Abstract
Treatment with mesenchymal stem cells (MSCs) has been revealed to suppress CD4+ T cells and autoimmunity in both mouse models and patients with primary Sjögren syndrome (pSS); however, the underlying mechanism remains unclear. MicroRNAs (miRNAs or miRs) mediate CD4+ T cell activation, but the mechanism is not understood, particularly for CD4+ T cells treated with MSCs. Characterization of miRNAs may reveal pSS pathogenesis, guide MSC treatment and provide more personalized management options. The present study aimed to perform an miRNome analysis of quiescent and T cell receptor (TCR)-activated CD4+ T cells treated with MSCs via miRNA profiles and bioinformatics. Following 72 h of co-culture, MSCs inhibited TCR-induced CD4+ T cell activation and decreased IFN-γ levels. The numbers of aberrant miRNAs in pSS naïve (vs. healthy naïve), pSS activation (vs. pSS naïve), MSC treatment and pre-IFN-γ MSC treatment (vs. pSS activation) groups were 42, 55, 27 and 32, respectively. Gene enrichment analysis revealed that 259 pathways were associated with CD4+ T cell stimulation, and 240 pathways were associated with MSC treatment. Increased miRNA-7150 and miRNA-5096 and decreased miRNA-125b-5p and miRNA-22-3p levels in activated CD4+ T cells from patients with pSS were reversed by MSC treatment. Notably, the proliferation of CD4+ T cells and CD4+ IFN-γ+ cells, expression levels of miRNA-125b-5p and miRNA-155 in CD4+ T cells and supernatant IFN-γ secretion were associated with disease activity. miRNA may play a vital role in MSC treatment for activated CD4+ T cells. The results indicated that the expression levels of miRNA-125b-5p and miRNA-155 in TCR-activated CD4+ T cells from patients with pSS may provide insight regarding autoimmune diseases and offer a novel target for prospective treatment. Therefore, these results may be crucial in providing MSC treatment for pSS.
Keywords: CD4+ T cell, mesenchymal stem cells, microRNA, primary Sjögren syndrome, T cell receptor pathway
Introduction
Primary Sjögren syndrome (pSS) is an autoimmune disease that can attack the exocrine glands, causing symptoms such as xerostomia and keratoconjunctivitis sicca (1). However, treatments for patients with pSS, such as stimulating drugs and artificial saliva, are ineffective and only symptomatic (2).
Mesenchymal stem cells (MSCs), such as human umbilical cord mesenchymal stem cells (hUCMSCs), offer a promising treatment for pSS due to their low immunogenicity and immunoregulatory potential (3). MSCs have been reported to exert inhibitory functions on activated lymphoid cells, including CD4+ T cells (4). However, the underlying mechanisms, such as direct cell contact and secretion of soluble mediators, including prostaglandin E2 (PGE2), IL-10, TGF-β and hepatic growth factor (5), are contradictory. However, the regulatory mechanisms underlying CD4+ T cell activation by MSCs are still unclear due to their multiplicity, for example, subtle gene regulation. The microRNA (miRNA or miR) pathway may be involved in gene regulation for CD4+ T cell activation (6).
miRNAs have been reported to control T cell activation (6). miRNA microarray has been used to identify unique miRNAs associated with glandular inflammation and dysfunction from patients with pSS (7). Furthermore, pathway analysis of miRNAs predicted to target Ro/SSA and La/SSB autoantigens revealed differential miRNA expression levels in the salivary glands and peripheral blood mononuclear cells (PBMCs) from patients with pSS (7).
In light of miRNA function in CD4+ T cells and pSS pathogenesis, MSCs may exert immunomodulatory effects on CD4+ T cells and offer a potential treatment for pSS. Therefore, the present study aimed to perform an miRNome analysis of quiescent and T cell receptor (TCR)-activated CD4+ T cells treated with MSCs via miRNA profiling and bioinformatics. The interaction between miRNAs and regulatory pathways (particularly the TCR pathway) was studied in TCR-activated CD4+ T cells to provide a novel understanding of pSS progression and MSC treatment mechanisms.
Materials and methods
Isolation, cultivation, immunophenotyping and labeling of hUCMSCs
The present study was approved (approval no. K-2012-006) by the Research Ethics Committee of Tongji Hospital of Tongji University (Shanghai, China). hUCMSCs were isolated from full-term infants after obtaining parental written consent (8). Briefly, Wharton's jelly tissue was separated from UCs, digested with 1 mg/ml collagenase type I (Sigma-Aldrich; Merck KGaA) and then plated in fresh culture medium. Following expansion for 1 week, adherent cells were obtained and replated in DMEM (Gibco; Thermo Fisher Scientific, Inc.) at 37°C and 5% CO2.
At passage 4, hUCMSCs were identified by flow cytometry with a FACSCalibur II flow cytometer (BD Biosciences), using antibodies (all from eBioscience; Thermo Fisher Scientific, Inc.) against MSCs [CD13-APC (cat. no. 47-0138-42), CD54-APC (cat. no. 17-0542-82), CD73-PE (cat. no. 25-0739-42), CD166-PE (cat. no. 12-1668-42) and CD90-APC (cat. no. 47-0909-41)], hematopoietic cells [CD14-FITC (cat. no. MHCD1401), CD19-FITC (cat. no. 11-0193-82), CD34-PE (cat. no. 12-0349-41), CD45-PE-Cy7 (cat. no. MHCD4512) and CD117-APC (cat. no. 47-1171-80)], integrins [CD29-APC (cat. no. 17-0291-80)], extracellular matrix receptors [CD44-FITC (cat. no. 11-0441-86)] and major histocompatibility complexes [HLA-DR-PerCP (cat. no. 46-9952-41) and HLA-ABC-FITC (cat. no. 11-9983-41)]. MSCs were stained with primary human albumin-FITC (cat. no. CLFAG2140; Cedarlane Laboratories) and pan-cytokeratin-FITC (cat. no. 130-119-141; Miltenyi Biotec GmbH), and then with secondary FITC rabbit anti-human albumin (cat. no. A18904; eBioscience; Thermo Fisher Scientific, Inc.). MSCs were inductively cultured to assay adipogenic, osteoplastic and chondrogenic differentiation to assess their multipotency, as previously described (9).
Patients and controls
Venous blood was collected from inpatients with pSS at the Department of Rheumatology and Immunology, Tongji Hospital of Tongji University (Shanghai, China) between January 2013 and December 2016. The pSS diagnosis complied with the American-European Consensus Group criteria (10). The patients had no other autoimmune diseases and took no immunosuppressive drugs. Healthy controls (HCs) were recruited from the Examination Department, Tongji Hospital of Tongji University. The present study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients and HCs. Clinical features are presented in Table I. The pSS activity was evaluated using the EULAR Sjögren's syndrome disease activity index (ESSDAI) (11).
Table I.
Clinical characteristics of patients with pSS and healthy controls.
| Clinical characteristics | Patients with pSS, n=13 | Healthy controls, n=13 |
|---|---|---|
| Age, years (mean ± SEM) | 19–68 (47.21±8.33) | 20–65 (48.42±9.75) |
| Female, % | 100 | 100 |
| Mean disease duration, years (SEM) | 6.09 (3.54) | – |
| Anti-SSA positive, % | 100 | 0 |
| Anti-SSB positive, % | 100 | 0 |
| Antinuclear antibody positive, % | 100 | 0 |
| Lymphocytic focus score ≥1 foci, % | 100 | – |
| IgG >16 g/l, % | 100 | 0 |
pSS, primary Sjögren syndrome.
Peripheral CD4+ T cell separation
Venous blood was collected in EDTA tubes for PBMC isolation within 4 h using Ficoll-Hypaque density configuration (Sigma-Aldrich; Merck KGaA). CD4+ T cells stained at 4°C for 20 min with FITC anti-human CD4 (cat. no. 300506; BioLegend, Inc.) were sorted on a FACSCalibur flow cytometer.
CD4+ T cell and MSC co-culture experiments
Following isolation, the CD4+ T cells were divided into five groups: Healthy naïve (CD4+ T cells from HC), pSS naïve (CD4+ T cells from patients with pSS), pSS activation [CD4+ T cells from patients with pSS stimulated by anti-CD3 antibody and anti-CD28 antibody (BioLegend, Inc.) for 72 h], MSC treatment (stimulated CD4+ T cells from patients with pSS co-cultured with MSCs for 72 h) and pre-IFN-γ MSC treatment [stimulated CD4+ T cells from patients with pSS co-cultured with MSCs (pre-stimulated by IFN-γ) for 72 h]. CD4+ T cell proliferation was analyzed using flow cytometry with CellTrace™ CFSE cell proliferation kit (cat. no. C34554; Thermo Fisher Scientific, Inc.). The CFSE plot consisted of certain characteristic ridges demonstrating cell proliferation following stimulation. CD4+ T cell division was denoted by the mean generation number (MGN). Cells were gated in compliance with their forward- and side-scatter characteristics for the purpose of excluding dead cells and debris. For flow cytometry, primary antibodies [CD4-PE (cat. no. 565999) and IFN-γ-FITC (cat. no. 561057; both from BD Biosciences)] were added to the cells at 4°C for 20 min. The cells were operated on a FACS Calibur and studied using CellQuest™ Pro software (BD Biosciences). The co-culture supernatants were tested by ELISA, according to the manufacturer's instructions (Shanghai Westang Bio-Tech).
miRNA microarray
The microarray assay was performed using a facilitator (LC Sciences). The 3′ end of the micromolecular RNAs (4 µg) was elongated by adding a poly (A) tail and ligated with pCp-Cy3 dyes. Hybridization was carried out at 4°C for 20 h on a µParaflo microfluidic array. Following RNA hybridization, fluorescence signals were scanned using a laser scanner (GenePix 4000B; Molecular Devices LLC), analyzed with Array-Pro image analysis software version X3 (Media Cybernetics, Inc.) and then standardized with a locally weighted scatterplot smoothing filter as previously described (12).
Reverse transcription-quantitative (RT-q)PCR
The miRNAs were confirmed via stem-loop RT-PCR. Total RNA from CD4+ T cells was extracted with TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. cDNA was synthesized from 0.5 µg RNA, using a reverse transcription kit (Takara Bio, Inc.), according to the manufacturer's protocols. miRNAs were reverse-transcribed using a specific primer (Table SI). qPCR was run with a specific primer (Table SI) and SYBR Premix Ex Taq (Takara Bio, Inc.) on an ABI PRISM 7500 Real-Time PCR system (Applied Biosciences; Thermo Fisher Scientific, Inc.). PCR was performed as follows: 95°C for 10 sec, followed by 40 cycles of denaturation at 95°C for 5 sec, and annealing/extension at 60°C for 20 sec. The relative changes were calculated using the 2−ΔΔCq method (13); healthy naïve individuals were used as the control.
Pathway and miRNA gene network
Two databases [TargetScan (targetscan.org/) and miRanda (microrna.org)] were used to predict the combined target genes of aberrant miRNAs in the two groups. Gene Ontology (GO) was applied to predict the primary function of target genes based on the microarray data obtained as aforementioned (14,15). A pathway study was used to identify the significant pathway of differential genes in accordance with Kyoto Encyclopedia of Genes and Genomes (KEGG), Biocarta and Reactome analysis (15,16). Furthermore, Fisher's exact test was used to select the crucial pathway, and the ingate was determined using the P and false discovery rate values. The enrichment Re was calculated as described previously (17–19), which was as follows by: Re=(nf/n)/(Nf/N), where nf is the number of differential genes within the particular category, n is the total number of genes within the same category, while Nf is the number of differential genes in the entire microarray, and N is the total number of genes in the microarray. The association between miRNAs and targets was acquired based on differential levels and their associations in the Sanger miRBase Release 20.0 (sanger.ac.uk/Software/Rfam/mirna/) for miRNAs to construct an miRNA gene network (19).
Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS, Inc.) for Windows, followed by two-tailed, unpaired or paired Student's t-test for significant differences. Data are presented as the mean ± SEM of three independent repeats. P<0.05 was considered to indicate a statistically significant difference.
Results
Isolation and identification of hUCMSCs
The primary hUCMSC culture took 5–10 days to reach sub-confluence. Flow cytometry revealed that the cells did not exhibit hematopoietic progenitor labels (for example, CD45, CD34, CD14 and HLA-DR) but expressed MSC markers such as CD73, CD105, CD166 and CD90 (data not shown). After 10 days, the attached cells were fibroblast-like. MSCs also differentiated into adipocytes, chondrocytes and osteocytes (data not shown).
MSCs inhibit proliferation of CD4+ T cells
Following co-culture for 72 h, MSCs suppressed CD3/CD28-stimulated CD4+ T cell multiplication under a dose-dependent mode, as determined by a decrease in the CFSE peak generation number (Table II). Under co-culture through cell-cell contact, MGN decreased in terms of MSC:CD4+ ratio (1:10 and 1:5; MSC treatment vs. pSS activation). This confirmed previous studies describing dose-dependent MSC inhibition of T cell proliferation (20,21). IFN-γ has previously been reported to trigger the MSC inhibitory effects on T cell proliferation (22,23), but in the present study, MSC pretreatment with IFN-γ did not result in increased CD4+ T cell inhibition.
Table II.
MSCs inhibit mitogenic CD4+ T cell proliferation.
| MSC treatment (MSC:CD4+ T) | Pre-IFN-γ MSC treatment (MSC:CD4+ T) | ||||||
|---|---|---|---|---|---|---|---|
| MGN statistics | pSS activation | 1:5 | 1:10 | 1:20 | 1:5 | 1:10 | 1:20 |
| MGN | 6.62±1.28 | 2.89±0.53 | 3.36±0.69 | 5.97±0.81 | 3.14±0.78 | 3.79±0.46 | 6.58±0.95 |
| q-value | – | 3.67 | 6.33 | 2.09 | 4.27 | 5.99 | 1.76 |
| P-value | – | <0.05a | <0.01b | >0.05c | <0.01a | <0.01b | >0.05c |
CFSE-labeled CD4+ T cells were activated by CD3/CD28 agonists and co-cultured with different MSC concentrations for 72 h. CD4+ T cell inhibition, as revealed by decreased MGN, was dependent on MSC:T cell ratio. MSC induced significant inhibition of T cell proliferation at high ratios (1:5 and 1:10). n=8.
P vs. pSS activation
P vs. 1:10
P vs. 1:20. MSC, mesenchymal stem cells; pSS, primary Sjögren syndrome; MGN, mean generation number.
Activated CD4+ T cells and MSC treatment demonstrate different miRNA signatures
A genome-wide survey of miRNA expression levels was performed using miRNA microarray for 2,578 human miRNA sequences enumerated in the Sanger database. After separating signals from noise, the numbers of distinct miRNAs in pSS naïve (vs. healthy naïve), pSS activation (vs. pSS naïve), MSC treatment (vs. pSS activation) and pre-IFN-γ MSC treatment (vs. MSC treatment) groups were 42, 55, 27 and 32, respectively (Fig. 1; Table SII). Compared with the pSS naïve group, 26 of 55 miRNAs were upregulated in the pSS activation group. The top 10 upregulated miRNAs were miRNA-30c-1-3p, −155-5p, −1246, −1273g-3p, −1275, −4472, −4638-5p, −5096, −7150 and −7641. The top downregulated miRNAs were miRNA-15a-5p, −30d-5p, −30e-5p, −140-3p, −181a-5p, −451a, −3607-5p, −4443, −4734 and −6510-5p. miRNA-15a-5p, −30d-5p, −451a, −1246, −1275, −4734, −5096 and −6510-5p were also differentially expressed between pSS and healthy naïve groups (Fig. 1; Table SII). These data indicated that these miRNAs were involved in pSS pathogenesis.
Figure 1.
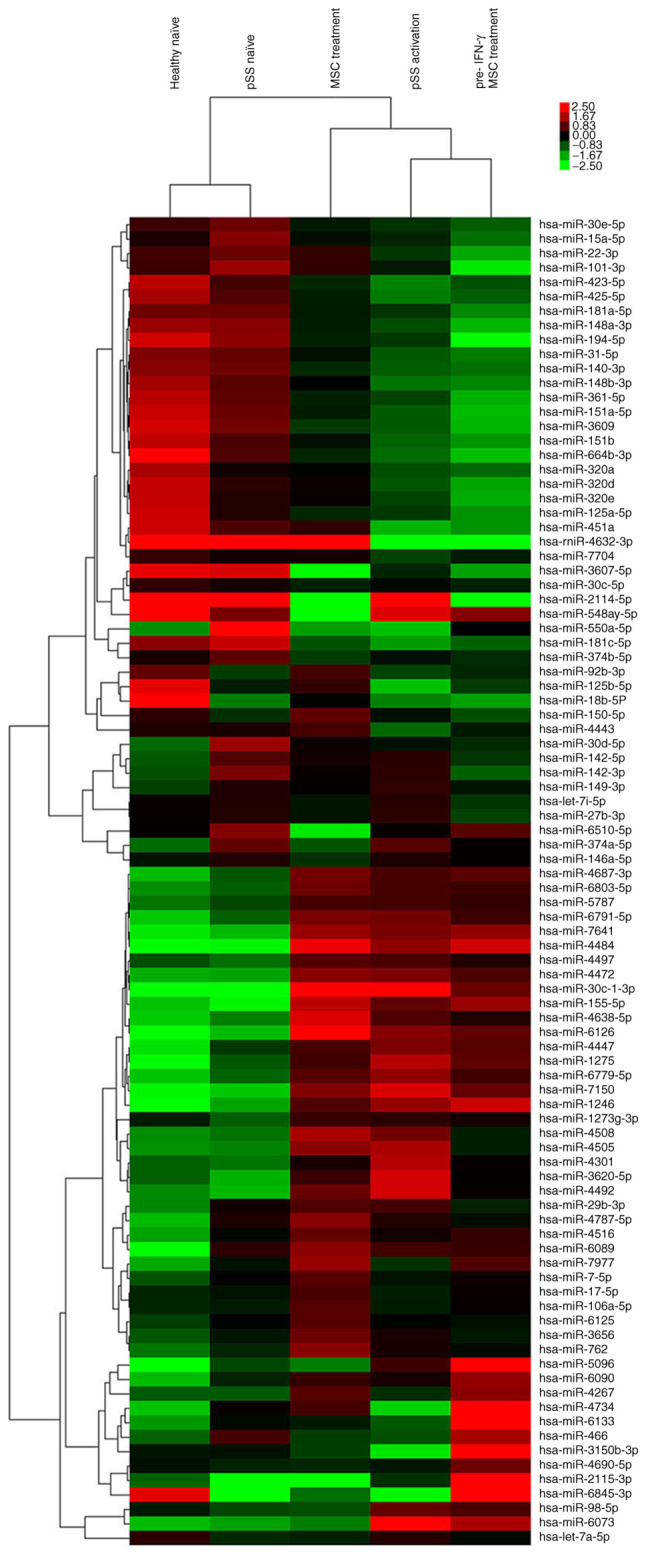
Expression level profiles of miRNAs in CD4+ T cells from healthy and pSS naïve, pSS activation, MSC treatment and pre-IFN-γ MSC treatment groups were detected using miRNA microarray. Top bar represents signal levels of miRNA expression from −2.5 (green) to +2.5 (red). Individual >2-fold dysregulated miRNAs are presented on the right. miRNA, microRNA; pSS, primary Sjögren syndrome; MSC, mesenchymal stem cell.
Given the unique miRNA profiling in stimulated CD4+ T cells from patients with pSS, the effect of MSC treatment alone on miRNome patterns of activated CD4+ T cells was further investigated. Compared with pSS activation, 13 of 27 differentially expressed miRNAs were upregulated following MSC treatment (Fig. 1; Table SII), including miRNA-92b-3p, −125b-5p, −150-5p, −155-5p, −451a, −3150b-3p, −4267, −4443, −4484, −4638-5p, −4734, −6126 and −7977. The downregulated miRNAs were miRNA-98-5p, −146a-5p, −374a-5p, −1246, −1275, −3607-5p, −3620-5p, −4301, −4492, −5096, −6073, −6510-5p and −7150. Moreover, the upregulation of miRNA-98-5p, −1246, −1275, −3620-5p, −4301, −4492, −5096, −6073 and −7150 and miRNA-155-5p, −4484, −4638-5p and −6216 in pSS activation was reversed or promoted by MSC treatment, respectively. Downregulation of miRNA-125b-5p, −451a, −3150b-3p, −4443 and −4734 and miRNA-3607-5p and −6510-5p in pSS activation was reversed or promoted by MSC treatment, respectively.
Although MSC pretreatment by IFN-γ did not inhibit CD4+ T cell proliferation more potently compared with MSC treatment alone, 32 differentially expressed miRNAs existed between the two groups (Fig. 1; Table SII). The primary upregulated miRNAs in the pre-IFN-γ MSC treatment group included miRNA-146a-5p, −466, −1246, −3150b-3p, −4267, −4690-5p, −4734, −5096, −6090 and −6133, whereas the downregulated miRNAs included miRNA-22-3p, −30c-1-3p, −150-5p, −451a, −762, −3656, −4508, −4638-5p and −6126.
miRNA target prediction by two databases
In light of the impact of pSS activation and MSC treatment on CD4+ T cell proliferation, TargetScan and miRanda were used to predict the combined target genes of aberrant miRNAs in the two groups. A total of 3,124 and 2,127 target genes were predicted for the 55 miRNAs in the pSS activation group and 27 miRNAs in the MSC treatment group, respectively (Tables SIII and SIV).
miRNA action on activated CD4+ T cells and MSC treatment via bioinformatics
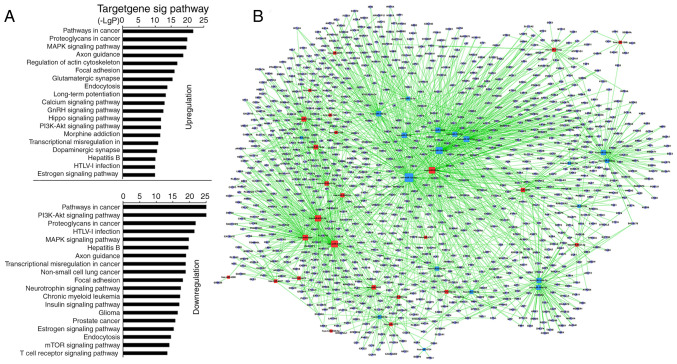
KEGG predicted that 55 miRNAs in the pSS activation group significantly upregulated 128 and downregulated 131 GO terms (Table SV). Among the upregulated GOs, the top 10 were associated with ‘pathways in cancer’, ‘proteoglycans in cancer’, ‘MARK signaling pathway’, ‘regulation of actin cytoskeleton’, ‘axon guidance’, ‘focal adhesion’, ‘glutamatergic synapse’, ‘endocytosis’, ‘long-term potentiation’ and ‘calcium signaling pathway’ (Fig. 2A; Table SV). Among the downregulated GOs, the top 10 were associated with ‘PI3K-AKT signaling pathway’, ‘pathways in cancer’, ‘HTLV–I infection’, ‘proteoglycans in cancer’, ‘hepatitis B’, ‘MAPK signaling pathway’, ‘axon guidance’, ‘non-small-cell lung cancer’, ‘transcriptional misregulation in cancer’ and ‘focal adhesion’ (Fig. 2A; Table SV). ‘TCR signaling pathway’, which is stimulated directly by the anti-CD3 antibody and anti-CD28 antibody, was significantly downregulated (Fig. 2A; Table SV). The miRNA-mRNA network via bioinformatics predicted that the top 20 GO terms showing a high enrichment degree were upregulated by miRNA-5787, −98-5p, −6791-5p, −4505, −7150, −6779-5p, −30c-1-3p, −155-5p and −5096, and downregulated by miRNA-15a-5p, −181a-5p, −181c-5p, −22-3p, −140-3p, −3609, −30e-5p, −148b-3p, −101-3p, −148a-3p and −30d-5p (Fig. 2B and Table SVI). Notably, miRNA-155-5p, −98-5p, −5096, −5787, −181a-5p, −15a-5p, −148b-3p, −140-3p, −7150 and −3609 participated in ‘TCR signaling pathway’ (Table SVII). miRNA-155 was predicted to regulate the TCR signaling pathway via targeting key genes [such as Fos, p21 (RAC1) activated kinase (PAK)2, MAP3K14, and PIK3R1] whereas miRNA-181a-5p was predicted to target Fos and tumor necrosis factor. Other high-degree miRNAs were also associated with ‘TCR signaling pathway’ [miRNA-5096 and −148b-3p targeted SOS1; miRNA-15a-5p targeted KRAS; miRNA-7150 targeted 3-phosphoinositide-dependent protein kinase 1, MAP2K4 and P73; miRNA-98-5p targeted AKT2, CBL, RAS guanyl releasing protein 1 (RASGPR1), VAV3 and PAK1; Table SVII]. The microarray analysis indicated that miRNA-92b-3p, −125b-5p and −150-5p exhibited the highest upregulation, and miRNA-146a-5p, −374a-5p and −1246 exhibited the greatest downregulation. However, bioinformatics demonstrated that the aforementioned miRNAs were not involved in ‘TCR signaling pathway’.
Figure 2.
Prediction of signaling pathways and the mRNA-miRNA network in CD4+ T cells from the pSS activation group. (A) miRNA-Kyoto Encyclopedia of Genes and Genomes network. Vertical axis is the pathway category; horizontal axis is the P-value of each pathway. Lower P-values indicate more miRNAs regulate the pathways and the pathways serve more important roles in activated CD4+ T cells. (B) miRNA-mRNA-network. Red squares represent upregulated miRNAs; yellow squares represent downregulated miRNAs; blue circles represent genes; lines represent the association between the miRNA and the gene. The network is for the assessment of regulatory status of miRNAs and genes. The degree (size) of squares indicates regulatory functionality of miRNA (i.e. bigger degree indicates more functions). Similarly, the degree of the blue circle is consistent with the network linkage (bigger circle indicates greater regulation by miRNAs). miRNA, microRNA; pSS, primary Sjögren syndrome.
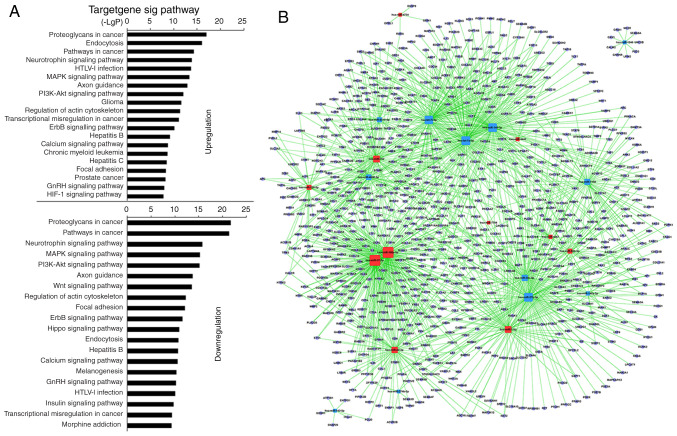
Analysis using KEGG revealed that 27 miRNAs in MSC-treated CD4+ T cells upregulated 117 and downregulated 123 GO terms significantly (Table SVIII). Among the upregulated GO terms, the top 10 were associated with ‘proteoglycans in cancer’, ‘endocytosis’, ‘pathways in cancer’, ‘neurotrophin signaling pathway’, ‘HTLV–I infection’, ‘PI3K-AKT signaling pathway’, ‘MAPK signaling pathway’, ‘axon guidance’, ‘glioma’ and ‘regulation of actin cytoskeleton’. Among the downregulated GO terms, the top 10 were associated with ‘proteoglycans in cancer’, ‘pathways in cancer’, ‘neurotrophin signaling pathway’, ‘PI3K-AKT signaling pathway’, ‘MAPK signaling pathway’, ‘axon guidance’, ‘regulation of actin cytoskeleton’, ‘Wnt signaling pathway’, ‘focal adhesion’ and ‘ErbB signaling pathway’ (Fig. 3A; Table SVIII). Furthermore, GO terms, including ‘proteoglycans in cancer’, ‘endocytosis, pathways in cancer’, ‘MAPK signaling pathway’, ‘HTLV–I infection’, ‘PI3K-AKT signaling pathway’, ‘axon guidance’, ‘regulation of actin cytoskeleton’ and ‘focal adhesion’, also showed statistically significant enrichment in the pSS activation group. ‘TCR signaling pathway’ remained unchanged in the MSC treatment group (Table SIX). The miRNA-mRNA network revealed that, of miRNAs with high enrichment degree, miRNA-92b-3p, −7704, −762, −7-5p, −4734, −4443, −4267, −22-3p, −17-5p, −150-5p and −106a-5p were upregulated, and miRNA-7150, −6510-5p, −4690-5p, −374b-5p, −30c-5p, −27b-3p, −149-3p, −146a-5p, −142-5p, −1246, -let-7i-5p and -let-7a-5p were downregulated (Fig. 3B; Table SX). miRNA-22-3p and −7150 exhibited a high enrichment degree in the pSS activation group and miRNA-7150 participated in ‘TCR signaling pathway’.
Figure 3.
Prediction of signaling pathways and the mRNA-miRNA network in CD4+ T cells from MSC treatment group. (A) miRNA-Kyoto Encyclopedia of Genes and Genomes network. Horizontal axis is the P-value of each pathway. Lower P-values indicate more miRNAs regulate the pathways and the pathways serve more important roles in MSC-treated CD4+ T cells. (B) miRNA-mRNA-network. Red squares represent upregulated miRNAs; yellow squares represent downregulated miRNAs; blue circles represent genes; lines represent association between the miRNA and the gene. The network is for the assessment of regulatory status of miRNAs and genes. The degree (size) of square represents regulatory functionality of miRNA (i.e. bigger degree indicates more functions). Similarly, the degree of the blue circle is consistent with the network linkage (bigger circle indicates greater regulation by miRNAs). miRNA, microRNA; MSC, mesenchymal stem cell.
qPCR validation in the miRNA microarray
Based on miRNA function classification, certain miRNAs in the miRNA microarray were validated by qPCR. Of the eight aberrant miRNAs in the pSS activation group (Fig. 4), the expression levels of miRNA-155-5p, −7150 and −5096 were upregulated in activated CD4+ T cells while those of miRNA-15a-5p, −181a, −125b-5p, −140-3p and −22-3p were downregulated. Furthermore, upregulation of miRNA-7150 and −5096 and downregulation of miRNA-125b-5p and −22-3p were reversed in the MSC treatment group. Moreover, miRNA-5096 was upregulated in the pSS naïve (vs. healthy naïve) group whereas miRNA-125b-5p was downregulated (Fig. 4). All these results coincided with the microarray results. Although the microarray indicated that miRNA-155-5p had no 2-fold difference in the pSS activation or MSC treatment groups, qPCR validated a 1.5-fold increase in the MSC treatment group compared with the pSS activation group.
Figure 4.
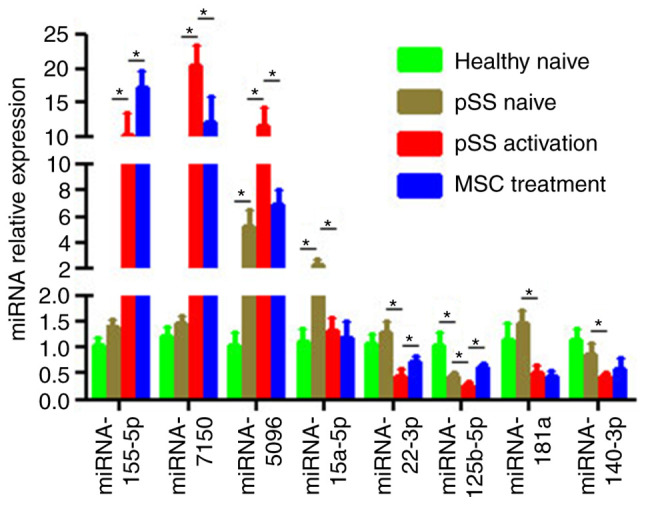
Confirmation of differential expression levels of miRNAs via RT-qPCR. Expression levels of mature miRNA-155-5p, −7150, −5096, −15a-5p, −181a, −125b-5p, −140-3p and −22-3p in CD4+ T cells from healthy and pSS naïve, pSS activation and MSC treatment groups were determined using RT-qPCR. U6 snRNA expression levels were used for normalization. Data are presented as the mean ± SEM of three independent repeats. *P<0.05. miRNA, microRNA; RT-q, reverse transcription-quantitative; pSS, primary Sjögren syndrome; MSC, mesenchymal stem cell.
MSCs suppress IFN-γ production in activated CD4+ T cells from patients with pSS
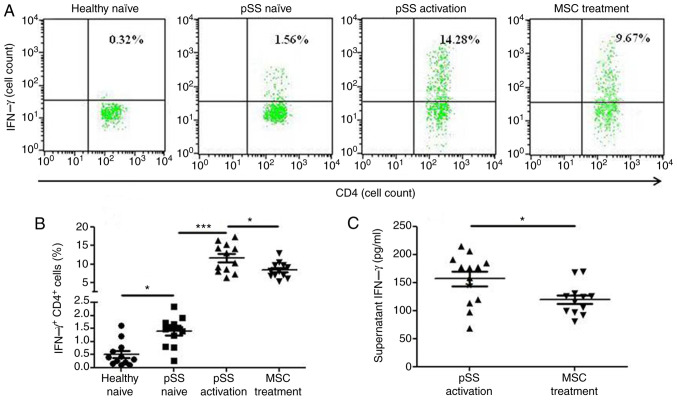
CD4+ IFN-γ+ cells were more prevalent in the pSS activation group than in the pSS naïve group following 72-h co-culture, and MSCs suppressed the levels of CD4+ IFN-γ+ cells (Fig. 5A and B). Moreover, MSCs decreased the levels of supernatant IFN-γ (Fig. 5C).
Figure 5.
IFN-γ expression levels in CD4+ T cells from healthy and pSS naïve, pSS activation and MSC treatment groups. (A) Representative flow cytometric analysis of CD4+IFNγ+ cells from four groups. (B) Percentage of CD4+IFNγ+ cells was calculated (n=12). (C) Supernatant IFN-γ secretion from pSS activation and MSC treatment groups was assessed by ELISA (n=12). Data are presented as the mean ± SEM. *P<0.05; ***P<0.001. pSS, primary Sjögren syndrome; MSC, mesenchymal stem cell.
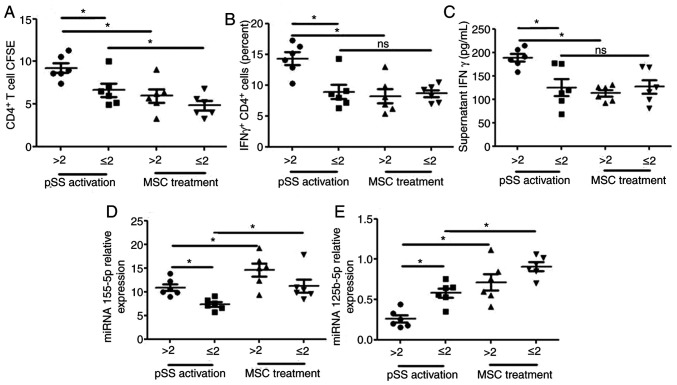
Association between disease activity and miRNA-155-5p/miRNA-125b-5p in activated CD4+ T cells from patients with pSS
In light of activated CD4+ cells in PBMCs from patients with pSS, the present study investigated whether miRNA-155-5p/miRNA-125b-5p in activated CD4+ T cells was associated with pSS disease activity. Patients were classified into two groups based on ESSDAI score: ESSDAI ≤2 (n=6) was classified as inactive and ESSDAI >2 (n=6) was considered to be active. The ESSDAI score was associated with alterations in proliferation of CD4+ T cells and CD4+ IFN-γ+ cells, the expression levels of miRNA-125b-5p and miRNA-155 in CD4+ T cells and supernatant IFN-γ secretion (Fig. 6). The proliferation of CD4+ T and CD4+ IFN-γ+ cells, the expression levels of miRNA-155 in CD4+ T cells and supernatant IFN-γ secretion were notably increased and the expression levels of miRNA-125b-5p in CD4+ T cells were significantly decreased in patients with active pSS. MSC treatment reversed these effects on the proliferation of CD4+ T cells and CD4+ IFN-γ+ cells, the expression levels of miRNA-125b-5p in CD4+ T cells and supernatant IFN-γ secretion and promoted the expression levels of miRNA-155 in CD4+ T cells from patients with active pSS. However, MSC treatment did not reverse the effects on the proliferation of CD4+ IFN-γ+ cells and supernatant IFN-γ secretion in CD4+ T cells from patients with inactive pSS.
Figure 6.
Association between disease activity and miRNA-155-5p/miRNA-125b-5p in activated CD4+ T cells from patients with pSS. (A) Proliferation of CD4+ T cells, (B) number of CD4+ IFN-γ+ cells, (C) supernatant IFN-γ secretion and expression levels of (D) miRNA-155-5p and (E) miRNA-125b-5p in CD4+ T cells were in patients with inactive (ESSDAI ≤2; n=6) and active disease (ESSDAI >2; n=6) from pSS activation and MSC treatment groups. Data are presented as the mean ± SEM. *P<0.05. miRNA, microRNA; pSS, primary Sjögren syndrome; ESSDAI, EULAR Sjögren's syndrome disease activity index; MSC, mesenchymal stem cell; ns, not significant.
MSC-secreted cytokines
MSCs regulate T cells via soluble factors (24). Therefore, potential cytokines for MSC modulation of CD4+ T cells were investigated. Activated CD4+ T cells alone in culture were revealed to secrete numerous cytokines such as PGE2, indoleamine 2,3-dioxygenase (IDO), IL-6 and −10 and TGF-β1. When MSCs were co-cultured with activated CD4+ T cells, the expression levels of PGE2, IDO and IL-6 significantly increased (Fig. 7).
Figure 7.
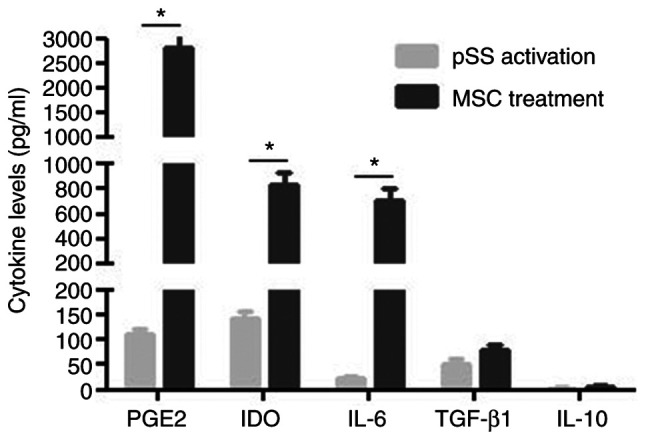
Cytokine secretion profiles of CD4+ T cells/MSCs from pSS activation and MSC treatment groups as measured by ELISA. Data are presented as the mean ± SEM of 3 independent experiments. *P<0.05. MSC, mesenchymal stem cells; pSS, primary Sjögren's syndrome; PGE2, prostaglandin E2; IDO, indoleamine 2,3-dioxygenase.
Discussion
To the best of our knowledge, the present study was novel in revealing genome-wide miRNAs in CD4+ T cells from patients with pSS following activation and MSC treatment. CD4+ T cells relied on signaling pathways that maintain homeostasis between activation and quiescence. Specific miRNAomics detected 55 differential miRNAs between the pSS activation and naïve groups. Teteloshvili et al (25) reported that CD4+ T cells of healthy individuals stimulated by CD3/CD28 antibodies exhibited significant activation-induced changes in 12 miRNAs, including upregulation of miRNA-155, miR-21 and miR-146a. The present miRNA array comprised 38 new miRNAs in the T-lymphocyte function, including upregulated 128 and 131 downregulated GO terms. Moreover, ‘TCR signaling pathway’ also changed directly, which was targeted by miRNAs such as miRNA-155-5p, −98-5p, −5096, −5787, −181a-5p, −15a-5p, −148b-3p, −140-3p, −7150 and −3609. The present study investigated certain known miRNAs in the T-lymphocyte function. For example, miRNA-155 has been revealed to upregulate the susceptibility of CD4+ T cells to natural regulatory T cell-mediated inhibition (26); miRNA-1246 is predominantly expressed in both naïve and memory regulatory T cells (Tregs) (27); and miRNA-15a-5p is displayed in naïve natural Tregs from patients at high risk of type 1 diabetes (28). The loss of miRNA-181a-5p has been demonstrated to alleviate experimental autoimmune encephalomyelitis, attenuate basal TCR signaling in peripheral T cells and decrease their migration from lymph to lesions (29).
MSCs inhibit T cell proliferation and activation and suppress IFN-γ production in CD4+ T cells in patients with pSS, but the underlying mechanism remains unclear. In the present study, the effect of MSCs on miRNA expression levels in activated CD4+ T cells in patients with pSS was studied; a total of 27 differential miRNAs between the pSS activation and MSC treatment groups was identified. These miRNAs were involved in 117 upregulated and 123 downregulated GO terms. Although the TCR signaling pathway remained unchanged, certain TCR-targeted miRNAs in the pSS activation group, such as miRNA-98-5p, −5096, −7150 or miRNA-155-5p, were reversed or promoted by MSC treatment. Notably, the expression levels of miRNA-155-5p are increased in PBMCs of patients with pSS (7). Upregulated miRNA-155-5p in the pSS activation group was promoted by MSC treatment. Grigoryev et al (30) revealed that knockdown of miRNA-155-5p resulted in significant proliferation of CD4+ T cells, confirming that the miRNA-155-5p serves an antiproliferative role during activation. The present findings indicated that MSCs may inhibit mitogenic CD4+ T cell proliferation via upregulation of miRNA-155-5p. In addition, although miRNA-125b-5p did not target the TCR signaling pathway directly, both miRNA microarray and qPCR demonstrated that downregulation of miRNA-125b-5p in the pSS naïve group further decreased activation, whereas these effects were reversed by MSC treatment. miRNA-125b-5p was reported to regulate genes associated with T cell differentiation, such as IL2RB, IFNG, PR/SET domain 1 and IL10RA (31); overexpression of miRNA-125b-5p in naïve lymphocytes may inhibit differentiation to effector lymphocytes. miRNA-125b-5p may indirectly participate in TCR activation of CD4+ T cells, pSS pathogenesis and MSC treatment for pSS.
The association between ESSDAI and miRNA-155-5p/miRNA-125b-5p in activated CD4+ T cells was analyzed. The activated CD4+ T cells from patients with active pSS exhibited increased expression of the IFN-γ+ phenotype, characterized by the overexpression of IFN-γ and miRNA-155-5p and low expression levels of miRNA-125b-5p. MSC treatment did not change IFN-γ levels in activated CD4+ T cells from patients with inactive pSS but did change miRNA-155-5p and miRNA-125b-5p levels in activated CD4+ T cells from patients with inactive pSS. miRNA-155-5p or miRNA-125b-5p may be a more suitable biomarker than IFN-γ when patients with inactive pSS are treated using MSCs. However, further studies are required to confirm this due to the small cohort of patients with pSS.
As for the underlying mechanisms by which MSCs change the miRNome patterns of activated CD4+ T cells, MSCs may affect the alloimmune response via either direct contact or secretory cytokines such as PGE2, TGF-β, IL-10, matrix metalloproteinases and IDO. MSCs suppressed CD4+ T cell proliferation in patients with pSS, which was consistent with the findings of a previous study (3). However, exposure to IFN-γ 24 h before co-culture did not induce greater MSC inhibitory effects on CD4+ T cell proliferation, which was different from the findings of a previous study (22). This may be because the inhibitory effects of exogenous IFN-γ pretreatment were insufficient to overcome the effects of endogenous IFN-γ in activated CD4+ T cells.
MSCs alone or co-cultured with activated CD4+ T cells were revealed to secrete a number of factors, which were reported to affect the miRNA expression levels directly, such as PGE2 (32,33), TGF-β1 (34,35) and IL-10 (36,37), indicating the critical role of these soluble factors of MSCs in miRNA profiles.
The present study had certain limitations. Firstly, the association between miRNA-155/125-5p and pSS pathogenesis was only investigated via co-culturing MSCs with pSS CD4+ T cells. Future studies should use transiently silenced miRNA-155 and/or −125-5p in pSS CD4+ T cells to support the present results. Secondly, miRNA-125b and miRNA-155 changes in systematic RNA array were not significant. This may because be only one RNA array was performed for each group, therefore the miRNA-125b and miRNA-155 was verified by qPCR. Finally, the present study did not demonstrate whether soluble factors or MSCs themselves affect CD4+ activation and cytokine production. This requires confirmation via further experiments, such as RNA interference against Cox-2 in MSCs.
In summary, the present study identified miRNome changes in CD4+ T cells from patients with pSS following activation and MSC treatment, which presented with different miRNA profiles. These miRNAs changed GO terms significantly and were associated with CD4+ T cell proliferation and activation. The TCR signaling pathway induced directly by anti-CD3 and anti-CD28 antibody was affected more profoundly in the pSS activation group than in the MSC treatment group. Upregulation of miRNA-5096 and miRNA-7150 and downregulation of miRNA-22-3p and miRNA-125b-5p in the pSS activation group were reversed by MSC treatment; miRNA-5096 was upregulated and miRNA-125b-5p was downregulated in the pSS naïve group compared with the healthy naïve group, indicating that the two miRNAs may serve a key role in pSS pathogenesis and MSC treatment. Moreover, upregulated miRNA-155-5p was further increased by MSC treatment, implying that MSCs may exhibit immunosuppressant effects in activated CD4+ T cells via the miRNA-155-5p antiproliferative mechanism. The findings demonstrated a key role of miRNAs in CD4+ T cells from patients with pSS following activation and MSC treatment; miRNA-5096, −125b-5p or −155-5p contributed to CD4+ T cell proliferation and activation, which may be crucial for pSS pathogenesis and MSC treatment. Moreover, miRNA-125b-5p and miRNA-155 levels in activated CD4+ T cells from patients with pSS indicated that pSS disease activity could offer a novel biomarker for future MSC management.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MSCs
mesenchymal stem cells
- pSS
primary Sjögren syndrome
- GO
Gene Ontology
- PGE2
prostaglandin E2
- IDO
indoleamine 2,3-dioxygenase
- UCs
umbilical cords
- KEGG
Kyoto Encyclopedia of Genes and Genomes
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81273295, 81671598 and 81801601), the China International Medical Exchange Foundation (grant no. Z-2014-06-2-1620), the Shanghai Wu Mengchao Medical Foundation (grant no. 17YF1417200), the Clinical Research Key Program of Tongji Hospital Tongji University (grant no. ITJZD1909) and the Research Training Foundation of Tongji Hospital (grant no. GJPY1805).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JT have made substantial contributions to conception and design of the present study, and acquisition, analysis and interpretation of data. BG was involved in data acquisition and drafting the manuscript. ZL, LZ, JH, JP, SP, MZ and JL contributed to data acquisition. JL also made substantial contributions to conception and design of the present study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Healthy controls and patients with pSS signed informed consent from Tongji Hospital, Tongji University School of Medicine (Shanghai, China). The participants' rights were protected. All procedures with blood samples and MSCs were confirmed by the Ethics Committee of Tongji Hospital (approval no. K-2012-006; 25 February 2012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mariette X, Criswell LA. Primary sjögren's syndrome. N Engl J Med. 2018;378:931–939. doi: 10.1056/NEJMcp1702514. [DOI] [PubMed] [Google Scholar]
- 2.Brito-Zerón P, Sisó-Almirall A, Bové A, Kostov BA, Ramos-Casals M. Primary Sjögren syndrome: An update on current pharmacotherapy options and future directions. Expert Opin Pharmacother. 2013;14:279–289. doi: 10.1517/14656566.2013.767333. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical sjögren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss AR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191. doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 6.Podshivalova K, Salomon DR. MicroRNA regulation of T-lymphocyte immunity: Modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit Rev Immunol. 2013;33:435–476. doi: 10.1615/CritRevImmunol.2013006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alevizos I, Alexander S, Turner RJ, Illei GG. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in sjögren's syndrome. Arthritis Rheum. 2011;63:535–544. doi: 10.1002/art.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Tondreau T, Lagneaux L, Dejeneffe M, Delforge A, Massy M, Mortier C, Bron D. Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: Phenotype, proliferation kinetics and differentiation potential. Cytotherapy. 2004;6:372–379. doi: 10.1080/14653240410004943. [DOI] [PubMed] [Google Scholar]
- 10.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, et al. Classification criteria for sjögren's syndrome: A revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, Gottenberg JE, Bootsma H, Mariette X, Vitali C, EULAR Sjögren's Task Force EULAR sjögren's task force. EULAR sjogren's syndrome disease activity index: Development of a consensus systemic disease activity index for primary sjogren's syndrome. Ann Rheum Dis. 2010;69:1103–1109. doi: 10.1136/ard.2009.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Pfaffl MW, Lange IG, Daxenberger A, Meyer HH. Tissue-specific expression pattern of estrogen receptors (ER): Quantification of ER alpha and ER beta mRNA with real-time RT-PCR. APMIS. 2001;109:345–355. doi: 10.1034/j.1600-0463.2001.090503.x. [DOI] [PubMed] [Google Scholar]
- 14.The Gene Ontology Consortium: The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma'ayan A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford) 2016;2016:baw100. doi: 10.1093/database/baw100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A, Sidiropoulos K, Cook J, Gillespie M, Haw R, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. doi: 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi M, Horton JD, Cohen JC, Hobbs HH, Stephens RM. WholePathwayScope: A comprehensive pathway-based analysis tool for high-throughput data. BMC Bioinformatics. 2006;19:30. doi: 10.1186/1471-2105-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Lee YT, Hong JM, Hwang YI. Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression. Anat Cell Biol. 2013;46:262–271. doi: 10.5115/acb.2013.46.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. doi: 10.1186/ar2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 25.Teteloshvili N, Smigielska-Czepiel K, Kroesen BJ, Brouwer E, Kluiver J, Boots AM, van den Berg A. T-Cell activation induces dynamic changes in miRNA expression patterns in CD4 and CD8 T-cell subsets. Microrna. 2015;4:117–122. doi: 10.2174/2211536604666150819194636. [DOI] [PubMed] [Google Scholar]
- 26.Lind EF, Ohashi PS. Mir-155, a central modulator of T-cell responses. Eur J Immunol. 2014;44:11–15. doi: 10.1002/eji.201343962. [DOI] [PubMed] [Google Scholar]
- 27.Smigielska-Czepiel K, van den Berg A, Jellema P, van der Lei RJ, Bijzet J, Kluiver J, Boots AM, Brouwer E, Kroesen BJ. Comprehensive analysis of miRNA expression in T-cell subsets of rheumatoid arthritis patients reveals defined signatures of naive and memory tregs. Genes Immun. 2014;15:115–125. doi: 10.1038/gene.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Feng ZP, Naselli G, Bell F, Wettenhall J, Auyeung P, Ellis JA, Ponsonby AL, Speed TP, Chong MM, Harrison LC. MicroRNAs in CD4+ T cell subsets are markers of disease risk and T cell dysfunction in individuals at risk for type 1 diabetes. J Autoimmun. 2016;68:52–61. doi: 10.1016/j.jaut.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Schaffert SA, Loh C, Wang S, Arnold CP, Axtell RC, Newell EW, Nolan G, Ansel KM, Davis MM, Steinman L, Chen CZ. Mir-181a-1/b-1 modulates tolerance through opposing activities in selection and peripheral t cell function. J Immunol. 2015;195:1470–1479. doi: 10.4049/jimmunol.1401587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grigoryev YA, Kurian SM, Hart T, Nakorchevsky AA, Chen C, Campbell D, Head SR, Yates JR, III, Salomon DR. MicroRNA regulation of molecular networks mapped by global microRNA, mRNA, and protein expression in activated T lymphocytes. J Immunol. 2011;187:2233–2243. doi: 10.4049/jimmunol.1101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi RL, Rossetti G, Wenandy L, Curti S, Ripamonti A, Bonnal RJ, Birolo RS, Moro M, Crosti MC, Gruarin P, et al. Distinct microRNA signatures in human lymphocyte subsets and enforcement of the naive state in CD4+ T cells by the microRNA miR-125b. Nat Immunol. 2011;12:796–803. doi: 10.1038/ni.2057. [DOI] [PubMed] [Google Scholar]
- 32.Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol. 2013;190:5809–5817. doi: 10.4049/jimmunol.1203274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshima H, Oshima M. The role of PGE2-associated inflammatory responses in gastric cancer development. Semin Immunopathol. 2013;35:139–150. doi: 10.1007/s00281-012-0353-5. [DOI] [PubMed] [Google Scholar]
- 34.Domingo-Gonzalez R, Wilke CA, Huang SK, Laouar Y, Brown JP, Freeman CM, Curtis JL, Yanik GA, Moore BB. Transforming growth factor-β induces microRNA-29b to promote murine alveolar macrophage dysfunction after bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2015;308:L86–L95. doi: 10.1152/ajplung.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci USA. 2013;110:11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer JS, Montufar-Solis D, Vigneswaran N, Klein JR. Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10-/-mice precedes expression in the colon. J Immunol. 2011;187:5834–5841. doi: 10.4049/jimmunol.1100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.