Abstract
Background
The Patient-Reported Outcomes Measurement Information SystemⓇ (PROMISⓇ) is a dynamic system of psychometrically sound patient-reported outcome (PRO) measures. There has been a recent increase in the use of PROMIS measures, yet little has been written about the reporting of these measures in the field of orthopedics. The purpose of this study was to conduct a systematic review to determine the uptake of PROMIS measures across orthopedics and to identify the type of PROMIS measures and domains that are most commonly used in orthopedic research and practice.
Methods
We searched PubMed, Embase, and Scopus using keywords and database-specific subject headings to capture orthopedic studies reporting PROMIS measures through November 2018. Our inclusion criteria were use of PROMIS measures as an outcome or used to describe a population of patients in an orthopedic setting in patients ≥ 18 years of age. We excluded non-quantitative studies, reviews, and case reports.
Results
Our final search yielded 88 studies published from 2013 through 2018, with 57% (50 studies) published in 2018 alone. By body region, 28% (25 studies) reported PROMIS measures in the upper extremity (shoulder, elbow, hand), 36% (32 studies) reported PROMIS measures in the lower extremity (hip, knee, ankle, foot), 19% (17 studies) reported PROMIS measures in the spine, 10% (9 studies) reported PROMIS measures in trauma patients, and 6% (5 studies) reported PROMIS measures in general orthopedic patients. The majority of studies reported between one and three PROMIS domains (82%, 73 studies). The PROMIS Computerized Adaptive Test (CAT) approach was most commonly used (81%, 72 studies). The most frequently reported PROMIS domains were physical function (81%, 71 studies) and pain interference (61%, 54 studies).
Conclusion
Our review found an increase in the reporting of PROMIS measures over the recent years. Utilization of PROMIS measures in orthopedic populations is clinically appropriate and can facilitate communication of outcomes across different provider types and with reduced respondent burden.
Registration
The protocol for this systematic review was designed in accordance with the PRISMA guidelines and is registered with the PROSPERO database (CRD42018088260).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-020-02068-9.
Keywords: PROMIS, Patient-reported outcome measures, Orthopedics, Physical function, Pain
Introduction
In order to determine if a patient has achieved a meaningful outcome, it is insufficient to evaluate treatment results solely on medical history, physical findings, laboratory tests, or imaging findings [1]. Patient-reported outcome (PRO) measures are a useful tool to quantify and communicate a patient’s health status to healthcare providers that directly incorporates the patient’s voice. Change in PROs can be one of the measures of “success” from a patient’s perspective after an orthopedic procedure [2]. PROs are increasingly being used as part of the clinical encounter to guide treatment decisions and determine the effectiveness of interventions [3], but PROs have presented challenges with implementation and measure selection.
In orthopedic practice and research, there is great variability in the number of PRO measures available. As a result, there is confusion among orthopedic providers about which PRO measure is most appropriate given a patient population and how to appropriately interpret a patient’s score to enhance treatment recommendations. Subsequently, in orthopedics, there has been a recent increase in the adoption of a universally accepted set of PRO measures: the Patient-Reported Outcomes Measurement Information System® (PROMIS®). PROMIS has been compared against conventional general health and disease-specific PRO measures and regularly has been found to improve coverage of the relevant health domain, increase reliability, and reduce respondent burden [4].
PROMIS measures were developed with support by the National Institutes of Health (NIH) as an effort to address the need for more valid, reliable, and generalizable measures of clinical outcomes that are important to patients [5]. PROMIS is a set of psychometrically sound measures to assess a patient’s physical, mental, and social health across multiple conditions or diseases, including orthopedic conditions. PROMIS measures overcome the limitations of traditional PRO measures used in orthopedic research and practice by scoring all PROMIS domains using a common metric of a T-score that is normalized to the U.S. general population. PROMIS provides access to both fixed-length measures (e.g., 6-item measure of fatigue) and computerized adaptive testing (CAT) that tailors the measure for each individual to allow for efficient assessment when response burden is of concern [6].
In recent years, a proliferation of studies have reported the association of PROMIS measures with traditional measures and have demonstrated the reliability and performance of PROMIS measures in orthopedic populations. While there have been a few systematic reviews about the use of PROMIS measures in certain disciplines within orthopedics [7–10], these reviews do not describe how the measures have been reported neither in the literature nor the general uptake of PROMIS measures within orthopedic research and practice. Thus, we sought to evaluate the adoption of PROMIS measures in orthopedics by describing how the measures are used and reported on, including the PROMIS domains evaluated, the type of PROMIS instrument used, and other traditional measures that were reported along with PROMIS measures.
Methods
Review design
The protocol for this systematic review was designed in accordance with the PRISMA guidelines [11] and is registered with the PROSPERO database (CRD42018088260) [12]. We collaborated with a research librarian (LL) to develop an appropriate search strategy and management of the literature review.
Data sources and search strategy
We performed a literature search of PubMed, Embase, and Scopus from inception to November 4, 2018, using a combination of keywords and database-specific subject headings to capture studies done in an orthopedic setting and/or procedures that reported a PROMIS measure as an outcome (Additional file 1). We added search filters to exclude case studies or reports, editorials, letters to the editor, and studies not written in English.
Inclusion and exclusion criteria
Inclusion criteria included the use of PROMIS measures in studies conducted in orthopedic settings for clinical care purposes or studies that used PROMIS measures to assess an outcome from an orthopedic intervention. Our exclusion criteria were study population < 18 years of age; non-orthopedic interventions, settings, or providers performing the intervention; and qualitative studies, commentaries, or systematic reviews. All included studies were peer-reviewed, reported at least one PROMIS measure, and used an experimental, quasi-experimental, or observational design. Two authors screened articles (MH and SZG) and a third author (ER) resolved any conflicts.
Study selection and data extraction
After databases were searched, titles and abstracts of studies were uploaded into Covidence, a systematic review management software [13]. The article selection process was done in two phases. In the first phase, two authors (MH and SZG) performed independent reviews of titles and abstracts in Covidence using the predefined inclusion and exclusion criteria. Articles were moved to full-text review if one or both authors found the article potentially relevant. In the second phase, the same two authors independently reviewed full-text articles for eligibility. Any conflicts were resolved by the third author.
Data analysis
Included studies were evaluated from November 2018 to June 2020. The primary purpose of this review was to describe the uptake of PROMIS measures in orthopedic research and practice through qualitative synthesis, and then rate the quality of included studies. Therefore, we did not perform a meta-analysis of data. For the qualitative synthesis, we described the studies by publication year, clinical population, study type, and sample size. We evaluated the reporting of PROMIS measures by recording the PRO domains reported in each study and the type of PROMIS measures used (i.e., domain-specific fixed short forms, multiple domain profile short forms, or CAT). Last, we described the frequency in which PROMIS measures were reported alongside traditional measures by the clinical population. Traditional measures are non-PROMIS established measures used in orthopedics.
Quality assessment
We used the Newcastle-Ottawa Scale (NOS) to assess the quality of included studies (Additional file 2). Because this review included a heterogeneous group of studies with a wide variety of methodologies, there is likely no single risk of bias tool to perfectly evaluate study quality across such a diverse group. The NOS was developed to assess the quality of nonrandomized studies, and evaluates studies within three domains: the selection of study groups, the comparability between these groups, and the determination of the outcome of interest. We used a version of the NOS specifically adapted for cross-sectional studies [14] and for case control and cohort studies [15]. The NOS scoring of seven or more stars is generally considered high quality, though no ranges have been officially reported in the literature [16].
Results
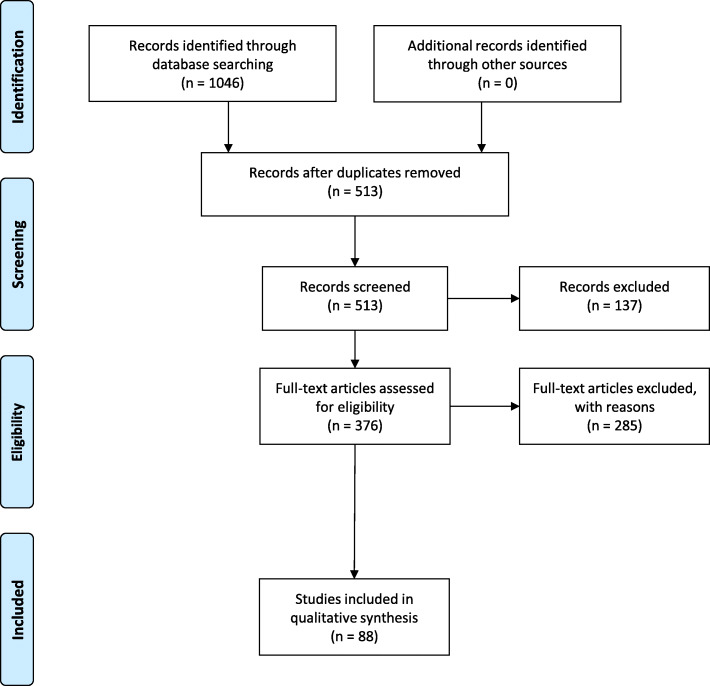
Our preliminary search yielded 1046 citations, and after duplicates were removed, 513 citations were reviewed by their titles and abstracts. Of those, 376 were moved forward to the full-text review stage, and 88 articles remained for inclusion in the systematic review [3, 17–103] (Fig. 1). After conflicts were resolved by the third author, we calculated an 81.6% agreement between the authors performing full-text review.
Fig. 1.
PRISMA literature flow diagram [11]
Study characteristics
Table 1 shows the characteristics of included studies by year, clinical population, study type, and sample size.
Table 1.
Study characteristics
| Year | Author | Orthopedic population | Study design | N |
|---|---|---|---|---|
| 2013 | Hung et al. [17] | Lower extremity patients | Cross-sectional study | 288 |
| 2014 | Hung et al. [18] | Lower extremity patients | Cohort study (prospective observational study) | 311 |
| 2014 | Hung et al. [19] | Lower extremity patients | Cross-sectional study | 126 |
| 2014 | Hung et al. [3] | Trauma patients | Cross-sectional study | 153 |
| 2014 | Hunt et al. [20] | Lower extremity patients | Cohort study (prospective observational study) | 140 |
| 2014 | Papuga et al. [21] | Lower extremity patients | Cohort study (prospective observational study) | 106 |
| 2014 | Tyser et al. [22] | Upper extremity patients | Cross-sectional study | 134 |
| 2015 | Beckmann et al. [24] | Upper extremity patients | Cross-sectional study | 187 |
| 2015 | Mellema et al. [23] | Upper extremity patients | Cohort study (prospective observational study) | 136 |
| 2015 | Morgan et al. [25] | Trauma patients | Cross-sectional study | 47 |
| 2015 | Overbeek et al. [103] | Upper extremity patients | Cross-sectional study | 93 |
| 2015 | Stuart et al. [26] | Trauma patients | Cross-sectional study | 55 |
| 2016 | Beckmann et al. [29] | Upper extremity patients | Cross-sectional study | 379 |
| 2016 | Dasa et al. [33] | Lower extremity patients | Retrospective cohort | 100 |
| 2016 | Fuchs et al. [27] | Lower extremity patients | Retrospective cohort | 93 |
| 2016 | Hermanussen et al. [37] | Upper extremity patients | Cross-sectional study | 111 |
| 2016 | Ho et al. [28] | Lower extremity patients | Cohort study (prospective observational study) | 61 |
| 2016 | Nota et al. [30] | Upper extremity patients | Cross-sectional study | 193 |
| 2016 | Oak et al. [34] | Lower extremity patients | Cohort study (prospective observational study) | 45 |
| 2016 | Papuga et al. [35] | Spine patients | Cross-sectional study | 319 |
| 2016 | Parrish et al. [31] | Upper extremity patients | Cross-sectional study | 112 |
| 2016 | Peters et al. [32] | Upper extremity patients | Cross-sectional study | 115 |
| 2016 | van Leeuwen et al. [36] | Trauma patients | Cross-sectional study | 124 |
| 2017 | Anthony et al. [46] | Upper extremity patients | Cross-sectional study | 70 |
| 2017 | Anthony et al. [45] | Upper extremity patients | Cross-sectional study | 82 |
| 2017 | Beleckas et al. [41] | Upper extremity patients | Cohort study (prospective observational study) | 5202 |
| 2017 | Dowdle et al. [47] | Upper extremity patients | Cross-sectional study | 53 |
| 2017 | Hancock et al. [43] | Lower extremity patients | Cross-sectional study | 107 |
| 2017 | Henn et al. [53] | Upper extremity patients | Cohort study (prospective observational study) | 300 |
| 2017 | Kaat et al. [52] | Trauma patients | Cohort study (prospective observational study) | 132 |
| 2017 | Kazmers et al. [54] | Upper extremity patients | Cross-sectional study | 1299 |
| 2017 | Kleimeyer et al. [50] | Spine patients | Cohort study (prospective observational study) | 88 |
| 2017 | Koltsov et al. [38] | Lower extremity patients | Cohort study (prospective observational study) | 191 |
| 2017 | Nixon et al. [39] | Lower extremity patients | Cross-sectional study | 85 |
| 2017 | Oh et al. [40] | Upper extremity patients | Cross-sectional study | 125 |
| 2017 | Purvis et al. [49] | Spine patients | Cohort study (prospective observational study) | 148 |
| 2017 | Sheean et al. [42] | Lower extremity patients | Cross-sectional study | 42 |
| 2017 | St John et al. [55] | Upper extremity patients | Cross-sectional study | 722 |
| 2018 | Alvarez-Nebreda et al. [102] | Trauma patients | Cohort study (prospective observational study) | 273 |
| (101) | Anderson et al. [100] | Lower extremity patients | Cohort study (prospective observational study) | 61 |
| 2018 | Anderson et al. [101] | Lower extremity patients | Retrospective cohort | 88 |
| 2018 | Austin et al. [99] | Lower extremity patients | Retrospective cohort | 2308 |
| 2018 | Beleckas et al. [97] | General orthopedics | Cross-sectional study | 14679 |
| 2018 | Beleckas et al. [98] | General orthopedics | Retrospective cohort | 3339 |
| 2018 | Beleckas et al. [58] | Upper extremity patients | Cross-sectional study | 3315 |
| 2018 | Bernholt et al. [96] | Lower extremity patients | Retrospective cohort | 75 |
| 2018 | Bernstein et al. [95] | Lower extremity patients | Cohort study (prospective observational study) | 500 |
| 2018 | Bhatt et al. [94] | Spine patients | Cohort study (prospective observational study) | 78 |
| 2018 | Boody et al. [56] | Spine patients | Cohort study (prospective observational study) | 59 |
| 2018 | Cavallero et al. [93] | Trauma patients | Retrospective cohort | 56 |
| 2018 | Chen et al. [92] | Lower extremity patients | Retrospective cohort | 233 |
| 2018 | Crijns et al. [91] | Upper extremity patients | Retrospective cohort | 4511 |
| 2018 | Fisherauer et al. [59] | Upper extremity patients | Cross-sectional study | 105 |
| 2018 | Fram et al. [90] | Upper extremity patients | Retrospective cohort | 11 |
| 2018 | Gausden et al. [89] | Lower extremity patients | Cohort study (prospective Observational study) | 132 |
| 2018 | Gausden et al. [88] | Trauma patients | Cohort study (prospective observational study) | 174 |
| 2018 | Hancock et al. [87] | Lower extremity patients | Cross-sectional study | 100 |
| 2018 | Haskell et al. [61] | General orthopedics | Cross-sectional study | 4524 |
| 2018 | Haws et al. [86] | Spine patients | Retrospective cohort | 74 |
| 2018 | Hung et al. [85] | Lower extremity patients | Cohort study (prospective Observational study) | 785 |
| 2018 | Hung et al. [44] | Lower extremity patients | Cohort study (prospective observational study) | 983 |
| 2018 | Hung et al. [83] | Lower extremity patients | Cohort study (prospective observational study) | 2226 |
| 2018 | Hung et al. [84] | Lower extremity patients | Cohort study (prospective observational study) | 3069 |
| 2018 | Hung et al. [81] | Spine patients | Cohort study (prospective observational study) | 763 |
| 2018 | Hung et al. [82] | Spine patients | Cohort study (prospective observational study) | 1945 |
| 2018 | Hung et al. [60] | Upper extremity patients | Cross-sectional study | 1759 |
| 2018 | Kadri et al. 3 [80] | General orthopedics | Cross-sectional study | 841 |
| 2018 | Kagan et al. [79] | Lower extremity patients | Cohort study (prospective observational study) | 91 |
| 2018 | Karns et al. [78] | Lower extremity patients | Retrospective cohort | 434 |
| 2018 | Khechen et al. [77] | Spine patients | Retrospective cohort | 41 |
| 2018 | Kleimeyer et al. [76] | Spine patients | Retrospective cohort | 75 |
| 2018 | Kohring et al. [75] | Lower extremity patients | Retrospective cohort | 271 |
| 2018 | Kohring et al. [74] | Lower extremity patients | Retrospective cohort | 540 |
| 2018 | Kootstra et al. [73] | Upper extremity patients | Cross-sectional study | 126 |
| 2018 | Medina et al. [72] | General orthopedics | Cross-sectional study | 937 |
| 2018 | Meredith et al. [71] | Lower extremity patients | Cross-sectional study | 383 |
| 2018 | Merrill et al. [51] | Spine patients | Cohort study (prospective observational study) | 111 |
| 2018 | Nixon et al. [70] | Lower extremity patients | Retrospective cohort | 159 |
| 2018 | Owen et al. [48] | Spine patients | Cohort study (prospective observational study) | 60 |
| 2018 | Patel et al. [69] | Spine patients | Cohort study (prospective observational study) | 98 |
| 2018 | Patterson et al. [68] | Upper extremity patients | Cross-sectional study | 164 |
| 2018 | Patton et al. [67] | Lower extremity patients | Retrospective cohort | 680 |
| 2018 | Purvis et al. [66] | Spine patients | Cohort study (prospective observational study) | 231 |
| 2018 | Raad et al. [65] | Spine patients | Cohort study (prospective observational study) | 76 |
| 2018 | Rubery et al. [64] | Spine patients | Retrospective cohort | 78 |
| 2018 | Schwartz et al. [63] | Spine patients | Cohort study (prospective observational study) | 167 |
| 2018 | Stoop et al. [57] | Upper extremity patients | Cross-sectional study | 122 |
| 2018 | Vincent et al. [62] | Trauma patients | Cohort study (prospective Observational study) | 101 |
Year
Studies included in this review were published from 2013 through 2018. The number of publications reporting PROMIS measures notably increased across time: 2013 (1%, 1 study), 2014 (7%, 6 studies), 2015 (6%, 5 studies), 2016 (13%, 11 studies), 2017 (17%, 15 studies). The majority of studies were published in 2018 (57%, 50 studies).
Clinical population
PROMIS measures were reported in orthopedic studies across multiple clinical populations. For reporting, we grouped the studies by body region rather than specific diagnosis. The majority of studies (36%, 32 studies) reported PROMIS measures in lower extremity disorders (hip, knee, ankle, foot), followed by upper extremity disorders (shoulder, elbow, hand) (28%, 25 studies), spine disorders (19%, 17 studies), orthopedic trauma (10%, 9 studies). Few studies (6%, 5 studies) reported PROMIS measures in general orthopedic patients.
Study type and sample size
The studies in this review varied in the study design used to assess outcomes. The largest percentage of studies were cohort studies (59%, 52 studies). Most of these were prospective observational designs (38%, 33 studies), and 22% (19 studies) were retrospective observational designs. Many studies (41%, 36 studies) used a cross-sectional study design to analyze the psychometric properties of PROMIS or to validate in a patient population. No randomized controlled trials were reported using PROMIS measures as an outcome measure. Sample sizes in the studies ranged from 11 patients to 14,679 patients, with 133 patients as the median number reported. Five studies included patients from registries including the American Orthopedic Foot and Ankle Society’s National Orthopedic Foot and Ankle Research Outcomes Network and the Maryland Orthopedic Registry.
Reporting of PROMIS measures
The most frequently reported PROMIS domains in the studies included in this review were physical function (81%, 71 studies), pain interference (61%, 54 studies), depression (31%, 28 studies), physical function-upper extremity (18%, 16 studies), physical function-lower extremity (3%, 3 studies), and anxiety (15%, 13 studies) (Table 2). Most studies (75%, 66 studies) reported more than one PROMIS domain. Approximately a third of studies (32%, studies) reported two PROMIS domains, 25% (22 studies) reported three PROMIS domains, 9% (8 studies) reported four PROMIS domains, and the remainder (9%, 8 studies) reported between 5 and 9 PROMIS domains. Only a quarter (25%, 22 studies) reported one PROMIS domain. Of the type of PROMIS instrument used (i.e., CAT, short form, or profile), the vast majority of studies (81%, 71 studies) reported using the PROMIS CAT approach. A small percentage of studies reported only fixed-length instruments (15%, 13 studies) and (4%, 4 studies) reported a combination of CAT and fixed-length questionnaires.
Table 2.
Reporting of PROMIS measures
| Domain | Studies reporting domain | % CAT instrument format |
|---|---|---|
| Physical function | 81% (71) | 93% |
| Pain interference | 61% (54) | 85% |
| Pain behavior | 4% (4) | 100% |
| Emotional distress - depression | 32% (28) | 85% |
| Physical function - upper extremity | 18% (16) | 69% |
| Physical function - lower extremity | 3% (3) | 100% |
| Physical function - mobility | 1% (1) | 100% |
| Emotional support | 1% (1) | 100% |
| Psychological illness | 2% (2) | 100% |
| Instrumental support | 1% (1) | 100% |
| Sleep disturbance | 4% (4) | 50% |
| Emotional distress - anger | 1% (1) | 100% |
| Emotional distress - anxiety | 13% (12) | 83% |
| Fatigue | 8% (7) | 71% |
| Ability to participate in social roles and activities | 1% (1) | 100% |
| Satisfaction with participation in social roles | 10% (9) | 78% |
| Global health | 7% (6) | 0% |
| Pain intensity | 4% (4) | 0% |
| Emotional distress | 1% (1) | 0% |
PROMIS and traditional PROs
Fourteen studies in this review reported PROMIS as the sole outcome measure. Of those 14 studies, 9 were published in 2018 alone. Widely reported traditional measures were reported alongside PROMIS measures in all studies. Traditional measures included measuring the constructs of pain, disability, psychosocial comorbidity, and quality of life. Table 3 describes the reporting of traditional measures alongside PROMIS measures by body region.
Table 3.
PROMIS domains and traditional PROs by body region
| PROMIS domains/constructs | Traditional PRO measures |
|---|---|
| General orthopedics | |
| Physical function | International Knee Documentation Committee |
| Pain interference | American Shoulder and Elbow Surgeons Shoulder Score |
| Emotional distress—depression | Musculoskeletal Outcomes Data Evaluation and Management System |
| Emotional distress—anxiety | Tegner Activity Scale |
| Fatigue | Marx Activity Rating Scales |
| Satisfaction with participation in social roles | Brief Michigan Hand Questionnaire |
| Physical function—upper extremity | International Physical Activity Questionnaire |
| Pain intensity | Numeric Pain Scale—Global |
| Global health | Numeric Pain Scale—Local |
| Physical functional—lower extremity | |
| Lower extremity | |
| Physical function | Knee Injury and Osteoarthritis Outcome Score |
| Pain interference | Western Ontario and McMaster Universities Arthritis Index |
| Emotional distress—depression | Hip Disability and Osteoarthritis Outcome Score |
| Emotional distress—anxiety | Knee Injury and Osteoarthritis Outcome Score for Joint Replacement |
| Emotional distress—anger | Hip Disability and Osteoarthritis Outcome Score for Joint Replacement |
| Pain intensity | GAITRite Walk Testing |
| Fatigue | International Knee Documentation Committee |
| Satisfaction with participation in social roles | Oxford Knee Score |
| Sleep disturbance | Short Form 12 |
| Pain behavior | Numeric Pain Scale—Global |
| Ability to participate in social roles and activities | Numeric Pain Scale—Local |
| Global health | Musculoskeletal Outcomes Data Evaluation and Management System |
| Physical function—mobility | Tegner Activity Scale |
| Physical function—upper extremity | Marx Activity Rating Scales |
| Physical functional—lower extremity | Short Form 36 |
| EuroQol EQ-5D | |
| Douleur Neuropathique 4 (DN4-I) | |
| Visual Analog Scale | |
| International Physical Activity Questionnaire | |
| Press Ganey Outpatient Medical Practice Survey | |
| Veterans RAND 12 (VR-12) | |
| Modified Harris Hip Score | |
| Posture Assessment Scale for Stroke | |
| Olerud-Molander Ankle Score | |
| Foot and Ankle Ability Measure | |
| Foot Function Index | |
| Foot and Ankle Outcome Score | |
| Short Form 36 | |
| International Hip Outcome Tool (iHOT-33) | |
| Single Assessment Numeric Evaluation | |
| American Society of Anesthesiologists classification | |
| Spine | |
| Physical function | Oswestry Disability Index |
| Pain interference | Neck Disability Index |
| Emotional distress—depression | Modified Japanese Orthopedic Association Scale |
| Emotional distress—anxiety | Short Form 12 |
| Pain behavior | Global Rating of Change |
| Satisfaction with participation in social roles | Visual Analog Scale |
| Fatigue | EuroQol EQ-5D |
| Sleep disturbance | Scoliosis Research Society (SRS-22r) |
| Pain intensity | Generalized Anxiety Disorder (GAD-7) |
| Emotional distress | Patient Health Questionnaire for Depression Scale (PHQ-8) |
| Short Form 36 (Rand-36 / SF-36) | |
| Zurich Claudication Questionnaire | |
| Brief Pain Inventory | |
| North America Spine Society Patient Satisfaction Index | |
| Coccygodynia Disability Index (CDI) | |
| Trauma | |
| Physical function | Visual Analog Scale (VAS) |
| Pain intensity | Disabilities of the Arm, Shoulder, and Hand (DASH) |
| Physical function—upper extremity | Quick Disability of the Arm, Shoulder, and Hand (QuickDASH) |
| Satisfaction with participation in social roles | Constant Shoulder Score |
| Psychological illness | Short Musculoskeletal Functional Assessment (SMFA) |
| Timed Up and Go | |
| Short Form 36 (Rand-36/SF-36) | |
| Injustice Experience Questionnaire | |
| Patient Health Questionnaire for Depression short form (PHQ-2) | |
| Pain Self-Efficacy Questionnaire short form (PSEQ-2) | |
| Pain Catastrophizing | |
| FRAIL Questionnaire | |
| UCLA Shoulder Score | |
Quality of studies and risk of bias
A majority of studies assessed had a low risk of bias. All cohort and cross-sectional studies scored seven or above in their respective versions of the NOS quality assessment tool, and, with one exception, all case-control studies scored eight or above. Table 4 describes the risk of bias summary for individual studies included in this review, and Additional file 2 contains detailed results of the quality assessment.
Table 4.
Risk of bias summary table
| # Studies | % Studies | |
|---|---|---|
| Low (7 or above) | 87 | 98.8% |
| Moderate to high (6 or below) | 1 | 1.2% |
Discussion
In this review, we evaluated the uptake of PROMIS measures in orthopedic research and practice by describing how PROMIS measures were reported in published studies. The number of studies reporting the use of PROMIS measures increased exponentially from 2013 through 2017, with a spike in studies reporting PROMIS measures in 2018 alone (57% of total studies). This large increase in studies potentially indicates that PROMIS measures are being more widely adopted within orthopedic research and practice as an outcome measure. This increase may be due to the evolution of PROMIS measures from the short form, fixed instrument to the CAT instrument. Additionally, progress has been made with the availability and integration of PROMIS measures into Electronic Health Record (EHR) systems, allowing easier use of PROMIS CAT in the clinical setting [104, 105]. However, in relation to the increase in reporting of PROMIS measures in the literature, the vast majority of studies in our review reported the use of traditional measures alongside PROMIS measures [106]. This finding supports that, while PROMIS measures are gaining traction within orthopedics, researchers and clinicians may not be ready to abandon traditional measures in favor of PROMIS measures, despite evidence that the PROMIS domains of physical function and pain interference outperform traditional measures [107]. The reasons for this hesitancy may be related to familiarity with traditional measures, participation in registries that do not have PROMIS measures as part of the core set of measures, or a perceived lack of applicability in their patient populations. However, it may be noted that any new PRO measure should be considered experimental; thus, established measures are included both for validation purposes and to gain more understanding of how they relate to each other.
Our review also found that the use of PROMIS measures across clinical populations varied, with 37% of studies examining lower extremity conditions, followed by upper extremity (28%) and spine conditions (19%). This finding is consistent with the supporting literature where the use of PROMIS measures in lower, upper, and spine is increasing as a primary measure across clinical populations [1, 4, 108]. Last, most studies in our review reported the use of CAT-based assessments as the PROMIS assessment type. This finding is not surprising, as the primary benefits of the PROMIS CAT measures are the decrease in patient burden and the precision of the estimate. The majority of studies reported between one and three PROMIS domains. Unsurprisingly, the most commonly reported PROMIS domains were physical function and pain interference, which are validated and compared to many traditional measures. Of the psychological domains, depression was reported more frequently than anxiety. While the field of orthopedics is focused on improved functioning and reduced pain, we would encourage a more holistic view of the patient by incorporating more psychological constructs that may affect patient prognosis. This review provides evidence that the prevalence and support for use of PROMIS measures is growing in orthopedics and that PROMIS is being recognized as a PRO measure of choice for clinical trials [109].
Limitations
Our systematic review has some limitations. First, we aimed to describe the prevalence and use of PROMIS measures within orthopedic practice and research rather than to compare outcomes or exposures in the studies. Our review had broad inclusion criteria, and thus there was high variability, with study designs often considered less rigorous. The majority of studies were retrospective and prospective cohort studies. No studies in our review were randomized clinical trials; however, this is likely because of the relative unavailability of PROMIS measures until recently. It will take some time before clinical trials that use PROMIS measures as endpoints are published.
Second, we reported on the PROMIS domains but did not perform meta-analyses to examine the effects of treatment or compare the performance of PROMIS measures with other reported measures. Last, many studies included in the review examined the reliability and validity of PROMIS measures in orthopedic populations, so the studies that reported PROMIS measures as the primary outcomes were less frequent, potentially leading to the impression that there is a higher prevalence of reporting PROMIS measures in the literature.
Conclusions
PROMIS measures have been increasingly reported in orthopedic research and practice and present a new era of PRO measurement for clinical practice and scientific dissemination. Our findings are relevant for orthopedic researchers and clinicians who are using, or considering using, PROMIS measures. Our findings can provide guidance for stakeholders about the selection and administration of PRO measures, supporting value-based decisions both in clinics and prostheses procurement [110]. The domains of physical function and pain interference are the most commonly reported PROMIS domains, and these measure similar constructs to the traditional, body region-specific measures. Considerations about which PROMIS measures to administer in clinical populations should be made by determining what constructs are most important and whether PROMIS measures are sufficient alone or if traditional measures are needed to supplement the PROMIS measures. Given the evidence for the validity and reliability of PROMIS in orthopedics, we expect a decrease in the use of other established PRO measures in order to reduce respondent burden.
The implications for future research and practice in orthopedics support that PROMIS measures are versatile, reliable, and valid for orthopedic research and practice. Further, PROMIS measures provide distinct advantages over traditional measures, particularly, when the study population is heterogeneous. Multiple recent studies indicate that widespread variability exists in the particular PROs used in studies of the same diagnosis, thereby significantly limiting the translatability of many of these high-impact studies [6, 8, 111, 112]. Future research on the use of PROMIS measures in orthopedics should focus on the use of PROMIS measures as the primary outcome measure, particularly in studies that examine heterogeneous patient populations. Last, PROMIS measures hold immense potential for improving patient and provider communication, particularly across specialties.
Supplementary information
Search Strategies.
Quality Assessment.
Acknowledgements
None
Authors’ contributions
All authors meet the criteria for authorship based on the International Committee of Medical Journal Editors. MH was responsible for the design of the study, reviewing articles, and preparing the manuscript; SG for reviewing articles and preparing the manuscript; ER for the design of the study, reviewing articles, and preparing the manuscript; BR for participating in the writing of the manuscript; and LC for reviewing articles and preparing the manuscript. All authors have reviewed the manuscript prior to publication. The author(s) read and approved the final manuscript.
Funding
None
Availability of data and materials
A limited data set with fields reported in this paper is available upon request via email to the corresponding author, with no limitations on the reuse of the data.
Ethics approval and consent to participate
This study was exempt from the Institutional Review Board at Duke University.
Consent for publication
All authors consent to the publication of the data in this manuscript.
Competing interests
None to report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones RS, Stukenborg GJ. Patient-Reported Outcomes Measurement Information System (PROMIS) use in surgical care: a scoping study. J Am Coll Surg. 2017;224(3):245–254. doi: 10.1016/j.jamcollsurg.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. 2015;350:g7818. doi: 10.1136/bmj.g7818. [DOI] [PubMed] [Google Scholar]
- 3.Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. 2014;28:J Orthopaedic Trauma, 439–43. 10.1097/bot.0000000000000059. [DOI] [PubMed]
- 4.Brodke DJ, Saltzman CL, Brodke DS. PROMIS for orthopaedic outcomes measurement. J Am Acad Orthop Surg. 2016;24(11):744–749. doi: 10.5435/JAAOS-D-15-00404. [DOI] [PubMed] [Google Scholar]
- 5.Ader DN. Developing the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45:S1–S2. doi: 10.1097/01.mlr.0000260537.45076.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella D, Gershon R, Lai J-S, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):133–141. doi: 10.1007/s11136-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 7.Gulledge CM, Lizzio VA, Smith DG, Guo E, Makhni EC. What are the floor and ceiling effects of patient-reported outcomes measurement information system computer adaptive test domains in orthopaedic patients? A systematic review. Arthroscopy. 2020. 10.1016/j.arthro.2019.09.022. [DOI] [PubMed]
- 8.Makhni EC, Meadows M, Hamamoto JT, Higgins JD, Romeo AA, Verma NN. Patient Reported Outcomes Measurement Information System (PROMIS) in the upper extremity: the future of outcomes reporting? J Shoulder Elbow Surg [Internet]. 2017;26(2):352–357. doi: 10.1016/j.jse.2016.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JA, Schwartz CE. Patient-reported outcomes in spine surgery: past, current, and future directions. J Neurosurg Spine. 2019;31(2):155–164. doi: 10.3171/2019.1.SPINE18770. [DOI] [PubMed] [Google Scholar]
- 10.Hunt KJ, Lakey E. Patient-reported outcomes in foot and ankle surgery. Orthop Clin North Am [Internet]. 2018;49(2):277–289. doi: 10.1016/j.ocl.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sideri S, Papageorgiou SN, Eliades T. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J Clin Epidemiol. 2018;100:103–110. doi: 10.1016/j.jclinepi.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Innovation VH. Covidence systematic review software. Veritas Health Innovation Melbourne, VIC. 2017. [Google Scholar]
- 14.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen P-A, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47(3-4):181–191. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 17.Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ, et al. Validation of PROMIS ® physical function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466–3474. doi: 10.1007/s11999-013-3097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung M, Baumhauer JF, Brodsky JW, Cheng C, Ellis SJ, Franklin JD, et al. Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int [Internet]. 2014;35(6):592–599. doi: 10.1177/1071100714528492. [DOI] [PubMed] [Google Scholar]
- 19.Hung M, Franklin JD, Hon SD, Cheng C, Conrad J, Saltzman CL. Time for a paradigm shift with computerized adaptive testing of general physical function outcomes measurements. Foot Ankle Int. 2014;35(1):1–7. doi: 10.1177/1071100713507905. [DOI] [PubMed] [Google Scholar]
- 20.Hunt KJ, Alexander I, Baumhauer J, Brodsky J, Chiodo C, Daniels T, et al. The Orthopaedic Foot and Ankle Outcomes Research (OFAR) network: feasibility of a multicenter network for patient outcomes assessment in foot and ankle. Foot Ankle Int. 2014;35(9):847–854. doi: 10.1177/1071100714544157. [DOI] [PubMed] [Google Scholar]
- 21.Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793–801. doi: 10.1002/jor.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyser AR, Beckmann J, Franklin JD, Cheng C, Hon SD, Wang A, et al. Evaluation of the PROMIS physical function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047–2051. doi: 10.1016/j.jhsa.2014.06.130. [DOI] [PubMed] [Google Scholar]
- 23.Mellema JJ, O’Connor CM, Overbeek CL, Hageman MG, Ring D. The effect of feedback regarding coping strategies and illness behavior on hand surgery patient satisfaction and communication: a randomized controlled trial. Hand. 2015;10(3):503–511. doi: 10.1007/s11552-015-9742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckmann JT, Hung M, Bounsanga J, Wylie JD, Granger EK, Tashjian RZ. Psychometric evaluation of the PROMIS Physical Function Computerized Adaptive Test in comparison to the American Shoulder and Elbow Surgeons score and Simple Shoulder Test in patients with rotator cuff disease. J Shoulder Elbow Surg. 2015;24(12):1961–1967. doi: 10.1016/j.jse.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JH, Kallen MA, Okike K, Lee OC, Vrahas MS. PROMIS physical function computer adaptive test compared with other upper extremity outcome measures in the evaluation of proximal humerus fractures in patients older than 60 years. J Orthop Trauma. 2015;29(6):257–263. doi: 10.1097/BOT.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 26.Stuart AR, Higgins TF, Hung M, Weir CR, Kubiak EN, Rothberg DL, et al. Reliability in measuring preinjury physical function in orthopaedic trauma. J Orthop Trauma. 2015;29(12):527–532. doi: 10.1097/BOT.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs DJ, Ho BS, LaBelle MW, Kelikian AS. Effect of arthroscopic evaluation of acute ankle fractures on PROMIS intermediate-term functional outcomes. Foot Ankle Int. 2016;37(1):51–57. doi: 10.1177/1071100715597657. [DOI] [PubMed] [Google Scholar]
- 28.Ho B, Houck JR, Flemister AS, Ketz J, Oh I, DiGiovanni BF, et al. Preoperative PROMIS scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37(9):911–918. doi: 10.1177/1071100716665113. [DOI] [PubMed] [Google Scholar]
- 29.Beckmann JT, Hung M, Voss MW, Crum AB, Bounsanga J, Tyser AR. Evaluation of the patient-reported outcomes measurement information system upper extremity computer adaptive test. J Hand Surg Am. 2016;41(7):739–744. doi: 10.1016/j.jhsa.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Nota SPFT, Spit SA, Oosterhoff TCH, Hageman MGJS, Ring DC, Vranceanu A-M. Is social support associated with upper extremity disability? Clin Orthop Relat Res. 2016;474(8):1830–1836. doi: 10.1007/s11999-016-4892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrish RC, 2nd, Menendez ME, Mudgal CS, Jupiter JB, Chen NC, Ring D. Patient satisfaction and its relation to perceived visit duration with a hand surgeon. J Hand Surg Am. 2016;41(2):257–262. doi: 10.1016/j.jhsa.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Peters RM, Menendez ME, Mellema JJ, Ring D, Vranceanu A-M. Sleep disturbance and upper-extremity disability. Arch Bone Jt Surg. 2016;4(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 33.Dasa V, Lensing G, Parsons M, Harris J, Volaufova J, Bliss R. Percutaneous freezing of sensory nerves prior to total knee arthroplasty. Knee. 2016;23(3):523–528. doi: 10.1016/j.knee.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Oak SR, Strnad GJ, Bena J, Farrow LD, Parker RD, Jones MH, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 Questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714. doi: 10.1177/2325967116674714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papuga MO, Mesfin A, Molinari R, Rubery PT. Correlation of PROMIS physical function and pain CAT instruments with Oswestry Disability Index and Neck Disability Index in spine patients. Spine. 2016;41(14):1153–1159. doi: 10.1097/BRS.0000000000001518. [DOI] [PMC free article] [PubMed]
- 36.van Leeuwen WF, van der Vliet QMJ, Janssen SJ, Heng M, Ring D, Vranceanu A-M. Does perceived injustice correlate with pain intensity and disability in orthopaedic trauma patients? Injury. 2016;47(6):1212–1216. doi: 10.1016/j.injury.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Hermanussen HH, Menendez ME, Chen NC, Ring D, Vranceanu A-M. Predictors of upper-extremity physical function in older adults. Arch Bone Jt Surg. 2016;4(4):359–365. [PMC free article] [PubMed] [Google Scholar]
- 38.Koltsov JCB, Greenfield ST, Soukup D, Do HT, Ellis SJ. Validation of patient-reported outcomes measurement information system computerized adaptive tests against the foot and ankle outcome score for 6 common foot and ankle pathologies. Foot Ankle Int. 2017;38(8):870–878. doi: 10.1177/1071100717709573. [DOI] [PubMed] [Google Scholar]
- 39.Nixon DC, McCormick JJ, Johnson JE, Klein SE. PROMIS pain interference and physical function scores correlate with the Foot and Ankle Ability Measure (FAAM) in patients with hallux valgus. Clin Orthop Relat Res. 2017;475(11):2775–2780. doi: 10.1007/s11999-017-5476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh Y, Drijkoningen T, Menendez ME, Claessen FMAP, Ring D. The influence of psychological factors on the Michigan Hand Questionnaire. Hand. 2017;12(2):197–201. doi: 10.1177/1558944716642765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beleckas CM, Padovano A, Guattery J, Chamberlain AM, Keener JD, Calfee RP. Performance of Patient-Reported Outcomes Measurement Information System (PROMIS) upper extremity (UE) versus physical function (PF) computer adaptive tests (CATs) in upper extremity clinics. J Hand Surg Am. 2017;42(11):867–874. doi: 10.1016/j.jhsa.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheean AJ, Schmitz MR, Ward CL, Barrow AE, Tennent DJ, Roach CJ, et al. Assessment of disability related to femoroacetabular impingement syndrome by use of the Patient-Reported Outcome Measure Information System (PROMIS) and objective measures of physical performance. Am J Sports Med [Internet]. 2017;45(11):2476–2482. doi: 10.1177/0363546517708793. [DOI] [PubMed] [Google Scholar]
- 43.Hancock KJ, Glass N, Anthony CA, Hettrich CM, Albright J, Amendola A, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954–958. doi: 10.2106/JBJS.16.00848. [DOI] [PubMed] [Google Scholar]
- 44.Hung M, Saltzman CL, Greene T, Voss MW, Bounsanga J, Gu Y, et al. Evaluating instrument responsiveness in joint function: the HOOS JR, the KOOS JR, and the PROMIS PF CAT. J Orthop Res. 2018;36(4):1178–1184. doi: 10.1002/jor.23739. [DOI] [PubMed] [Google Scholar]
- 45.Anthony CA, Glass N, Hancock K, Bollier M, Hettrich CM, Wolf BR. Preoperative performance of the patient-reported outcomes measurement information system in patients with rotator cuff pathology. Arthroscopy. 2017;33(10):1770–1774. doi: 10.1016/j.arthro.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Anthony CA, Glass NA, Hancock K, Bollier M, Wolf BR, Hettrich CM. Performance of PROMIS instruments in patients with shoulder instability. Am J Sports Med. 2017;45(2):449–453. doi: 10.1177/0363546516668304. [DOI] [PubMed] [Google Scholar]
- 47.Dowdle SB, Glass N, Anthony CA, Hettrich CM. Use of PROMIS for patients undergoing primary total shoulder arthroplasty. Orthop J Sports Med. 2017;5(9):2325967117726044. doi: 10.1177/2325967117726044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen RJ, Zebala LP, Peters C, McAnany S. PROMIS physical function correlation with NDI and mJOA in the surgical cervical myelopathy patient population. Spine [Internet]. 2018;43(8):550–555. doi: 10.1097/BRS.0000000000002373. [DOI] [PubMed] [Google Scholar]
- 49.Purvis TE, Andreou E, Neuman BJ, Riley LH, 3rd, Skolasky RL. Concurrent validity and responsiveness of PROMIS health domains among patients presenting for anterior cervical spine surgery. Spine. 2017;42(23):E1357–E1365. doi: 10.1097/BRS.0000000000002347. [DOI] [PubMed] [Google Scholar]
- 50.Kleimeyer JP, Wood KB, Lønne G, Herzog T, Ju K, Beyer L, et al. Surgery for refractory coccygodynia: operative versus nonoperative treatment. Spine. 2017;42(16):1214–1219. doi: 10.1097/BRS.0000000000002053. [DOI] [PubMed] [Google Scholar]
- 51.Merrill RK, Zebala LP, Peters C, Qureshi SA, McAnany SJ. Impact of depression on patient-reported outcome measures after lumbar spine decompression. Spine. 2018;43(6):434–439. doi: 10.1097/BRS.0000000000002329. [DOI] [PubMed] [Google Scholar]
- 52.Kaat AJ, Rothrock NE, Vrahas MS, OʼToole RV, Buono SK, Zerhusen T Jr, et al. Longitudinal validation of the PROMIS physical function item bank in upper extremity trauma. J Orthop Trauma [Internet]. 2017;31(10):e321–e326. doi: 10.1097/BOT.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 53.Henn RF, 3rd, Dubina AG, Jauregui JJ, Smuda MP, Tracy JK. The Maryland Orthopaedic Registry (MOR): design and baseline characteristics of a prospective registry. J Clin Orthop Trauma. 2017;8(4):301–307. doi: 10.1016/j.jcot.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kazmers NH, Hung M, Rane AA, Bounsanga J, Weng C, Tyser AR. Association of physical function, anxiety, and pain interference in nonshoulder upper extremity patients using the PROMIS platform. J Hand Surg Am [Internet]. 2017;42(10):781–787. doi: 10.1016/j.jhsa.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St John MJ, Mitten D, Hammert WC. Efficacy of PROMIS pain interference and Likert pain scores to assess physical function. J Hand Surg Am. 2017;42(9):705–710. doi: 10.1016/j.jhsa.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Boody BS, Bhatt S, Mazmudar AS, Hsu WK, Rothrock NE, Patel AA. Validation of Patient-Reported Outcomes Measurement Information System (PROMIS) computerized adaptive tests in cervical spine surgery. J Neurosurg Spine. 2018;28(3):268–279. doi: 10.3171/2017.7.SPINE17661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoop N, Menendez ME, Mellema JJ, Ring D. The PROMIS Global Health Questionnaire correlates with the QuickDASH in patients with upper extremity illness. Hand. 2018;13(1):118–121. doi: 10.1177/1558944717691127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beleckas CM, Wright M, Prather H, Chamberlain A, Guattery J, Calfee RP. Relative prevalence of anxiety and depression in patients with upper extremity conditions. J Hand Surg Am. 2018;43(6):571.e1–571.e8. doi: 10.1016/j.jhsa.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischerauer SF, Talaei-Khoei M, Vissers FL, Chen N, Vranceanu A-M. Pain anxiety differentially mediates the association of pain intensity with function depending on level of intolerance of uncertainty. J Psychiatr Res. 2018;97:30–37. doi: 10.1016/j.jpsychires.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Hung M, Voss MW, Bounsanga J, Gu Y, Granger EK, Tashjian RZ. Psychometrics of the Patient-Reported Outcomes Measurement Information System Physical Function instrument administered by computerized adaptive testing and the Disabilities of Arm, Shoulder and Hand in the orthopedic elbow patient population. J Shoulder Elbow Surg. 2018;27(3):515–522. doi: 10.1016/j.jse.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haskell A, Kim T. Implementation of Patient-Reported Outcomes Measurement Information System data collection in a private orthopedic surgery practice. Foot Ankle Int [Internet]. 2018;39(5):517–521. doi: 10.1177/1071100717753967. [DOI] [PubMed] [Google Scholar]
- 62.Vincent HK, Hagen JE, Zdziarski-Horodyski LA, Patrick M, Sadasivan KK, Guenther R, et al. Patient-Reported Outcomes Measurement Information System outcome measures and mental health in orthopaedic trauma patients during early recovery. J Orthop Trauma. 2018;32(9):467–473. doi: 10.1097/BOT.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz CE, Zhang J, Rapkin BD, Finkelstein JA. Reconsidering the minimally important difference: evidence of instability over time and across groups. Spine J. 2019;19(4):726–734. doi: 10.1016/j.spinee.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Rubery PT, Houck J, Mesfin A, Molinari R, Papuga MO. Preoperative Patient Reported Outcomes Measurement Information System scores assist in predicting early postoperative success in lumbar discectomy. Spine [Internet]. 2019;44(5):325–333. doi: 10.1097/BRS.0000000000002823. [DOI] [PubMed] [Google Scholar]
- 65.Raad M, Jain A, Huang M, Skolasky RL, Sciubba DM, Kebaish KM, et al. Validity and responsiveness of PROMIS in adult spinal deformity: the need for a self-image domain. Spine J. 2019;19(1):50–55. doi: 10.1016/j.spinee.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Purvis TE, Neuman BJ, Riley LH, 3rd, Skolasky RL. Discriminant ability, concurrent validity, and responsiveness of PROMIS health domains among patients with lumbar degenerative disease undergoing decompression with or without arthrodesis. Spine. 2018;43(21):1512–1520. doi: 10.1097/BRS.0000000000002661. [DOI] [PubMed] [Google Scholar]
- 67.Patton RS, Runner RP, Lyons RJ, Bradbury TL. Clinical outcomes of patients with lateral femoral cutaneous nerve injury after direct anterior total hip arthroplasty. J Arthroplasty. 2018;33(9):2919–2926. doi: 10.1016/j.arth.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 68.Patterson BM, Orvets ND, Aleem AW, Keener JD, Calfee RP, Nixon DC, et al. Correlation of Patient-Reported Outcomes Measurement Information System (PROMIS) scores with legacy patient-reported outcome scores in patients undergoing rotator cuff repair. J Shoulder Elbow Surg. 2018;27(6S):S17–S23. doi: 10.1016/j.jse.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 69.Patel AA, Dodwad S-NM, Boody BS, Bhatt S, Savage JW, Hsu WK, et al. Validation of Patient Reported Outcomes Measurement Information System (PROMIS) computer adaptive tests (CATs) in the surgical treatment of lumbar spinal stenosis. Spine. 2018;43(21):1521–1528. doi: 10.1097/BRS.0000000000002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nixon DC, Cusworth BM, McCormick JJ, Johnson JE, Klein SE. Patient-reported allergies do not predict poorer PROMIS function, pain, and depression scores following foot and ankle surgery. Foot Ankle Int. 2018;39(8):949–953. doi: 10.1177/1071100718769667. [DOI] [PubMed] [Google Scholar]
- 71.Meredith SJ, Nadarajah V, Jauregui JJ, Smuda MP, Medina SH, Bennett CH, et al. Preoperative opioid use in knee surgery patients. J Knee Surg. 2019;32(7):630–636. doi: 10.1055/s-0038-1666868. [DOI] [PubMed] [Google Scholar]
- 72.Medina SH, Nadarajah V, Jauregui JJ, Smuda MP, Foster M, Meredith SJ, et al. Orthopaedic surgery patients who use recreational marijuana have less pre-operative pain. Int Orthop. 2019;43(2):283–292. doi: 10.1007/s00264-018-4101-x. [DOI] [PubMed] [Google Scholar]
- 73.Kootstra TJM, Wilkens SC, Menendez ME, Ring D. Is physician empathy associated with differences in pain and functional limitations after a hand surgeon visit? Clin Orthop Relat Res. 2018;476(4):801–807. doi: 10.1007/s11999.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kohring JM, Pelt CE, Anderson MB, Peters CL, Gililland JM. Press Ganey Outpatient Medical Practice Survey scores do not correlate with patient-reported outcomes after primary joint arthroplasty. J Arthroplasty. 2018;33(8):2417–2422. doi: 10.1016/j.arth.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 75.Kohring JM, Erickson JA, Anderson MB, Gililland JM, Peters CL, Pelt CE. Treated versus untreated depression in total joint arthroplasty impacts outcomes. J Arthroplasty. 2018;33(7S):S81–S85. doi: 10.1016/j.arth.2018.01.065. [DOI] [PubMed] [Google Scholar]
- 76.Kleimeyer JP, Cheng I, Alamin TF, Hu SS, Cha T, Yanamadala V, et al. Selective anterior lumbar interbody fusion for low back pain associated with degenerative disc disease versus nonsurgical management. Spine. 2018;43(19):1372–1380. doi: 10.1097/BRS.0000000000002630. [DOI] [PubMed] [Google Scholar]
- 77.Khechen B, Haws BE, Patel DV, Bawa MS, Elboghdady IM, Lamoutte EH, et al. PROMIS physical function score strongly correlates with legacy outcome measures in minimally invasive lumbar microdiscectomy. Spine. 2019;44(6):442–446. doi: 10.1097/BRS.0000000000002841. [DOI] [PubMed] [Google Scholar]
- 78.Karns MR, Jones DL, Todd DC, Maak TG, Aoki SK, Burks RT, et al. Patient- and procedure-specific variables driving total direct costs of outpatient anterior cruciate ligament reconstruction. Orthop J Sports Med. 2018;6(8):2325967118788543. doi: 10.1177/2325967118788543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kagan R, Anderson MB, Christensen JC, Peters CL, Gililland JM, Pelt CE. The recovery curve for the patient-reported outcomes measurement information system patient-reported physical function and pain interference computerized adaptive tests after primary total knee arthroplasty. J Arthroplasty. 2018;33(8):2471–2474. doi: 10.1016/j.arth.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 80.Kadri O, Jildeh TR, Meldau JE, Blanchett J, Borowsky P, Muh S, et al. How long does it take for patients to complete PROMIS scores?: an assessment of PROMIS CAT Questionnaires administered at an ambulatory sports medicine clinic. Orthop J Sports Med. 2018;6(8):2325967118791180. doi: 10.1177/2325967118791180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung M, Saltzman CL, Voss MW, Bounsanga J, Kendall R, Spiker R, et al. Responsiveness of the Patient-Reported Outcomes Measurement Information System (PROMIS), Neck Disability Index (NDI) and Oswestry Disability Index (ODI) instruments in patients with spinal disorders. Spine J. 2019;19(1):34–40. doi: 10.1016/j.spinee.2018.06.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hung M, Saltzman CL, Kendall R, Bounsanga J, Voss MW, Lawrence B, et al. What Are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res. 2018;476(10):2027–2036. doi: 10.1097/CORR.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hung M, Bounsanga J, Voss MW, Saltzman CL. Establishing minimum clinically important difference values for the Patient-Reported Outcomes Measurement Information System Physical Function, hip disability and osteoarthritis outcome score for joint reconstruction, and knee injury and osteoarthritis outcome score for joint reconstruction in orthopaedics. World J Orthop. 2018;9(3):41–49. doi: 10.5312/wjo.v9.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hung M, Baumhauer JF, Licari FW, Voss MW, Bounsanga J, Saltzman CL. PROMIS and FAAM minimal clinically important differences in foot and ankle orthopedics. Foot Ankle Int [Internet]. 2019;40(1):65–73. doi: 10.1177/1071100718800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hung M, Baumhauer JF, Licari FW, Bounsanga J, Voss MW, Saltzman CL. Responsiveness of the PROMIS and FAAM instruments in foot and ankle orthopedic population. Foot Ankle Int. 2019;40(1):56–64. doi: 10.1177/1071100718799758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haws BE, Khechen B, Guntin JA, Cardinal KL, Bohl DD, Singh K. Validity of PROMIS in minimally invasive transforaminal lumbar interbody fusion: a preliminary evaluation. J Neurosurg Spine. 2018;29(1):28–33. doi: 10.3171/2017.11.SPINE17989. [DOI] [PubMed] [Google Scholar]
- 87.Hancock KJ, Glass N, Anthony CA, Wolf BR, Hettrich CM, Albright J, et al. PROMIS: a valid and efficient outcomes instrument for patients with ACL tears. Knee Surg Sports Traumatol Arthrosc. 2019;27(1):100–104. doi: 10.1007/s00167-018-5034-z. [DOI] [PubMed] [Google Scholar]
- 88.Gausden EB, Levack AE, Sin DN, Nwachukwu BU, Fabricant PD, Nellestein AM, et al. Validating the Patient Reported Outcomes Measurement Information System (PROMIS) computerized adaptive tests for upper extremity fracture care. J Shoulder Elbow Surg. 2018;27(7):1191–1197. doi: 10.1016/j.jse.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Gausden EB, Levack A, Nwachukwu BU, Sin D, Wellman DS, Lorich DG. Computerized adaptive testing for patient reported outcomes in ankle fracture surgery. Foot Ankle Int. 2018;39(10):1192–1198. doi: 10.1177/1071100718782487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fram B, Wall LB, Gelberman RH, Goldfarb CA. Surgical transposition for chronic instability of the extensor carpi ulnaris tendon. J Hand Surg Eur Vol. 2018;43(9):925–930. doi: 10.1177/1753193418773036. [DOI] [PubMed] [Google Scholar]
- 91.Crijns TJ, Bernstein DN, Ring D, Gonzalez R, Wilbur D, Hammert WC. Factors associated with a discretionary upper-extremity surgery. J Hand Surg Am. 2019;44(2):155.e1–155.e7. doi: 10.1016/j.jhsa.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 92.Chen RE, Papuga MO, Voloshin I, Nicandri GT, Goldblatt JP, Bronstein RD, et al. Preoperative PROMIS scores predict postoperative outcomes after primary ACL reconstruction. Orthop J Sports Med. 2018;6(5):2325967118771286. doi: 10.1177/2325967118771286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cavallero M, Rosales R, Caballero J, Virkus WW, Kempton LB, Gaski GE. Locking plate fixation in a series of bicondylar tibial plateau fractures raises treatment costs without clinical benefit. J Orthop Trauma. 2018;32(7):333–337. doi: 10.1097/BOT.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 94.Bhatt S, Boody BS, Savage JW, Hsu WK, Rothrock NE, Patel AA. Validation of patient-reported outcomes measurement information system computer adaptive tests in lumbar disk herniation surgery. J Am Acad Orthop Surg. 2019;27(3):95–103. doi: 10.5435/JAAOS-D-17-00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bernstein DN, Kelly M, Houck JR, Ketz JP, Flemister AS, DiGiovanni BF, et al. PROMIS pain interference is superior vs numeric pain rating scale for pain assessment in foot and ankle patients. Foot Ankle Int. 2019;40(2):139–144. doi: 10.1177/1071100718803314. [DOI] [PubMed] [Google Scholar]
- 96.Bernholt D, Wright RW, Matava MJ, Brophy RH, Bogunovic L, Smith MV. Patient reported outcomes measurement information system scores are responsive to early changes in patient outcomes following arthroscopic partial meniscectomy. Arthroscopy. 2018;34(4):1113–1117. doi: 10.1016/j.arthro.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 97.Beleckas CM, Prather H, Guattery J, Wright M, Kelly M, Calfee RP. Anxiety in the orthopedic patient: using PROMIS to assess mental health. Qual Life Res. 2018;27(9):2275–2282. doi: 10.1007/s11136-018-1867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beleckas CM, Guattery J, Chamberlain AM, Khan T, Kelly MP, Calfee RP. Using Patient-reported Outcomes Measurement Information System measures to understand the relationship between improvement in physical function and depressive symptoms. J Am Acad Orthop Surg. 2018;26(24):e511–e518. doi: 10.5435/JAAOS-D-17-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Austin DC, Torchia MT, Moschetti WE, Jevsevar DS, Keeney BJ. Patient outcomes after total knee arthroplasty in patients older than 80 years. J Arthroplasty [Internet]. 2018;33(11):3465–3473. doi: 10.1016/j.arth.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 100.Anderson MR, Houck JR, Saltzman CL, Hung M, Nickisch F, Barg A, et al. Validation and generalizability of preoperative PROMIS scores to predict postoperative success in foot and ankle patients. Foot Ankle Int. 2018;39(7):763–770. doi: 10.1177/1071100718765225. [DOI] [PubMed] [Google Scholar]
- 101.Anderson MR, Baumhauer JF, DiGiovanni BF, Flemister S, Ketz JP, Oh I, et al. Determining success or failure after foot and ankle surgery using Patient Acceptable Symptom State (PASS) and Patient Reported Outcome Information System (PROMIS) Foot Ankle Int. 2018;39(8):894–902. doi: 10.1177/1071100718769666. [DOI] [PubMed] [Google Scholar]
- 102.Alvarez-Nebreda ML, Heng M, Rosner B, McTague M, Javedan H, Harris MB, et al. Reliability of proxy-reported Patient-reported Outcomes Measurement Information system physical function and pain interference responses for elderly patients with musculoskeletal injury. J Am Acad Orthop Surg. 2019;27(4):e156–e165. doi: 10.5435/JAAOS-D-17-00644. [DOI] [PubMed] [Google Scholar]
- 103.Overbeek CL, Nota SPFT, Jayakumar P, Hageman MG, Ring D. The PROMIS physical function correlates with the QuickDASH in patients with upper extremity illness. Clin Orthop Relat Res. 2015;473(1):311–317. doi: 10.1007/s11999-014-3840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wagner LI, Schink J, Bass M, Patel S, Diaz MV, Rothrock N, et al. Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer. 2015;121(6):927–934. doi: 10.1002/cncr.29104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fidai MS, Saltzman BM, Meta F, Lizzio VA, Stephens JP, Bozic KJ, et al. Patient-Reported Outcomes Measurement Information System and legacy patient-reported outcome measures in the field of orthopaedics: a systematic review. Arthroscopy. 2018;34:605–614. doi: 10.1016/j.arthro.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 107.Brodke DS, Goz V, Voss MW, Lawrence BD, Spiker WR, Hung M. PROMIS PF CAT outperforms the ODI and SF-36 physical function domain in spine patients. Spine. 2017;42(12):921–929. doi: 10.1097/BRS.0000000000001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Hara NN, Richards JT, Overmann A, Slobogean GP, Klazinga NS. Is PROMIS the new standard for patient-reported outcomes measures in orthopaedic trauma research? Injury [Internet] 2019. [DOI] [PubMed] [Google Scholar]
- 109.Shalhoub H, Reaney M. PROMIS® tools as endpoints in clinical trials: what should you know? A review of PROMIS® capabilities and the current regulatory space. Int J Clin Trials. 2016;3(4):174–179. doi: 10.18203/2349-3259.ijct20163953. [DOI] [Google Scholar]
- 110.Pennestrì F, Lippi G, Banfi G. Pay less and spend more-the real value in healthcare procurement. Ann Transl Med. 2019;7(22):688. doi: 10.21037/atm.2019.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makhni EC, Padaki AS, Petridis PD, Steinhaus ME, Ahmad CS, Cole BJ, et al. High variability in outcome reporting patterns in high-impact ACL literature. J Bone Joint Surg Am [Internet]. 2015;97(18):1529–1542. doi: 10.2106/JBJS.O.00155. [DOI] [PubMed] [Google Scholar]
- 112.Galeoto G, Piepoli V, Ciccone E, Mollica R, Federici C, Magnifica F, et al. Musculoskeletal Health Questionnaire: translation, cultural adaptation and validation of the Italian version (MSK-HQ-I). Muscles Ligaments Tendons J (MLTJ). 2019;9(2). 10.32098/mltj.02.2019.20.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategies.
Quality Assessment.
Data Availability Statement
A limited data set with fields reported in this paper is available upon request via email to the corresponding author, with no limitations on the reuse of the data.