Abstract
Introduction
Poly-(ADP)-ribose polymerase (PARP) inhibitors are successfully used for treatment of BRCA-mutated (mut) breast cancers and are under extensive evaluation for BRCA- and PALB2-mutated pancreatic ductal adenocarcinoma (PDAC). However, the optimal treatment regimen for BRCA/PALB2-mutated PDCA has yet to be established. Moreover, limited data are available on the association of BRCA/PALB2 gene alterations with other comutations and immunological biomarkers.
Material and methods
Tumour samples of 2818 patients with PDAC were analysed for BRCA1/2 PALB2 mutations and other genes by next-generation sequencing (NGS) (MiSeq on 47 genes, NextSeq on 592 genes). TMB was calculated based on somatic non-synonymous missense mutations. MSI-H/dMMR was evaluated by NGS, and PD-L1 expression was determined using immunohistochemistry.
Results
In 4.2% (n=124) of all PDAC samples BRCA mutations have been detected. BRCA2 mutations were more commonly observed than BRCA1 mutations (3.1%(n=89) vs 1.1% [n=35], p<0.0001). BRCA2 mutation was associated with an older age (64 vs 61 years for wild-type (wt), p=0.002) and PALB2 mutation was observed more frequently in female than in male patients. BRCA and PALB2 mutations were associated with MSI-H/dMMR compared with wt (BRCA: 4.8% vs 1.2%, p=0.002; PALB2: 6.7% vs 1.3 %, p=0.18), PD-L1 expression of >1.0% (BRCA: 21.8% vs wt 11.2%, p<0.001, PALB2: 0.0% vs 12.4 %, p=0.38) and high TMB (BRCA: mean 8.7 vs 6.5 mut/MB, p<0.001; PALB2: 10.6 mut/Mb vs 6.6 mut/Mb, p=0.0007). Also, PD-L1 expression and TMB differed between BRCA and PALB2 mutation and wt samples in MSS tumours (p<0.05). BRCA-mutated and PALB2-mutated PDACs were characterised by a different mutational profile than was observed in wt tumours.
Conclusions
BRCA and PALB2 mutations were found in a significant subgroup of PDACs. These mutations were associated with a distinct molecular profile potentially predictive for response to immune-checkpoint inhibitor therapy. Therefore, these data provide a rationale to evaluate PARP inhibitors in combination with immune-checkpoint inhibitors in patients with BRCA/PALB2-mutated PDAC.
Keywords: pancreatic cancer, BRCA, PALB2, molecular profile
Key questions.
What is already known about this subject?
Patients with pancreatic ductal adenocarcinoma (PDAC) have an overall poor prognosis and new therapeutic strategies are needed. BRCA and PALB2 mutations have been described in a subset of pancreatic cancer patients but their molecular landscape is unknown. Especially the correlation of BRCA and PALB2 mutations with immune-related biomarkers are missing.
What does this study add?
We present a large study investigating the molecular landscape of patients with BRCA-mutated and PALB2-mutated pancreatic cancer. These subgroups appear to be characterised by a distinct molecular profile. Of note, cancers that carry theses mutations are associated with the expression of biomarkers that are potentially associated with response to immunotherapy such as TMB, PD-L1 expression and MSI status.
How might this impact on clinical practice?
This study addresses a current topic, since the recent approval of maintenance olaparib has completely modified the therapeutic strategy of BRCA-mutated PDACs during the last 2 years. Additionally, our findings suggest an intriguing speculation regarding the promising efficacy of testing Poly-(ADP)-ribose polymerase (PARP)-inhibitors with immune-checkpoint inhibitors in this poor-prognosis tumour. Thus, clinical trials testing both PARP-inhibitors with immune-checkpoint inhibitors are clearly warranted in the near future.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth-leading cause of cancer-related death worldwide.1 In early-stage disease, the 5-year overall survival for patients with stage IA PDAC is about 14%. In the metastatic stage only <1% of patients are alive at 5 years after diagnosis.2 The identification of molecular mechanisms leading to the development of PDAC is of utmost importance for understanding the nature of pancreatic cancer in order to elucidate new therapeutic options. For PDACs, four subtypes have been defined and characterised as stable, locally rearranged, scattered, unstable. The unstable subtype describes PDACs with genomic instability due to defects in DNA repair.3 The family of DNA damage response (DDR) proteins includes, besides others, BRCA and PALB2. A family history of PDAC can be found in 5%–10% of PDAC patients,4 and is associated with hereditary ovarian and breast cancer syndromes.5 6 BRCA1 and BRCA2 are the most common of the known germline mutations involved in familial pancreatic cancer.7 Somatic BRCA1 and BRCA2 mutations, on the other hand, are found in 1%–4% of PDACs.3 8 Similarly, germline mutations in PALB2 have been associated with an increased risk of PDAC development.9 PALB2 encodes a protein essential for double strand break repair and homologous recombination by serving as a bridging molecule, which connects the BRCA complex and stimulates the strand invasion of RAD51.10
Targeting BRCA1/2 and PALB2 is considered an attractive therapeutic concept in various cancers, since resistance to genotoxic therapies has been associated with increased DDR signalling and many cancers harbour defects in components of this system.11 Preclinical and early phase clinical trials have demonstrated promising effects.12 13 Consequently, this provides justification for the development of clinical trials to test DDR targeting agents either as single agents or in combination. Several preclinical studies provided a rationale for the use of Poly-(ADP)-ribose polymerase (PARP) inhibitors in patients with mutations in DNA-repair proteins such as BRCA1/2 and PALB2. In BRCA-mutated breast or ovarian cancer patients, the introduction of such targeted agents has resulted in improved outcomes in the first-line setting and beyond.14 15 Only recently, a phase III trial investigating the PARP inhibitor olaparib in PDAC with BRCA mutations has been published.16 According to this study, maintenance treatment with olaparib after chemotherapy lead to a significantly longer progression-free survival compared with placebo (7.4 months vs 3.8 months, p=0.004, HR=0.53). Moreover, a study investigating rucaparib, another PARP inhibitor, showed promising efficacy in patients with BRCA as well as PALB2 mutations.17
In this study, we aimed to analyse the molecular portrait of BRCA1/2-mutated and PALB2-mutated PDACs to support rational decision making for the emerging treatment options in this entity.
Material and methods
Samples
Formalin-fixed paraffin-embedded specimens derived from patients from around the world with PDAC were sent for analysis. Between April 2015 and January 2018, 2818 tumour tissue specimens were analysed in a commercial CLIA-certified laboratory (Caris Life Sciences, Phoenix, Arizona, USA). Patients agreed to submission of their tumour specimen alone, but no clinical data on survival or treatment were submitted to Caris Life Science. The only information that was available was basic demographic data such as age, sex, origin and date of the tumour sample. The analysis of this study was performed following the guidelines of the Declaration of Helsinki, Belmont report and US Common rule. Deidentified, retrospective data were used in keeping with 45 CFR 46.101(b)(4). As such, no patient consent was necessary as this study is considered IRB exempt.
Next-generation sequencing and immunohistochemistry
Immunohistochemistry and next-generation sequencing was performed on 2818 tumour samples. The methods used for testing were already extensively described in our recent publication.18
Statistical analysis
All statistical analyses were performed with JMP V.10.0 (SAS Institute), or R V.3.5.1 (R Development Core Team). The comparisons of continuous data were performed using Student’s t-test, and those of categorical data were done using Fisher’s exact test.
Results
Patient characteristics
In total, 2818 patients with histologically confirmed PDAC were molecularly profiled. BRCA1, BRCA2 and PALB2 mutations were detected in 1.3% (n=37), 3.1% (n=89) and 0.6% (n=15), respectively (see table 1). No patient with a BRCA1/2 mutation showed a concomitant PALB2 mutation and vice versa. BRCA2-mutated (mut)PDAC patients were significantly older than wild-type (wt) cases (64 vs 61 years, p=0.002). Whereas age was similar in BRCA1-mut or PALB2-mut PDAC compared with wt patients. In PALB2-mut PDAC a female predominance was observed whereas there was no significant association with gender for BRCA1-mut and BRCA2-mut PDAC.
Table 1.
Patients characteristics
| BRCA1 | P value | BRCA2 | P value | PALB2 | P value | ||||
| Mutation | Wild-type | Mutation | Wild-type | Mutation | Wild-type | ||||
| Total cases No (%) |
37 (1.3) | 2818 (98.7) | 89 (3.1) | 2754 (96.9) | 15 (0.6) | 2405 (99.4) | |||
| Median Age (years) |
64 | 60 | 0.055 | 64 | 61 | 0.002 | 67 | 65 | 0.5426 |
| Gender (%) | |||||||||
| Female | 15 (40.5) | 1318 (46.8) | ns | 41 (46.1) | 1289 (46.8) | ns | 11 (73.3) | 1114 (46.3) | 0.0265 |
| Male | 22 (59.5) | 1500 (53.2) | 48 (53.9) | 1465 (53.2) | 4 (26.7) | 1291 (53.7) | |||
ns, not significant.
BRCA1/2-mutated and PALB2-mutated PDACs have a distinct molecular profile
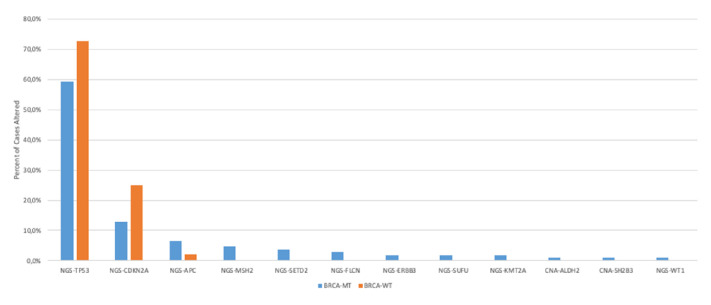
To investigate genetic co-alterations in BRCA1/2 as well as PALB2 mutations we investigated the molecular portrait and compared them with wt cases. Since the molecular profile of BRCA1-mut and BRCA2-mut PDACs was similar, we combined these cohorts for further analysis. Figure 1 depicts the most significantly altered genetic alterations in BRCA-mut versus BRCA-wt PDACs (see also online supplemental table 1).
Figure 1.
The genetic landscape of BRCA-mutated PDAC (p<0.05). NGS, next-generation sequencing; PDAC, Pancreatic ductal adenocarcinoma.
esmoopen-2020-000942supp001.pdf (40.2KB, pdf)
The mutational landscape of BRCA-mut tumours differed significantly from BRCA-wt cases: TP53 (59.3% vs 72.8%, p=0.001), CDKN2A (12.9% vs 24.9%, p=0.005), APC (6.5% vs 2.2%, p=0.008), SETD2 (3.7% vs 0.4%, p=0.001), FLCN (2.8% vs 0.2%, p=0.003), ERBB3 (1.9% vs 0.2%, p=0.025), SUFU (1.9% vs 0.0%, p=0.006), WT1 (1.0% vs 0.0%, p=0.043) as well as KMT2A (1.9% vs 0.2%, p=0.037).
Significant differences in copy number alterations between BRCA-mut and BRCA-wt were observed for ALDH2 (1.0% vs 0.0%, p=0.044) and SH2B3 (1.0% vs 0.0%, p=0.044).
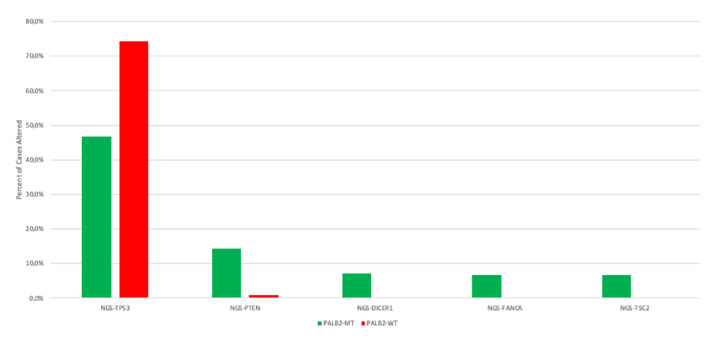
Concerning PALB2 mutations, significant differences in concomitant gene alterations as compared with PALB2-wt were observed in TP53 (46.7% vs 74.3 % p=0.03), PTEN (14.3% vs 0.8 %, p=0.007), DICER1 (7.1% vs 0.2 %, p=0.035), FANCA (6.7% vs 0.2%, p=0.03) and TSC2 (6.7% vs 0.0%, p=0.01). Other frequently altered genes were KRAS (66.7% vs 83.5 %, p=0.08), ARID1A (50.0% vs 19.9%, p=0.18) and CDKN2A (30.8% vs 24.4 %, p=0.53) (figure 2, online supplemental table 2). With regard to copy number alterations, no significant difference were observed.
Figure 2.
The molecular landscape of PALB2-mutated PDAC (p<0.05). NGS, next-generation sequencing; PDAC, Pancreatic ductal adenocarcinoma.
esmoopen-2020-000942supp002.pdf (39.2KB, pdf)
BRCA/PALB2 mutations are associated with biomarkers predicting response to immune-checkpoint inhibitors
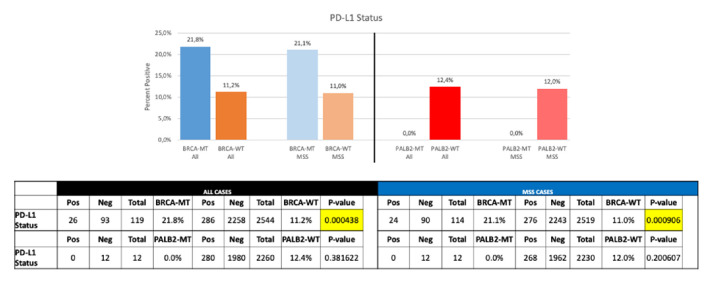
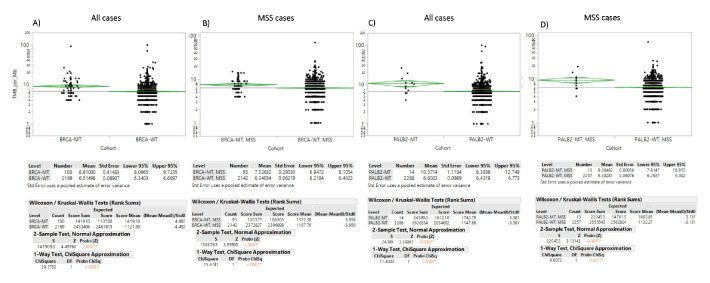
Mutations in BRCA1/2 as well as in PALB2 were associated with a higher programmed-death ligand 1 (PD-L1) expression compared with wt samples (figure 3). PD-L1 expression of more than 1% was detected in 21.8% of BRCA-mut samples as compared with 11.2% in BRCA-wt PDAC (p<0.0001). No difference in PD-L1 staining >1% between PALB2-mut and PALB2-wt were observed. Mean tumour mutational burden (TMB) was 8.91 mut/Mb and 6.51 mut/Mb (median=7 mut/MB vs 6 mut/MB) for BRCA-mut and BRCA-wt PDAC, respectively (p<0.0001; figure 4A). Similarly, in PALB2-mut PDAC a significant higher TMB was found than in PALB2-wt (median 9.5 vs 6 mut/MB, mean 10.57 vs 6.6 mt/MB; p=0.0007) (figure 4C).
Figure 3.
PD-L1 staining in PALB2/BRCA wild-type and mutated cases.
Figure 4.
Mean TMB in BRCA and PALB2 mutated versus wild-type PDAC: (A) BRCA mutations in all cases, (B) BRCA mutations in MSS, (C) PALB2 mutations in all cases, (D) PALB2 mutations in MSS. TMB, Tumour mutational burden; MSS, microsatellite status stable.
BRCA/PALB2-mutated pancreatic cancer is related to MSI-H/dMMR status
In 1.3% of tested PDAC samples a microsatellite instability-high (MSI-H)/damaged mismatch repair (dMMR) was observed. 4.8% of BRCA-mut tumours showed an MSI-H/dMMR, whereas 1.2% of BRCA-wt samples yielded a MSI-H/dMMR status (p=0.01; figure 5). MSI-H/dMMR in PALB2-mut PDAC specimens was detected in 6.7% compared with 1.3% in PALB2-wt samples (p=0.18).
Figure 5.
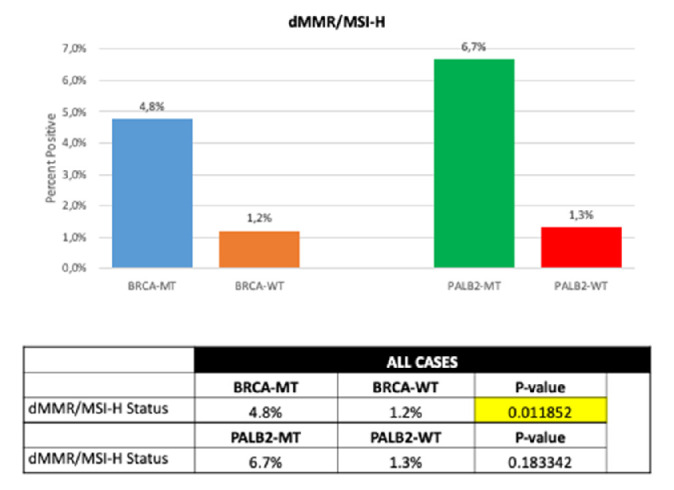
dMMR/MSI-H in PALB2/BRCA wild-typeversus mutated patients. MSI-H/dMMR, Microsatellite instability-high / damaged mismatch repair.
Within the MSI-H/dMMR subgroup, BRCA-mut PDACs yielded a higher TMB (median 7 mt/Mb; mean 7.52 mt/Mb) as compared with BRCA-wt (median 6 mt/Mb; mean 6.34 mt/Mb; p≤0.001). TMB was also higher in the MSS group (median 7 vs 6 mt/MB; mean 7.4 vs 6.3 mt/Mb; p<0.0001, figure 4B).
A numerically higher frequency of MSI-H/dMMR (6.7% vs 1.3%, p=0.18) and higher TMB (median 9.5 vs 6 mt/MB, mean 10.57 vs 6.6 mt/Mb, p=0.0007) was observed in PALB2-mut compared with PALB2-wt cancers. In the MSS subgroup, PALB2-mut tumours had a TMB of median 9 vs 6 mt/MB (mean of 9.57 mt/Mb) in contrast to 6.38 mt/Mb in PALB2-wt cases (figure 4D).
Discussion
In this study, we aimed to delineate the genetic profile of BRCA1/2-mut and PALB2-mut PDACs. To the best of our knowledge, this is the largest study investigating the mutational profile of BRCA1/2-mut as well as PALB2-mut PDACs. BRCA1, BRCA2 and PALB2 mutations were found in 1.3%, 3.1%, and 0.6% of the patients, which is in line with previous series.3 8
The therapeutic implications of identifying mutations in these genes, either germline or somatic, have increased dramatically over the past few years. The inhibiting of PARP to generate a synthetic lethal effect in cancer cells with DDR-gene mutations has led to promising outcomes in many cancer entities.19 PARP inhibitors cause multiple double strand breaks, and in tumours with BRCA and PALB2 mutations, these double strand breaks cannot be efficiently repaired, leading to the death of cancer cells. In contrast, normal cells do not replicate their DNA as often as cancer cells, and they frequently have other DDR genes, which allow them to survive the inhibition of PARP.20
Only recently the PARP inhibitor olaparib has been tested within the phase III POLO trial as a maintenance treatment in BRCA-mut PDAC after platinum-based therapy. A prolonged progression-free survival (7.4 vs 3.8 months) and higher response rates (RR; 23% vs 12 %) were observed within the cohort treated in the olaparib arm compared with placebo.16 A randomised multicentre phase II trial investigated the combination of gemcitabine and cisplatin with or without veliparib in 52 patients with metastatic BRCA/PALB2-mut PDAC. The addition of veliparib, however, failed to improve patient outcomes in this study, although, the RR in both treatment arms were encouraging (65.2% and 74.2%), indicating that patients with PDAC with alterations in DDR genes may benefit from platinum-based treatment regimens.21 This finding is in line with data from another study by Golan et al investigating the effect of neo-adjuvant FOLFIRINOX in BRCA2-mut PDACs, which lead to complete pathological responses in 44.4% of BRCA2-mut patients vs 10% in BRCA2-wt patients.22 A meta-analysis including six trials confirmed the strategy of intitial treatment with platinum-based chemotherapy in patients with a germline BRCA mutation. At the same time, the authors called for more and higher-quality trials in this setting.23
Further studies are desirable to evaluate PARP inhibitors in different treatment settings in DDR-altered PDACs.
Taking into consideration the broadly accepted model of PDAC development, which postulates stepwise mutations in KRAS, CDKN2A, TP53 and SMAD4,24 it revealed that BRCA-mut as well as PALB2-mut PDACs are associated with a distinct molecular profile as compared with wt cases. In our cohort, BRCA-mut samples had significantly fewer mutations in TP53 and CDKN2A than BRCA-wt specimens. However, no differences were found in the prevalence of KRAS and SMAD4 alterations. In PALB2-mut samples, a lower mutational rate of TP53 was observed. BRCA1/2 mutations lead to DDR deficiency driving their susceptibility to cancer development.3 25 Therefore, our data support the hypothesis that carcinogenesis in BRCA1/2 and PALB2 patients differs from patients with PDAC-wt tumours.
The DNA damage caused by PARP inhibition leads to increased neoantigen and tumour-associated antigen expression.26 This reshapes the tumour microenvironment and has the potential to restore the antitumour immune response, which can be further enhanced by treatment with immune-checkpoint inhibitors. Additionally, preclinical data suggest PARP inhibition may have immunomodulatory potential.27 Two prostate carcinoma cell lines, one with a known BRCA2 mutation and the other without mutations in BRCA1/2 were exposed to olaparib. Natural killer cell-mediated lysis increased in both cell lines regardless of BRCA phenotype.28 Of note, we detected a higher prevalence of MSI-H/dMMR status in BRCA-mut than BRCA-wt samples (4.8% vs 1.2%) and a numerically higher MSI-H/dMMR status in PALB2-mut than in PALB2-wt specimens (6.7% vs 1.3%). Compared with another series conducted by Hu and colleagues the prevalence of MSI-H/dMMR appears higher, since in that study a prevalence of only 0.8% in PDAC was observed.29 Of note, only germline variants were reported in that study, whereas in our cohort we included both germline variants and somatic mutations. Due to the study design that included only tumour DNA of the available specimens we were unable to make a distinction between germline and somatic mutations.
The association of BRCA mutations and an MSI-H/dMMR status may have implications on the use of immune-checkpoint inhibitors in BRCA-mut and PALB2-mut PDAC, as pembrolizumab has been approved as a site agnostic drug for use in any tumour manifesting MSI-H/dMMR tumours by the FDA.30 Furthermore, immune-related biomarkers such as TMB and PD-L1 expression levels were highly expressed in BRCA-mut tumours. This is in line with data coming from other tumours, showing that PDL-1 status correlates with "BRCAness", TMB as well as MSI-H/dMMR status.31 32 However, the best predictive marker for response to immunotherapy is still to be defined. Of note, recently Shim et al33 presented a human leucocyte antigen corrected TMB, that included loss of heterozygosity and enabled a better prediction of response to immune-checkpoint inhibitors. Moreover, the value of PD-L1 expression as a predictive biomarker for the treatment with PD-1/PD-L1 inhibitors in PDAC remains inconclusive.34 35
For PALB2-mut PDAC we observed a numerical difference in PD-L1 staining in contrast to the findings in tumours that were PALB2-wt. However, this finding did not reach statistical significance. Taking into consideration the high rate of MSI-H/dMMR and the low rate of PD-L1 positivity in PALB2-mut PDACs, further studies are required to elucidate how PALB2 mutations influence the immune microenvironment in PDAC and other entities. This can help to understand how PALB2 mutations may affect the efficiency of treatment for these patients with an immune-checkpoint inhibitor.
Further limitations in our study need to be mentioned as well. Due to the retrospective nature of our study, a selection bias may be present. Moreover, no clinical and personal data, such as survival, treatment protocols and patient’s outcome are available. Therefore, we are not able to analyse the prognostic and predictive value of BRCA and PALB2 mutations in PDAC.
Since our results show that a subset of BRCA-mut PDACs express immuno-related biomarkers, it might be reasonable to test PARP-inhibitor with immune-checkpoint inhibitors.26 Such combinations are currently being tested in several other tumour types. Promising combinations include those tested in the TOPACIO trial (NCT 02657889)36 that combines pembrolizumab with niraparib in triple-negative breast and ovarian cancer, and in the MEDIOLA trial (NCT 02734004) which combines durvalumab with olaparib in advanced solid tumours. Single-agent checkpoint inhibition showed only low activity in PDAC, so far.37 However, when looking on MSI-H/dMMR status, response rates increased from 0% to approximately 18%.30
Conclusion
To the best of our knowledge, this is the largest study describing the molecular portrait of BRCA1/2- and PALB2-mutated PDACs. We showed that these mutations are associated with a distinct genetic profile and are associated with predictive immunotherapy-related biomarkers suggesting a potential rationale to combine PARP-inhibitors with immune-checkpoint inhibitors. The hypothesis generated in this study should be evaluated further and must be proven in investigational trials.
Footnotes
Twitter: @agrothey
AS and KZ contributed equally.
CF and GS contributed equally.
Contributors: All authors contributed significantly to this work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JX, AE and WMK are employers of Caris Life Sciences. JLM and AS are consultants for Caris Life Sciences. RMG received research and travel support from Caris Life Sciences. AFS received research, travel, and speaking fees from Caris Life Sciences. AG, HL and AS received travel support from Caris Life Sciences.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Waddell N, Pajic M, Patch A-M, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495–501. 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog 2012;51:14–24. 10.1002/mc.20855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132–8. 10.1038/bjc.2014.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedenson B. Brca1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed 2005;7:60. [PMC free article] [PubMed] [Google Scholar]
- 7.Leung K, Saif MW. BRCA-associated pancreatic cancer: the evolving management. JOP 2013;14:149–51. 10.6092/1590-8577/1462 [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu, Cancer Genome Atlas Research Network . Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017;32:e13:185–203. 10.1016/j.ccell.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi S, Doi M, Ikari N, et al. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci Rep 2018;8:8105. 10.1038/s41598-018-26526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Zhou J, Zhang K, et al. Molecular mechanisms of PALB2 function and its role in breast cancer management. Front Oncol 2020;10:301. 10.3389/fonc.2020.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber AM, Ryan AJ. Atm and ATR as therapeutic targets in cancer. Pharmacol Ther 2015;149:124–38. 10.1016/j.pharmthera.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Hengel SR, Spies MA, Spies M. Small-Molecule inhibitors targeting DNA repair and DNA repair deficiency in research and cancer therapy. Cell Chem Biol 2017;24:1101–19. 10.1016/j.chembiol.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandsma I, Fleuren EDG, Williamson CT, et al. Directing the use of DDR kinase inhibitors in cancer treatment. Expert Opin Investig Drugs 2017;26:1341–55. 10.1080/13543784.2017.1389895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggio F, Bruzzone M, Ceppi M, et al. Single-agent PARP inhibitors for the treatment of patients with BRCA-mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open 2018;3:e000361. 10.1136/esmoopen-2018-000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomao F, Bardhi E, Di Pinto A, et al. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: an updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat Rev 2019;80:101909. 10.1016/j.ctrv.2019.101909 [DOI] [PubMed] [Google Scholar]
- 16.Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019;381:317–27. 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binder KAR, Mick R, O'Hara M, et al. Abstract CT234: a phase II, single arm study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic mutation in BRCA1, BRCA2 or PALB2. Cancer Res 2019;79 10.1158/1538-7445.AM2019-CT234 [DOI] [PubMed] [Google Scholar]
- 18.Spizzo G, Puccini A, Xiu J, et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open 2020;5:e000682. 10.1136/esmoopen-2020-000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi M, Dong B, Qin S, et al. Advances and perspectives of PARP inhibitors. Exp Hematol Oncol 2019;8:29. 10.1186/s40164-019-0154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord CJ, Ashworth A. Parp inhibitors: synthetic lethality in the clinic. Science 2017;355:1152–8. 10.1126/science.aam7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Reilly EM, Lee JW, Zalupski M, et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J Clin Oncol 2020;38:1378–88. 10.1200/JCO.19.02931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golan T, Barenboim A, Lahat G, et al. Increased rate of complete pathologic response after neoadjuvant Folfirinox for BRCA mutation carriers with borderline resectable pancreatic cancer. Ann Surg Oncol 2020;27:3963–70. 10.1245/s10434-020-08469-8 [DOI] [PubMed] [Google Scholar]
- 23.Rebelatto TF, Falavigna M, Pozzari M, et al. Should platinum-based chemotherapy be preferred for germline breast cancer genes (BRCA) 1 and 2-mutated pancreatic ductal adenocarcinoma (PdaC) patients? A systematic review and meta-analysis. Cancer Treat Rev 2019;80:101895. 10.1016/j.ctrv.2019.101895 [DOI] [PubMed] [Google Scholar]
- 24.Pelosi E, Castelli G, Testa U. Pancreatic cancer: molecular characterization, clonal evolution and cancer stem cells. Biomedicines 2017;5. 10.3390/biomedicines5040065. [Epub ahead of print: 18 Nov 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy R, Chun J, Powell SN. Brca1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer 2011;12:68–78. 10.1038/nrc3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li A, Yi M, Qin S, et al. Prospects for combining immune checkpoint blockade with PARP inhibition. J Hematol Oncol 2019;12:98. 10.1186/s13045-019-0784-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov 2019;9:646–61. 10.1158/2159-8290.CD-18-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenerty KE, Padget M, Wolfson B, et al. Immunotherapy utilizing the combination of natural killer- and antibody dependent cellular cytotoxicity (ADCC)-mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition. J Immunother Cancer 2018;6:133. 10.1186/s40425-018-0445-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res 2018;24:1326–36. 10.1158/1078-0432.CCR-17-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with Noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato H, Niimi A, Yasuhara T, et al. Dna double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun 2017;8:8. 10.1038/s41467-017-01883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeber A, Puccini A, Xiu J, et al. Association of BRCA -mutant pancreatic cancer with high tumor mutational burden (TMB) and higher PD-L1 expression. JCO 2019;37:4133 10.1200/JCO.2019.37.15_suppl.4133 [DOI] [Google Scholar]
- 33.Shim JH, Kim HS, Cha H, et al. HLA-corrected tumor mutation burden and homologous recombination deficiency for the prediction of response to PD-(L)1 blockade in advanced non-small-cell lung cancer patients. Ann Oncol 2020;31:902–11. 10.1016/j.annonc.2020.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Guo C-Y, Tou F-F, et al. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. Int J Cancer 2020;147:116–27. 10.1002/ijc.32744 [DOI] [PubMed] [Google Scholar]
- 35.Macherla S, Laks S, Naqash AR, et al. Emerging role of immune checkpoint blockade in pancreatic cancer. Int J Mol Sci 2018;19. 10.3390/ijms19113505. [Epub ahead of print: 07 Nov 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-Arm phases 1 and 2 trial of Niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol 2019. 10.1001/jamaoncol.2019.1048. [Epub ahead of print: 13 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott PA, Bang Y-J, Piha-Paul SA, et al. T-Cell-Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol 2019;37:318–27. 10.1200/JCO.2018.78.2276 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000942supp001.pdf (40.2KB, pdf)
esmoopen-2020-000942supp002.pdf (39.2KB, pdf)