Abstract
Patients with cancer are at high risk of venous thromboembolic events, and this risk can be further increased in patients with certain cancer types and by cancer treatments. Guidelines on the prevention of cancer-associated thrombosis (CAT) recommend thromboprophylaxis for hospitalised patients; however, this is not routinely recommended for ambulatory patients receiving chemotherapy and is limited to specified high-risk patients. Identification of the ambulatory patients at risk of CAT who would most benefit from anticoagulant therapy is therefore critical to reduce the incidence of this complication. For patients receiving thromboprophylaxis for CAT, treatment options include low molecular weight heparin, acetylsalicylic acid, warfarin or direct oral anticoagulants (apixaban or rivaroxaban), dependent on the cancer type and cancer treatment regimen. This review discusses emerging clinical trial data and their potential clinical impact.
Keywords: anticoagulant, cancer-associated thrombosis, direct oral anticoagulant, low molecular weight heparin, venous thromboembolism, thromboprophylaxis
INTRODUCTION AND BACKGROUND
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication of cancer and is termed as cancer-associated thrombosis (CAT). The risk of VTE is increased fourfold to sevenfold in patients with cancer compared with those without malignancy.1 2 However, the risk of VTE in patients with cancer is variable and is influenced by a range of factors, which can be grouped into four broad categories: tumour related (eg, type and stage of cancer), treatment related (eg, anti-cancer therapy), patient related (eg, patient age or history of VTE) and biomarkers (eg, D-dimer levels).3 A detailed overview of factors within these categories is provided in table 1.3–12
Table 1.
Examples of VTE risk factors in patients with cancer by risk factor group3–12
| Tumour-related factors | Treatment-related factors |
| Site of cancer (eg, lung, gastric, ovarian) | Anti-angiogenic agents (eg, thalidomide, lenalidomide) |
| Histological stage (I–IV) | Hormonal therapies (eg, tamoxifen) |
| Cancer stage (localised, regional, distant) | Chemotherapy agents (eg, gemcitabine or platinum-based therapies) |
| Time since diagnosis | Cancer surgery |
| Erythropoiesis-stimulating agents | |
| Erythrocyte and platelet transfusions | |
| Central venous catheters | |
| Prolonged hospitalisation | |
| Patient-related factors | Biomarkers |
| Genetic factors (eg, hereditary thrombophilia) | Blood related (eg, levels of platelets, haemoglobin, leucocytes) |
| Medical illnesses/comorbidities | Platelet and clotting activation related (D-dimer, soluble P-selectin, prothrombin fragment 1+2, thrombin generation) |
| Very high or very low body weight | Clotting factor-related (eg, FVIII, CRP) |
| Prior VTE | NET formation (eg, citrullinated histone H3) |
| Varicose veins | TF-MP* |
| Age and gender | Podoplanin† |
| CCL3 | |
| sVEGF |
*Association between elevated TF-MP activity and future VTE has been observed only in patients with pancreatic cancer.
†Association between podoplanin expression and occurrence of VTE has been shown only in patients with brain cancer.
CCL3, chemokine (C-C motif) ligand 3; CRP, C reactive protein; FVIII, factor VIII; NET, neutrophil extracellular trap; sVEGF, soluble vascular endothelial growth factor; TF-MP, tissue factor bearing microparticles; VTE, venous thromboembolism.
Tumour-related VTE risk factors
Certain cancers are more strongly associated with VTE than others. In a cohort study of over 6500 patients with active cancer who experienced a first episode of VTE, lung, stomach, ovarian, pancreatic and brain cancers were associated with higher incidence rates of VTE (10.1–14.6 per 100 person-years) than bladder, breast, prostate, haematological, colon and uterine cancers (2.7–7.0 per 100 person-years).6 Furthermore, studies have shown that even within cancer types, variations can exist; for example, VTE rates are particularly high in patients with glioblastoma versus astrocytoma.13 It is also important to keep in mind that absolute numbers of patients with low-risk cancer types and venous thromboembolic events may be high due to the high prevalence of those cancer types, such as breast or prostate cancer.
Advanced cancer is associated with a higher risk of VTE than localised or early stage disease. One meta-analysis of patients with breast, lung, colorectal, prostate, brain, bone, pancreatic or haematological cancer reported first VTE rates of 68 per 1000 person-years in high-risk patients (defined as those with high-grade or metastatic disease, and/or receiving anti-cancer treatments considered to be high risk).14 In contrast, those deemed to be at a lower level of risk had first VTE rates of 13 per 1000 person-years.14 In the Vienna Cancer and Thrombosis Study (CATS), 832 patients with solid tumours were assessed for association between cancer stage at diagnosis (local, regional or distant) and VTE occurrence.15 Regionally advanced and distant cancers were found to be associated with significantly higher rates of VTE occurrence than localised cancers (regional: HR 3.7, 95% CI 1.5 to 9.6; distant: HR 5.4, 95% CI 2.3 to 12.9).15 Furthermore, when tumours were graded based on the American Joint Committee on Cancer (AJCC) group-level grading system (G1=well differentiated; G2=moderately differentiated; G3=poorly differentiated; G4=undifferentiated) in the same study, high-grade tumours (G3 and G4) were associated with a significantly higher risk of VTE than low-grade tumours (G1 and G2; HR 2.0; 95% CI 1.1 to 3.5; p=0.015).16
Cancer treatment-related VTE risk factors
Anti-neoplastic therapies or supportive care treatments (chemotherapy, anti-angiogenic therapy, hormonal therapy and erythropoiesis-stimulating agents (ESAs)) are also thrombogenic and several of these agents have been shown to be associated with an increased risk of VTE.4 17 18 A likely underlying mechanism is vascular damage.19 20 A US population-based study found that compared with patients without cancer, chemotherapy in patients with malignant neoplasms was associated with an OR of 6.5 (95% CI 2.1 to 20.2) for VTE, whereas those with malignant neoplasms not receiving chemotherapy had an OR of 4.1 (95% CI 1.9 to 8.5).21
Hormonal therapies can also increase VTE risk. Patients with breast cancer given tamoxifen experienced higher rates of VTE than those who did not receive hormonal therapy (5-year risks of 1.2% and 0.5%, respectively). The prothrombotic effect was predominantly in the first 2 years after initiating treatment (relative risk (RR) 3.5; 95% CI 2.1 to 6.0, compared with RR 1.5; 95% CI 0.88 to 2.5 after 2 years) and was more pronounced in older women.22 The risks associated with tamoxifen therapy were further increased when used in combination with chemotherapy. This was shown in a study of 705 postmenopausal patients randomised to tamoxifen, or tamoxifen and chemotherapy, with VTE incidences of 1.4% and 10.8%, respectively.23
The thrombogenic properties of agents with anti-angiogenic and immunomodulatory properties have been demonstrated in several studies. Patients with newly diagnosed multiple myeloma treated with thalidomide monotherapy had a risk of VTE of 1.3 (95% CI 0.4 to 7.2) per 100 patient-cycles.24 This risk was amplified by coadministration with the corticosteroid dexamethasone, with a VTE risk of 4.1 (95% CI 2.8 to 5.9) per 100 patient-cycles. Lenalidomide plus dexamethasone was associated with a risk of VTE of 0.8 (95% CI 0.07 to 2.0) per 100 patient-cycles.24 Anti-angiogenic therapy in combination with a cytotoxic agent also elevated the risk of developing VTE in an analysis of European and North American patients with advanced cancer treated in phase I studies. Anti-angiogenic plus cytotoxic therapy was associated with a VTE incidence of 8.9% compared with 3.5% in patients treated with other regimens.25 Similarly, patients with multiple myeloma who received thalidomide and doxorubicin have been shown to be at high risk of developing DVT.26
ESAs used to treat anaemia in patients with cancer also increase the risk of VTE. A meta-analysis of over 12 000 patients demonstrated that patients with cancer who received ESAs had a higher risk of VTE than those who did not (RR 1.75; 95% CI 1.50 to 2.05).4 In addition, the ESA-related risk of VTE was highest in patients with ovarian and cervical cancers (RR 2.45; 95% CI 1.12 to 5.33); patients with haematological malignancies were at greater risk than those with solid tumours. Newer targeted-therapy agents have also been associated with risk of VTE in cancer, including anti-vascular epidermal growth factor therapy, immunotherapy and cyclin-dependent kinase inhibitors.27 28
In addition to medications for patients with cancer, the risk of VTE is elevated in patients undergoing major cancer surgical procedures. In a meta-analysis of ~1.5 million patients who had undergone oncologic surgery, the overall incidence of VTE was estimated to be 2.3% (95% CI 2.1% to 2.5%).29 The risk of postoperative VTE was highest in those with bone and soft tissue cancer (10.6%; 95% CI 2.9% to 18.2%) and lung cancer (8.1%; 95% CI 3.7% to 12.6%).29
VTE risk factors in patients with cancer
Various patient-related factors increase VTE risk in cancer. Recent data from the UK Clinical Practice Research Datalink showed that elderly patients with cancer had the highest incidence of a first venous thromboembolic event.6 Furthermore, the point of highest VTE incidence differed by sex, with the peak occurring during the sixth decade for male patients and in the eighth decade for women.6 Prior VTE and family history of VTE have been associated with an increased risk of VTE in multiple cancer types,30 31 and several studies suggest a link between prothrombotic genetic risk factors such as Factor V Leiden and cancer-related VTE.1 32 Body weight also influences VTE risk in patients with active cancer, with one US population-based retrospective case–control study demonstrating an increase in incident VTE in patients with active cancer and low body mass index (BMI; <18.5 kg/m2, OR 1.9) or high BMI (≥35 kg/m2, OR 4.0) versus controls.5
Biomarkers and cancer-related VTE risk
Several biomarkers have been shown to correlate with the occurrence of VTE in cancer. Parameters such as increased leucocyte and platelet counts and decreased haemoglobin have been shown to be good predictors of VTE risk in patients with cancer.33–35 Additionally, associations between elevated concentrations of prothrombin fragment 1+2, soluble P-selectin, clotting Factor VIII and D-dimer and increased VTE occurrence in cancer have been reported.36–38
Despite the elevated risk of VTE in patients with cancer, thromboprophylaxis is not routinely administered to those treated for cancer as outpatients. This narrative review discusses existing guidelines and data on VTE prevention in ambulatory cancer care, methods for identification of patients in this setting who could benefit from thromboprophylaxis, and emerging data that provide further insights into the safety and efficacy of thromboprophylaxis for management of these patients.
GUIDELINES FOR THE PREVENTION OF CANCER-ASSOCIATED THROMBOSIS IN AMBULATORY PATIENTS
A number of guidelines and guidance on the prevention of CAT are available from major societies and organisations, such as the American Society of Clinical Oncology (ASCO), the British Society for Haematology (BSH), the European Society for Medical Oncology (ESMO), the International Initiative on Thrombosis and Cancer (ITAC), the International Society on Thrombosis and Haemostasis (ISTH) and the National Comprehensive Cancer Network (NCCN).39–44 These guidelines provide recommendations on thromboprophylaxis in different populations of patients with cancer including hospitalised patients, ambulatory patients and those undergoing surgery for cancer. However, they have not all been updated to incorporate the latest clinical trial data from recent trials investigating the prevention of CAT with direct oral anticoagulants (DOACs).
Although thromboprophylaxis is widely recommended for most hospitalised patients with cancer and in those undergoing cancer-related surgery, it is not routinely recommended for ambulatory patients with cancer receiving chemotherapy. This is despite most patients with cancer receiving their treatment in the outpatient setting. However, the guidelines state that thromboprophylaxis may be considered for selected ambulatory patients at intermediate-to-high risk of VTE.39–44 Low molecular weight heparin (LMWH) is generally the agent recommended in patients with solid tumours receiving chemotherapy. In those with multiple myeloma receiving pomalidomide-based, thalidomide-based or lenalidomide-based regimens with chemotherapy and/or dexamethasone, the recommendation is either acetylsalicylic acid, LMWH or warfarin.39–42 44 Most of the guidelines advise the use of the Khorana risk score to assess VTE risk (the Khorana score is discussed in more detail in the Identifying patients who would benefit from thromboprophylaxis section).39–44 A newer class of anticoagulants, the DOACs (including apixaban, dabigatran, rivaroxaban and edoxaban), were not considered suitable for use in previous guidelines for CAT prevention in ambulatory patients with cancer because the writing of most guidelines preceded the publication of recent randomised trials. However, the recently updated ASCO, ISTH, NCCN and ITAC guidelines now recommend thromboprophylaxis with apixaban, rivaroxaban or LMWH in selected high-risk ambulatory patients with cancer and no significant risk factors for bleeding and no concerns regarding drug–drug interactions.41–44
STUDIES AND POTENTIAL LIMITATIONS OF THROMBOPROPHYLAXIS IN AMBULATORY PATIENTS WITH CANCER
Several phase IIb and phase III studies have explored thromboprophylaxis with heparin derivatives versus placebo in ambulatory patients with cancer. The study populations included patients with a range of solid tumour types (PROTECHT, SAVE-ONCO, TOPIC-1 and TOPIC-2) or advanced pancreatic cancer (FRAGEM, CONKO-004).45–49 The generally consistent observation across these studies was that thromboprophylaxis with various heparin derivatives significantly reduced VTE risk versus placebo without a significant increase in the risk of major bleeding.
The PROTECHT study investigated the thromboprophylactic effect of nadroparin once daily (od) versus placebo in patients with lung, gastrointestinal, pancreatic, breast, ovarian, or head and neck cancers.45 Nadroparin significantly reduced the rate of the primary composite endpoint of symptomatic venous or arterial thromboembolic events versus placebo (p=0.02), and a lower rate of VTE was specifically observed in patients receiving nadroparin versus placebo (1.4% and 2.9%, respectively). SAVE-ONCO investigated semuloparin od versus placebo in a similar population of patients with metastatic or locally advanced solid tumours.46 A significant reduction in the incidence of the primary endpoint of VTE (composite of symptomatic DVT, non-fatal PE or death related to VTE) was observed with LMWH (HR 0.36; 95% CI 0.21 to 0.60; p<0.001) with an approximately threefold reduction in the absolute event rate (1.2% and 3.4% in patients receiving LMWH or placebo, respectively). Similar results were seen in trials with LMWH in patients with breast cancer, non-small-cell lung carcinoma and advanced pancreatic cancer. Table 2 provides an overview of the key features of the patient populations, study designs and main outcomes of these trials.45–53
Table 2.
Key features of major trials of heparin derivatives and DOACs in ambulatory patients with cancer
| Study | Tumour type(s) | Treatment | Comparator | Treatment duration | Randomised patients | Primary efficacy outcome | Principal safety outcome |
| PROTECHT (2009)45 | Lung, GI, pancreatic, breast, ovarian, or head and neck cancers | Nadroparin 3800 IU anti-Factor Xa od | Placebo | 4 months | 1166 (779 nadroparin, 387 placebo) |
Composite of symptomatic venous or arterial thromboembolic events: 2.0% vs 3.9%; p=0.02 |
Major bleeding: 0.7% vs 0%; p=0.18 |
| SAVE-ONCO (2012)46 | Metastatic or locally advanced solid tumours | Semuloparin 20 mg od | Placebo | Intended minimum 3 months | 3212 (1608 semuloparin, 1604 placebo) |
Composite of any symptomatic DVT, any non-fatal PE and VTE-related death: 1.2% vs 3.4%; p<0.001 |
Clinically relevant bleeding: 2.8% vs 2.0%: p=NS |
| TOPIC-1 (2012)47 | Metastatic breast carcinoma | 3000 IU certoparin od | Placebo | 6 months | 353 (174 certoparin, 179 placebo) | Symptomatic or asymptomatic VTE: 4% vs 4%; p=NS |
Bleeding events: 5.2% vs 1.7%; p=0.084 |
| TOPIC-2 (2012)47 | Stage III/IV non-small-cell lung carcinoma | 3000 IU certoparin od | Placebo | 6 months | 547 (273 certoparin, 274 placebo) | Symptomatic or asymptomatic VTE: 4.5% vs 8.3%; p=NS |
Bleeding events: 13.6% vs 7.3%; p=0.024 |
| FRAGEM (2012)48 | Advanced pancreatic cancer | Weight-adjusted dalteparin | Placebo | 12 weeks | 123 (60 dalteparin, 63 placebo) | All-type VTE during the study period: 3.4% vs 23%; p=0.002 |
NR |
| CONKO-004 (2015)49 | Advanced pancreatic cancer | Enoxaparin | Placebo | 3 months | 312 (160 enoxaparin, 152 placebo) |
First event rate of symptomatic VTE within 3 months after randomisation: 1.3% vs 9.9%; p=0.01 |
Major bleeding: 3.3% vs 4.4%; p=1.0 |
| PHACS (2017)50 | All | Dalteparin 5000 IU od | Observation | 12 weeks | 98 (50 dalteparin, 48 on observation) |
All VTE: 12% vs 21%; unstratified HR 0.64; 95% CI 0.22 to 1.72 |
Clinically relevant bleeding events: 14% vs 2%; p=0.025 |
| CASSINI (2018)51 52 75 | Solid tumours or lymphoma | Rivaroxaban 10 mg od | Placebo | 6 months | 841 (420 rivaroxaban, 421 placebo) | Composite of symptomatic or asymptomatic lower extremity, proximal DVT, symptomatic upper extremity or distal DVT, symptomatic or incidental PE; and VTE-related death: 6.0% vs 8.8%; p=0.10 |
Major bleeding: 2.0% vs 1.0%; p=0.26 |
| AVERT (2018)53 | All newly diagnosed cancers except basal cell carcinoma, squamous cell carcinoma, acute leukaemia or myeloproliferative neoplasms | Apixaban 2.5 mg two times per day | Placebo | 6 months | Target enrolment 574 | Objectively documented VTE over a follow-up period of 180 days: 4.2% vs 10.2%; p<0.001 |
Major bleeding: 3.5% vs 1.8%; p<0.05 |
DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; GI, gastrointestinal; IU, international units; NR, not reported; NS, not significant; od, once daily; PE, pulmonary embolism; VTE, venous thromboembolism.
As mentioned, VTE prophylaxis for all ambulatory patients with cancer is currently not endorsed by guidelines. This is largely because of the low rates in patients randomised to placebo, low absolute reduction in the risk of VTE resulting in a high number of patients needed to treat to avoid one VTE and an unclear benefit–risk profile in unselected patients. There are also limitations for thromboprophylaxis with LMWH associated with prolonged use, such as reductions in bone mineral density,54 skin and allergic reactions,55 liver transaminase elevations,56 the inconvenience of repeated injections and cost.
IDENTIFYING PATIENTS WHO WOULD BENEFIT FROM THROMBOPROPHYLAXIS
Various methods and risk models have been proposed for the identification of groups of patients with cancer that would benefit most from VTE prophylaxis. These risk models comprise various factors that influence their predictive value: tumour size, location, stage, type, time since cancer diagnosis and comorbidities. Examples of these include the Khorana score, Vienna CATS score, PROTECHT score, CONKO score (table 3)33 57–60 and COMPASS-CAT risk assessment model (table 4).61
Table 3.
VTE risk scoring systems for patients with cancer63
| Score name and year published | Khorana score33 (2008) |
Vienna CATS score57 (2010) |
PROTECHT score58 (2012) |
CONKO score59 (2013) |
| Parameter | ||||
| Very high-risk tumours (pancreatic, gastric) | 2 | 2 | 2 | 2 |
| High-risk tumours (lung, gynaecological, lymphoma, bladder, testicular) | 1 | 1 | 1 | 1 |
| Pre-chemotherapy haemoglobin <10 g/dL or use of an ESA | 1 | 1 | 1 | 1 |
| Pre-chemotherapy white blood cell count >11×109/L | 1 | 1 | 1 | 1 |
| Pre-chemotherapy platelet count ≥350×109/L | 1 | 1 | 1 | 1 |
| Body mass index ≥35 kg/m2 | 1 | 1 | 1 | – |
| D-dimer >1.44 µg/L | – | 1 | – | – |
| Soluble P-selectin >53.1 ng/L | – | 1 | – | – |
| Gemcitabine chemotherapy | – | – | 1 | – |
| Platinum-based chemotherapy | – | – | 1 | – |
| WHO performance status ≥2 | – | – | – | 1 |
Each number indicates the number of points assigned to each parameter if it is present; – indicates that the parameter is not part of the respective scoring system.
CATS, Cancer and Thrombosis Study; ESA, erythropoiesis-stimulating agent; VTE, venous thromboembolism.
Table 4.
Simplified COMPASS-CAT scoring system for the prediction of VTE in ambulatory patients with common cancers on anti-cancer therapy (2017)61
| Parameter | Score* |
| Cancer-related risk factors | |
| Anti-hormonal therapy† | 6 |
| Time since cancer diagnosis ≤6 months | 4 |
| Central venous catheter use | 3 |
| Advanced stage of cancer | 2 |
| Predisposing risk factors | |
| CV risk factors (at least 2 of: personal history of PAD, ischaemic stroke, CAD, hypertension, hyperlipidaemia, diabetes or obesity) | 5 |
| Recent hospitalisation for acute medical illness | 5 |
| Personal history of VTE | 1 |
| Biomarkers | |
| Platelet count ≥350×109/L | 2 |
*Low/intermediate risk: 0–6; high risk: ≥7.
†For women with hormone receptor-positive breast cancer or on anthracycline treatment.
CAD, coronary artery disease; CAT, cancer-associated thrombosis; CV, cardiovascular; PAD, peripheral artery disease; VTE, venous thromboembolism.
The Khorana risk assessment score, which has been validated in multiple settings and is recommended by the guidelines,39–44 identified five parameters associated with CAT: site of primary cancer; BMI ≥35 kg/m2; pre-chemotherapy leucocyte count >11×109/L; pre-chemotherapy platelet count ≥350×109/L; and haemoglobin <10 g/dL or use of an ESA. Each parameter present is assigned a score of 1 point, except for the site of primary cancer: a score of 2 points is assigned for very high-risk cancers (stomach and pancreas) and 1 point is assigned for high-risk cancers (lung, lymphoma, gynaecological, bladder and testicular). A score of 0 indicates that the patient is at low risk of VTE, a score of 1–2 indicates medium VTE risk and a score of ≥3 indicates high risk (the maximum score is 6); these correspond to symptomatic VTE risks of 0.3%–1.5%, 1.8%–4.8% and 6.7%–12.9%, respectively.62
The Vienna CATS, PROTECHT and CONKO scores have been developed to improve the VTE risk discrimination capabilities of the Khorana score with additional parameters such as biomarker measurements (eg, D-dimer concentration) or type of chemotherapy (platinum-based or gemcitabine-based chemotherapy), or removal and replacement of existing variables (BMI for WHO performance status). A recent prospective multinational cohort analysis comparing the predictive performance of the four scoring systems in patients with advanced cancer who were receiving chemotherapy was conducted.63 The results suggest that the Vienna CATS and PROTECHT scores might be more precise at distinguishing between high-risk and low-risk patients, but require further refinement and validation before they can be introduced into clinical practice.63 This study, however, enrolled patients up to several months after starting systemic therapy for cancer, which can affect many variables included in most risk tools, such as haemoglobin, leucocyte and platelet counts.63
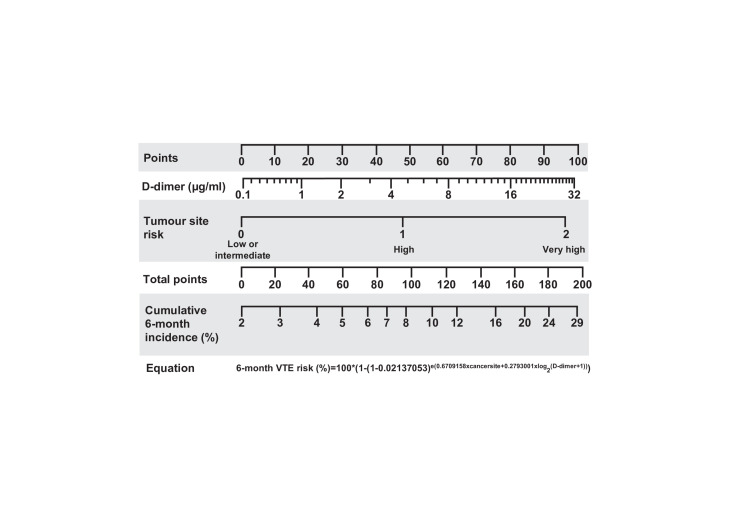
Recently, a clinical prediction model for CAT that includes only the tumour site (the most important component of the Khorana score) and the biomarker D-dimer was developed and externally validated.60 Unlike the Khorana score, which stratified patients into low risk, intermediate risk or high risk for VTE, this simplified model uses a nomogram based on one clinical factor and one biomarker, allowing for more precise and individualised approach to VTE risk assessment.33 60 Compared with the other VTE risk scores, the clinical model showed considerable improvement in the prediction of VTE in ambulatory patients with solid cancers. The model also benefits from a 6-month prediction window, which covers the period this patient population is most likely to develop VTE. This helps identify patients with a 10%–15% risk of developing VTE who may benefit from thromboprophylaxis and protects low-VTE-risk patients from unnecessary bleeding complications. This clinical prediction model is available as a paper-based nomogram (figure 1) and is available as an online risk calculator (http://catscore.meduniwien.ac.at).60
Figure 1.
Nomogram and equation for predicting the 6-month risk of venous thromboembolism (VTE) in ambulatory patients with solid cancer.
Coagulation and cancer are recognised as dynamic processes which can be influenced by patient-related and treatment-related VTE risk factors that change over time (table 1). One shortcoming of the currently available VTE prediction scores is that these are all based on a single measurement at a baseline time point and do not consider the fluctuating VTE risk throughout a patient’s cancer journey.63 64 A recent prospective cohort study looked at whether dynamic predictions of VTE in patients with cancer could be based on individual longitudinal D-dimer trajectories. Results showed that D-dimer levels increased before the onset of VTE but were unchanged in patients with cancer who did not develop VTE. These findings suggest that changes in D-dimer levels over time may improve personalised prediction of CAT by providing dynamic prognostic information on CAT beyond a single D-dimer measurement in time.64
As an example of the practical application of VTE risk scoring systems, the PHACS study applied the Khorana score to study patients with cancer who were receiving ambulatory care and were at high risk of VTE as determined by a Khorana score ≥3 (table 2).50 Patients were randomised to either LMWH (dalteparin) or no prophylactic anticoagulation. However, the study was terminated early owing to low enrolment numbers (117 patients were enrolled). The analysis of the 98 patients who were randomised showed a non-significant reduction in the risk of VTE (12% of patients on dalteparin (n=6/50) vs 21% of patients on observation (n=10/48); center-stratified cause-specific HR 0.69, 95% CI 0.23 to 1.89, p=0.28) and a significant increase in the risk of clinically relevant bleeding (seven patients on dalteparin vs one patient on observation; HR 7.02; 95% CI 1.24 to 131.6; p=0.025). The investigators noted that the study was underpowered due to the enrolment issues, but that it provides some evidence of the usefulness of risk-based approaches in identifying patients at high risk of VTE and a rationale for using VTE risk evaluation in future studies to combat the VTE burden associated with cancer and its treatment. These findings were confirmed when these data were pooled with results from the PROTECHT and SAVE-ONCO studies (RR 0.41; 95% CI 0.22 to 0.78; p=0.006),50 and in a meta-analysis of seven trials investigating LMWH for thromboprophylaxis, when patients with lung cancer were excluded from the analysis.65
In addition to the parameters in these risk evaluation scoring systems, other variables may also help to identify patients with cancer at higher VTE risk. The recent ONCOlogie et Chambres ImPlantables prospective multicentre cohort study in France assessed VTE rates in patients with cancer with implantable intravenous ports.31 The catheter-related risk factors for thrombosis differed from non-catheter-related VTE.
DOACs AND CANCER-ASSOCIATED THROMBOSIS
DOACs are currently approved for the treatment and secondary prevention of VTE in a broad range of patients based on the results of phase III studies.66–73 DOACs offer several advantages over traditional VTE therapies such as LMWH and warfarin, including oral routes of administration, simple dosing regimens, lack of requirements for initial parenteral injections (rivaroxaban and apixaban), lack of need for routine monitoring and dose adjustment, and the limited number of known drug–drug interactions.
Prevention of cancer-associated thrombosis with DOACs
There may be a potential role for the DOACs in the prevention of CAT. Safety of apixaban (5, 10 or 20 mg od) versus placebo was assessed in a phase II pilot trial of 125 patients with metastatic cancer who were receiving chemotherapy.74 Apixaban was well tolerated and was also associated with a VTE incidence of 1.1% vs 13.8% with placebo. However, any benefit of DOACs in patients with cancer must be balanced against the increased risk of bleeding in some tumour types, which highlights the need to identify whom of these patients would benefit most from thromboprophylaxis with DOACs. Two clinical trials of DOACs as thromboprophylaxis in ambulatory cancer populations have been published recently (table 2).
CASSINI
The CASSINI study (NCT02555878) is part of the CALLISTO programme and the results were published in 2019.75 CASSINI was a multinational, randomised, placebo-controlled phase III superiority study comparing the efficacy and safety of rivaroxaban with that of placebo for primary prophylaxis of VTE in ambulatory adult participants with various cancer types and a Khorana score ≥2, who were scheduled for systemic cancer therapy.52 75 A total of 841 patients with no evidence of DVT at screening from over 180 treatment centres in North America and Europe were randomised to rivaroxaban 10 mg od or placebo for up to 6 months. During the study, patients had lower-extremity ultrasound scans every 8 weeks. The VTE primary efficacy endpoint was the composite of objectively confirmed symptomatic or asymptomatic lower-extremity, proximal DVT; symptomatic upper-extremity or distal DVT; symptomatic or incidental PE; and VTE-related death, and the principal safety endpoint was major bleeding. The intention-to-treat analysis up to day 180 of the observation period comprised the primary analysis population, and VTE or VTE-related death occurred in 25 out of 420 patients (6.0%) in the rivaroxaban group compared with 37 out of 421 patients (8.8%) in the placebo group (HR 0.66; 95% CI 0.40 to 1.09; p=0.10).75 In the prespecified on-treatment analysis, VTE or VTE-related death occurred in 11 out of 420 patients (2.6%) in the rivaroxaban group compared with 27 out of 421 (6.4%) in the placebo group (HR 0.40; 95% CI 0.20 to 0.80).75 Total thromboembolic events, including VTE, myocardial infarction, stroke and systemic embolism, were also lower in patients treated with rivaroxaban versus placebo, occurring in 29 out of 420 (6.9%) and 49 out of 421 (11.6%) of patients, respectively (HR 0.57; 95% CI 0.36 to 0.90; p=0.014).76 Overall, 8 out of 405 patients (2.0%) and 4 out of 404 patients (1.0%) in the rivaroxaban and placebo groups, respectively, had a major bleeding event (HR 1.96; 95% CI 0.59 to 6.49; p=0.26).75 Non-major clinically relevant bleeding, adverse events and all-cause mortality were also similar between the rivaroxaban and the placebo groups.75
AVERT
AVERT (NCT02048865) was a randomised, placebo-controlled phase III superiority trial that compared the efficacy and safety of apixaban 2.5 mg two times per day with that of placebo in patients receiving chemotherapy who were at high risk of VTE based on a Khorana score ≥2.53 77 78 The study was conducted in 13 centres in Canada, with an enrolment of 574 patients. The VTE primary efficacy outcome was the first episode of objectively documented symptomatic or incidental VTE during the first 6 months after the initiation of apixaban or placebo. Secondary safety outcomes included major bleeding, clinically relevant non-major bleeding and overall survival.
Of the 574 patients randomised, 563 were included in a modified intention-to-treat analysis. The most common types of primary cancer in patients in AVERT were gynaecological (25.8%), lymphoma (25.3%) and pancreatic (13.6%). VTE was observed in 12 out of 288 patients (4.2%) who received apixaban and 28 out of 275 patients (10.2%) in the placebo group (HR 0.41; 95% CI 0.26 to 0.65; p<0.001). During the on-treatment period, VTE occurred in 3 out of 288 patients (1.0%) in the apixaban group and 20 out of 275 patients (7.3%) in the placebo group (HR 0.14; 95% CI 0.05 to 0.42). In the modified intention-to-treat analysis, there were 10 (3.5%) major bleeding events in the apixaban group and five (1.8%) in the placebo group (HR 2.00; 95% CI 1.01 to 3.95; p=0.046). However, during the on-treatment period, major bleeding occurred in 6 out of 288 patients (2.1%) in the apixaban group and in 3 out of 275 patients (1.1%) in the placebo group (HR 1.89; 95% CI 0.39 to 9.24).53
The findings of CASSINI and AVERT are generally consistent with the earlier SAVE-ONCO and PROTECHT studies of LMWH in mixed cancer populations, which used the on-treatment period for their primary endpoints. However, although the risk reduction observed in CASSINI during the on-treatment period was similar to that in these LMWH studies, and a greater risk reduction was observed in AVERT versus SAVE-ONCO and PROTECHT, the absolute risk reduction was twofold to threefold higher in the DOAC studies (4% for CASSINI and 6.3% for AVERT compared with 2.2% for SAVE-ONCO and 1.9% for PROTECHT). The safety profile of apixaban and rivaroxaban observed in these studies is also likely to be reassuring to physicians, given the low absolute rates of bleeding and similarity to the outcomes in patients who received placebo. However, the differences in the study outcomes (ie, screening for and inclusion of symptomatic and asymptomatic VTE in CASSINI versus symptomatic events only in AVERT), study designs and patient populations must be kept in mind when interpreting the data.75 78 Some key interstudy distinctions to consider include: patients with primary brain cancer or cerebral metastases were excluded from CASSINI but not AVERT; CASSINI also excluded patients with DVT, and all patients were required to undergo a baseline screening ultrasound prior to randomisation to select out individuals with existing thrombosis; results of the AVERT study were based on modified intention-to-treat analysis, including only patients who received at least one dose of study drug.52 53 78 79 Overall, direct comparisons between AVERT and CASSINI should not be made.
Both CASSINI and AVERT have the potential to support the use of the DOACs in thromboprophylaxis in selected patients with cancer at increased risk of VTE.53 75 80 Although both studies incorporated the Khorana score to identify patients at high VTE risk, they used a lower cut-off score (≥2) than in the original validation study (≥3).33 52 53 This lower cut-off score was chosen independently by the CASSINI and AVERT trial investigators based on prospective findings from the 2010 Vienna study, which showed that the 6-month cumulative incidence of VTE in patients with a Khorana score of 2 was nearly 10%, and this was deemed high enough to require thromboprophylaxis.57 Consequently, the latest ASCO clinical guidelines for the treatment of CAT use this trial-specific Khorana score cut-off of ≥2 to define patients with cancer at high risk of VTE.42 The reduced burden associated with the DOACs has the potential to have a substantial positive impact on the daily care of patients with cancer, especially given the limitations around long-term use of LMWH. International guidance has been updated to incorporate these data.41–43
CONCLUSIONS
Cancer is associated with a substantial risk of VTE, and the level of risk is affected by a range of factors including the type and stage of cancer, cancer treatment approach, and the individual patient’s demographic and clinical characteristics. Current guidelines do not recommend routine thromboprophylaxis in all ambulatory patients with cancer, but do advise considering anticoagulation in selected ambulatory patients with cancer and an intermediate-to-high-risk of VTE. For patients who are eligible, LMWH and more recently the DOACs apixaban and rivaroxaban (based on the AVERT and CASSINI trials, respectively) are the recommended options in recently updated guidelines, whereas LMWH, acetylsalicylic acid or warfarin are advised in certain patients with myeloma. Further subgroup and exploratory analyses of the DOAC trials as well as continued refinement of risk-stratification methods may further improve the identification of outpatients at a high risk of VTE that may benefit from thromboprophylaxis. How these developments impact future clinical practice will be of interest to a variety of physicians involved in management of patients with cancer in an ambulatory care setting.
Footnotes
Contributors: All authors contributed to this manuscript and approved the final version.
Funding: Kate Weatherall from Chameleon Communications International provided editorial assistance with the preparation of the manuscript, with funding from Bayer AG, in accordance with Good Publication Practices.
Competing interests: AK has received non-financial support from AngioDynamics, grants from Array, Bristol-Myers Squibb, Merck and Leap, and personal fees from AngioDynamics, Leo Pharma, Medscape, PharmaCyte and TriSalus. AC has received non-financial support from Bayer AG during the conduct of the study, and personal fees from AbbVie, Boehringer Ingelheim, Janssen, Ono Pharmaceuticals and Portola Pharmaceuticals, and grants and personal fees from Bristol-Myers Squibb, Daiichi Sankyo and Pfizer outside the submitted work. MC has received grants from Bayer AG, Bristol-Myers Squibb, Leo Pharma and Octapharma, honoraria from Bayer AG, Boehringer Ingelheim, Leo Pharma and Pfizer, and served on advisory boards for Leo Pharma and Sanofi. GM has received non-financial support from Bayer AG, Bristol-Myers Squibb/Pfizer, Leo Pharma and Stago, and grant support from Bristol-Myers Squibb/Pfizer. IP has received honoraria from Bayer AG, Boehringer Ingelheim, Daiichi Sankyo and Pfizer. PW has received personal fees from Bayer AG, Bristol-Myers Squibb/Pfizer, Itreas, Janssen, Medscape Servier Canada and Sanofi, and grant support fees from Bayer AG and Bristol-Myers Squibb/Pfizer. PK has nothing to disclose.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
References
- 1.Blom JW, Doggen CJM, Osanto S, et al. . Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715–22. 10.1001/jama.293.6.715 [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Mohr DN, Silverstein MD, et al. . Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000;160:761–8. 10.1001/archinte.160.6.761 [DOI] [PubMed] [Google Scholar]
- 3.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 2017;117:219–30. 10.1160/TH16-08-0615 [DOI] [PubMed] [Google Scholar]
- 4.Gao S, Ma J-J, Lu C. Venous thromboembolism risk and erythropoiesis-stimulating agents for the treatment of cancer-associated anemia: a meta-analysis. Tumour Biol 2014;35:603–13. 10.1007/s13277-013-1084-5 [DOI] [PubMed] [Google Scholar]
- 5.Ashrani AA, Gullerud RE, Petterson TM, et al. . Risk factors for incident venous thromboembolism in active cancer patients: a population based case-control study. Thromb Res 2016;139:29–37. 10.1016/j.thromres.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen AT, Katholing A, Rietbrock S, et al. . Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost 2017;117:57–65. 10.1160/TH15-08-0686 [DOI] [PubMed] [Google Scholar]
- 7.Königsbrügge O, Pabinger I, Ay C. Risk factors for venous thromboembolism in cancer: novel findings from the Vienna Cancer and Thrombosis Study (CATS). Thromb Res 2014;133 Suppl 2:S39–43. 10.1016/S0049-3848(14)50007-2 [DOI] [PubMed] [Google Scholar]
- 8.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood 2013;122:2011–8. 10.1182/blood-2013-04-460147 [DOI] [PubMed] [Google Scholar]
- 9.Mauracher L-M, Posch F, Martinod K, et al. . Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost 2018;16:508–18. 10.1111/jth.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017;130:1499–506. 10.1182/blood-2017-03-743211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir Seyed Nazari P, Marosi C, Moik F, et al. . Low systemic levels of chemokine C-C motif ligand 3 (CCL3) are associated with a high risk of venous thromboembolism in patients with glioma. Cancers 2019;11. 10.3390/cancers11122020. [Epub ahead of print: 14 Dec 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posch F, Thaler J, Zlabinger G-J, et al. . Soluble vascular endothelial growth factor (sVEGF) and the risk of venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study (CATS). Clin Cancer Res 2016;22:200–6. 10.1158/1078-0432.CCR-14-3358 [DOI] [PubMed] [Google Scholar]
- 13.Brandes AA, Scelzi E, Salmistraro G, et al. . Incidence and risk of thromboembolism during treatment of high-grade gliomas: a prospective study. Eur J Cancer 1997;33:1592–6. 10.1016/S0959-8049(97)00167-6 [DOI] [PubMed] [Google Scholar]
- 14.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med 2012;9:e1001275. 10.1371/journal.pmed.1001275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickmann B, Ahlbrecht J, Ay C, et al. . Regional lymph node metastases are a strong risk factor for venous thromboembolism: results from the Vienna Cancer and Thrombosis Study. Haematologica 2013;98:1309–14. 10.3324/haematol.2012.073338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahlbrecht J, Dickmann B, Ay C, et al. . Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 2012;30:3870–5. 10.1200/JCO.2011.40.1810 [DOI] [PubMed] [Google Scholar]
- 17.Kuzel T, Esparaz B, Green D, et al. . Thrombogenicity of intravenous 5-fluorouracil alone or in combination with cisplatin. Cancer 1990;65:885–9. [DOI] [PubMed] [Google Scholar]
- 18.Levine MN, Gent M, Hirsh J, et al. . The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N Engl J Med 1988;318:404–7. 10.1056/NEJM198802183180703 [DOI] [PubMed] [Google Scholar]
- 19.Chow AY, Chin C, Dahl G, et al. . Anthracyclines cause endothelial injury in pediatric cancer patients: a pilot study. J Clin Oncol 2006;24:925–8. 10.1200/JCO.2005.03.5956 [DOI] [PubMed] [Google Scholar]
- 20.de Vos FYFL, Willemse PHB, de Vries EGE, et al. . Endothelial cell effects of cytotoxics: balance between desired and unwanted effects. Cancer Treat Rev 2004;30:495–513. 10.1016/j.ctrv.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Heit JA, Silverstein MD, Mohr DN, et al. . Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809–15. 10.1001/archinte.160.6.809 [DOI] [PubMed] [Google Scholar]
- 22.Hernandez RK, Sørensen HT, Pedersen L, et al. . Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer 2009;115:4442–9. 10.1002/cncr.24508 [DOI] [PubMed] [Google Scholar]
- 23.Pritchard KI, Paterson AH, Paul NA, et al. . Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol 1996;14:2731–7. 10.1200/JCO.1996.14.10.2731 [DOI] [PubMed] [Google Scholar]
- 24.Carrier M, Le Gal G, Tay J, et al. . Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost 2011;9:653–63. 10.1111/j.1538-7836.2011.04215.x [DOI] [PubMed] [Google Scholar]
- 25.Mandalà M, Clerici M, Corradino I, et al. . Incidence, risk factors and clinical implications of venous thromboembolism in cancer patients treated within the context of phase I studies: the 'SENDO experience'. Ann Oncol 2012;23:1416–21. 10.1093/annonc/mdr524 [DOI] [PubMed] [Google Scholar]
- 26.Zangari M, Siegel E, Barlogie B, et al. . Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: implications for therapy. Blood 2002;100:1168–71. 10.1182/blood-2002-01-0335 [DOI] [PubMed] [Google Scholar]
- 27.Nalluri SR, Chu D, Keresztes R, et al. . Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300:2277–85. 10.1001/jama.2008.656 [DOI] [PubMed] [Google Scholar]
- 28.Dimopoulos MA, Leleu X, Palumbo A, et al. . Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia 2014;28:1573–85. 10.1038/leu.2014.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Guo Q, Hu W, Incidence HW. Incidence, risk factors, and outcomes of venous thromboembolism after oncologic surgery: a systematic review and meta-analysis. Thromb Res 2019;173:48–56. 10.1016/j.thromres.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 30.Zöller B, Palmer K, Li X, et al. . Family history of venous thromboembolism and risk of hospitalized thromboembolism in cancer patients: a nationwide family study. Thromb Res 2015;136:573–81. 10.1016/j.thromres.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 31.Decousus H, Bourmaud A, Fournel P, et al. . Cancer-associated thrombosis in patients with implanted ports: a prospective multicenter French cohort study (ONCOCIP). Blood 2018;132:707–16. 10.1182/blood-2018-03-837153 [DOI] [PubMed] [Google Scholar]
- 32.Pabinger I, Ay C, Dunkler D, et al. . Factor V Leiden mutation increases the risk for venous thromboembolism in cancer patients - results from the Vienna Cancer And Thrombosis Study (CATS). J Thromb Haemost 2015;13:17–22. 10.1111/jth.12778 [DOI] [PubMed] [Google Scholar]
- 33.Khorana AA, Kuderer NM, Culakova E, et al. . Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902–7. 10.1182/blood-2007-10-116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simanek R, Vormittag R, Ay C, et al. . High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost 2010;8:114–20. 10.1111/j.1538-7836.2009.03680.x [DOI] [PubMed] [Google Scholar]
- 35.Khorana AA, Francis CW, Culakova E, et al. . Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104:2822–9. 10.1002/cncr.21496 [DOI] [PubMed] [Google Scholar]
- 36.Ay C, Simanek R, Vormittag R, et al. . High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008;112:2703–8. 10.1182/blood-2008-02-142422 [DOI] [PubMed] [Google Scholar]
- 37.Ay C, Vormittag R, Dunkler D, et al. . D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 2009;27:4124–9. 10.1200/JCO.2008.21.7752 [DOI] [PubMed] [Google Scholar]
- 38.Vormittag R, Simanek R, Ay C, et al. . High factor VIII levels independently predict venous thromboembolism in cancer patients: the Cancer and Thrombosis Study. Arterioscler Thromb Vasc Biol 2009;29:2176–81. 10.1161/ATVBAHA.109.190827 [DOI] [PubMed] [Google Scholar]
- 39.Watson HG, Keeling DM, Laffan M, et al. . Guideline on aspects of cancer-related venous thrombosis. Br J Haematol 2015;170:640–8. 10.1111/bjh.13556 [DOI] [PubMed] [Google Scholar]
- 40.Mandalà M, Falanga A, Roila F, et al. . Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22:vi85–92. 10.1093/annonc/mdr392 [DOI] [PubMed] [Google Scholar]
- 41.Farge D, Frere C, Connors JM, et al. . 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2019;20:e566–81. 10.1016/S1470-2045(19)30336-5 [DOI] [PubMed] [Google Scholar]
- 42.Key NS, Khorana AA, Kuderer NM, et al. . Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2020;38:496–520. 10.1200/JCO.19.01461 [DOI] [PubMed] [Google Scholar]
- 43.Wang T-F, Zwicker JI, Ay C, et al. . The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost 2019;17:1772–8. 10.1111/jth.14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Comprehensive Cancer Network Cancer-associated venous thromboembolic disease, version 1.2020. National Comprehensive Cancer Network, Inc, 2020. Available: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf [Accessed 6 May 2020]. [DOI] [PubMed]
- 45.Agnelli G, Gussoni G, Bianchini C, et al. . Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 2009;10:943–9. 10.1016/S1470-2045(09)70232-3 [DOI] [PubMed] [Google Scholar]
- 46.Agnelli G, George DJ, Kakkar AK, et al. . Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601–9. 10.1056/NEJMoa1108898 [DOI] [PubMed] [Google Scholar]
- 47.Haas SK, Freund M, Heigener D, et al. . Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost 2012;18:159–65. 10.1177/1076029611433769 [DOI] [PubMed] [Google Scholar]
- 48.Maraveyas A, Waters J, Roy R, et al. . Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012;48:1283–92. 10.1016/j.ejca.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 49.Pelzer U, Opitz B, Deutschinoff G, et al. . Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol 2015;33:2028–34. 10.1200/JCO.2014.55.1481 [DOI] [PubMed] [Google Scholar]
- 50.Khorana AA, Francis CW, Kuderer NM, et al. . Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res 2017;151:89–95. 10.1016/j.thromres.2017.01.009 [DOI] [PubMed] [Google Scholar]
- 51.Khorana AA, Soff GA, Kakkar AK, et al. . Rivaroxaban thromboprophylaxis in high-risk ambulatory cancer patients receiving systemic therapy: results of a randomized clinical trial (CASSINI). Blood 2018;132:LBA-1 10.1182/blood-2018-120738 [DOI] [Google Scholar]
- 52.Khorana AA, Vadhan-Raj S, Kuderer NM, et al. . Rivaroxaban for preventing venous thromboembolism in high-risk ambulatory patients with cancer: rationale and design of the CASSINI trial. Thromb Haemost 2017;117:2135–45. 10.1160/TH17-03-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimpton M, Wells PS, Carrier M. Apixaban for the prevention of venous thromboembolism in high-risk ambulatory cancer patients receiving chemotherapy: rational and design of the AVERT trial. Thromb Res 2018;164 Suppl 1:S124–9. 10.1016/j.thromres.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 54.Gajic-Veljanoski O, Phua CW, Shah PS, et al. . Effects of long-term low-molecular-weight heparin on fractures and bone density in non-pregnant adults: a systematic review with meta-analysis. J Gen Intern Med 2016;31:947–57. 10.1007/s11606-016-3603-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005;106:401–7. 10.1182/blood-2005-02-0626 [DOI] [PubMed] [Google Scholar]
- 56.Rodger MA, Hague WM, Kingdom J, et al. . Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet 2014;384:1673–83. 10.1016/S0140-6736(14)60793-5 [DOI] [PubMed] [Google Scholar]
- 57.Ay C, Dunkler D, Marosi C, et al. . Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377–82. 10.1182/blood-2010-02-270116 [DOI] [PubMed] [Google Scholar]
- 58.Verso M, Agnelli G, Barni S, et al. . A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med 2012;7:291–2. 10.1007/s11739-012-0784-y [DOI] [PubMed] [Google Scholar]
- 59.Pelzer U, Sinn M, Stieler J, et al. . [Primary pharmacological prevention of thromboembolic events in ambulatory patients with advanced pancreatic cancer treated with chemotherapy]. Dtsch Med Wochenschr 2013;138:2084–8. 10.1055/s-0033-1349608 [DOI] [PubMed] [Google Scholar]
- 60.Pabinger I, van Es N, Heinze G, et al. . A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5:e289–98. 10.1016/S2352-3026(18)30063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerotziafas GT, Taher A, Abdel-Razeq H, et al. . A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: the prospective COMPASS-Cancer-Associated Thrombosis study. Oncologist 2017;22:1222–31. 10.1634/theoncologist.2016-0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khorana AA. Cancer and coagulation. Am J Hematol 2012;87 Suppl 1:S82–7. 10.1002/ajh.23143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Es N, Di Nisio M, Cesarman G, et al. . Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica 2017;102:1494–501. 10.3324/haematol.2017.169060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Posch F, Riedl J, Reitter E-M, et al. . Dynamic assessment of venous thromboembolism risk in patients with cancer by longitudinal D-dimer analysis: a prospective study. J Thromb Haemost 2020;18:1348–56. 10.1111/jth.14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Es N, Ventresca M, Di Nisio M, et al. . The Khorana score for prediction of venous thromboembolism in cancer patients: an individual patient data meta-analysis. J Thromb Haemost 2020;18:1940–51. 10.1111/jth.14824 [DOI] [PubMed] [Google Scholar]
- 66.Schulman S, Kearon C, Kakkar AK, et al. . Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–52. 10.1056/NEJMoa0906598 [DOI] [PubMed] [Google Scholar]
- 67.The EINSTEIN Investigators Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. 10.1056/NEJMoa1007903 [DOI] [PubMed] [Google Scholar]
- 68.The EINSTEIN–PE Investigators Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. 10.1056/NEJMoa1113572 [DOI] [PubMed] [Google Scholar]
- 69.Agnelli G, Buller HR, Cohen A, et al. . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. 10.1056/NEJMoa1302507 [DOI] [PubMed] [Google Scholar]
- 70.Agnelli G, Buller HR, Cohen A, et al. . Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708. 10.1056/NEJMoa1207541 [DOI] [PubMed] [Google Scholar]
- 71.Weitz JI, Lensing AWA, Prins MH, et al. . Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017;376:1211–22. 10.1056/NEJMoa1700518 [DOI] [PubMed] [Google Scholar]
- 72.The Hokusai-VTE Investigators Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15. 10.1056/NEJMoa1306638 [DOI] [PubMed] [Google Scholar]
- 73.Schulman S, Kearon C, Kakkar AK, et al. . Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18. 10.1056/NEJMoa1113697 [DOI] [PubMed] [Google Scholar]
- 74.Levine MN, Gu C, Liebman HA, et al. . A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost 2012;10:807–14. 10.1111/j.1538-7836.2012.04693.x [DOI] [PubMed] [Google Scholar]
- 75.Khorana AA, Soff GA, Kakkar AK, et al. . Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 2019;380:720–8. 10.1056/NEJMoa1814630 [DOI] [PubMed] [Google Scholar]
- 76.Khorana AA, McNamara MG, Kakkar AK, et al. . Assessing full benefit of rivaroxaban prophylaxis in high-risk ambulatory patients with cancer: thromboembolic events in the randomized CASSINI trial. TH Open 2020;4:e107–12. 10.1055/s-0040-1712143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parker A, Peterson E, Lee AYY, et al. . Risk stratification for the development of venous thromboembolism in hospitalized patients with cancer. J Thromb Haemost 2018;16:1321–6. 10.1111/jth.14139 [DOI] [PubMed] [Google Scholar]
- 78.Carrier M, Abou-Nassar K, Mallick R, et al. . Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711–9. 10.1056/NEJMoa1814468 [DOI] [PubMed] [Google Scholar]
- 79.Rossel A, Robert-Ebadi H, Marti C. Preventing venous thromboembolism in ambulatory patients with cancer: a narrative review. Cancers 2020;12:612. 10.3390/cancers12030612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agnelli G. Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N Engl J Med 2019;380:781–3. 10.1056/NEJMe1816060 [DOI] [PubMed] [Google Scholar]