Abstract
Toothache is a common painful consequence of damage to the teeth, particularly when coupled to infection. Clinical restoration of tooth integrity, sometimes involving physical and chemical sterilization of the tooth with nerve fiber ablation (i.e., endodontic therapy), generally alleviates pain and allows long-lasting dental function. These observations raise questions regarding the biological role of tooth-innervating fibers. Here, we determined the transcriptomic diversity of the sensory neurons that can be retrogradely labeled from mouse molar teeth. Our results demonstrate that individual molars are each targeted by a dedicated population of about 50 specialized trigeminal neurons. Transcriptomic profiling identifies the majority of these as expressing markers of fast-conducting neurons, with about two-thirds containing nociceptive markers. Our data provide a new view of dental innervation, extending previous reports that used candidate gene approaches. Importantly, almost all retrogradely labeled neurons, including nociceptors, express the recently characterized mechanosensor Piezo2, an ion channel that endows cells with sensitivity to gentle touch. Intriguingly, about a quarter of the labeled neurons do not appear to be nociceptors, perhaps insinuating a role for them in discriminative touch. We hypothesize that dental neurons are capable of providing mechanosensitive information to drive rapid behavioral responses and protect teeth from damage. Damage to the teeth and exposure of the large population of molar nociceptors may trigger prolonged or abnormal activation driving toothache. Future studies examining the responses of these transcriptomically defined classes of neurons will help define their significance in oral sensation.
Keywords: neuroscience/neurobiology, pulp biology, gene expression, pain, receptors, mechanotransduction
Introduction
Sensory input provides salient information about the environment that can be crucial for survival. Somatosensation, encompassing our senses of touch, temperature, and proprioception, relies on neurons with cell bodies in the trigeminal and dorsal root ganglia that innervate the periphery, with the trigeminal system targeting the head and neck. Nociceptors are tuned to detect noxious stimuli, trigger pain, and elicit protective responses, whereas other somatosensors are activated by low-threshold stimuli providing discriminative input. Reflecting these differential roles, the sensory neurons exhibit diversity in soma sizes, conduction velocities, anatomic specializations, peripheral and central targets, as well as gene expression profiles (Le Pichon and Chesler 2014). Recently, single-cell (sc) transcriptomics defined about a dozen major transcriptomic classes related to functional differences among the cells (Usoskin et al. 2015; Nguyen et al. 2017; Nguyen et al. 2019). We developed an in situ hybridization (ISH)–based approach for identifying these classes at a cellular level in tissue sections (von Buchholtz et al. 2020). By coupling this technique with retrograde tracing, the types of neurons innervating specific targets can be assessed without the bias of selecting candidate genes (Chaudhary et al. 2001; Gibbs et al. 2011; Won et al. 2017).
Trigeminal neurons innervating the teeth can drive toothache, a very common type of pain (Scully 2013). Although there is consensus that many neurons innervating teeth are nociceptors (Fried and Gibbs 2014), the great range reported to express specific markers makes it difficult to assess their specialization. For example, the neuropeptide calcitonin gene–related peptide (CGRP), generally considered a marker for nociceptors, has been reported to be present in 15% to 60% of tooth-projecting neurons (Fried et al. 1989; Gibbs et al. 2011; Michot et al. 2018). Estimates for other nociceptive channels and transduction molecules are similarly variable (Chaudhary et al. 2001; Ichikawa and Sugimoto 2001; Stenholm et al. 2002; Kvinnsland et al. 2004; Park et al. 2006; Hermanstyne et al. 2008; Chung et al. 2011; Kim et al. 2011). These discrepancies make it difficult to assess what sensory input an animal would normally receive. Nonetheless, it is commonly believed that activation of the sensory neurons targeting the teeth almost always results in pain, often reflecting damage to a tooth (Fried and Gibbs 2014). Given the importance of teeth for effective intake of nutrients (Nowjack-Raymer and Sheiham 2003, 2007), we reasoned that tooth sensation more likely evolved to fulfill a protective need (e.g., signaling mechanical threats to tooth integrity). To better understand how innervation defines tooth sensation, we set out to characterize the transcriptomic profiles of trigeminal neurons that could be retrogradely labeled from the teeth. Our results reveal that in mice, each molar is richly innervated by neurons with fast-conducting gene expression profiles. Finally, although we confirm that the majority of tooth-projecting neurons are nociceptors, each molar is selectively targeted by about a dozen cells likely to signal discriminative touch.
Materials and Methods
See Appendix Expanded Methods for full description of experimental procedures used.
Animal experiments were carried out in strict accordance with the guidelines of the US National Institutes of Health for the care and use of laboratory animals and were approved by the National Institute of Dental and Craniofacial Research’s Animal Care and Use Committee. All experiments used adult male and female FVB/N mice.
Retrograde tracers (Thermo Fisher Scientific) were reconstituted in Milli-Q water and used for molar neuron labeling (Song et al. 2017): shallow occlusal cavitations into dentin were prepared with a 1/4 round dental bur and a micromotor drill. All-in-one bonding agent (Kerr) was applied to axial surfaces of the tooth and surrounding tissues to create a hydrophobic barrier. Various retrograde tracers have been used to explore the peripheral targeting of sensory neurons, including wheat germ agglutinin (WGA) and cholera toxin B-subunit (CTB), which can be conjugated to different fluorophores. We found that WGA and CTB conjugates performed exceptionally well as compared with other labels and provided robust neuronal labeling within 24 h of application. These tracers were applied and allowed to dry: for WGA, application was repeated for >20 min; for CTB, >5 min. The labeled teeth were then covered (Flow-It ALC B2; Pentron) to seal the preparation. Initially, we explored the depth of preparations and found that pulp exposure led to inconsistencies with detection of small numbers of neurons in some animals, whereas shallow cavitation into dentin resulted in reliable labeling with minimal damage to tooth structure. For the palate, 2 µL of tracer was injected into the rugae of the hard palate across multiple sites. Trigeminal ganglia were dissected 16 to 20 h following tracer application and immediately processed for clearing or ISH.
3DISCO cleared ganglia (Launay et al. 2015) were imaged with light sheet microscopy. Reconstructions were generated with Vision4D software (Arivis) and used for quantitation.
ISH and automatic transcriptomic class assignment was carried out as described previously (von Buchholtz et al. 2020).
Results
Identifying Trigeminal Neurons Projecting to the Molar Teeth
To better understand the significance of sensory innervation of the teeth, we selectively labeled dental pulp afferent neurons in mice (Fig. 1A) and characterized their transcriptomic profile using multigene ISH (von Buchholtz et al. 2020) with the goal of achieving their comprehensive and unbiased characterization.
Figure 1.
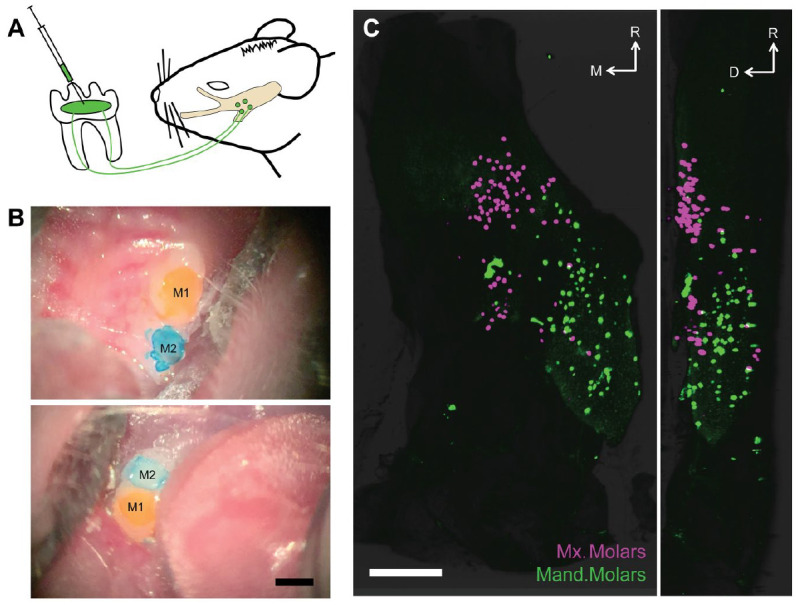
Retrograde labeling of the trigeminal sensory neurons reveals rich and selective innervation of molar teeth. (A) Schematic depicting our labeling paradigm: briefly, we prepared shallow surgical cavitations in the mouse molars, applied retrograde tracer, and observed robust labeling in somata of trigeminal sensory neurons from 16 to 72 h after labeling. (B) Images of maxillary (top) and mandibular molars (bottom) after application of distinct retrograde tracers CTB-488 (yellow) and CTB-647 (blue) and placement of dental composite. Scale bar: 500 μm. CTB, cholera toxin B-subunit. M1 and M2 denote first and second molar within each arch. (C) Representative light sheet microscopy of an optically cleared right trigeminal ganglion with sensory neurons labeled from application of spectrally distinct CTB conjugates to ipsilateral maxillary (magenta) and mandibular (green) arches (the first and second molars in each quadrant are labeled). Two-dimensional maximum projection images of the whole ganglion from the dorsal (left panel) and lateral (right panel) views with rostral orientation toward the top. Scale bar: 500 μm. D, dorsal; M, medial; R, rostral. See Appendix Movie 1 for 3-dimensional reconstruction of these data.
Mouse dentition features 1 incisor and 3 molars per quadrant. In rodents, incisors continue to erupt throughout life, making them quite dissimilar to their counterparts in most other animals. Molars, however, are generally conserved among mammals and likely share patterns of innervation across species. Therefore, we concentrated on defining neurons innervating the larger 2 mouse molar teeth (molar neurons) in each quadrant of the mouth. As illustrated (Fig. 1B), we could selectively target adjacent teeth in the maxillary and mandibular arches. Figure 1C shows typical robust staining of molar neurons in the ipsilateral trigeminal ganglion cleared via the 3DISCO approach (Launay et al. 2015). Note that when maxillary and mandibular molars were differentially labeled, large groups of sensory neurons targeting a given arch were broadly distributed in the ganglion, but overlapped minimally with those from the other arch. As expected, the maxillary and mandibular molars received innervation from their respective trigeminal divisions, even though these divisions exhibit poorly defined boundaries (von Buchholtz et al. 2020). Furthermore, we observed segregation along the dorsoventral axis (Appendix Movies 1–3), which was consistent among mice.
Each Molar Is Innervated by a Distinct Population of Sensory Neurons
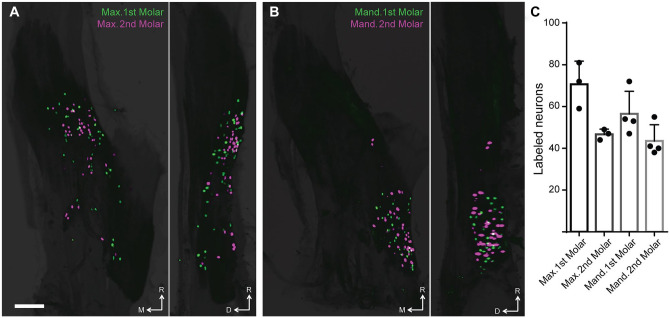
Given the number of neurons innervating teeth, we hypothesized that an individual tooth would be targeted by dedicated neurons to allow discrimination at the single tooth level. We therefore applied distinct CTB fluorescent conjugates to adjacent molars and quantitated neurons labeled by 1 or both dyes. Light sheet microscopy revealed that almost all molar neurons targeted a single tooth, with 738 of 745 (99%) of molar neurons selectively labeled by just 1 fluorophore (Fig. 2A, B). Colabeled neurons (7 of 745, <1%) may innervate the 2 adjacent teeth or reflect technical limitations. Quantitation of neurons innervating each tooth (Fig. 2C) illustrates the consistency of labeling among animals and reveals that each molar is targeted by approximately 50 sensory neurons (loosely correlated to molar size; Fig. 1B). The quantitative precision among animals underscores 3 important points. First, the approach is reproducible, presumably reflecting the encapsulated nature of the tooth. Second, most molar neurons that can be targeted by CTB are labeled, since otherwise greater variation would have been observed. Third, the number of neurons innervating a given tooth is highly conserved among animals. Our data also revealed that neurons targeting adjacent molars in a single arch were interspersed, as well as confirming the distinct distribution of maxillary and mandibular molar neurons.
Figure 2.
Adjacent mouse molars are targeted by distinct populations of sensory neurons. (A, B) Representative collapsed images (maximum projection light sheet) of optically cleared right trigeminal ganglia with sensory neurons labeled from application of spectrally distinct CTB conjugates to first (green) and second (magenta) molars. View of the whole ganglion from the dorsal (left panel) and lateral (right panel) views with the rostral orientation toward the top. (a) Maxillary and (b) mandibular teeth were labeled in different animals. Scale bar: 500 μm. CTB, cholera toxin B-subunit; D, dorsal; M, medial; R, rostral. (C) Bar graph representing the number of trigeminal sensory neurons labeled following application of single tracer to each tooth. Data are mean ± SEM from 3 mice for maxillary teeth and 4 mice from mandibular teeth. See Appendix Movies 2 and 3 for 3-dimensional reconstruction of these data.
Strikingly, the CTB-labeled molar neurons appeared large and relatively uniform in diameter (Fig. 2A, B), suggesting that very few polymodal nociceptors (small diameter neurons) were labeled with our approach. To investigate if this was indeed the case, we next used a powerful multigene ISH platform to determine the transcriptomic profiles of CTB-labeled molar neurons. Notably, as we had predicted, almost all molar neurons (385 of 401, 96%) were characterized by the expression of S100b—a marker for 3 distinct types of large myelinated sensory neurons (von Buchholtz et al. 2020). By contrast, few labeled cells expressed transcripts commonly found in polymodal nociceptors (Trpa1, Trpm8, Mrgprd, or Nppb). Of note, staining revealed that approximately 20% of S100b-positive molar neurons express the gene Calca (Fig. 3A), which encodes CGRP. Neurons expressing S100b in combination with Calca represent Aδ nociceptors (Nguyen et al. 2017) in keeping with the concept that the teeth have rich innervation by sensory neurons that directly evoke pain (Fried and Gibbs 2014). Importantly, the constellation of gene expression patterns found in CTB-targeted neurons suggests that the molars receive rich innervation by 2 transcriptomic classes of fast-conducting cells (Fig. 3B). Since a typical molar is innervated by approximately 50 neurons (Fig. 2C), only 2 small-diameter neurons innervating a tooth were retrogradely labeled by CTB. This is in stark contrast to the overall classification of trigeminal neurons or other craniofacial structures, such as the eye or meninges, which are instead targeted by a variety of neuronal classes (von Buchholtz et al. 2020).
Figure 3.
Multigene in situ hybridization (ISH) reveals that CTB-labeled molar neurons are dominated by 2 transcriptomic classes of large A-type sensory neurons. (A) Representative images from retrograde labeling of molar neurons and multigene ISH for a single section of a trigeminal ganglion. CTB–Alexa 555 was applied to the maxillary first and second molars; retrograde-labeled neurons were imaged (red); and the section was subjected to 3 rounds of ISH (green). Genes (left to right, beginning in the top row): S100 calcium-binding protein B (S100b), calcitonin gene-related peptide (CGRP; Calca), FXYD domain-containing ion transport regulator 2 (Fxyd2), natriuretic polypeptide B (Nppb), transmembrane protein 233 (Tmem233), mas-related G protein–coupled receptor member D (Mrgprd); transient receptor potential cation channel, subfamily A, member 1 (Trpa1); and transient receptor potential cation channel, subfamily M, member 8 (Trpm8). To aid identification of molar neurons, the retrograde labeling was overlaid on individual ISH images. Scale bar: 100 μm. CTB, cholera toxin B-subunit. Transcriptomic class of 401 molar neurons from 6 mice was determined per an automated UNet-based algorithm. Data are from 55 fields. (B) Stacked bar charts comparing the transcriptomic profile of molar neurons (left) with a general population of trigeminal sensory neurons from the whole ganglion (right). (C) Algorithm criteria for transcriptomic class assignment.
CTB and WGA Provide Unbiased Comprehensive Labeling of the Classes of Sensory Neurons Innervating the Molar Teeth
One concern is that CTB has been suggested to preferentially label large-diameter neurons whereas WGA better targets small-diameter cells (Robertson and Arvidsson 1985; Lamotte et al. 1991). Therefore, we explored if these 2 labels identified distinct populations of molar neurons. As a first step, an admixture of spectrally distinct CTB and WGA fluorescent conjugate tracers were applied to a single maxillary molar. Whole mount imaging (Fig. 4A, B) revealed nearly complete overlap of WGA and CTB fluorescence (153 of 165, 93% of neurons). Thus, when applied in excess to the encapsulated structure of a molar tooth, these water-soluble and diffusible tracers target a highly overlapping population of cells. As a control, we injected the same mix of retrograde tracers into the attached gingiva of the hard palate. Notably, a range of small and large neurons innervating this tissue was labeled (Fig. 4C) with much less overlap among probes. This difference likely reflects the greater spread of retrograde tracers in the palate where no defined physical barrier prevents diffusion.
Figure 4.
WGA and CTB tracing reveal similar comprehensive labeling and classification of molar neurons. (A) Collapsed image (maximum projection light sheet) of optically cleared right trigeminal ganglia with sensory neurons labeled from application of WGA–Alexa 488 (green) and CTB–Alexa 647 (magenta) to the maxillary first molar. Dorsal view with rostral orientation toward the left. Scale bar: 500 μm. L, lateral; R, rostral. (B) Panels highlight extensive overlap of CTB and WGA labeling. The magnified field is indicated by a white rectangle in panel A. Scale bar: 100 μm. (C) Images are shown for comparison from an optically cleared trigeminal ganglion with sensory neurons labeled by injection of spectrally distinct WGA and CTB tracers into the attached gingiva covering the hard palate. Note the larger number of small-diameter cells and the lower degree of overlap between the labels. Scale as shown in panel B. Similar results were obtained for these types of colabeling in 2 animals for each site of application. (D) Pie charts representing the transcriptomic classification of WGA- and CTB-labeled neurons innervating the palate (1635 neurons, 2 groups of 3 mice, 55 fields) and first and second maxillary molars (812 neurons, 4 groups of 3 mice, 97 fields) highlight that, whereas many classes of sensory neuron target the palate, the molar teeth are innervated primarily by C4 and C6 cells. (E) For cells targeting the molars (black solid bars) and the palate (gray open bars), fold enrichment of each class of neurons was calculated relative to prevalence in the whole ganglion (see Fig. 3B). Bar graph shows the mean + SEM (n = 4 groups, molars; 2 groups, palate) and the individual results from WGA (red dots) and CTB labeling (green dots). All classes other than C4 and C6 innervate the teeth less frequently than what would be expected (dashed line). See Appendix Table 2 for statistical analysis. (F) Distribution of diameters for CTB- and WGA-labeled cells targeting the molars (black solid bars) and the palate (gray open bars) as quantitated in tissue sections. CTB, cholera toxin B-subunit; WGA, wheat germ agglutinin.
We then characterized the transcriptomic profile of the neurons innervating the palate using ISH analysis. Our results (Fig. 4D) revealed that a broad array of neuronal classes targets this region of the oral cavity, with prominent representation of C-type nociceptors (classes C7 to C10) as well as cool-sensing neurons (C1 and C2). By contrast, the combined results from labeling molar neurons with both retrograde tracers confirmed that they are almost exclusively large-diameter A-type neurons. Comparison of labeling between mice and tracers 1) showed that in our study CTB and WGA were similarly effective at labeling different classes of sensory neurons, 2) demonstrated the consistency of our approach, and 3) substantiated the specialized nature of molar neurons (Fig. 4E) and their large diameter, particularly when compared with neurons labeled from the palate (Fig. 4F).
Large-Diameter Neurons Innervating the Teeth Include a Complex Assortment of Putative Nociceptors
Classification of trigeminal neurons is primarily based on the original scRNA sequence data where the large-diameter C4 and C6 classes were underrepresented (Nguyen et al. 2017). More recently, single nuclear (sn) sequencing has divided both these classes into a number of transcriptomic subgroups (Nguyen et al. 2019) that can be identified by expression of additional markers (see Appendix Table 1). We reasoned that these genes could be valuable for extending our classification of molar neurons. We adapted the multigene approach to probe the expression pattern of 10 additional transcripts (18 in total), including 5 that mark or have roles in subsets of nociceptors. WGA-labeled molar neurons were first classified with the standard 8 genes (Fig. 5A, top 2 rows). As expected, most molar neurons (580 of 668, 87%) expressed S100b, of which about a third also expressed Calca (188 of 668, 28%), defining them as predominantly C4 (59%) and C6 (28%) cells. Importantly, molar neurons were also positive for several of the new probes that we selected, helping define these cells in the context of the snRNA sequencing (Nguyen et al. 2019).
Figure 5.

Molars are innervated by distinct populations of large-diameter sensory neurons involved in nociception and discriminative touch. (A) Representative images are shown from retrograde tracing (WGA) and 6 rounds of multigene in situ hybridization for a single section of a trigeminal ganglion. Expression patterns of a panel of genes in molar neurons labeled by application of WGA–Texas red to the maxillary first and second molars allowed expanded classification by transcriptomic profile. Fluorescence microscopy images show a region of the ganglion with retrogradely labeled molar neurons (WGA). Red circles outline neurons labeled by WGA, allowing visual identification of their gene expression pattern. WGA staining and the genes used for 8-probe classification are shown in the top 2 rows. Other genes that map to subsets of C4 and C6 cells in single nuclear (sn) RNA sequencing (Nguyen et al. 2019) were examined (see Appendix Table 1 for details of their expression and properties). Genes were as follows: 5-hydroxytryptamine (serotonin) receptor 3A (Htr3a); transient receptor potential cation channel, subfamily V, member 1 (Trpv1); sodium channel, voltage-gated, type X, alpha-Nav1.8 (Scn10a); teashirt zinc finger family member 2 (Tshz2); neurofilament, heavy polypeptide-NF200 (Nefh); piezo-type mechanosensitive ion channel component 2 (Piezo2); protocadherin 7 (Pcdh7); bone morphogenetic protein receptor 1b (Bmpr1b); netrin G1 (Ntng1); and calretinin (Calb2). Data are from 44 fields. Scale bar: 100 μm. (B) Venn diagram indicates number and percentage of neurons expressing selected genes in the S100b-positive labeled cells (87%; 580 of 668 total molar neurons). Note that a fraction of the C6 cells were positive for Trpv1 and that Htr3a and Scn10a, genes involved in nociception, labeled large groups of C4 molar neurons. (C) Venn diagram depicting the expression of Piezo2 in neuron populations found in panel B. CTB, cholera toxin B-subunit; WGA, wheat germ agglutinin.
Trpv1 is generally considered a marker for polymodal nociceptors but is also expressed in a small population of Aδ fibers (Mitchell et al. 2010), which would be more consistent with the reported medium to large diameter of Trpv1-positive molar neurons (Fried et al. 1989; Ichikawa and Sugimoto 2001; Gibbs et al. 2011). Interestingly, we found that a subset of the C6 (Aδ nociceptors) expresses Trpv1 and innervates the teeth, with an estimated 3 neurons per molar. In addition, retrograde labeling revealed that 1 or 2 Trpv1-expressing C-fibers target an individual tooth (Fig. 3) but the majority of molar neurons did not express this heat-sensitive channel. By contrast, we observed that another ion channel that is crucial for pain perception in mice (Abrahamsen et al. 2008) and humans (Faber et al. 2012), Nav1.8 (Scn10a), was present in the majority of labeled neurons (Fig. 5A), including all C6 cells and approximately half of the C4 neurons (Fig. 5B). A fourth gene linked to nociception, Htr3a (Zeitz et al. 2002), was also prominently expressed in C6 cells and a subset of the Scn10a-positive C4 neurons. Together, these results imply that a range of fast-conducting nociceptors targets the molars and accounts for about 65% of retrogradely labeled cells.
Most other WGA-positive neurons were large diameter and likely included low-threshold mechanosensors (Nguyen et al. 2017). In this regard, we studied the expression profile of Piezo2, which encodes the ion channel responsible for gentle touch detection (Ranade et al. 2014; Chesler et al. 2016). Piezo2 is highly expressed in molar neurons (Fig. 5A), labeling almost all neurons that we identified as targeting the teeth (Fig. 5C). Among these neurons, the specialized nociceptors expressing Trpv1 (a thermosensor) were all positive for this sensitive mechanosensory channel. Intriguingly, however, a subset of the Scn10a-negative C4 neurons did not express this gene.
Discussion
The tooth and dental pulp are prominently innervated by trigeminal sensory neurons. Tooth damage and exposure of dentin or inflammation of the pulp can trigger excruciating pain in humans, suggesting that many of these neurons may be nociceptive. However, people are normally unaware of tooth sensation, raising questions about the biological significance of dental innervation. We reasoned that far from simply signaling injury and damage, sensory innervation of the teeth must have evolved to serve a protective function. In this scenario, minor changes to the expected sensory input from the teeth (e.g., inadvertently biting on a grain of sand) would help protect animals from potentially catastrophic damage to the teeth, providing strong selective pressure shaping dental innervation. Here we expose 4 properties of sensory innervation of the teeth that substantiate this hypothesis. First, we demonstrated that a single molar is targeted by at least 50 different neurons, meaning that a large fraction of trigeminal neurons (2% to 3%; Shimeld et al. 1999; LaGuardia et al. 2000) detects input from the teeth. Second, each molar has its own dedicated sensory neurons capable of providing tooth-level resolution. Third, labeled neurons were almost exclusively transcriptomically classified as large diameter and myelinated (i.e., fast-conducting cells) ideal for rapid signaling. Finally, about 70% of molar neurons were nociceptors and thus likely hardwired to trigger protective behavioral responses.
Previous studies based on targeted approaches to study the sensory innervation of the teeth (Fried et al. 1989; Gibbs et al. 2011; Chung et al. 2012; Michot et al. 2018) have shown that a subset expresses CGRP, a prominent marker of several types of nociceptor (Le Pichon and Chesler 2014). Although there is considerable variation in the proportion of molar neurons reported to contain this neuropeptide (15% to 60%), these studies are consistent with our demonstration that about 30% of them are Calca (i.e., CGRP) positive. Similarly, it has been reported that a large subset of molar neurons expresses Scn10a (Park et al. 2006; Won et al. 2017) in keeping with our results. However, for other markers (e.g., Trpa1 or Trpm8), reverse transcription polymerase chain reaction (RT-PCR)–based approaches provided much higher estimates for the presence of these genes (Park et al. 2006; Hermanstyne et al. 2008; Kim et al. 2011) and thus C-fiber nociceptors than we showed here. It is possible that our method for retrograde labeling missed some C-fiber innervation. However, the mouse-to-mouse consistency, similarity between WGA and CTB (which are diffusible and were applied to teeth at high concentration), and the large number of neurons labeled all suggest that this is unlikely. Thus, one explanation for this difference is that the high sensitivity of RT-PCR allows amplification of trace mRNA, whereas the lower sensitivity of ISH provides a true classification of the molar neurons. Notably, similar studies of Trpv1 suggested that a huge range (8% to 87%) of molar neurons (Chaudhary et al. 2001; Stenholm et al. 2002; Park et al. 2006; Chung et al. 2011; Gibbs et al. 2011; Kim et al. 2011; Won et al. 2017) expresses this channel, which is primarily found in polymodal nociceptors. Our data are in close agreement with the lower estimates (Ichikawa and Sugimoto 2001; Kvinnsland et al. 2004; Chung et al. 2011; Gibbs et al. 2011), which typically did not rely on RT-PCR and show that these cells include specialized Aδ nociceptors. Similarly, we find very few Mrgprd-expressing C13 molar neurons, confirming findings with mice where these cells were marked by genetic targeting (Chung et al. 2012). In summary, our results provide a comprehensive analysis of molar neurons labeled by retrograde tracers, and they define a large population of molar nociceptors with the transcriptomic properties of fast-conducting A-type neurons. The gene expression profiles of these molar nociceptors highlight the ion channels Nav1.8 and Htr3a as potential therapeutic targets.
The mammalian tooth is a rugged structure capable of withstanding extreme forces (Jansen van Vuuren et al. 2020) generated during mastication (Shimada et al. 2012). Its specialized anatomy includes an outer layer of enamel, the hardest known biomaterial (Engel 2017), in keeping with a need to preserve its functional integrity. Here we demonstrate that teeth are richly innervated by a mix of specialized nociceptors and neurons that may have a more discriminative role. Most of the time, we are not aware of any sensory input from our teeth and appreciate this rich innervation only when something is awry (McCormick et al. 2013), but the presence of obstinate particulate food debris is a common and unmistakable annoyance arising from subtle alterations in the occlusal surfaces and dental articulation (Siirilä and Laine 1963). This type of sensitivity may protect teeth from damage. Interestingly, the majority of molar neurons express the mechanoreceptor Piezo2, suggesting that they could contribute information about gentle types of touch on an intact tooth (Won et al. 2017) and complement sensory information provided by innervation of the periodontal ligament. Functional in vivo studies in genetically targeted mice will be informative in determining the role of Piezo2 in molar neuron sensation. Moreover, the identification of human subjects with loss-of-function alleles of this gene (Chesler et al. 2016) means that in the future it should be possible to interrogate the functional role of PIEZO2 in human tooth perception.
Author Contributions
J.J. Emrick, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; L.J. von Buchholtz, contributed to data analysis and interpretation, critically revised the manuscript; N.J.P. Ryba, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520941837 for Transcriptomic Classification of Neurons Innervating Teeth by J.J. Emrick, L.J. von Buchholtz and N.J.P. Ryba in Journal of Dental Research
Acknowledgments
We are grateful to Claire Le Pichon and Hanna Silberberg for assistance with 3DISCO clearing and light sheet microscopy, Carolyn Smith and the Microscopy and Imaging Core of the National Institute of Child Health and Human Development, and Ted Usdin and the Systems Neuroscience Imaging Resource of the National Institute of Mental Health. We also thank Alexander Chesler and Kyle Tomek for their helpful comments on the manuscript as well as Mark Hoon and other members of our laboratory for help and advice.
Footnotes
A supplemental appendix to this article is available online.
This work was supported by the Intramural Program (N.J.P.R.) and Clinical Research Fellowship Program (Division of Intramural Research; J.J.E.) of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: J.J. Emrick  https://orcid.org/0000-0001-5619-1078
https://orcid.org/0000-0001-5619-1078
L.J. von Buchholtz  https://orcid.org/0000-0002-8882-8333
https://orcid.org/0000-0002-8882-8333
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. 2008. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 321(5889):702–705. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Martenson ME, Baumann TK. 2001. Vanilloid receptor expression and capsaicin excitation of rat dental primary afferent neurons. J Dent Res. 80(6):1518–1523. [DOI] [PubMed] [Google Scholar]
- Chesler AT, Szczot M, Bharucha-Goebel D, Čeko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, et al. 2016. The role of PIEZO2 in human mechanosensation. N Engl J Med. 375(14):1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Jue SS, Dong X. 2012. Projection of non-peptidergic afferents to mouse tooth pulp. J Dent Res. 91(8):777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee J, Duraes G, Ro JY. 2011. Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res. 90(9):1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. 2017. Enamel is the hardest biomaterial known. In: Engel J, editor. A critical survey of biomineralization: control, mechanisms, functions and material properties. Cham, Switzerland: Springer International Publishing; p. 17–27. [Google Scholar]
- Faber CG, Lauria G, Merkies IS, Cheng X, Han C, Ahn HS, Persson AK, Hoeijmakers JG, Gerrits MM, Pierro T, et al. 2012. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A. 109(47):19444–19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried K, Arvidsson J, Robertson B, Brodin E, Theodorsson E. 1989. Combined retrograde tracing and enzyme/immunohistochemistry of trigeminal ganglion cell bodies innervating tooth pulps in the rat. Neuroscience. 33(1):101–109. [DOI] [PubMed] [Google Scholar]
- Fried K, Gibbs JL. 2014. Dental pulp innervation. In: Goldberg M, editor. The dental pulp: biology, pathology, and regenerative therapies. Berlin (Germany): Springer Berlin Heidelberg; p. 75–95. [Google Scholar]
- Gibbs JL, Melnyk JL, Basbaum AI. 2011. Differential TRPV1 and TRPV2 channel expression in dental pulp. J Dent Res. 90(6):765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanstyne TO, Markowitz K, Fan L, Gold MS. 2008. Mechanotransducers in rat pulpal afferents. J Dent Res. 87(9):834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. 2001. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 890(1):184–188. [DOI] [PubMed] [Google Scholar]
- Jansen van Vuuren L, Broadbent JM, Duncan WJ, Waddell JN. 2020. Maximum voluntary bite force, occlusal contact points and associated stresses on posterior teeth. J R Soc N Z. 50(1):132–143. [Google Scholar]
- Kim HY, Chung G, Jo HJ, Kim YS, Bae YC, Jung SJ, Kim JS, Oh SB. 2011. Characterization of dental nociceptive neurons. J Dent Res. 90(6):771–776. [DOI] [PubMed] [Google Scholar]
- Kvinnsland IH, Luukko K, Fristad I, Kettunen P, Jackson DL, Fjeld K, Von Bartheld CS, Byers MR. 2004. Glial cell line–derived neurotrophic factor (GDNF) from adult rat tooth serves a distinct population of large-sized trigeminal neurons. Eur J Neurosci. 19(8):2089–2098. [DOI] [PubMed] [Google Scholar]
- LaGuardia JJ, Cohrs RJ, Gilden DH. 2000. Numbers of neurons and non-neuronal cells in human trigeminal ganglia. Neurol Res. 22(6):565–566. [DOI] [PubMed] [Google Scholar]
- Lamotte CC, Kapadia SE, Shapiro CM. 1991. Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoid-HRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP). J Comp Neurol. 311(4):546–562. [DOI] [PubMed] [Google Scholar]
- Launay PS, Godefroy D, Khabou H, Rostene W, Sahel JA, Baudouin C, Melik Parsadaniantz S, Reaux-Le Goazigo A. 2015. Combined 3DISCO clearing method, retrograde tracer and ultramicroscopy to map corneal neurons in a whole adult mouse trigeminal ganglion. Exp Eye Res. 139:136–143. [DOI] [PubMed] [Google Scholar]
- Le Pichon CE, Chesler AT. 2014. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat. 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AP, Abubaker AO, Laskin DM, Gonzales MS, Garland S. 2013. Reducing the burden of dental patients on the busy hospital emergency department. J Oral Maxillofac Surg. 71(3):475–478. [DOI] [PubMed] [Google Scholar]
- Michot B, Lee CS, Gibbs JL. 2018. TRPM8 and TRPA1 do not contribute to dental pulp sensitivity to cold. Sci Rep. 8(1):13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Bates BD, Keller JM, Lopez M, Scholl L, Navarro J, Madian N, Haspel G, Nemenov MI, Iadarola MJ. 2010. Ablation of rat TRPV1-expressing adelta/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol Pain. 6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, Le Pichon CE, Ryba N. 2019. Stereotyped transcriptomic transformation of somatosensory neurons in response to injury. Elife. 8:e49679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, Wu Y, Bonilla LS, von Buchholtz LJ, Ryba NJP. 2017. Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLoS One. 12(9):e0185543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowjack-Raymer RE, Sheiham A. 2003. Association of edentulism and diet and nutrition in us adults. J Dent Res. 82(2):123–126. [DOI] [PubMed] [Google Scholar]
- Nowjack-Raymer RE, Sheiham A. 2007. Numbers of natural teeth, diet, and nutritional status in us adults. J Dent Res. 86(12):1171–1175. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim MS, Fang Z, Li HY, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB. 2006. Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem. 281(25):17304–17311. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. 2014. PIEZO2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 516(7529):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B, Arvidsson J. 1985. Transganglionic transport of wheat germ agglutinin-HRP and choleragenoid-HRP in rat trigeminal primary sensory neurons. Brain Res. 348(1):44–51. [DOI] [PubMed] [Google Scholar]
- Scully C. 2013. Pain. In: Scully C, editor. Oral and maxillofacial medicine. 3rd ed. Edinburgh (Scotland): Churchill Livingstone; p. 125–135. [Google Scholar]
- Shimada A, Yamabe Y, Torisu T, Baad-Hansen L, Murata H, Svensson P. 2012. Measurement of dynamic bite force during mastication. J Oral Rehabil. 39(5):349–356. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Easty DL, Hill TJ. 1999. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and cytokines. J Virol. 73(3):1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siirilä HS, Laine P. 1963. The tactile sensibility of the parodontium to slight axial loadings of the teeth. Acta Odontol Scand. 21(5):415–429. [DOI] [PubMed] [Google Scholar]
- Song M, Kim S, Kim T, Park S, Shin K-H, Kang M, Park N-H, Kim R. 2017. Development of a direct pulp-capping model for the evaluation of pulpal wound healing and reparative dentin formation in mice. J Vis Exp. (119):54973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm E, Bongenhielm U, Ahlquist M, Fried K. 2002. VR1- and VRL-1-like immunoreactivity in normal and injured trigeminal dental primary sensory neurons of the rat. Acta Odontol Scand. 60(2):72–79. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. 2015. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 18(1):145–153. [DOI] [PubMed] [Google Scholar]
- von Buchholtz LJ, Lam RM, Emrick JJ, Chesler AT, Ryba NJP. 2020. Assigning transcriptomic class in the trigeminal ganglion using multiplex in situ hybridization and machine learning. Pain [epub ahead of print 2 May 2020]. doi: 10.1097/j.pain.0000000000001911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J, Vang H, Lee PR, Kim YH, Kim HW, Kang Y, Oh SB. 2017. Piezo2 expression in mechanosensitive dental primary afferent neurons. J Dent Res. 96(8):931–937. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. 2002. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 22(3):1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520941837 for Transcriptomic Classification of Neurons Innervating Teeth by J.J. Emrick, L.J. von Buchholtz and N.J.P. Ryba in Journal of Dental Research