Abstract
Introduction
Pancreatic cancer (PC), even in the absence of metastatic disease, has a dismal prognosis. One-third of them are borderline resectable (BRPC) or locally advanced unresectable PC (LAUPC) at diagnosis. There are limited prospective data supporting the best approach on these tumours. Neoadjuvant chemotherapy (ChT) is being increasingly used in this setting.
Methods
This is a retrospective series of consecutive patients staged as BRPC or LAUPC after discussion in the multidisciplinary board (MDB) at an academic centre. All received neoadjuvant ChT, followed by chemoradiation (ChRT) in some cases, and those achieving enough downstaging had a curative-intent surgery. Descriptive data about patient’s characteristics, neoadjuvant treatments, toxicities, curative resections, postoperative complications, pathology reports and adjuvant treatment were collected. Overall survival (OS) and progression-free survival was calculated with Kaplan-Meier method and log-rank test.
Results
Between August 2011 and July 2019, 49 patients fulfilled the inclusion criteria, and all of them received neoadjuvant ChT. Fluorouracil+folinic acid, irinotecan and oxaliplatin was the most frequently used scheme (77%). The most prevalent grade 3 or 4 toxicities were neutropenia (26.5%), neurotoxicity (12.2%), diarrhoea (8.2%) and nausea (8.2%). 18 patients (36.7%) received ChRT thereafter. In total, 22 patients (44,9%) became potentially resectable and 19 of them had an R0 or R1 pancreatic resection. One was found to be unresectable at surgery and two refused surgery. A vascular resection was required in 7 (35%). No postoperative deaths were observed. Postoperative ChT was given to 12 (66.7%) of resected patients. Median OS of the whole cohort was 24,9 months (95% CI 14.1 to 35.7), with 30.6 months for resected and 13.1 months for non-resected patients, respectively (p<0.001).
Conclusion
A neoadjuvant approach in BRPC and LAUPC was well tolerated and allowed a curative resection in 38.8% of them with a potential improvement on OS.
Keywords: borderline resectable, locally advanced unresectable, pancreatic cancer, neoadjuvant therapy, FOLFIRINOX
Key questions.
What is already known about this subject?
Neoadjuvant chemotherapy and chemoradiation are extensively used in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma, aiming at downsizing the primary tumour and favouring a curative-intent surgery. However, prospective data from randomised trials are scarce on this setting.
What does this study add?
This study presents a consecutive cohort from a dedicated academic institution in the management of pancreatic cancer, where a multidisciplinary approach with neoadjuvant chemotherapy plus chemoradiation in some cases allowed a shift to a potential resectability in 44.9% of them with a curative resection rate of almost 40%.
How might this impact on clinical practice?
This work confirms that fluorouracil+folinic acid, irinotecan and oxaliplatin or gemcitabine plus nab-paclitaxel, widely used on metastatic pancreatic cancer, are tolerable with manageable toxicities. They induce tumour downstaging and a complete tumour resection in some cases, potentially leading to an improvement in overall survival.
Introduction
Pancreatic cancer (PC) is characterised by a high lethality and a poor prognosis with a 5-year survival rate of less than 6%, being the seventh leading cause of cancer death worldwide.1 Only 15%–20% of patients present with localised disease susceptible to surgical resection. The radicality of surgery is a major prognostic factor with a marked increase in the chance of long-term survival for patients who underwent radical surgery.2 Free resection margins are associated with better locorregional control of the disease, although its impact on overall survival (OS) is controversial.3
Locally advanced, non-metastatic PC occurs in approximately 35% of newly diagnosed patients and includes borderline resectable (BRPC) and locally advanced unresectable pancreatic cancer (LAUPC).4 BRPC and LAUPC are defined according to anatomic, biological or conditional criteria.5 BRPC represents a major risk for positive margin resection due to close proximity or involvement of major vascular structures. In this regard, preoperative therapy would represent a valuable strategy for downsizing.6 LAUPCs are the majority of these localised tumours in which conversion to resectability would be the ultimate goal of treatment, but unfortunately many will not achieve enough downstaging. In those cases, extending survival preserving quality of life will be the main goal.
Despite the importance of accurately classifying a localised PC according to those definitions, a uniformly accepted set of criteria that define patients with BRPC or LAUPC does not exist. This subdivision has been defined based on vascular involvement, specifically of the venous (particularly portal vein or upper mesenteric vein) and arterial (upper mesenteric artery or branches of coeliac tripod) vascular system. This classification was made in accordance with the National Comprehensive Cancer Network (NCCN), the joint consensus conference of the Americas Hepato-Pancreato-Biliary Association, the Society of Surgical Oncology and the Society for Surgery of the Alimentary Tract.7 8 These patients are at an increased risk of non-radical surgical resection and therefore would be candidates for chemotherapy (ChT) with or without radiotherapy (RT).9
In recent years, the medical treatment of PC has improved considerably with some regimens that showed greater activity than conventional ones. In particular, a combination of 5-fluorouracil+folinic acid, irinotecan and oxaliplatin (FOLFIRINOX) demonstrated an increase in median survival and an objective response rate of over 30%.10 On the other hand, the combination of gemcitabine and nab-paclitaxel also demonstrated better outcomes in patients with metastatic disease.11 These combinations of drugs have been used in locally advanced disease aiming at improving downstaging, and therefore, increasing the radical resection rate. The role of RT on this setting is controversial, but it may contribute to better locoregional control.
Based on these considerations, we conducted a single-centre retrospective analysis, within a multidisciplinary pancreatic-biliary group, to evaluate the clinical outcomes of conversion surgery after neoadjuvant treatment (NAT) in patients with BRPC and LAUPC and this paper reports our findings.
Methods
Patient population
Retrospective study with a series of consecutive patients from the Hospital Clínico Universitario of Valencia, Spain, an academic centre. All were diagnosed of pancreatic adenocarcinoma with confirmed histological diagnosis by endoscopic ultrasonography-guided fine-needle aspiration, endoscopic retrograde cholangiopancreatography or percutaneous biopsy. All these patients should have been staged as BRPC or LAUPC based on CT staging after discussion in the multisciplinary board (MDB), and an NAT was offered to all of them with the intention of obtaining enough downstaging to convert to resectable disease. Patients were identified through the MDB reports and data were obtained from the medical records. The exclusion criteria were: resectable PC, metastatic disease, coexisting comorbidities that contraindicated an NAT or concomitant diagnosis of cancer in the past 5 years. The study cut-off date was March 2020. All patients provided a signed informed consent.
Staging
A contrast-enhanced CT was performed in all the cases, and a portal venous, arterial and pancreatic phases using a pancreatic protocol was performed in some. Several radiologists with expertise on PC staging reviewed the images in the MDB meeting stablishing the diagnosis of BRPC/LAUPC according to the published definitions. Two dedicated radiologists reviewed the baseline CT scans to confirm locoregional staging according to the NCCN criteria. Details on vascular involvement (venous, arterial or both) and degrees of vascular involvement were recorded. Online supplemental table 1 shows the proforma used on the review of the CT scans.
esmoopen-2020-000929supp001.pdf (43KB, pdf)
Chemotherapy
FOLFIRINOX and gemcitabine plus nab-paclitaxel were the ChT schedules of choice in most of the cases. After 2–3 months of treatment, the patients had a first assessment of response with a CT scan and were discussed in the MDB meeting. When potentially resectable, patients were sent to surgery. If considered unresectable, they went on with ChT and every 2–3 months a new CT evaluation with MDB discussion was done. After surgery, some patients received adjuvant ChT. All treatment-emergent adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events V.4.03.12
Radiotherapy
After approximately 6 months of ChT, if yet considered unresectable, some cases received long-course chemoradiation (ChRT) over the lymph nodes in fractions of 180 cGy/day until 45 Gy and over the pancreatic tumour with a total dose of 50–54 Gy in fractions of 200 cGy/day, concomitant with oral ChT (capecitabine).
Surgery
Some patients were considered potentially resectable after achieving tumour downstaging. The surgical technique performed depended on the location and extension of the pancreatic tumour, and a vascular resection and reconstruction was needed in some cases. A team of dedicated pancreato-biliary surgeons performed surgery 6–8 weeks after ending Ch(RT).
Pathology report
The surgical specimen was evaluated by a dedicated pathologist according to a standard protocol.13–15 A proforma report was used to favour a systematic and complete data collection. The pathology report was elaborated on the criteria of the seventh edition of the TNM classification. Information regarding the involvement of resection margins, presence of lymphovascular and/or perineural invasion and degree of regression was also reported. An R0 resection was defined as a complete tumour resection with negative margins (more than 1 mm), and an R1 resection when there was a microscopic involvement of the resection margin at less or equal than 1 mm. The tumour regression grade was reported according to the modified Ryan classification.16
Outcomes
The patients were followed to detect relapses (in those resected) or until death or lost to follow-up. Progression-free survival (PFS) of all the cohort was defined as the time from the diagnosis to the date of progression or death due to any cause. Non-progressing patients were censored at the date of the last follow-up visit. OS of all the cohort was defined as the time from the diagnosis to the date of death due to any cause. Patients alive were censored at the date of the last follow-up visit.
Statistical analysis
The statistical analysis was performed with the software IBM SPSS Statistics for Windows, V.23.0. Since this was a retrospective analysis, no formal statistical assumption was carried out. Patients with missing data were excluded from the analysis. No variables in the datasets exceeded 5% of missing values.
All descriptive analyses were expressed as number and percentage on categorical variables, and median and range on numerical variables. The mean survival time was calculated by Kaplan-Meier analysis, as well as the comparison of survival between groups using the log-rank test, considering a p≤0.05 statistically significant.
Results
Between August 2011 and July 2019, 49 consecutive patients fulfilled the inclusion criteria of BRPC or LAUPC and had no contraindication to receive neoadjuvant ChT. Online supplemental figure 1 shows the flow chart of the selection process. Table 1 summarises patient’s characteristics. Median age was 59 and more than 90% had an ECOG performance status (PS) of 0 or 1. The majority of tumours were located in the head of the pancreas and about 40% of the patients required the placement of a biliary stent. A contrast-enhanced CT scan determined only venous involvement in 31.8% of cases, only arterial involvement in 4.5% and both venous and arterial in the remaining 56.8%. In one case, a neoadjuvant approach was recommended due to an infiltration of duodenum.
Table 1.
Patient characteristics (n:49)
| Gender (N%) | |
| Female | 25 (51%) |
| Male | 24 (49%) |
| Median age years (range) |
59 (33–78) |
| ECOG performance status (PS) N (%) | |
| PS 0 | 11 (22.4%) |
| PS 1 | 34 (69.4%) |
| PS 2 | 4 (8.2%) |
| Histology N (%) | |
| Adenocarcinoma | 47 (96%) |
| Mucinous Adc. | 1 (2%) |
| Poorly dif. Adc. | 1 (2%) |
| Grade N (%) | |
| Unknown | 45 (91.8%) |
| G1 | 0 |
| G2 | 2 (4.1%) |
| G3 | 2 (4.1%) |
| Location N (%) | |
| Head/ UP | 29 (63.2%) |
| Neck/Body | 17 (34.8%) |
| Tail | 1 (2%) |
| Ca 19.9 (U/L) Median (range) |
171.5 (1–16404) |
| Albumin (mg/dL) Median (range) |
4.0 (2.2–4.7) |
| Haemoglobin (g/dL) Median (range) |
12.4 (7.2–15.5) |
| Biliary stent N (%) | |
| No | 28 (57.1%) |
| Yes | 20 (40.8%) |
| Unknown | 1 (2%) |
| Imaging technique used for staging N (%) | |
| CT-scan | 49 (100%) |
| MRI | 7 (14.3%) |
| Endoscopic US | 41 (83.7 %) |
| Staging after review* N (%) | |
| Resectable | 4 (8.2%) |
| Borderline | 19 (38.8%) |
| Locally advanced | 21 (42.9%) |
| Unknown (no CT available) | 5 (10.2%) |
| Vascular involvement N (%) | |
| No vascular infiltration | 3 (6.8%) |
| Venous infiltration | 14 (31.8%) |
| Arterial infiltration | 2 (4.5%) |
| Both venous and arterial infiltration | 25 (56.8%) |
*Available CTs from the diagnosis were retrospectively reviewed by two radiologists according to the NCCN criteria.
Adc, adenocarcinoma; BRPC, borderline pancreatic cancer; Dif, differentiated; ECOG, Eastern Cooperative Oncology Group; LAUPC, locally advanced unresectable pancreatic cancer; UP, uncinated process; US, ultrasonography.
esmoopen-2020-000929supp002.pdf (419.1KB, pdf)
FOLFIRINOX was used in 38 patients (77.6%) and gemcitabine/nab-paclitaxel in 10 (20.4%). Table 2 shows the characteristics and toxicities of NAT. Neoadjuvant ChT was well tolerated. A reduced dose was required in 34 patients (69.4%) at start or during ChT, and 27 patients (55.1%) received granulocyte-colony stimulating factors support. The most prevalent grade 3 or 4 toxicities were neutropenia (14.3% and 12.2%, respectively) and asthenia (6.1% and 2%, respectively). Moreover, ChRT was given to 18 patients (36.7%).
Table 2.
Neoadjuvant treatment: characteristics and related toxicities (n:49)
| ChT N (%) | ||||
| FOLFIRINOX | 38 (77.6%) | |||
| Gemcitabine+nab-paclitaxel | 10 (20.4%) | |||
| Gemcitabine | 1 (2%) | |||
| Reduced dose N (%) | ||||
| Yes* | 34 (69.4%) | |||
| No | 15 (30.6%) | |||
| G-CSF N(%) | ||||
| Yes | 27 (55.1%) | |||
| No | 22 (44.9%) | |||
| Number cycles of ChT Median (range) | ||||
| FOLFIRINOX | 8 (2–21) | |||
| Gemcitabine + abraxane | 5 (1–11) | |||
| ChRT N (%) | ||||
| No | 31 (63.3%) | |||
| Yes | 18 (36.7%) | |||
| Toxicity during (n:49) of ChT N (%) | ||||
| Yes | 45 (91.8%) | |||
| No | 4 (8.2%) | |||
| Toxicities on ChT | ||||
| Grade† | ||||
| No | G1 | G2 | G3 | G4 |
| Neutropenia N (%) | ||||
| 24 (49%) | 3 (6.1%) | 9 (18.4%) | 7 (14.3%) | 6 (12.2%) |
| Asthenia N (%) | ||||
| 15 (30.6%) | 8 (16.3%) | 22 (44.9%) | 3 (6.1%) | 1 (2%) |
| Neurotoxicity N (%) | ||||
| 19 (38.8%) | 16 (32.7%) | 8 (16.3%) | 6 (12.2%) | 0 |
| Diarrhoea N (%) | ||||
| 27 (55.1%) | 7 (14.3%) | 11 (22.4%) | 4 (8.2%) | 0 |
| Nausea N (%) | ||||
| 26 (53.1%) | 9 (18.4%) | 10 (20.4%) | 4 (8.2%) | 0 |
| Thrombopenia N (%) | ||||
| 42 (85.7%) | 3 (6.1%) | 1 (2%) | 3 (6.1%) | 0 |
| Hepatotoxicity N (%) | ||||
| 40 (81.6%) | 4 (8.2%) | 3 (6.1%) | 2 (4.1%) | 0 |
| Anaemia N (%) | ||||
| 42 (85.7%) | 2 (4.1%) | 3 (6.1%) | 2 (4.1%) | 0 |
| Fever N (%) | ||||
| No | 37 (75.5%) | |||
| Yes | 12 (24.5%) | |||
*11 patients (22.4%) ChT dose reduced from the beginning/23 patients (47%) ChT dose reducedon the course of treatment
†All treatment-emergent adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events V.4.0
ChRT, chemoradiation; ChT, chemotherapy; FOLFIRINOX, fluorouracil+folinic acid, irinotecan and oxaliplatin; G-CSF, granulocyte-colony stimulating factor.
Overall, after NAT with or without ChRT, 22 patients (44.9%) were considered potentially resectable. Two refused the surgery. One had only an exploratory laparotomy due to unresectable tumour. However, in 19 (38.8%) an R0 or R1 pancreatic resection was achieved. No postoperative deaths were observed and 5 (25%) resected patients had some postoperative complications. Adjuvant ChT was given to 12 (63.5%) of them. Table 3 summarises some details of surgical techniques, morbidity and adjuvant treatment, as well as the pathology report details. The pathology assessment of surgical specimens showed a complete response in one case (5.3%) and a moderate response in four cases (21.1%). Thirteen patients (68.4%) had ypT1 or ypT2 tumours and 10 patients (52.6%) were ypN0. An R0 resection was achieved in 9 (47.4%).
Table 3.
Details on surgery, pathological outcomes and adjuvant treatment in resected patients
| Resectability (n:49) | |
| Yes | 20 (40.8%) |
| No | 26 (53.1%) |
| Patients refused surgery | 2 (4.1%) |
| Other* | 1 (2%) |
| Reasons for unresectability (n:26) | |
| Progressive disease | 15 (57.7%) |
| Persistence of vascular involvement | 9 (34.8%) |
| Toxicity | 2 (7.7%) |
| Surgical procedure (n:20) | |
| Whipple | 11 (55%) |
| Total pancreatectomy | 6 (30%) |
| Distal pancreatectomy | 2 (10%) |
| Exploratory laparotomy | 1 (5%) |
| Vascular resection (n:19) | |
| No | 12 (63.2%) |
| Yes | 7 (36.8%) |
| Surgical morbidity (n:20) | |
| No | 15 (75%) |
| Yes | 5 (25%) |
| Pathology assessment of surgical specimens (n:19) | |
| Histology N (%) | |
| Ductal Adc. | 17 (89.5%) |
| Mucinous | 1 (5.3%) |
| ypT0 ypN0 | 1 (5.3%) |
| Grade N (%) | |
| G1 | 6 (31.6%) |
| G2 | 8 (42.1%) |
| G3 | 4 (21.1%) |
| NA | 1 (5.3%) |
| ypT N (%) | |
| T1 | 7 (36.8%) |
| T2 | 6 (31.6%) |
| T3 | 2 (10.5%) |
| T4 | 3 (15.8%) |
| T0 | 1 (5.3%) |
| ypN N (%) | |
| N0 | 11 (57.9%) |
| N1 | 6 (31.6%) |
| N2 | 2 (10.5%) |
| Tumour regression grade† N (%) | |
| 0 (pCR) | 1 (5.3%) |
| 1 (Moderate) | 4 (21.1%) |
| 2 (Minimum) | 5 (26.3%) |
| 3 (Poor) | 9 (47.4%) |
| Vascular/lymphatic/perineural invasion N (%) | |
| Yes | 14 (73.7%) |
| No | 5 (26.3%) |
| Involvement of surgical margin R1 N (%) | |
| Yes | 9 (47.4%) |
| No | 9 (47.4%) |
| Not reported | 1 (5.3%) |
| Adjuvant ChT (n:19) | |
| No | 7 (36.8%) |
| Yes: FOLFIRINOX: 2 (16.7%) Gemcitabine/capecitabine: 4 (33.3%) Gemcitabine: 6 (50%) |
12 (63.2%) |
*Not assessed due to clinical deterioration.
†Modified Ryan Scheme for Tumour Regression Score.11
Adc, adenocarcinoma; ChT, chemotherapy; FOLFIRINOX, fluorouracil+folinic acid, irinotecan and oxaliplatin; pCR, pathological complete response.
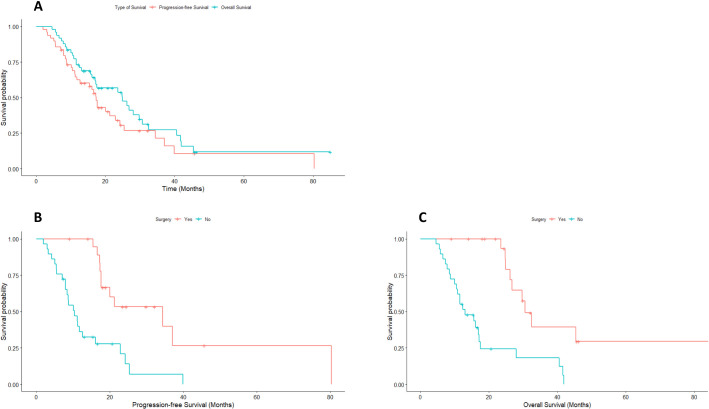
A relapse during follow-up was seen in 10/19 of the resected patients (52.6%). Location of relapses was locoregional, lymphonodal, and hepatic in three patients, peritoneal in two cases and pulmonary in one patient. At the time of the analysis, with a median follow-up of 45.7 months, 35 patients progressed and 33 died. The median PFS of the whole cohort was 17.4 months (95% CI 15.6 to 19.2) and 22% of them did not progress 3 years after diagnosis. When comparing resected with non-resected patients, median PFS was 34.4 months and 10.4 months, respectively (p<0.001). The median OS of the whole cohort was 24.9 months (95% CI 14.1 to 35.7), and 28% of them were alive 3 years after diagnosis. When comparing resected with non-resected patients, median OS was 30.6 months and 13.1 months, respectively (p<0.001). Figure 1A shows the Kaplan-Meier PFS and OS curves of the whole cohort. Figure 1B, C shows the Kaplan-Meier curves of PFS and OS, comparing resected and non-resected patients.
Figure 1.
(A) Kaplan-Meier curves showing progression-free survival (PFS) and overall survival (OS) for the whole cohort. (B) Kaplan-Meier curves of PFS and (C) OS, comparing resected and non-resected patients.
Table 4 shows the results of a univariable analyses of some relevant clinical or treatment characteristics with PFS and OS. Of note, only surgery did impact on both PFS (HR 4.0; p:<0.001) and OS (HR 4.96; p:<0.001) Due to the limited number of cases, a multivariable analysis was not performed.
Table 4.
Univariate Cox regression analyses for the association between PFS and OS and clinical characteristics
| Variables | PFS | OS | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex (female vs male) | 1.86 | 0.93 to 3.73 | NS | 1.29 | 0.64 to 2.61 | NS |
| Age (≤60 vs>60 years) | 0.85 | 0.43 to 1.69 | NS | 1.12 | 0.57 to 2.22 | NS |
| PS (0 vs 1, 2) | 1.37 | 0.32 to 5.77 | NS | 2.32 | 0.68 to 7.92 | NS |
| Tumour location (head/UP vs body/tail) | 0.79 | 0.39 to 1.62 | NS | 0.89 | 0.44 to 1.81 | NS |
| Staging | ||||||
| Resectable | 1 | 1 | ||||
| BRPC | 2.05 | 0.27 to 15.9 | NS | 1.92 | 0.25 to 14.85 | NS |
| LAUPC | 2.0 | 0.26 to 15.53 | NS | 2.15 | 0.28 to 16.53 | NS |
| CA 19.9 (normal vs high) | 0.71 | 0.34 to 1.47 | NS | 0.97 | 0.45 to 2.10 | NS |
| Albumin (normal vs low) | 0.81 | 0.33 to 1.97 | NS | 0.66 | 0.25 to 1.70 | NS |
| Surgery (yes vs no) | 4.0 | 1.88 to 8.55 | <0.001 | 4.96 | 2.18 to 11.29 | <0.001 |
BRPC, borderline resectable pancreatic cancer; CA, Carbohydrate antigen 19-9 (CA 19-9); LAUPC, locally advanced unresectable pancreatic cancer; L/N, lymphocyte/ neutrophil; NS, non-significant; OS, overall survival; PFS, progression-free survival; PS, performance status; UP, Uncinated process.;
Discussion
Our series shows that neoadjuvant ChT mainly with FOLFIRINOX or gemcitabine plus nab-paclitaxel could convert into resectable a significant proportion of patients initially defined as BRPC and LAUPC. This approach may be feasible and tolerable, leading to some patients, in whom the primary tumour could be completely resected, into prolonged disease control and survival. Almost 45% of our patients could be converted to a potential surgical resection and actually 38% of them had an R0–R1 pancreatic resection. Moreover, median OS for the whole cohort is above 25 months and for those achieving a complete surgical resection went over 32 months with a 28% of them being alive at 3 years after starting treatment.
One of the main issues in classifying locally advanced PC is the use of a standardised classification to differentiate BRPC from LAUPC. The one used in our patients has been the one proposed by NCCN.7 Most classifications define BRPC as those in which the superior mesenteric artery is contacting the tumour less than 180°, allowing potentially further resection with vascular reconstruction, if downsizing is eventually induced by neoadjuvant therapy.17 On the other hand, LAUPC is essentially defined when a vascular reconstruction is not possible whatever the anatomic reason is. This lack of a common criteria may produce different patient selection and makes different series difficult to compare. A multidisciplinary discussion is the cornerstone of the management of BRPC or LAUPC and the presence of a dedicated radiologist is essential to define regional staging.18 19 In our series all locoregional staging procedures were independently reviewed by two dedicated radiologists and our report provides detailed information on vascular involvement in 90% of our cases. Arterial involvement with or without venous infiltration was observed in 61% of our cases, indicating quite extensive locoregional features.
There are few prospective data from randomised clinical trials on NAT on BRPC or LAUPC.20–22 However, in absence of metastatic disease and after several courses of ChT, if a response has been achieved, initially unresectable tumours can be converted into resectable ones.23 The two most active treatments considered gold standard in metastatic disease, FOLFIRINOX and gemcitabine plus nab-paclitaxel, have been used as conversion therapy with some favourable outcomes. A recently reported phase II randomised trial compared in 90 patients with BRPC direct surgery as standard of care with three experimental arms of NAT (FOLFIRINOX, gemcitabine plus capecitabine or ChRT). The primary endpoint was R0 and R1 resection rate and no significant differences were found among the study arms. However, OS was improved in patients allocated to NAT, despite presenting only short-term follow-up.20
The role of RT in the treatment algorithm is still contentious. The PREOPANC-1 phase III trial compared neoadjuvant ChRT with gemcitabine as single agent with immediate surgery in resectable and BRPC patients and demonstrated an increase in R0 resection rate (40% vs 71%, p<0.001). Patients receiving ChRT presented more frequently downstaging and a more prolonged disease-free survival and locoregional failure-free interval. However, OS was not modified by NAT (median 14.3 vs 16 months; HR 0.78; p=0.096) with neoadjuvant ChRT.24 A retrospective series of patients treated at the Johns Hopkins Hospital showed no difference on postoperative complications among BRPC and LAUPC treated with conventional ChRT in comparison to stereotactic RT, although the later treatment was more frequently performed in more locally advanced tumours and required more often vascular resection.25 Some patients in our series received conventional ChRT with good tolerance and no significant toxicities. ChRT was only indicated if tumours were still defined as unresectable after conversion ChT in the MDB discussion.
The role of adjuvant ChT after NAT and conversion surgery is unclear, as there is no scientific evidence to support its use. However, the international guidelines recommend delivering adjuvant treatment on this setting due to the fact that most of the patients will develop an early recurrence or die within 12 months after conversion surgery.26 27 On the other hand, many patients are not able to receive adjuvant treatment for different reasons, such as PS after surgery, postoperative complications, and whether or not the patient was able to complete a total NAT before surgery.
Assessment of response and resectability throughout NAT treatment is key for BRPC or LAUPC management, as the goal is to perform at some moment a curative resection. However, the accuracy of restaging with the current imaging techniques is limited.28 29 All the patients from our centre were evaluated in the MDB, showing the importance of an expert radiologist in determining the potential resectability of each patient. In fact, only 1 out of 20 patients who went to surgery had no curative resection.
The pathology report on the surgical specimen has to be detailed in describing relevant prognostic features such as TNM (Tumor, Node, Metastases) stage, involvement of the resection margin or lymphatic/vascular or perineural involvement. Moreover, after NAT some changes in the surgical specimen are likely to be observed, such as fibrosis or necrosis. Those findings also challenge pathologists to define regression grades.30 Those observations should be reflected in the pathology report, but to date there are several grading systems and a standardised score has not been established yet.31 In general, a good biological response, indicated by a reduction in levels of CA 19-9, together with a major or complete pathological response, are considered important factors defining better prognosis.32 33
This study has many limitations due to its retrospective nature and single centre data. Potential selection bias cannot be controlled in this type of studies. However, it offers a real-life experience in PRPC and LAUPC, where scarce data from phase 3 trials exists in order to guide the treatment decisions. This work shows the importance of a multidisciplinary team approach in dedicated centres to implement a sequential approach able to eventually achieve a curative-intent surgery, in cases that some years ago would have been considered only for palliative.
Conclusions
Patients diagnosed of BR or LAU pancreatic adenocarcinoma were offered an NAT approach after a thorough evaluation in a MDB. Almost half of the patients achieved a complete tumour resection with improved survival in comparison to non-resected tumours.
Footnotes
Twitter: @MarinaGarces_, @tfleitask
SR and CP contributed equally.
LS and AC contributed equally.
Contributors: AC and LS did equally contributed as senior authors.
Funding: This study was supported by grants from the Instituto de Salud Carlos III (PI18/01909 to AC and DR; PI18/01508 to TF and PI19/00250 to LS). VG was supported by Rio Hortega contract CM18/00241 from the Carlos III Health Institute; TTF-K is supported by Joan Rodes contract 17/00026 from the Carlos III Health Institute. DR was supported by Joan Rodes contract 16/00040 from the Instituto de Salud Carlos III.
Competing interests: AC declares institutional research funding from Genentech, Merck Serono, BMS, MSD, Roche, Beigene, Bayer, Servier, Lilly, Novartis, Takeda, Astellas, Natera and Fibrogen and advisory board orspeaker fees from Merck Serono, Roche, Servier, Takeda, Natera and Astellas in the last 5 years. All remaining authors have declared no conflict of interest. LS has participated in the development of teaching resources, advisory and educational programs for Abbott, Mylan, Johnson and Johnson, Medtronic, Baxter and Prim.
Patient consent for publication: Not required.
Ethics approval: This research was approved by the Ethics Committee of the HCUV.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing not applicable as no datasets generated and/or analysed for this study. Data are available on reasonable request. Data are available on request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery 2012;152:S43–9. 10.1016/j.surg.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiura T, Uesaka K, Mihara K, et al. Margin status, recurrence pattern, and prognosis after resection of pancreatic cancer. Surgery 2013;154:1078–86. 10.1016/j.surg.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 4.Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. JCO 2015;33:1770–8. 10.1200/JCO.2014.59.7930 [DOI] [PubMed] [Google Scholar]
- 5.Sabater L, Muñoz E, Roselló S, et al. Borderline resectable pancreatic cancer. challenges and controversies. Cancer Treat Rev 2018;68:124–35. 10.1016/j.ctrv.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 6.Katz MHG, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787–95. 10.1245/s10434-013-2886-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN NCCN guidelines pancreatic adenocarcinoma. Available: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf [Accessed 13 Apr 2020].
- 8.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727–33. 10.1245/s10434-009-0408-6 [DOI] [PubMed] [Google Scholar]
- 9.Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol 2013;24:2484–92. 10.1093/annonc/mdt239 [DOI] [PubMed] [Google Scholar]
- 10.Conroy T, Desseigne F, Ychou M, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services Common terminology criteria for adverse events (CTCAE) version 4.03. 2010. USA: National Institutes of Health, National Cancer Institute, 2016. [Google Scholar]
- 13.Gómez-Mateo MC, Sabater-Ortí L, Ruiz-Montesinos I. Pathology reporting of resected pancreatic/periampullary cancer specimen : Surgery for pancreatic and periampullary cancer. Springer, 2018: 247–80. [Google Scholar]
- 14.Sabater L, Gómez-Mateo MdelC, López-Sebastián J, et al. Prognostic implications of the standardized study of resection margins in pancreatic cancers. Cir Esp 2014;92:532–8. 10.1016/j.ciresp.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 15.Sabater L, Cugat E, Serrablo A, et al. Does the artery-first approach improve the rate of R0 resection in pancreatoduodenectomy?: a multicenter, randomized, controlled trial. Ann Surg 2019;270:738–46. 10.1097/SLA.0000000000003535 [DOI] [PubMed] [Google Scholar]
- 16.Ryan R, Gibbons D, Hyland JMP, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141–6. 10.1111/j.1365-2559.2005.02176.x [DOI] [PubMed] [Google Scholar]
- 17.Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg 2019;270:340–7. 10.1097/SLA.0000000000002753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of abdominal radiology and the American pancreatic association. Radiology 2014;270:248–60. 10.1148/radiol.13131184 [DOI] [PubMed] [Google Scholar]
- 19.Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2–11. 10.1016/j.pan.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 20.Ghaneh P, Palmer DH, Cicconi S, et al. ESPAC-5F: Four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or Folfirinox or chemoradiotherapy (crt) in patients with borderline resectable pancreatic cancer. JCO 2020;38:4505 10.1200/JCO.2020.38.15_suppl.4505 [DOI] [PubMed] [Google Scholar]
- 21.Yoo C, Lee SS, Song KB, et al. Neoadjuvant modified Folfirinox followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a phase 2 study for clinical and biomarker analysis. Br J Cancer 2020;123:362–8. 10.1038/s41416-020-0867-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz MHG, Shi Q, Ahmad SA, et al. Preoperative modified Folfirinox treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial a021101. JAMA Surg 2016;151:e161137. 10.1001/jamasurg.2016.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michelakos T, Pergolini I, Castillo CF-D, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with Folfirinox. Ann Surg 2019;269:733–40. 10.1097/SLA.0000000000002600 [DOI] [PubMed] [Google Scholar]
- 24.Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020;38:1763–73. 10.1200/JCO.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair AB, Rosati LM, Rezaee N, et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: the impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery 2018;163:1090–6. 10.1016/j.surg.2017.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v56–68. 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol 2019;37:2082–8. 10.1200/JCO.19.00946 [DOI] [PubMed] [Google Scholar]
- 28.Katz MHG, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749–56. 10.1002/cncr.27636 [DOI] [PubMed] [Google Scholar]
- 29.Barreto SG, Loveday B, Windsor JA, et al. Detecting tumour response and predicting resectability after neoadjuvant therapy for borderline resectable and locally advanced pancreatic cancer. ANZ J Surg 2019;89:481–7. 10.1111/ans.14764 [DOI] [PubMed] [Google Scholar]
- 30.Verbeke C, Häberle L, Lenggenhager D, et al. Pathology assessment of pancreatic cancer following neoadjuvant treatment: time to move on. Pancreatology 2018;18:467–76. 10.1016/j.pan.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 31.Cacciato Insilla A, Vivaldi C, Giordano M, et al. Tumor regression grading assessment in locally advanced pancreatic cancer after neoadjuvant Folfirinox: interobserver agreement and prognostic implications. Front Oncol 2020;10:64. 10.3389/fonc.2020.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai S, George B, Wittmann D, et al. Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg 2020;271:740–7. 10.1097/SLA.0000000000003049 [DOI] [PubMed] [Google Scholar]
- 33.Truty MJ, Kendrick ML, DavidM N, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for Borderline/Locally advanced pancreatic cancer. Annals of surgery 9000. Available: https://journals.lww.com/annalsofsurgery/Fulltext/9000/Factors_Predicting_Response,_Perioperative.95162.aspx [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000929supp001.pdf (43KB, pdf)
esmoopen-2020-000929supp002.pdf (419.1KB, pdf)