Abstract
Background
For patients with metastatic renal cell carcinoma (mRCC) who progress on standard-of-care therapies, there is an unmet need for novel treatments. Phase I clinical trials are designed to test the safety, toxicity and optimal dosing of novel agents. Herein, we analysed the outcomes of patients with mRCC enrolled in phase I trials and assess the utility of prognostic scores.
Methods
Patients with all histologies of mRCC were included if they received treatment on a phase I clinical trial at MD Anderson Cancer Center (MDACC). Survival outcomes were calculated using Cox proportional hazard model. Prognostic value of the International Metastatic RCC Database Consortium (IMDC), Royal Marsden Hospital (RMH) and MDACC scores was assessed using the likelihood ratio (LR) χ2 test and the c-index.
Results
Among 82 patients with mRCC who received treatment, 21 patients participated in more than one trial, resulting in 106 trial participants (TP). Median prior therapies was two. For all TPs, median overall survival (OS) was 31.2 months, progression-free survival (PFS) was 5.9 months and objective response rate was 22%. Median OS and PFS were significantly shorter with increasing IMDC, RMH and MDACC scores. The RMH and MDACC scores outperformed the IMDC score for predicting OS (RMH LR χ2=8.64; MDACC LR χ2=7.74; IMDC LR χ2=2.36) and PFS (RMH LR χ2=17.5; MDACC LR χ2=20.3; IMDC LR χ2=4.28).
Conclusions
The RMH and MDACC prognostic scores can be used to predict OS for patients with mRCC in phase I trials and may guide patient selection. Patients with mRCC should be considered for phase I trials.
Keywords: metastatic renal cell carcinoma, phase 1, clinical trials, prognostic scores, Royal Marsden Hospital score
Summary box.
What is already known about this subject?
Phase I clinical trials were designed to test the safety, toxicity and maximum tolerated or optimal biological dose of new treatments. Patients with metastatic renal cell carcinoma (mRCC) may be referred to phase I clinical trials after progression on standard-of-care therapy.
Publication bias exists for reporting the results of clinical trials, so phase I clinical trial efficacy across all patients with mRCC is unknown.
Furthermore, appropriate patient selection for enrolment in a phase I clinical trial is essential, and could be guided by validated prognostic scoring systems.
What does this study add?
Our study reveals the efficacy of phase I clinical trials in all patients with mRCC who were enrolled in a phase I clinical trial at a tertiary cancer centre.
In this context, phase I clinical trials appear to have clinical and therapeutic benefit for patients with all histologies of mRCC.
Additionally, we show that prognostic risk scores improve patient selection for phase I clinical trials, and the Royal Marsden Hospital and MD Anderson Cancer Center scores performed better than the International Metastatic RCC Database Consortium score at time of enrolment on a phase I clinical trial.
How might this impact on clinical practice?
This study may impact practice by providing clinicians and patients with mRCC evidence of the clinical and therapeutic benefit of phase I clinical trials. More importantly, this study might guide selection of patients with mRCC for enrolment in phase I clinical trials, thereby improving clinical outcomes.
Introduction
Over the past decade, treatment options for patients with metastatic renal cell carcinoma (mRCC) have exponentially expanded to include vascular endothelial growth factor receptor (VEGFR)-targeted therapies, immune checkpoint inhibitors, mammalian target of rapamycin (mTOR) inhibitors and multi-target tyrosine kinase inhibitors.1 Accordingly, mRCC has become a disease with a large number of targeted therapies approved. While these therapies may prolong life, most patients with mRCC continue to progress and eventually die from their cancer. Many of the approved treatments for mRCC have overlapping mechanisms of action and patterns of resistance, so there remains an unmet need for novel, life-prolonging treatments for patients with mRCC. Furthermore, registration trials for the above therapies were limited to patients with metastatic clear cell RCC (ccRCC). Patients with metastatic non-ccRCC (nccRCC) experience limited benefit from these therapies and have an urgent need for novel therapies.2
Historically, phase I clinical trials were designed to test the safety, toxicity, maximum tolerated dose/recommended phase II dose, and/or optimal biological dose of new treatments. Patients with mRCC may be referred to phase I clinical trials after progression on standard-of-care therapy.3 The role for phase I clinical trials in drug development is evolving with the introduction of biomarker-guided trials, larger dose-expansion cohorts in early phase trials, and the US Food and Drug Administration’s approval of therapies based on results from expanded phase I trials.4–7 There is always debate about the clinical and therapeutic benefit for patients who are enrolled in phase I trials.8 9 The central tenet of drug development should be patient selection and offer ‘the right drug for the right patient at the right time’.10 In this setting, appropriate patient selection for enrolment in a phase I clinical trial is essential, and could be guided by validated prognostic scoring systems. Prognostic scoring systems, such as the Royal Marsden Hospital (RMH) score and MD Anderson Cancer Center (MDACC) score, have been validated in adult and paediatric patients enrolled in phase I trials in multiple tumour types.11–17 For patients with mRCC, the International Metastatic RCC Database Consortium (IMDC) risk score is a validated model to inform prognosis prior to first, second and third-line treatments.18–20
Herein, we analysed the outcomes of patients with mRCC enrolled in phase I trials and assessed the utility of established prognostic scores at the time of enrolment on a phase I clinical trial.
Methods
Patients
Patients with all histologies of mRCC were included if they were enrolled and received treatment on a phase I clinical trial at the University of Texas MD Anderson Cancer Center (MDACC, Houston, Texas, USA). Baseline patient characteristics and clinical outcomes were collected retrospectively, and the Institutional Review Board (IRB) of MDACC approved this study. The IRB also independently approved all phase I trials included in this analysis, and patients provided informed consent prior to initiation of treatment.
Endpoints and prognostic scores
Clinical endpoints of interest included objective response rate (ORR), progression-free survival (PFS) and overall survival (OS). ORR was defined as partial response plus complete response (CR), per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 or immune-related RECIST. PFS was defined as time from trial enrolment until time of progression, last follow-up or death. OS was defined as time from trial enrolment until time of death or last follow-up. For each patient with clinical data available, an mRCC-specific prognostic score, the IMDC score and two phase I clinical trial prognostic scores, the RMH prognostic score and the MDACC prognostic score, were assessed at trial enrolment. The IMDC score includes haemoglobin<lower limit of normal, platelets>upper limit of normal (ULN), absolute neutrophil count>ULN, corrected calcium>ULN, Karnofsky performance status<80% and <1 year from time of diagnosis to systemic therapy (online supplemental table 1).18 The RMH score uses albumin <3.5 g/dL, lactate dehydrogenase>ULN and the number of metastatic sites (≥3 sites, online supplemental table 1).21 For mRCC, the MDACC score is the RMH score plus Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥1 (online supplemental table 1).22
esmoopen-2020-001073supp001.pdf (97.7KB, pdf)
Statistical analysis
Median follow-up time was calculated with the reverse Kaplan-Meier method.23 Survival outcomes were calculated using Cox proportional hazard models. Statistical significance was defined as a p value <0.05. The prognostic values of the IMDC, RMH and MDACC scores were assessed with the likelihood ratio (LR) χ2 test and Harrell’s c-index.24 Although LR χ2 tests are the gold standard metric for model discrimination and are more sensitive than the c-index, the latter is presented for the sake of completeness.
Results
Baseline characteristics
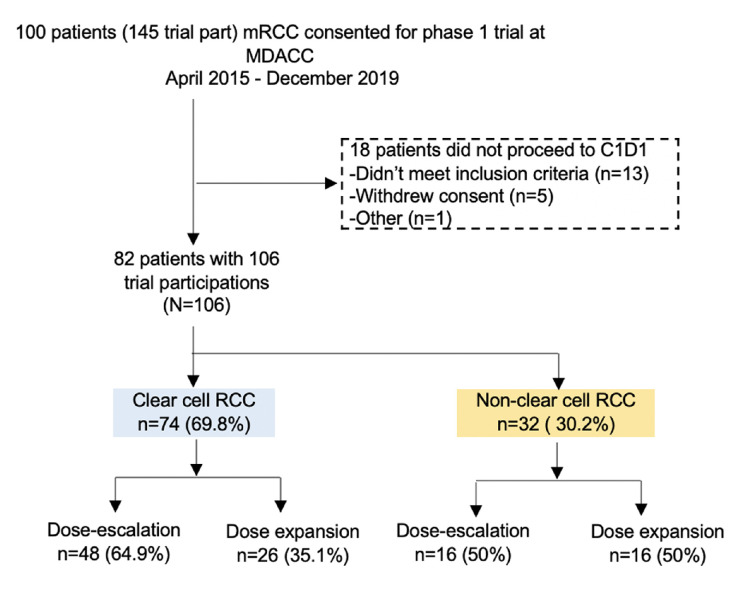
Between April 2015 and September 2019, 100 patients with mRCC were consented for a phase I clinical trial at MDACC, and 18 patients did not receive treatment on a phase I trial due to not meeting inclusion criteria or withdrawing consent (figure 1). Of the 82 patients with mRCC who received treatment, 59 patients had metastatic ccRCC, while 23 had metastatic nccRCC (table 1). The most common nccRCC histologies were papillary (n=7, 8.5%), renal medullary (n=4, 4.8%) and RCC with sarcomatoid dedifferentiation present (n=4, 4.8%, two had ccRCC with sarcomatoid dedifferentiation, one had RCC with sarcomatoid and rhabdoid dedifferentation, and one had mixed clear cell and papillary RCC with sarcomatoid dedifferentation). Twenty-one patients (25.6%) participated in more than phase I clinical trial, which resulted in a total of 106 trial participants (TP) for the 82 patients evaluated in our study. At the time of trial enrolment, the median age was 63 (range 23–77 years, IQR 19 years) and median number of prior treatments was two (range 0–9, table 1). At time of initiation of a phase I clinical trial, 63.2% of participants had IMDC intermediate risk disease and 17% had IMDC poor risk disease (table 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram. C1D1, cycle 1 day 1; part, participants; MDACC, MD Anderson Cancer Center; mRCC, metastatic renal cell carcinoma.
Table 1.
Baseline characteristics for patients with metastatic renal cell carcinoma enrolled on phase I clinical trials
| Characteristics | N=82 (106 trial participations, 40 unique trials) |
| Median age in years at trial enrolment (range; IQR in years) | 63 (23–77; 19) |
| Male (%) | 78 (73.6%) |
| Histology | n=82 (%) |
| Clear cell | 59 (72.0) |
| Papillary | 7 (8.5) |
| Renal medullary carcinoma | 4 (4.8) |
| RCC with sarcomatoid dediff | 4 (4.8) |
| Collecting duct carcinoma | 2 (2.4) |
| Unclassified | 2 (2.4) |
| Chromophobe | 1 (1.2) |
| Translocation | 1 (1.2) |
| Other non-clear cell | 2 (2.4) |
| Number of prior lines of therapy | N=106 (%), median=2, range=0–9 |
| 0 | 8 (7.5) |
| 1 | 29 (27.4) |
| 2 | 22 (20.8) |
| ≥3 | 47 (44.3) |
| IMDC risk score | N=106 (%) |
| Favourable | 9 (8.5) |
| Intermediate | 67 (63.2) |
| Unfavourable | 18 (17.0) |
| Not available | 12 (11.3) |
dediff, dedifferentation; IMDC, International metastatic renal cell carcinoma database consortium.
Efficacy of phase I clinical trials for mRCC
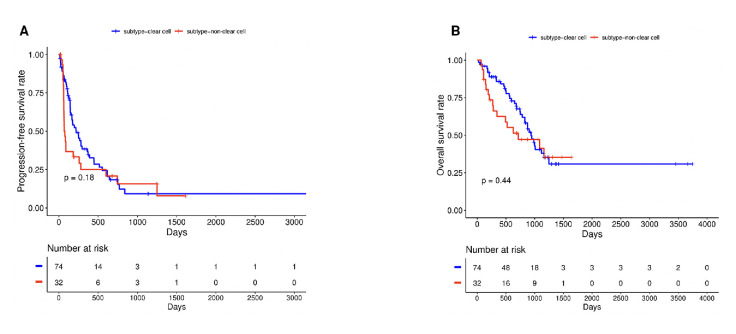
Across the 106 TPs, the median PFS was 5.9 months, median OS was 31.2 months and ORR was 22% with 2% of patients achieving a CR (table 2). When assessed by histology, patients with metastatic ccRCC had numerically longer PFS (7.3 vs 2.5 months, HR 1.39, 95% CI 0.86 to 2.25, p=0.18) and OS (31.6 vs 23.9 months, HR 1.26, 95% CI 0.71 to 2.23, p=0.44) with wide CIs indicating substantial uncertainty (table 2, figure 2A, B).
Table 2.
Efficacy of phase I clinical trials for all patients with metastatic renal cell carcinoma and by histology
| All mRCC (n=106) |
nccRCC (n=32) |
ccRCC (n=74) |
HR | P value | |
| PFS (95% CI) |
5.9 m (4.8 to 9.3 m) |
2.5 m (2.1 to 9.3 m) |
7.3 m (5.5 to 12.4 m) |
1.39 (0.86 to 2.25) |
0.19 |
| OS (95% CI) |
31.2 m (24.9 to 38.7 m) |
23.9 m (11.4 to NR) |
31.6 m (27.6 to 41.5 m) |
1.26 (0.71 to 2.23) |
0.44 |
| ORR (%) | 22 | 17 | 24 | – | – |
| CR (%) | 2 | 0 | 3 | – | – |
| PR (%) | 20 | 17 | 21 | – | – |
| SD (%) | 49 | 30 | 57 | – | – |
| PD (%) | 29 | 53 | 19 | – | – |
ccRCC, clear cell renal cell carcinoma; CR, complete response; m, months; mRCC, metastatic renal cell carcinoma; nccRCC, non-clear cell renal cell carcinoma; ORR, objective response rates; OS, overall survival; PD, progressive disease; PFS, progression free survival; PR, partial response; SD, stable disease.
Figure 2.
Kaplan-Meier curves for progression-free survival (A) and overall survival (B) by histology.
Efficacy of phase I clinical trials by trial type
Sixty-four TPs enrolled in a dose-escalation phase I trial, while 42 TPs enrolled in a dose-expansion phase I trial. Participants in dose-escalation trials had significantly longer PFS than their counterparts in dose-expansion trials (8.4 vs 3.6 months, HR 0.57, 95% CI 0.36 to 0.91, p=0.017, online supplemental figure 1A). Participants in dose-escalation trials also had significantly longer OS (38.7 vs 26.1 months, HR 0.44, 95% CI 0.25 to 0.75, p=0.003, online supplemental figure 1B). A detailed breakdown of the mechanisms of action of therapies that TPs received is available in online supplemental table 2.
esmoopen-2020-001073supp002.pdf (599.3KB, pdf)
Clinical utility of prognostic scores at time of trial enrollment
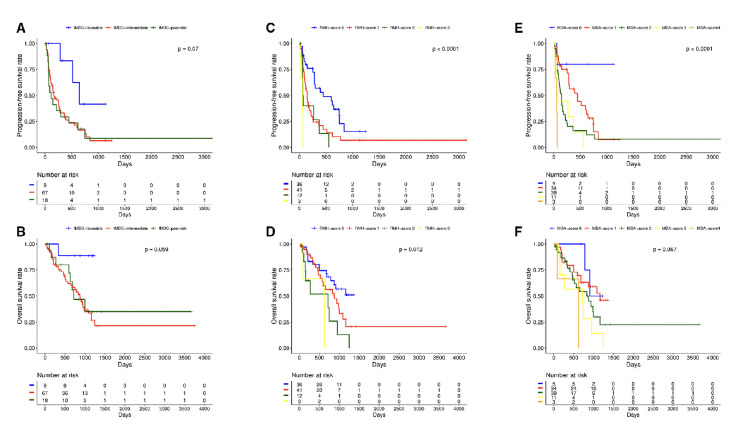
Table 3 lists the distribution of TPs across the IMDC, RMH and MDACC prognostic scores. Twelve TPs did not have the baseline laboratory values necessary to calculate their IMDC risk score at time of trial initiation, and the RMH and MDACC prognostic scores could not be calculated in 14 TPs. Median OS and PFS were significantly shorter with increasing IMDC, RMH and MDACC scores (table 3, figure 3A–F). The RMH (c-index=0.61, LR χ2=8.64, p=0.003) and MDACC scores (c-index=0.61, LR χ2=7.74, p=0.1) outperformed the IMDC score (c-index=0.57, LR χ2=2.36, p=0.10) in predicting OS. The IMDC, RMH and MDACC scores were also predictive of PFS, but the RMH (c-index=0.65, LR χ2=17.5, p<0.0001) and MDACC scores (c-index=0.65, LR χ2=20.3, p=<0.001) again outperformed the IMDC score (c-index=0.59, LR χ2=4.28, p=0.04).
Table 3.
Efficacy of phase I clinical trials by prognostic risk group*
| Median OS | HR (95% CI) |
P value | Median PFS | HR (95% CI) |
P value | |
| IMDC fav. (n=9) |
NR | Ref. | N/A | 21.4 m | Ref. | N/A |
| IMDC int. (n=67) |
29.1 m | 7.69 (1.05 to 56.10) |
0.04 | 5.6 m | 3.50 (1.09 to 11.27) |
0.04 |
| IMDC poor (n=18) |
23.9 m | 6.53 (0.82 to 52.31) |
0.08 | 3.7 m | 3.78 (1.09 to 13.06) |
0.04 |
| RMH 0 (n=36) |
29.1 m | Ref. | N/A | 14.9 m | Ref. | N/A |
| RMH 1 (n=41) |
29.2 m | 1.70 (0.87 to 3.31) |
0.12 | 4.8 m | 2.29 (1.32 to 3.97) |
0.003 |
| RMH 2 (n=12) |
23.9 m | 3.55 (1.54 to 8.18) |
0.003 | 2.3 m | 3.24 (1.46 to 7.20) |
0.004 |
| RMH 3 (n=3) |
20.9 | 3.58 (0.80 to 16.1) |
0.10 | 1.7 m | 15.07 (4.11 to 55.28) |
4.32e-05 |
| MDACC 0 (n=5) |
NR | Ref. | N/A | NR | Ref. | N/A |
| MDACC 1 (n=34) |
38.7 m | 1.45 (0.33 to 6.35) |
0.62 | 14.8 m | 3.58 (0.48 to 26.56) |
0.21 |
| MDACC 2 (n=39) |
29.2 m | 2.33 (0.54 to 10.0) |
0.26 | 4.8 m | 7.27 (0.99 to 53.49) |
0.05 |
| MDACC 3 (n=11) |
23.9 m | 4.16 (0.88 to 19.73) |
0.08 | 2.3 m | 9.00 (1.11 to 72.8) |
0.04 |
| MDACC 4 (n=3) |
20.9 m | 4.67 (0.64 to 33.88) |
0.13 | 1.7 m | 45.6 (4.5 to 462.22) |
0.001 |
fav., favourable; IMDC, International Metastatic RCC Database Consortium; int., intermediate; m, months; MDACC, MD Anderson Cancer Center; N/A, not available; NR, not reached; OS, overall survival; PFS, progression free survival; Ref., reference value; RMH, Royal Marsden Hospital.
Figure 3.
Kaplan-Meier curves for progression-free survival and overall survival by International Metastatic RCC Database Consortium (IMDC) subgroups (A, B), Royal Marsden Hospital (RMH) score (C, D) and MD Anderson Cancer Center (MDACC) phase I score (E, F).
Discussion
In a pooled phase I clinical trial experience for patients with mRCC from a large cancer centre, we demonstrate that phase I clinical trials may have therapeutic benefit for patients with mRCC, as the median OS, PFS and ORR compare favourably to historical controls for second and third-line treatment (online supplemental table 3).18 20 Because the therapeutic benefit of phase I clinical trials remains controversial, we sought to assess the outcomes of all patients with mRCC who received treatment on a phase I clinical trial at a tertiary cancer centre. By performing a pooled analysis of all our phase I clinical trials, the bias of only publishing positive early phase trials was limited. Of note, our findings suggest that patients with all histologies of mRCC may derive clinical benefit from phase I clinical trials, although patients with metastatic ccRCC did have numerically longer survival, consistent with the established poor prognosis of metastatic nccRCC.2 Unexpectedly, dose-escalation phase I trials had significantly longer OS and PFS than dose expansion trials. Based on the rationale for dose expansion cohorts, we expected to observe longer survival with dose expansion cohorts, but this finding reaffirms that improvements in the design of phase I trials have positively changed the therapeutic benefit of these studies.
Patient selection for referral to a phase I clinical trial is challenging for clinicians. Beyond biomarkers, next generation sequencing for actionable alterations and ECOG PS, clinicians need pragmatic clinical prognosticators For patients with mRCC, the IMDC risk score is a validated model to inform prognosis prior to first, second and third-line treatments.18–20 Alternatively, there are validated prognostic models for patients enrolling on a phase I clinical trial, such as the RMH and MDACC score.21 22 In this study, the IMDC, RMH and MDACC scores were all predictive of survival in patients with mRCC enrolling on a phase I clinical trial. However, the RMH and MDACC scores performed better than the IMDC score at time of enrolment on a phase I clinical trial, based on the much higher LR χ2 test. For comparison, the IMDC risk score was validated in the second-line setting with targeted therapy, where the c-index was 0.66, which is higher than its performance in the investigational setting after a median of two lines of therapy.18
For patients with mRCC who progress on contemporary, first-line treatment, standard-of-care options include cabozantinib, lenvatinib plus everolimus, nivolumab, everolimus or VEGFR-targeted therapy, and many of these options are limited by similar patterns of resistance to first-line treatment. In our experience, phase I clinical trials had comparable efficacy to approved salvage therapies for patients with mRCC. Median OS was 21.4 and 25.8 months for salvage cabozantinib and nivolumab in the METEOR and CheckMate-025 trials, respectively.25 26 In patients with a median of two prior lines of therapy, phase I clinical trials produced a median OS of 31.2 months and ORR of 22%. Similarly, the efficacy of phase I clinical trials compared favourably to population-based studies of third-line therapy. In the IMDC experience, median OS was 12.4 months with third-line VEGF-targeted therapies or mTOR inhibitors.20 These favourable comparisons reaffirm the therapeutic potential of early phase clinical trials for patients with mRCC. Yet, the significance of comparisons is limited due to a difference in time periods evaluated (2015–2019 in our study vs publications from 2015 and 2017).
Our study has several limitations due to its design. This study has limited generalisability because the data are from a single, tertiary academic centre where select faculty enrol patients on early phase trials. Also, detailed information regarding the treatments received is not available due to the pooled design of our analysis. Finally, there were a wide range of investigational therapies included in this analysis with heterogeneity in their mechanisms of action.
In conclusion, phase I clinical trials may confer clinical and therapeutic benefit for patients with all histologies of mRCC, and select patients with mRCC should be considered for phase I clinical trials. Prognostic risk scores, such as IMDC, RMH and MDACC, may help improve patient selection for phase I clinical trials, and the RMH and MDACC scores performed better than the IMDC score at time of enrolment on a phase I clinical trial.
Acknowledgments
We thank the patients and their families for their participation, and the investigators and site staff for their contributions.
Footnotes
Twitter: @onchahn, @Viveksubbiah
AWH and OA contributed equally.
Contributors: AWH, OA, PM, JR and VS designed the study. AWH, OA, EC and HL collected data for the analyses. JR performed all statistical analyses and PM provided guidance on statistical design. FM-B, AN, EJ, SP-P, DH, SP, TY, NT and VS enrolled patients who were included in this study. All authors contributed to drafting and critical revisions of the manuscript.
Funding: The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (NIH) Cancer Center Support (grant CA016672). The University of Texas MD Anderson Cancer Center clinical trials programme is supported in part by Cancer Prevention Research Institute of Texas (grant RP110584) and National Center for Advancing Translational Sciences (grant UL1 TR000371; Center for Clinical and Translational Sciences). VS is supported by the NIH National Cancer Institute (grant 1R01CA242845-01A1). PM is supported by a Young Investigator Award by the Kidney Cancer Association, a Career Development Award by the American Society of Clinical Oncology, by a Concept Award by the US Department of Defense and by the MD Anderson Khalifa Scholar award.
Competing interests: AWH, OA, EC, HL and JR have no conflicts to disclose. PM reports consultancy/honoraria to Pfizer, Bristol-Myers Squibb, Mirati, Exelixis and Axiom Healthcare Strategies; research funding to his institution from Mirati, Takeda, Bristol-Myers Squibb and Gateway for Cancer Research. FM-B reports consulting to Aduro BioTech, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche, Genentech, IBM Watson, Jackson Laboratory, Kolon Life Science, OrigiMed, PACT Pharma, Parexel International, Pfizer, Samsung Bioepis, Seattle Genetics, Tyra Biosciences, Xencor and Zymeworks. She reports honoraria from Chugai Biopharmaceuticals, Mayo Clinic and Rutgers Cancer Institute of New Jersey. She reports research funding to her institution from Aileron Therapeutics AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences, Curis, CytomX Therapeutics, Daiichi Sankyo, Debiopharm International, eFFECTOR Therapeutics, Genentech, Guardant Health, Millennium Pharmaceuticals, Novartis, Puma Biotechnology and Taiho Pharmaceutical. AN reports consulting to CytomX Therapeutics, Novartis, Kymab and Genome. He reports travel and accommodation expenses from ARMO BioSciences. He reports research funding to his institution from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor, Amplimmune, ARMO BioSciences, Eli Lilly, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, Bristol-Myers Squibb, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Kymab, PsiOxus and Immune Deficiency Foundation (Spouse). EJ reports research funding to his institution from Exelixis, Merck and Pfizer. He reports consulting for Eisai, Exelixis, Novartis, Merck, Pfizer, Roche. Sarina Piha-Paul reports research funding to her institution from AbbVie, ABM therapeutics, Acepodia, Alkermes, Aminex Therapeutics, Amphivena Therapeutics, BioMarin Pharmaceutical, Boehringer Ingelheim, Bristol Myers Squib, Chugai Pharmaceutical, Daichi Sankyo, Eli Lilly, Five Prime Therapeutics, Genmab A/S, GlaxoSmithKline, Helix BioPharma, Incyte, Jacobio Pharmaceuticals, Medimmune, Medivation, Merck Sharp and Dohme, Novartis Pharmaceuticals, Pieris Pharmaceuticals, Pfizer, Principia Biopharma, Puma Biotechnology, Rapt Therapeutics, Seattle Genetics, Taiho Oncology, Tesaro and TransThera Bio. NMT reports consultancy and honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, Nektar, Eisai, Ono Pharmaceutical, Eli Lilly, Oncorena, Ipsen, Surface Oncology and Neolukin Therapeutics. He reports research funding to his institution from Bristol-Myers Squibb, Exelixis, Pfizer, Nektar Therapeutics, Calithera Bioscience, Eli Lilly, Mirati Therapeutics, Arrowhead Pharmaceuticals, Takeda, Epizyme and Eisai. He reports travel expenses from Pfizer, Novartis, Nektar, Bristol-Myers Squibb, Eisai, Onco Pharmaceuticals, Oncorena, Surface Oncology, Lilly Oncology, Ipsen, and Neolukin Therapeutics. David S. Hong reports research funding to his institution from AbbVie, Adaptimmune, Aldi-Norte, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, GSK, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, MedImmune, Mirati, miRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, Takeda, and Turning Point Therapeutics. He reports travel expenses from Bayer, LOXO, miRNA, Genmab, AACR, ASCO, and SITC. He reports consulting for Alpha Insights, Amgen, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint, Infinity, Janssen, Merrimack, Medscape, Numab, Pfizer, Prime Oncology, Seattle Genetics, Takeda, Trieza Therapeutics, and WebMD. He reports ownership interests in Molecular Match (Advisor), OncoResponse (Founder), Presagia Inc (Advisor). Shubham Pant reports research funding to his institution from Mirati Therapeutics, Eli Lilly, Red Hill Biopharma Ltd., Xencor, Five Prime Therapeutics, Novartis, Rgenix, Sanofi-Aventis, Arqule, Bristol-Myers Squibb, Onco Response, Sanofi US Services Inc., and GlaxoSmithKline. Timothy Yap reports research funding to his institution from: AstraZeneca, Bayer, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius/Formation Biologics, F-Star, GlaxoSmithKline, Genentech, ImmuneSensor, Ipsen, Jounce, Karyopharm, Kyowa, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Sanofi, Seattle Genetics, Tesaro, and Vertex Pharmaceuticals. He reports consultancy from Almac, Aduro, AstraZeneca, Atrin, Axiom, Bayer, Calithera, Clovis, Cybrexa, EMD Serono, F-Star, Guidepoint, Ignyta, I-Mab, Jansen, Kyn Therapeutics, Merck, Pfizer, Roche, Seattle Genetics, and Zai Labs. Vivek Subbiah reports research funding to his institution from LOXO Oncology, Roche/Genentech, Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, Pharmamar, D3, Pfizer, Amgen, Multivir, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Medimmune, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, Turning Point Therapeutics, and Boston Pharmaceuticals. He reports consultancy for Helsinn, Incyte, and QED Pharma. He reports travel expenses from Helsinn, Incyte, ASCO and ESMO.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. The clinical data for this analysis is from numerous clinical trials and cannot be further shared, per the trial protocol agreements.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:804–34. 10.6004/jnccn.2017.0100 [DOI] [PubMed] [Google Scholar]
- 2.Kroeger N, Xie W, Lee J-L, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC database Consortium criteria. Cancer 2013;119:2999–3006. 10.1002/cncr.28151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook N, Hansen AR, Siu LL, et al. Early phase clinical trials to identify optimal dosing and safety. Mol Oncol 2015;9:997–1007. 10.1016/j.molonc.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postel-Vinay S, Soria J-C. Phase I trials in oncology: a new era has started. Ann Oncol 2015;26:7–9. 10.1093/annonc/mdu513 [DOI] [PubMed] [Google Scholar]
- 5.Adashek JJ, LoRusso PM, Hong DS, et al. Phase I trials as valid therapeutic options for patients with cancer. Nat Rev Clin Oncol 2019;16:773–8. 10.1038/s41571-019-0262-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chabner BA. Approval after phase I: ceritinib runs the three-minute mile. Oncologist 2014;19:577–8. 10.1634/theoncologist.2014-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuk MK, Chang JT, Theoret MR, et al. FDA approval summary: accelerated approval of pembrolizumab for second-line treatment of metastatic melanoma. Clin Cancer Res 2017;23:5666–70. 10.1158/1078-0432.CCR-16-0663 [DOI] [PubMed] [Google Scholar]
- 8.Kimmelman J. Is participation in cancer phase I trials really therapeutic? J Clin Oncol 2017;35:135–8. 10.1200/JCO.2016.67.9902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burris HA. Correcting the ASCO position on phase I clinical trials in cancer. Nat Rev Clin Oncol 2020;17:125. 10.1038/s41571-019-0311-4 [DOI] [PubMed] [Google Scholar]
- 10.Subbiah V, Kurzrock R. Challenging standard-of-care paradigms in the precision oncology era. Trends Cancer 2018;4:101–9. 10.1016/j.trecan.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groisberg R, Hong DS, Behrang A, et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer 2017;5:100. 10.1186/s40425-017-0301-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livingston JA, Hess KR, Naing A, et al. Validation of prognostic scoring and assessment of clinical benefit for patients with bone sarcomas enrolled in phase I clinical trials. Oncotarget 2016;7:64421–30. 10.18632/oncotarget.10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maymani H, Hess K, Groisberg R, et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer 2018;120:137–41. 10.1016/j.lungcan.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrales-Medina FF, Herzog C, Hess K, et al. Clinical characteristics and outcomes of pediatric oncology patients with aggressive biology enrolled in phase I clinical trials designed for adults: the University of Texas MD anderson cancer center experience. Oncoscience 2014;1:522–30. 10.18632/oncoscience.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subbiah V, Hess KR, Khawaja MR, et al. Evaluation of novel targeted therapies in aggressive biology sarcoma patients after progression from US FDA Approved therapies. Sci Rep 2016;6:35448. 10.1038/srep35448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sen S, Hess K, Hong DS, et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br J Cancer 2018;118:763–9. 10.1038/bjc.2017.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subbiah IM, Subbiah V, Tsimberidou AM, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget 2013;4:153–62. 10.18632/oncotarget.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko JJ, Xie W, Kroeger N, et al. The International metastatic renal cell carcinoma database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293–300. 10.1016/S1470-2045(14)71222-7 [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells JC, Stukalin I, Norton C, et al. Third-Line targeted therapy in metastatic renal cell carcinoma: results from the International metastatic renal cell carcinoma database Consortium. Eur Urol 2017;71:204–9. 10.1016/j.eururo.2016.05.049 [DOI] [PubMed] [Google Scholar]
- 21.Arkenau H-T, Barriuso J, Olmos D, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol 2009;27:2692–6. 10.1200/JCO.2008.19.5081 [DOI] [PubMed] [Google Scholar]
- 22.Wheler J, Tsimberidou AM, Hong D, et al. Survival of 1,181 patients in a phase I clinic: the MD anderson clinical center for targeted therapy experience. Clin Cancer Res 2012;18:2922–9. 10.1158/1078-0432.CCR-11-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark TG, Bradburn MJ, Love SB, et al. Survival analysis Part I: basic concepts and first analyses. Br J Cancer 2003;89:232–8. 10.1038/sj.bjc.6601118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FE HJ. Regression modeling strategies. 2nd ed Springer, 2015. [Google Scholar]
- 25.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917–27. 10.1016/S1470-2045(16)30107-3 [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-001073supp001.pdf (97.7KB, pdf)
esmoopen-2020-001073supp002.pdf (599.3KB, pdf)