Accurate intracellular monitoring of electrical activity in networks of excitable cells for long durations is crucial for both biophysical studies and pharmaceutical research. Work by the Spira lab1 was one of the first demonstrations of using minimally invasive extracellular tools to record synaptic- and action-potentials, with features similar to intracellular recordings. In that work, mushroom-shaped gold microelectrode arrays displayed cellular engulfment and promoted tight electrical coupling with neurons. Subsequently, phospholipid coating2 and electroporation methodologies3, 4 have been adopted for promoting intracellular entry and electrical recordings from nanowire or nanopillar-based tools. Most recently, the Angelis lab demonstrated that a combination of 3D nanoelectrodes and plasmonic optoporation5, which does not perturb spontaneous electrical activity, can allow for extra- and intracellular recordings from cardiac cells. In addition to the efforts toward intracellular electrical recording, significant advances have also been made in the areas of materials-based cellular modulation and structural studies of biointerfaces. For example, several nanostructured silicon6 and polymer7, 8 have been developed for the optical modulation of excitable and non-excitable cells, through photoelectrochemical, photocapacitive or photothermal effects. For structural studies, the Cui lab has extensively investigated the junctions between nano- and micro-structures and various cellular components, and has in particular shown effects of material curvature on the distribution of endocytic proteins9. These three elements of electrophysiology at biointerfaces (i.e., sensing, modulation, and structural control), however, still require new features such as scalability to achieve broad societal impact.
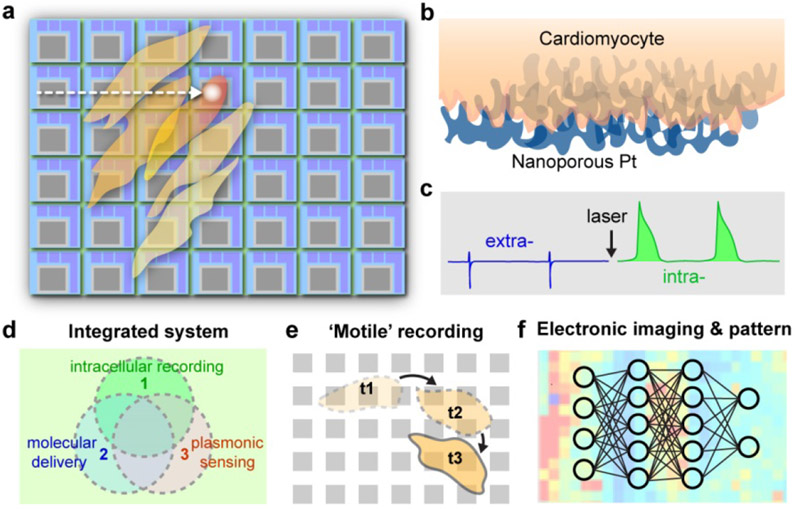
Here in Nature Nanotechnology, Dipalo et al.10 have integrated planar porous electrodes with plasmonic optoacoustic poration to perform intracellular recordings of action potentials from cardiac networks, using commercial CMOS-multi-electrode arrays (CMOS-MEAs) (Fig 1a-c). Specifically, the authors used a porous platinum meta-electrode surface with a high nanoscale roughness that can absorb near infrared light well, localize and amplify electromagnetic fields in the gaps between the bumps in the material, and form intimate contacts with cardiac cell membranes (Fig 1b). Human induced pluripotent stem cell derived cardiomyocytes (hiPSCs), primary rat cardiomyocytes, and HL-1 cardiomyocytes were cultured atop the CMOS-MEAs and transient poration in the cardiac cell membranes was induced by mechanical waves generated from 1 mW near infrared optical pulses, allowing for high quality intracellular recordings for up to 30 minutes (Fig 1c). Laser scanning across a 4096-electrode array was also performed, demonstrating the ability to record intracellular electrical signals from a network of thousands of cardiac cells in a minimally invasive manner. Moreover, authors showed efficacy of this laser scanning method for the mapping of electrical signals in cardiac circuits before and after treatment with pharmacological agents --- these results have implications for the advancement of drug screening techniques in both academia and the pharmaceutical industry. This work has presented a few advantages over existing intracellular recording technologies, such as a superb scalability and a compatibility with other semiconductor components, an avoidance of complex and expensive fabrication methods, and formation of biocompatible interfaces that include minimal protruding material elements (the physiological impacts of these protrusions are still largely unknown). .
Figure 1: Optoacoustic poration enables scalable intracellular electrophysiology.
Schematic diagrams of laser scanning over CMOS-MEAs (a), the biointerface (b) and switching from extra- and intra-cellular recording (c). Future directions are shown in d-f.
Future implications of this work include the ability to combine electrophysiological measurements with optoacoustic poration-enhanced molecular delivery and plasmonic biosensing in networks of cells (Fig 1d). The authors already demonstrated use of their device in pharmacological drug screening. With optoacoustic poration, a broad range of other biological effectors, such as nucleic acids, peptides and proteins, can be delivered to many cell types. A third feature, namely plasmonic biosensing of metabolites and disease biomarkers by the nanoporous metal, could be combined with the aforementioned intracellular recordings and molecular delivery in order to understand the coupling between biochemical signaling and electrophysiology in cellular networks (Fig 1d).
Another potential capability from this new technology is the ability to record intracellular electrical signals simultaneously from thousands of cells in a transient and minimally invasive manner, without perturbing cellular motility. This feature opens doors for studies in the field of developmental biology, allowing for the acquisition of intracellular electrical signals in excitable and non-excitable cells that are migrating and maturing across the CMOS-MEA substrate over time (Fig 1e). The ability to couple this method with optical microscopy, where specific cell types can be labeled, can allow for electrical signatures to be assigned to specific cells during different developmental stages. More generally, this new sensing platform, when integrated with other modulation methods, would help probe how electrical signaling can contribute to or be impacted by spatial organization of cells during the formation of a cellular network, at least in two dimensions.
Lastly, the network level information recorded from these CMOS-MEAs would allow for the classification of electrophysiological signatures of cellular circuits into various phenotypes. In such a scenario, high spatiotemporal electronic imaging of intracellular spiking dynamics in cellular networks can be produced by CMOS-MEAs, and the resultant electronic images can be correlated with specific phenotypes by machine-learning (ML) algorithms. A ML system can be taught to recognize what an electronic map of a specific disease looks like (i.e. a seizure in a neural network). After electrical signals from a cellular network cultured on the CMOS-MEA device in the presence or absence of a pharmacological agent are mapped, the electronic maps can be analyzed by the ML system to classify the behavior of the cellular network into a specific phenotype.
References:
- 1.Hai A, Shappir J & Spira ME Nature Methods 7, 200–U250 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Tian BZ et al. Science 329, 830–834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie C, Lin ZL, Hanson L, Cui Y & Cui BX Nature Nanotechnology 7, 185–190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson JT et al. Nature Nanotechnology 7, 180–184 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dipalo M et al. Nano Letters 17, 3932–3939 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parameswaran R et al. Nature Nanotechnology 13, 260–266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tortiglione C et al. Science Advances 3, e1601699 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sytnyk M et al. Nature Communications 8, 91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao WT et al. Nature Nanotechnology 12, 750–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dipalo M et al. Intracellular Recordings at network level on High-Density CMOS-MEAs by Plasmonic Meta-Electrodes. Nature Nanotechnology (2018). [DOI] [PubMed] [Google Scholar]