Abstract
Background
The aim of this study was to analyze the correlation of macular micro-field characteristics with vision and visual field in patients with non-arteritic anterior ischemic optic neuropathy.
Material/Methods
Retrospective case analysis was performed. Fifty-eight NAION patients with 62 affected eyes were included in the study. In addition, 54 eyes not affected by NAION from 54 patients among the 58 patients were included as controls. All eyes underwent best corrected visual acuity (BCVA) test, slit lamp biomicroscopy, indirect ophthalmoscopy, visual field examination, and microperimetry. BCVA was converted into logarithm of minimum angle of resolution (LogMAR) for statistical analysis. There was no significant difference in age, sex, eye type, or intraocular pressure between the 2 groups. The macular integrity assessment (MAIA) instrument was used for microperimetry. Mean light sensitivity (microMS) in the 10° macular region and the fixation rates for macular fovea 2° and 4° were recorded. Spearman correlation analysis was performed.
Results
The microMS values were significantly different between the control group and the affected eye group (t=−2.427, P=0.036). MicroMS was significantly correlated with logMAR BCVA (r=−0.802, P=−0.005) and with mean sensitivity (MS) and mean deviation (MD) (r=0.912, P=0.002; r=−0.905, P=0.002; P<0.05). MS and MD were not correlated with logMAR BCVA (r=−0.465, P=0.245; r=0.437, P=0.278).
Conclusions
The present study demonstrates that microMS of macular micro-visual field in NAION patients was significantly decreased at early stage, and was significantly correlated with and consistent with visual acuity and visual field.
MeSH Keywords: Ophthalmology; Optic Nerve; Optic Neuropathy, Ischemic
Background
Non-arteritic anterior ischemic optic neuropathy (NAION) is thought to be caused by dysfunction of the internal circulation of the optic nerve papilla, but the specific location and pathogenesis of vascular lesions have not been confirmed [1,2]. Although the blood flow of the short posterior ciliary artery is decreased in NAION patients, no evidence of thrombosis of the short posterior ciliary artery was found in anatomical and pathological studies of rare cases [3]. It is generally believed that this is due to poor systemic perfusion, and in some cases is caused by local capillary occlusion [3–5]. Fluorescein angiography in NAION patients seems to support this hypothesis [6]. Most of the previous studies focused on optic nerves of NAION [7]. In recent years, some studies have shown that macular ganglion cell complex (GCC) is associated with the prognosis of visual field and visual functions [8–10]. Microperimetry is a relatively new type of macular function testing technique, which can accurately measure macular retinal sensitivity and analyze macular functions [11–13]. In the present study, we assessed visual functions of NAION patients using microperimetry, and sought to understand visual impairment in NAION.
Material and Methods
Subjects
A total of 58 patients who were diagnosed with NAION at our hospital between June 2016 and June 2020 were enrolled in the study, including 62 eyes. All patients went to see the doctor within 10 days after the occurrence of NAION symptoms. All eyes underwent the best corrected visual acuity (BCVA) test, slit lamp biomicroscopy, indirect ophthalmoscopy, visual field examination, and microperimetry. The diagnostic criteria for NAION were set according to a previous report [13]. The inclusion criteria were: i) sudden painless visual loss; ii) optic disc edema with light color, and fissured bleeding on edges; iii) relative pupillary afferent blocking; iv) decreased color vision; v) visual field defect characteristics of NAION such as altitudinal field defect; and vi) normal intraocular pressure. The exclusion criteria were: i) arteritic anterior ischemic optic neuropathy; ii) spherical equivalent degree >3.00 D; iii) history of eye diseases such as cataract and macular diseases; iv) history of previous systemic diseases, medication and operation that might affect research results; v) poor fixation ability of both eyes. There were not significant differences in age (t=−0.657), sex, eye type (χ2=0.138, 0.089), or intraocular pressure (t=0.386) between the 2 groups (P>0.05). Ethics approval was obtained and the tenets of the Declaration of Helsinki were followed. The patients signed a consent form for inclusion in the study.
The BCVA test was performed using the international standard visual acuity chart, and the visual acuity was converted into logarithm of minimum angle of resolution (LogMAR) for statistical analysis. The visual threshold of 30° central visual field was measured using a Humphrey Field Analyzer (Zeiss, Oberkochen, Germany), and mean sensitivity (MS) and mean deviation (MD) were recorded. Patients with ametropia were measured wearing glasses. Macular integrity assessment (MAIA) instrument (Centervue, Icare, Raleigh, NC, USA) was used for microperimetry examination. The 4–2 threshold stimulus mode was selected. The test range was 10° in the macular area. The size of the cursor was Goldmann III. The brightness was 0–36 dB, and the background brightness was 1.27 cd/m2. The number of stimulation points was 37, and they were arranged in 3 concentric circles. The number of stimulation points in the central circle was 1 and that in the outer circle was 12. The reliability of the test was assessed by false positives, and those with false positive rate over 25% were excluded from the study. Mean light sensitivity (microMS) in the 10° macular region and the fixation rates for macular fovea 2° and 4° were recorded. The fixation rate for 2° macular fovea was represented by P1 with macular fovea as the center and 2° as the diameter. The fixation rate for 4° macular fovea was represented by P2 with macular fovea as the center and 4° as the diameter. Fixation stability was evaluated according to a previous report [13]. P1 ≥75% indicated stable fixation, P1 <75% and P2 ≥75% indicated relatively stable fixation, and P2 <75% indicated unstable fixation.
Statistics
The analysis of statistics was performed using SPSS software (version 17.0; IBM, Armonk, NY, USA). Measurement data were expressed as means±standard deviations. The microMS between 2 groups of eyes was compared using the t test. Spearman correlation was performed to analyze how microMS was correlated with MS, MD, or BCVA. P<0.05 indicated a statistically significant difference.
Results
The 58 patients included 25 males (25 eyes) and 33 females (37 eyes). The mean age was 56.68±7.14 years old. Average time from onset to treatment for all patients was 4.36±3.22 days. Four women had both eyes affected, and the remaining 54 patients each had 1 healthy eye, which was included in the control group. Among the 54 eyes in the control group from 54 patients, 25 eyes were from 25 male patients and 29 eyes were from 29 female patients. The mean age for the 54 patients was 57.23±6.22 years old (Table 1). The BCVA index for the affected eye group was less than 1.0. Among this group, 18 eyes had BCVA ≤0.3, accounting for 29.03%; 30 eyes had 0.4 ≤BCVA ≤0.6, accounting for 48.39%; 14 eyes had 0.7 ≤BCVA ≤1.0, accounting for 22.58%; and 4 eyes had BCVA=1.0. Mean logMAR BCVA for the affected eye group was 0.68±0.79. The eyes in the control group had BCVA between 0.8 and 1.0, and the mean logMAR BCVA was 0.07±0.06.
Table 1.
Demographic and ophthalmic findings of patients.
| Patient group | Control group | t | χ2 | ||
|---|---|---|---|---|---|
| Number of subjects | 58 | 54 | |||
| Number of eyes | 62 | 54 | |||
| Number of males | 25 | 25 | 0.138 | ||
| Number of females | 33 | 29 | |||
| Number of left eyes | 32 | 26 | 0.089 | ||
| Number of right eyes | 30 | 28 | |||
| Intraocular pressure (mmHg) | 12±0.57 | 14±0.32 | 0.386 | ||
| Age (years) | 56.68±7.14 | 57.23±6.22 | −0.657 | ||
| Number of days from onset to admission | 4.36±3.22 | ||||
| Vision (BVCA) ≤0.3 | 18 eyes (29.03%) | 0.8–1.0 | |||
| BVCA, 0.4–0.6 | 30 eyes (48.39%) | ||||
| BVCA, 0.7–1.0 | 14 eyes (22.58%) (four eyes with BCVA=1.0) | ||||
| logMAR BCVA | 0.68±0.79 | 0.07±0.06 | |||
| Temporal altitudinal field defect connected with optic disc | 16 eyes | ||||
| Inferior altitudinal field defect connected to the optic disc | 26 eyes | ||||
| Superior altitudinal field defect connected to optic disc | 8 eyes | ||||
| Nasal defects | 6 eyes | ||||
| Tubular vision, visual island, or diffuse visual field defects | 6 eyes | ||||
Ocular fundus examination showed that the affected eyes had a diffuse optic disc or fan-shaped edema, and their color was light red or slightly red.
Perimetry showed that all affected eyes had visual field defects. Among them, 16 eyes showed temporal altitudinal field defect connected with the optic disc, 26 eyes had inferior altitudinal field defect connected to the optic disc, 8 eyes exhibited superior altitudinal field defect connected to the optic disc, 6 eyes showed nasal defects, and 6 eyes only had tubular vision, visual island, or diffuse visual field defects. Microperimetry showed that the macular area sensitivity was decreased to various degrees.
Affected eyes had a MS value of 12.08±6.14 dB and a MD value of 15.59±5.86 dB. The microMS of affected eyes was significantly different from that of the control group (t=−2.427, P=0.036). Among the 62 affected eyes, 28 eyes (45.16%) had fixation stability, 17 eyes (27.42%) had relative fixation stability, and 17 eyes (27.42%) had fixation instability (Figure 1A–1D). The MS, MD, and microMS values in affected eyes were significantly different from those in the control group (P<0.05; Table 2).
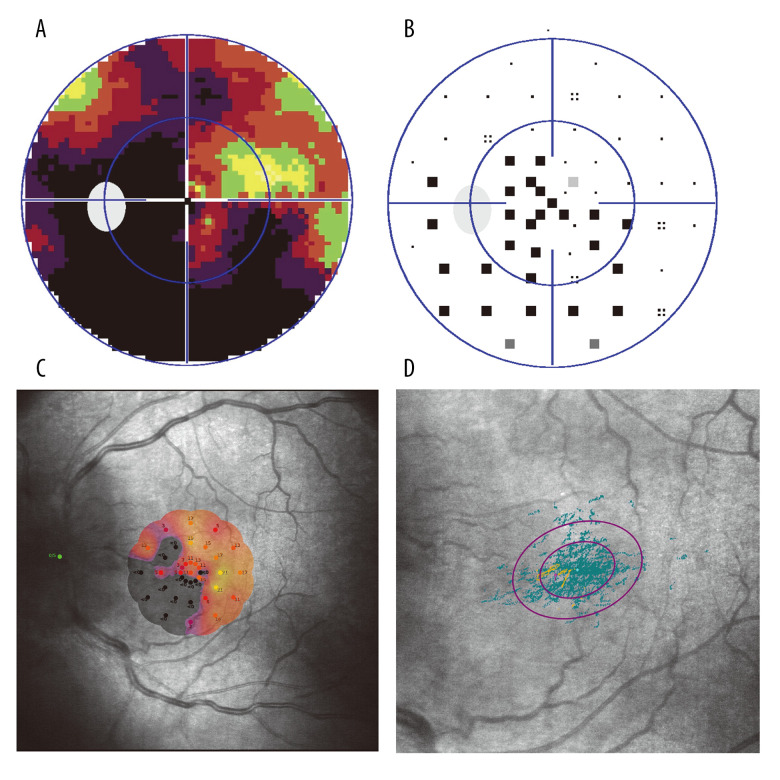
Figure 1.
(A) Gray-scale image of 30° central visual field observed by Humphrey perimetry from a NAION patient, showing a superior fan-shaped defect connected to the optic disc. (B) Correction map of the visual field of the same patient, showing a superior fan-shaped defect connected to the optic disc. (C) Macular micro-visual field changes corresponding to the visual field defect shown in (A). The microMS value was 7.1 dB. P1 and P2 were 94% and 100%, respectively. Fixation was relatively stable. (D) Fixed view of microperimetry. P1=41% and P2=80%.
Table 2.
Comparison of MS, MD, and microMS between healthy eyes and affected eyes from NAION patients.
| Groups | Number of eyes | MS (dB) | MD (dB) | microMS (μm) |
|---|---|---|---|---|
| Observation group | 62 | 12.08±6.14 | 15.59±5.86 | 13.7±9.76 |
| Control group | 54 | 18.76±6.86 | 9.7±5.24 | 28.4±2.08 |
| t value | – | 3.057 | 3.174 | 2.427 |
| P value | – | 0.003 | 0.024 | 0.036 |
Correlation analysis showed that microMS was significantly correlated with logMAR BCVA (r=−0.802, P=−0.005) and with MS or MD (r=0.912, P=0.002; r=−0.905, P=0.002; P<0.05). MS or MD had no correlation with logMAR BCVA (r=−0.465, P=0.245; r=0.437, P=0.278).
Discussion
The present study involved 58 NAION patients with 62 affected eyes, and these eyes had macular micro-visual field changes. We conducted detailed medical history inquiries on all patients included in the study and found that most patients had at least 1 potential vascular risk factor before or after disease onset. Hypertension and myocardial ischemia were the most common systemic diseases. Among the 25 male patients, 21 had a history of smoking, with an average duration of smoking of 24 years, and 2 women has a smoking history. Some of these patients did not undergo a systemic examination before the onset of NAION. For patients without systemic examination, we performed routine blood and urine tests, C-reactive protein test, and erythrocyte sedimentation rate test. The blood pressure of all patients was measured. NAION is a multifactorial disease, and many risk factors can induce NAION. Nocturnal hypotension and taking antihypertensive drugs before bedtime may be a risk factor for NAION [14]. Due to limited conditions, we regret that we were not able to carry out nighttime blood pressure monitoring for all patients. Patients with higher education were more likely to be able to detect the occurrence of NAION as soon as possible, and could accurately tell the specific time and symptoms of the disease. Our observation group contained slightly more female patients than male patients, and more female patients had both eyes affected. However, studies with larger sample sizes are needed to confirm these results.
NAION is a common acute optic nerve disease in middle-aged and older people [4]. Its pathogenic factors lead to optic disc nerve axoplasm stasis [15], retinal ganglion cell apoptosis, and secondary optic nerve injury, as well as demyelinating changes of the optic nerve [16]. As with other optic nerve diseases, visual field damage is a direct result of NAION. In the present study, the fundus optic disc was diffuse or fan-shaped with pale edema or congestion, and some of it was accompanied by peridiscal fissure or patchy hemorrhage. Severe optic disc edema was accompanied by macular neurosensory detachment. The detachment of the neurosensory layer in the macular area can heal with the disappearance of optic disc edema. In rare cases, after the edema subsided, stellate exudation was observed among the macular discs. Because of the ischemia of the local optic disc, compensatory dilation of capillaries in the non-ischemic region may subsequently occur. It is also speculated that mild inflammatory reaction may accompany the disease during the whole course.
In the affected-eye group, 4 eyes had best corrected visual acuity of 1.0, accounting for 6.45% of all affected eyes. All other affected eyes showed different degrees of decrease in central visual acuity. The visual field of all affected eyes showed different degrees of defects. For the evaluation of macular visual field, we used microperimetry.
Microperimetry is a functional visual field test to measure retinal sensitivity. It needs less training, the test process is less invasive, and fundus tracking is carried out at the same time, so it can accurately quantify the visual sensitivity at specific points of the retina. At present, there is no report about macular microperimetry for NAION-affected eyes. During our observation, even the eyes with 1.0 central visual acuity showed decreased sensitivity of the macular micro-visual field. Therefore, all patients in the present study had damaged macular area at the early stage of disease in addition to visual field damage. Of note, macular microperimetry was more sensitive for the detection of this damage. Previous reports show morphological changes of macular area in NAION eyes, but it is believed that the significant thinning of GCC in NAION eyes begins 1 month after the onset of NAION, and continue to progress within 1 year [8,9,17]. NAION animal models show that axonal degeneration of retinal ganglion cells (RGC) occurs within 1 week, and the apoptosis of RGC reaches the peak in 2–3 weeks [18]. In addition, the macular GCC is gradually thinned after 2 weeks [9,19]. In the present study, the sensitivity of macular micro-visual field in NAION eyes was decreased at an earlier time. The reasons may be that a double capillary system exists around the optic disc, but there is only 1 layer in the macular area, and, most importantly, almost half of the RGCs are located in the macula [20]. The acute decrease of perfusion in NAION makes the macula more vulnerable to ischemia and hypoxia. Macular electrophysiology is a complex process involving 3-level neurons and a variety of biochemical metabolisms. Macular microperimetry can detect abnormal changes at an earlier stage. The correlation of microMS with MS and MD suggests that the decrease of microMS in NAION is the result of the disease. Although some of the affected eyes had good central vision, they only had tubular vision or visual island. Therefore, it is suboptimal to only take vision or visual field as observation and evaluation indices, and the macular micro-visual field is well correlated with them both. We believe that the use of macular microperimetry may be better for the evaluation of NAION damage. We plan to perform research with a larger sample size to more deeply explore this topic. Because OCT is a structural test, NAION early optic disc edema may affect the OCT examination. Microperimetry is a functional examination that is not affected by edema, and is better able to reflect visual function. Therefore, it is of great significance to try to use microperimetry to study NAION and other optic neuropathy [21]. Interestingly, the MD of the control group seemed to be outside the normal limits (Table 2). The reason might be that the eyes in the control group were chosen from the contralateral eyes of the affected eyes, and NAION may not only be affected in the affected eyes, but also in the contralateral eyes. Although there was no obvious cataract and macular disease in the contralateral eyes, the increase of lens density or the thinning of retinal RPE caused by physiological factors may be the cause of the abnormal MD.
Conclusions
The present study demonstrates that microMS of the macular micro-visual field in NAION patients was significantly decreased at an early stage, and was significantly correlated and consistent with visual acuity and visual field. Microperimetry can be used for therapeutic evaluation and follow-up observation in NAION patients, because the results of microperimetry have a good correlation with vision and visual field. Visual field and micro-visual field data of patients during follow-up will be published in the future.
Acknowledgments
The authors wish to thank their department and research team for their help and dedication.
Footnotes
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye (London, England) 2015;29(1):65–79. doi: 10.1038/eye.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller NR. Current concepts in the diagnosis, pathogenesis, and management of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2011;31(2):e1–3. doi: 10.1097/WNO.0b013e31821f955c. [DOI] [PubMed] [Google Scholar]
- 3.Kaup M, Plange N, Arend KO, et al. Retrobulbar haemodynamics in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2006;90(11):1350–53. doi: 10.1136/bjo.2006.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayreh SS. Ischemic optic neuropathy. Progr Retin Eye Res. 2009;28(1):34–62. doi: 10.1016/j.preteyeres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Georgiou T, Wen YT, Chang CH, et al. Neuroprotective effects of Omega-3 polyunsaturated fatty acids in a rat model of anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2017;58(3):1603–11. doi: 10.1167/iovs.16-20979. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch T, Remky A, Plange N, et al. [Quantification of fluorescein angiography in patients with non-arteritic anterior ischemic optic neuropathy]. Ophthalmologe. 2011;108(8):728–32. doi: 10.1007/s00347-011-2387-9. [in Germna] [DOI] [PubMed] [Google Scholar]
- 7.Alasil T, Tan O, Lu AT, et al. Correlation of Fourier domain optical coherence tomography retinal nerve fiber layer maps with visual fields in nonarteritic ischemic optic neuropathy. Ophthalmic Surg Lasers Imaging. 2008;39(4 Suppl):S71–79. doi: 10.3928/15428877-20080715-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papchenko T, Grainger BT, Savino PJ, et al. Macular thickness predictive of visual field sensitivity in ischaemic optic neuropathy. Acta Ophthalmol. 2012;90(6):e463–69. doi: 10.1111/j.1755-3768.2012.02467.x. [DOI] [PubMed] [Google Scholar]
- 9.Goto K, Miki A, Araki S, et al. Time course of macular and peripapillary inner retinal thickness in non-arteritic anterior ischaemic optic neuropathy using spectral-domain optical coherence tomography. Neuroophthalmology. 2016;40(2):74–85. doi: 10.3109/01658107.2015.1136654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun MH, Liao YJ. Structure-function analysis of nonarteritic anterior ischemic optic neuropathy and age-related differences in outcome. J Neuroophthalmol. 2017;37(3):258–64. doi: 10.1097/WNO.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 11.Laishram M, Srikanth K, Rajalakshmi AR, et al. Microperimetry – a new tool for assessing retinal sensitivity in macular diseases. J Clin Diagn Res. 2017;11(7):NC08–11. doi: 10.7860/JCDR/2017/25799.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nittala MG, Velaga SB, Hariri A, et al. Retinal sensitivity using microperimetry in age-related macular degeneration in an amish population. Ophthalmic Surg Lasers Imaging Retina. 2019;50(9):e236–41. doi: 10.3928/23258160-20190905-15. [DOI] [PubMed] [Google Scholar]
- 13.Roh M, Laíns I, Shin HJ, et al. Microperimetry in age-related macular degeneration: Association with macular morphology assessed by optical coherence tomography. Br J Ophthalmol. 2019;103(12):1769–76. doi: 10.1136/bjophthalmol-2018-313316. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS. Controversies on neuroprotection therapy in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2020;104(2):153–56. doi: 10.1136/bjophthalmol-2019-314656. [DOI] [PubMed] [Google Scholar]
- 15.Khalilpour S, Latifi S, Behnammanesh G, et al. Ischemic optic neuropathy as a model of neurodegenerative disorder: A review of pathogenic mechanism of axonal degeneration and the role of neuroprotection. J Neurol Sci. 2017;375:430–41. doi: 10.1016/j.jns.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Guo Y, Slater BJ, et al. Axonal degeneration, regeneration and ganglion cell death in a rodent model of anterior ischemic optic neuropathy (rAION) Expe Eye Res. 2010;91(2):286–92. doi: 10.1016/j.exer.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedges TR, 3rd, Vuong LN, Gonzalez-Garcia AO, et al. Subretinal fluid from anterior ischemic optic neuropathy demonstrated by optical coherence tomography. Arch Ophthalmol. 2008;126(6):812–15. doi: 10.1001/archopht.126.6.812. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Mehrabian Z, Bernstein SL. The rodent model of nonarteritic anterior ischemic optic neuropathy (rNAION) J Vis Exp. 2016;(117):54504. doi: 10.3791/54504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson MA, Miller NR, Nolan T, et al. Peripapillary retinal nerve fiber layer swelling predicts peripapillary atrophy in a primate model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2016;57(2):527–32. doi: 10.1167/iovs.15-17880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, Kwon J, Shin JW, et al. Quantitative optical coherence tomography angiography of macular vascular structure and foveal avascular zone in glaucoma. PLoS One. 2017;12(9):e0184948. doi: 10.1371/journal.pone.0184948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elnahry GA, Elemary AM, Badr Eldin N, et al. Peripapillary microperimetry for the diagnosis and follow-up of papilledema in cases treated for idiopathic intracranial hypertension. Neurol Res. 2020 doi: 10.1080/01616412.2020.1820811. [Online ahead of print] [DOI] [PubMed] [Google Scholar]