of both paediatric (ages 4–17 years) and emerging young adult (18–25) patients undergoing lower urinary tract reconstruction and identification of an adequate control group to demonstrate clinically meaningful differences.
Several centres (Children’s Hospital Colorado, St Louis Children’s Hospital and Cincinnati Children’s Hospital Medical Center) already had ERAS protocols in place (or started them concurrently during study start-up) and randomisation by patient was felt to lack equipoise on the basis of pilot data showing substantial patient benefit. Randomisation by protocol item was deemed too complex and not feasible. Randomisation by surgeon was also felt to lack equipoise, although this is debatable from the standpoint that opposing views on ERAS implementation details may represent unknowable qualities of the intervention. Randomisation by centre would suffer similar pitfalls identified above. Blinding and allocation concealment are staples of RCTs, but are not possible in the setting of implementation of a complex protocol involving tens of interventions that touch nearly every aspect of the diffuse perioperative space. Furthermore, ERAS relies on standardisation of perioperative care, and having patients on either an ERAS protocol or ad hoc care would necessarily create an unwelcome opportunity for cross-contamination issues. A stepped wedge cluster randomised trial design was also considered and is being planned for a separate large multicentre effort in paediatric bowel resection for inflammatory bowel disease.22 Because two centres in this study group already had existing ERAS protocols, this was not compatible with this option.
The notion that RCT may not be appropriate in all circumstances, particularly within the realm of surgical procedures, is not new and has been discussed previously.23 There is an applicable framework for advancing surgical care through research and creation of evidence-based practices called the Idea, Development, Exploration, Assessment, and Long-term study (IDEAL) framework.24 In this classification, surgical innovation passes through several different stages. ERAS in paediatric urology is in the beginning stages and falls under stage 2 (Development) or stage 3 (Exploration), given the ground work shown in two small early studies.12 13 The IDEAL framework defines goals and methods that are best suited to each stage. At stage 3, prospective study is carried out in either an uncontrolled manner or in smaller size than a full-blown controlled trial. Because of the limitations posed by ERAS, equipoise and surgeon experience, the study group determined the best study would be a case–control study, with ERAS patients making up a prospective observational arm and propensity-matched controls coming from recent patients not exposed to the ERAS protocol.
After discussion, the study group defined two study phases. First, a pilot phase will assess study recruitment across sites, ERAS implementation, protocol adherence and study procedures. Second, an exploration phase will prospectively compare all patients on the ERAS protocol to recent historical controls matched on propensity to undergo surgery with utilisation of an ERAS protocol, should they have been treated presently, using clinically important covariates deemed most likely to affect recovery. Data from the pilot study will be fed forward into the exploratory study. The decision for a built-in pilot study allows each centre to build comfort level with study procedures along with maturation of the ERAS protocol. From a methodological standpoint, a pilot study is set up like a smaller version of the larger study without the need to define sample size or demonstrate clinically important outcomes but rather examine outcomes related to the set-up of the study itself.25 A built-in pilot component at each centre (first five patients) will allow ascertainment of treatment team perceptions of ERAS and barriers to protocol implementation. While 5 is a small number, high-volume centres only perform 10–15 of these cases per year.26
Centre eligibility and patient selection
Centres will be allowed to enrol patients in the study if the centre performed a minimum of five lower urinary tract reconstructive operations in the year prior to centre enrolment. This constitutes a baseline measure of quality and familiarity with the care of these complex patients perioperatively.
Surgeons and research assistants at each respective centre will be responsible for subject identification and recruitment through existing clinical relationships. Patients aged 4–25 years undergoing the following lower urinary tract reconstructive operations may be enrolled after providing informed consent (and assent, when applicable, see online supplemental files- Consent and Assent for examples): augmentation enterocystoplasty, creation of continent urinary channel (appendicovesicostomy, ileovesicostomy or colovesicostomy), creation of an antegrade continence enema channel and incontinent urinary diversions (ileal conduit with or without cystectomy or ileovesicostomy). Because some of these operations may be done with or without a bowel anastomosis—which is a major risk factor for surgical stress, increased operative time and risk of ileus—bowel anastomosis will be tracked and used for matching cases to controls as it is a strong effect modifier. Some providers noted patients with neurogenic bowel not on a bowel management programme (retrograde enemas, oral stool softeners or rectal suppositories) may be at increased risk prolonged return of bowel function, ileus, bowel obstruction or anastomotic bowel leak. For this reason, clinically constipated patients defined as Bristol 1 or 2 stools more than once per week, bowel movement interval greater than every other day, or palpable stool in >50% of colon on physical preoperative examination will be excluded from the study. Patients with these findings become eligible if their stooling pattern is addressed at least 4 weeks in advance of surgery with implementation of a bowel management programme continued up to the night before surgery.
bmjopen-2020-039035supp001.pdf (124.3KB, pdf)
bmjopen-2020-039035supp002.pdf (133.9KB, pdf)
The use of historical controls has long been felt to be controversial, secondary to the retrospective nature of their identification and data collection and potential biases. Using quality improvement methodology, in which historical controls are often used to compare outcomes to an intervention cohort, run diagrams and interrupted time-series analysis can provide insight into changes occurring over time with regard to either process, clinical outcome or balancing measures.27 This has the benefit of ensuring that the prospectively enrolled patient outcomes are attributed to the intervention (ERAS) and not to changes in patient care that were already underway prior to implementation. While use of prospective controls from non-ERAS institutions might serve as a better comparison (reduced bias, prospective data collection, parallel comparison of modern surgical patients undergoing similar operations), the study group felt that observational bias (Hawthorne effect) might influence malleable outcomes such as LOS.
Outcome measures
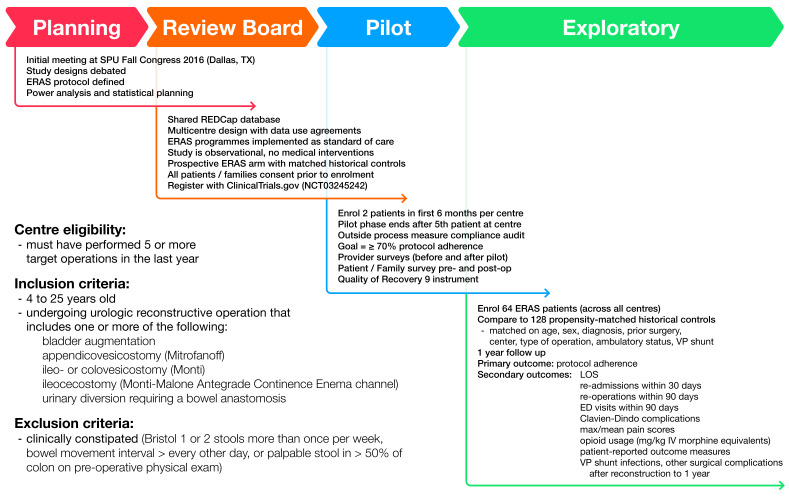
The pilot phase outcomes of interest include enrolling a minimum of two patients per centre in the first 6 months, and completing at least 90 days of follow-up on the first five enrolled patients (figure 1). A goal of ≥70% protocol item adherence (out of 20) at ≥75% of study centres was set. Finally, barriers to implementation will be identified and may determine if there is a need to optimise the protocol for wider application.
Figure 1.
Overview of study conception, implementation, centre eligibility, patient inclusion and exclusion criteria, and design methodology arrived at through group consensus. ED, emergency department; ERAS, enhanced recovery after surgery; IV, intravenous; LOS, length of stay; REDCap, Research Electronic Data Capture; SPU, Societies for Pediatric Urology; VP, ventriculoperitoneal.
The primary outcome of the exploratory phase is adherence to the ERAS protocol with number of items achieved (out of 20). Secondary outcomes include LOS; 30-day readmissions; 90-day reoperations; 90-day returns to the emergency room; 90-day complications by Clavien-Dindo classification (see Box 1 for full list of defined complications); number of long-term complications within 1 year (Box 2); minimum, median, maximum daily pain score during the first 7 days after surgery; and mean daily intravenous morphine equivalents (mg/kg) usage during the first 3 days after surgery.28 It is important to clarify that because this is an observational trial and the ERAS protocol is implemented as standard of care at each centre, the collection of complications here is a clinical outcome measure rather than one seen as a result of study intervention.
Box 1. List of pre-defined postoperative short-term complications.
90-Day Short-Term Complications
Clavien Grade I
electrolyte disturbance
fever (≥ 38°C) IV complication (infiltration)
nausea / vomiting neuropraxia (positioning complication)
transient elevation in Cr (acute kidney injury)
wound dehiscence
incisional seroma
other grade I
Clavien Grade II
blood transfusion
catheter manipulation, ACE
catheter manipulation, Mitrofanoff / Monti / urethral / suprapubic tube ± urinary retention
ileus requiring NG tube ± total parenteral nutrition + nausea / vomiting
infection / bacteremia treated with Abx ± fever
infection / pyelonephritis treated with Abx ± fever
infection / superficial wound treated with bedside drainage, Abx ± fever
infection / UTI treated with Abx ± fever
infection / GI infection with Abx ± fever ± diarrhea
venous thromboembolism
lymphocele or chylous ascites treated conservatively with diet changes
other grade II
Clavien Grade III
abdominal abscess requiring interventional radiology / operating room drainage
catheter malfunction / loss requiring placement in operating room
fascial dehiscence / evisceration treated in operating room
hemorrhage requiring embolization or operating room
small bowel obstruction treated surgically in operating room
urinoma requiring interventional radiology / operating room drainage
ureteral obstruction requiring percutaneous nephrostomy tube by interventional radiology / operating room
lymphocele or chyle leak requiring interventional radiology / operating room drainage or intervention
other grade III
Clavien Grade IV
respiratory failure requiring ventilation
renal failure
multiorgan failure
sepsis
other grade IV
Clavien Grade V
death
Box 2. List of pre-defined postoperative long-term complications.
1-Year Long-Term Complications
channel stenosis (any level) requiring revision
channel false passage
bowel obstruction
bladder stone formation
bladder perforation
incisional hernia
new onset metabolic acidosis
new onset chronic kidney disease
new onset renal scarring
VP shunt externalization
VP shunt infection (positive shunt tip and cerebral spinous fluid cultures)
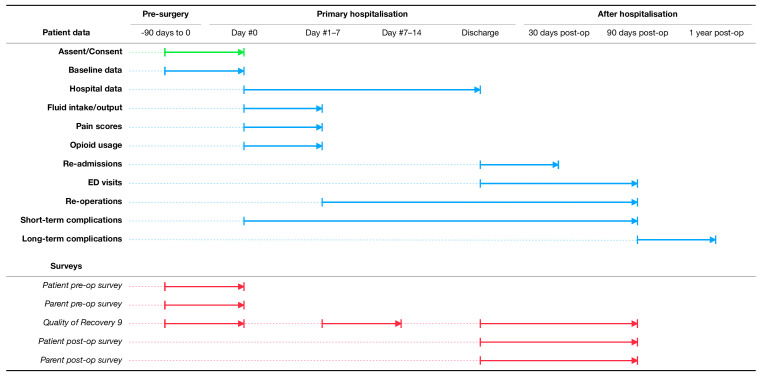
In addition to the objective clinical outcomes listed, patient and family-reported outcome measures will be administered to assess the impact of surgery on work and school (eg, missed days of each) and adjustment time at home needed to return to ‘normal’ (ie, daily routines not impacted heavily by having had surgery). These instruments include open-ended, non-validated parent and child surveys to be given preoperatively and postoperatively, and a Quality of Recovery 9 questionnaire to assess overall return of function (given before and after surgery, and again at clinic follow-up).29 Figure 2 demonstrates which outcome measures will be tracked over time with respect to the index surgery. Patients will be followed for 1 year, specifically to ensure that patients with a ventriculoperitoneal (VP) shunt do not experience increased rates of externalisation, infection or revision which has long been a concern of the community.30
Figure 2.
Timeline of patient enrolment, data collection including process and balancing measures, clinical outcomes and patient-reported outcome measures during the Pediatric Urology Recovery After Surgery Endeavor (PURSUE) study. ED, emergency department.
Data collection
A shared REDCap database has been designed, tested and implemented for use for this study. Data use agreements have been executed between centres and data sharing language was incorporated into patient consent to allow sharing of deidentified data sets maintaining patient confidentiality. The majority of perioperative process, outcome and balancing measures are charted within the medical record as part of standard of care. Where possible, these will be automatically abstracted electronically as five out of the six study centres use the Epic electronic health record system. In cases where data are not normative or where it requires clinical interpretation or cannot be abstracted electronically, manual chart review by research assistants trained by the study team will be done. Continuous data quality checks will be completed quarterly, including analysis of missing required data and any discrepancies. The study commenced enrolment in 2017 and aims to conclude in 2021.
After primary accrual is completed, the study committee plans to transition the shared database into a shared clinical registry for ongoing data collection to continue to study ERAS and further refinements to the care pathway.
Statistical analysis
A total of 64 ERAS patients will be needed to detect a decrease in mean overall LOS by 2 days, with type I error of 5% (false positive) and type II error of 20% (false negative) based on data from the pilot study showing mean LOS of 8.0 days (SD 7.3) for historical patients versus 5.7 days (SD 5.1) for patients who were treated under the ERAS protocol. Patients will be propensity score matched on likelihood to have been treated under an ERAS protocol 1:2 to recent historical controls from 5 years prior to the initiation of the ERAS protocol. Propensity matching controls for measured baseline covariates before analysis of the outcomes to reduce confounding. Based on pilot data (mean 2.1 complications/patient (SD 1.9) historically and vs 1.3 complications/patient (SD 1.2) under ERAS), this study will also be powered to detect a decrease in any grade complications per patient by 50%. Patients will be divided into two strata: those who underwent and did not undergo a bowel anastomosis as part of the index operation. Propensity score matching within the two strata using nearest-neighbour algorithm (also referred to as greedy matching) will occur on the following variables: age, sex, chronic kidney disease, presence of VP shunt, planned bladder augmentation, history of prior abdominal surgery (other than VP shunt), diagnosis of myelomeningocele, ambulatory status and centre. Bowel anastomosis was determined by the study group to be a strong effect modifier and thus patients will be exactly matched on that variable (creating two strata) and propensity matched on remaining covariates to avoid overfitting.
Because of the nature of propensity-matched data, care must be taken for comparison of historical controls and ERAS cases. Differences in baseline characteristics between matched groups will be assessed using methods that are not influenced by sample size and that do not refer to a hypothetical population (ie, standardised differences).31 The Mantel-Haenszel test will be used to compare proportions, and generalised linear modelling with generalised estimating equations to adjust for the matching design will be used to assess association of outcomes and predictors.32 Two-tailed p values <0.05 will be considered significant. No interim analyses are planned.
Study committee
Given the importance of a strong implementation serving as a foundation for success, the study group has created several committees, including an organising committee and audit committee. The organising committee is charged with overseeing data collection, arranging study conference calls and meetings and adjudicating authorship for subsequent papers laid out through a set of by-laws. The organising committee serves as a backstop to proper trial conduct under the purview of study (KOR) and site primary investigators (ACS, GJV, RC, DIC, RSZ). The audit committee arguably serves a more important role, overseeing regular clinical audits of ERAS protocol compliance. The committee is charged with meeting after each centre’s pilot phase (five patients) and ad hoc thereafter, and they will review overall compliance and serve as an external study group as part of plan/do/study/act quality improvement methodology to identify challenging areas and suggest solutions that may be novel for that centre. This highlights the point that the ERAS clinical pathway sets high-level goals, but leaves implementation details and specifics to each centre. This creates heterogeneity that mirrors real-world quality improvement projects, improving the generalisability of the project, but can lead to maladaptive internal centre processes. The audit committee’s goal is to help each centre identify issues early in the implementation and find creative solutions.
Strengths and limitations of the study design
Strengths of this study design include its multicentre nature, which the authors aim to use to demonstrate feasibility of ERAS implementation in a variety of geographically diverse paediatric-focused settings. Prospective data collection, a priori definitions of protocol elements and an exhaustive list of potential short and long-term complications also lend strengths to its design. The Standard Protocol Items: Recommendations for Interventional Trials checklist was used when preparing this report.33 Potential limitations of this study include variation in protocol implementation, unsuccessful attempts at protocol implementation, unobserved patient characteristics or other biases affecting outcome measures and use of historical, retrospective controls. The study group notes that there is very little level I evidence for protocol items in paediatric patients. Some are extrapolated from adult evidence and may not hold true. Additionally, patient-reported outcomes in this population are lacking. Pain interference and validated general function measures are available but were not designed nor tested expressly to measure recovery after surgery. When examining clinical outcomes, propensity matching on clinically relevant patient characteristics will allow meaningful comparison, and run charts of patient care variables over time will shed light on any changes in care patterns or outcomes that may have already been underway.
Ethics and dissemination
This study was approved by each free-standing tertiary care children’s hospital’s respective Institutional Review Board (St Louis Children’s Hospital (201703081), Children’s Hospital of Pittsburgh (17070089), Children’s Hospital Colorado (17-0746), Cincinnati Children’s Hospital Medical Center (2017-3322), Ann & Robert H Lurie Children’s Hospital (2019-2566) and Children’s Hospital of Richmond at VCU (HM20015891)). Prospectively enrolled patients who meet inclusion criteria will be approached for inclusion by either a urologist or research assistant prior to the day of surgery. Study protocol does not allow for patients to be approached in the preoperative area to avoid patient or family coercion. No study activities will occur prior to obtaining consent. Patients under 18 years of age (and over specific ages that vary by centre) will assent to enrolment. Participants retain the right to withdraw at any point for any reason. Importantly, non-adherence to the ERAS protocol or ERAS protocol deviation is not grounds for removal from the study. Not every patient will meet clinical standards for every protocol item. Rather, the goal of the ERAS protocol is to maximise evidence-based strategies to return the patient to normal function. ERAS protocol changes will only be made after completing primary accrual and analysis of results in conjunction with a thorough literature review by the study committee.
Patient and public involvement
ERAS, in many respects, is a patient-focused quality improvement project. While no patients or families were directly involved in the design of this study or recruitment of potential subjects, families expressed interest in being notified of the study results and this will occur. Patient and families will be engaged in future revisions of the underlying ERAS clinical pathway that result from evidence gathered through this study.
In conclusion, PURSUE (ClinicalTrials.gov) is a multicentre, prospective, propensity-matched, case–control cohort study that will examine outcomes in paediatric and emerging young adult patients undergoing lower urinary tract reconstruction who receive care under an ERAS pathway.34 Results will be published in peer-reviewed journals by study group members. This protocol marks the first phase of a collaborative quality improvement effort within the paediatric urology community to improve and standardise care of patients undergoing urological reconstructive surgery.
Supplementary Material
Footnotes
Twitter: @kylerove
Collaborators: PURSUE study group members: Douglas E Coplen (St Louis Children’s Hospital, St Louis, MO, USA), Paul F Austin (Texas Children’s Hospital, Houston, TX, USA), Erica J Traxel (St Louis Children’s Hospital, St Louis, MO, USA), Jacob AuBuchon (St Louis Children’s Hospital, St Louis, MO, USA), Robert P Moore (St Louis Children’s Hospital, St Louis, MO, USA), Vijaya M Vemulakonda (Children’s Hospital Colorado, Aurora, CO, USA), Brian T Caldwell (Children’s Hospital Colorado, Aurora, CO, USA), Carter J Sevick (Children’s Hospital Colorado, Aurora, CO, USA), Nicholas Burjek (Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL, USA), Elizabeth B Yerkes (Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL, USA), Yvonne Y Chan (Ann & Robert H Lurie Children's Hospital of Chicago, Chicago, IL, USA), C D Anthony Herndon (Children’s Hospital of Richmond at VCU, Richmond, VA, USA).
Contributors: KOR, Brian T Caldwell and DTW organised the initial meeting to discuss study protocol concepts in Dallas, Texas, USA in 2016. KOR, MAB, TPW, DIC, DTW and GJV developed and refined the 20-protocol ERAS pathway used in the study based on literature searches, screening and review. KOR led the protocol development and created patient recruitment tools. KOR, MAB and ACS chair the PURSUE organising committee that oversees study activities. KOR is the study primary investigator. KOR, ACS, RSZ, RC, DIC and GJV serve as site primary investigators. KOR performed the power analysis and statistical analysis plans with additional input, oversight and revision by DIC. KOR, ACS, DTW, GJV, TPW, BV, DIC, RC, RSZ, PURSUE Study Group and MAB were involved in the study conception, design, protocol manuscript drafting and critical revision. All authors approved the final version.
Funding: A Children’s Hospital Colorado Clinical and Operational Effectiveness and Patient Safety grant supports the implementation and study of an ERAS pathway. A Midwest Stone Institute grant supports PURSUE implementation at St Louis Children’s Hospital.
Disclaimer: Neither source of funding participated in the study design, collection, analysis or interpretation of data, in the writing of the manuscript, or in the decision to submit this manuscript for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
PURSUE Study group:
Douglas E. Coplen, Paul F. Austin, Erica J. Traxel, Jacob AuBuchon, Robert P. Moore, Vijaya M. Vemulakonda, Brian T. Caldwell, Carter J. Sevick, Nicholas Burjek, Elizabeth B. Yerkes, Yvonne Y. Chan, and C.D. Anthony Herndon
References
- 1. Schlomer BJ, Saperston K, Baskin L. National trends in augmentation cystoplasty in the 2000s and factors associated with patient outcomes. J Urol 2013;190:1352–8. 10.1016/j.juro.2013.04.075 [DOI] [PubMed] [Google Scholar]
- 2. Wang H-HS, Lloyd JC, Wiener JS, et al. . Nationwide trends and variations in urological surgical interventions and renal outcome in patients with spina bifida. J Urol 2016;195:1189–95. 10.1016/j.juro.2015.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weatherly DL, Szymanski KM, Whittam BM, et al. . Comparing inpatient versus outpatient bowel preparation in children and adolescents undergoing appendicovesicostomy. J Pediatr Urol 2018;14:50.e1–50.e6. 10.1016/j.jpurol.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292–8. 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 5. Fearon KCH, Ljungqvist O, Von Meyenfeldt M, et al. . Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466–77. 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 6. Thiele RH, Rea KM, Turrentine FE, et al. . Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg 2015;220:430–43. 10.1016/j.jamcollsurg.2014.12.042 [DOI] [PubMed] [Google Scholar]
- 7. Liu VX, Rosas E, Hwang J, et al. . Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg 2017;152:e171032. 10.1001/jamasurg.2017.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ERAS Compliance Group The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 2015;261:1153–9. 10.1097/SLA.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 9. Short HL, Heiss KF, Burch K, et al. . Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2018;53:1131–6. 10.1016/j.jpedsurg.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 10. Reismann M, von Kampen M, Laupichler B, et al. . Fast-Track surgery in infants and children. J Pediatr Surg 2007;42:234–8. 10.1016/j.jpedsurg.2006.09.022 [DOI] [PubMed] [Google Scholar]
- 11. Shinnick JK, Short HL, Heiss KF, et al. . Enhancing recovery in pediatric surgery: a review of the literature. J Surg Res 2016;202:165–76. 10.1016/j.jss.2015.12.051 [DOI] [PubMed] [Google Scholar]
- 12. Rove KO, Brockel MA, Saltzman AF, et al. . Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol 2018;14:252.e1–252.e9. 10.1016/j.jpurol.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 13. Haid B, Karl A, Koen M, et al. . Enhanced recovery after surgery protocol for pediatric urological augmentation and diversion surgery using small bowel. J Urol 2018;200:1100–6. 10.1016/j.juro.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 14. Daneshmand S, Ahmadi H, Schuckman AK, et al. . Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol 2014;192:50–6. 10.1016/j.juro.2014.01.097 [DOI] [PubMed] [Google Scholar]
- 15. Ahn JJ, Merguerian PA, Shnorhavorian M. Incidence and risk factors associated with 30-day post-operative venous thromboembolism: a NSQIP-pediatric analysis. J Pediatr Urol 2018;14:335.e1–335.e6. 10.1016/j.jpurol.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 16. Cairo SB, Lautz TB, Schaefer BA, et al. . Risk factors for venous thromboembolic events in pediatric surgical patients: defining indications for prophylaxis. J Pediatr Surg 2018;53:1996–2002. 10.1016/j.jpedsurg.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 17. Sherrod BA, McClugage SG, Mortellaro VE, et al. . Venous thromboembolism following inpatient pediatric surgery: analysis of 153,220 patients. J Pediatr Surg 2019;54:631–9. 10.1016/j.jpedsurg.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. study of wound infection and temperature group. N Engl J Med 1996;334:1209–15. 10.1056/NEJM199605093341901 [DOI] [PubMed] [Google Scholar]
- 19. Wong PF, Kumar S, Bohra A, et al. . Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg 2007;94:421–6. 10.1002/bjs.5631 [DOI] [PubMed] [Google Scholar]
- 20. Lenhardt R, Marker E, Goll V, et al. . Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997;87:1318–23. 10.1097/00000542-199712000-00009 [DOI] [PubMed] [Google Scholar]
- 21. Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect 2011;12:163–8. 10.1089/sur.2010.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clinical trial NCT04060303: enhancing recovery in children undergoing surgery for IBD (ENRICH-US). U.S. National Institutes of Health, 2019. Available: https://clinicaltrials.gov/ct2/show/NCT04060303 [Accessed 25 Mar 2020].
- 23. Wallis CJD, Detsky AS, Fan E. Establishing the effectiveness of procedural interventions: the limited role of randomized trials. JAMA 2018;320:2421–2. 10.1001/jama.2018.16329 [DOI] [PubMed] [Google Scholar]
- 24. McCulloch P, Altman DG, Campbell WB, et al. . No surgical innovation without evaluation: the ideal recommendations. Lancet 2009;374:1105–12. 10.1016/S0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 25. Thabane L, Ma J, Chu R, et al. . A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10:42 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bowlin P, Siparsky G, Wilcox D. 1187 nationwide review of bladder augmentation in pediatric hospitals. Journal of Urology 2011;185:e477 10.1016/j.juro.2011.02.820 [DOI] [Google Scholar]
- 27. EPOC Resources for review authors Cochrane effective practice and organisation of care (EPOC), 2017. Available: https://epoc.cochrane.org/resources/epoc-resources-review-authors [Accessed 27 Mar 2019].
- 28. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg 2004;240:205–13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myles PS, Hunt JO, Nightingale CE, et al. . Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg 1999;88:83–90. 10.1097/00000539-199901000-00016 [DOI] [PubMed] [Google Scholar]
- 30. Gundeti MS, Godbole PP, Wilcox DT. Is bowel preparation required before cystoplasty in children? J Urol 2006;176:1574–7. 10.1016/j.juro.2006.06.034 [DOI] [PubMed] [Google Scholar]
- 31. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fleiss JL, Levin B, Paik MC. The Analysis of Data from Matched Samples : Statistical Methods for Rates and Proportions. Hoboken, NJ: Wiley, 2004: 373–406. [Google Scholar]
- 33. Chan A-W, Tetzlaff JM, Altman DG, et al. . Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clinical trial NCT03245242: pediatric urology recovery after surgery endeavor (PURSUE). U.S. National Institutes of Health, 2017. Available: https://clinicaltrials.gov/ct2/show/NCT03245242 [Accessed 31 Aug 2017].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039035supp003.pdf (97.8KB, pdf)
bmjopen-2020-039035supp001.pdf (124.3KB, pdf)
bmjopen-2020-039035supp002.pdf (133.9KB, pdf)