Abstract
Background:
Epstein-Barr virus (EBV) positivity is associated with better gastric cancer prognosis and is found in a relatively fixed 9% of tumors worldwide.
Aims:
We aimed to examine the EBV status of gastric adenocarcinomas in a very high-incidence population and to compare prevalence between cardia and non-cardia anatomic subsites.
Methods:
We evaluated 1035 adult gastric adenocarcinoma cases presenting during 1997-2005 to the Shanxi Cancer Hospital in Taiyuan, Shanxi Province, China. EBV-encoded RNA was detected in alcohol-fixed paraffin-embedded tumor specimens by in situ hybridization. Associations were assessed in case-case comparisons using the chi-squared test for categorical variables and the Mann-Whitney U Test for continuous variables, with p-values < 0.05 considered statistically significant. Adjusted odds ratios were calculated using logistic regression, and mortality hazard ratios (HRs) were estimated by Cox proportional hazards regression.
Results:
Sixty-four percent of the evaluated cancers were found in the cardia. Cardia tumor localization was associated with male sex, advanced primary tumor stage, better differentiated histology, and intestinal-type Lauren classification. Four percent of the non-cardia and only 0.9% of cardia cancers were EBV-positive. EBV-positivity was associated with better overall survival (adjusted HR 0.30, 95% CI 0.14-0.63).
Conclusions:
Our study highlights unusually low EBV prevalence in gastric adenocarcinoma among a high-incidence population, particularly for cardia cancers. These findings suggest a unique risk factor profile for the high incidence of gastric cancer in this population.
Keywords: cardia, gastric cancer, Shanxi, Epstein-Barr Virus
Introduction
Gastric adenocarcinoma (GAC) is a relatively common malignancy worldwide, with low five-year survival rates at advanced stages [1–3]. GAC is often classified by its anatomical location within the stomach as either the more proximal cardia GAC (CGA) or more distal non-cardia GAC (NCGA). Epidemiologic studies suggest that CGA and NCGA are separate entities, and investigations aim to better understand cancer development in both subsites [4–7].
Shanxi Province in northern China is one of the world’s highest-incidence regions for both CGA and NCGA, with overall GAC rates in the area nearly ten times the global average [2, 8]. This gastric cancer excess has been a focus of etiologic inquiry, and known GAC risk factors incompletely explain the elevated incidence [9–12].
GAC’s association with Epstein-Barr virus (EBV) may help clarify our understanding of carcinogenesis in high-incidence GAC regions. EBV is present in the tumor cells of a relatively fixed 9% of GAC in both high- and low-incidence populations worldwide [13–16]. EBV-associated gastric cancers preferentially localize to the cardia [17], while most GAC overall is found in non-cardia subsites of the stomach [9, 17].
Here we report the EBV prevalence in both CGA and NCGA from Shanxi as a potential clue to better explain GAC rates in this high-incidence population.
Methods
GAC cases were eligible for this study if they presented to the Shanxi Cancer Hospital in Taiyuan, Shanxi, People’s Republic of China, and were diagnosed during 1997-2005. Enrolled cases were a systematic sample, representing all patients from selected days of selected weeks. Patients were included if they (i) were 20 years of age or older, (ii) had incident stomach cancer, (iii) resided in one of five geographic regions of Shanxi (Taiyuan, Linfen, Jinzhong, Chanzi, and Xinzhou), (iv) underwent resection of their tumors at the Shanxi Cancer Hospital, and (v) had their diagnosis histologically confirmed based on criteria from prior studies [11]. Of note, all patients in our study, including those who had evidence of distant disease at diagnosis, underwent resection. This surgical practice was reflective of the clinical approach to gastric cancer at the time in Shanxi. Histopathological diagnoses were made by pathologists at the Shanxi Cancer Hospital. Anatomic localization was categorized either as “cardia” (within the proximal three centimeters of the stomach) or “non-cardia” (within the remainder of the stomach). Primary tumor depth determined tumor stage, categorized as early (Tis, T1, or T2) or late (T3 or T4) [11]. Tumors were histologically categorized by the Lauren classification as diffuse- or intestinal-type and further graded as either well-, moderately- or poorly-differentiated. Regional lymph nodes were analyzed for the presence of tumor (positive or negative). Metastasis was assessed through clinical findings, and cases were analyzed for presence of distant spread (positive or negative).
Information regarding tobacco and alcohol use, pickled vegetable consumption, pickled vegetable juice consumption, and family history of cancer (both for any cancer and for specific upper gastrointestinal tract cancers) in first-degree relatives was collected by questionnaire. Cancer patients (or immediate family members) were periodically contacted by study research nurses to ascertain information about vital status and post-surgical chemotherapy and/or radiotherapy (received or not received) through the end of 2005. Deaths were recorded as they were detected, and patients still alive were censored as of the date of their last contact. Overall, 1035 GAC cases with EBV status available were included in our analysis.
The institutional review boards of the Shanxi Cancer Hospital, the National Cancer Institute, and the University of North Carolina approved the project, and written informed consent was obtained from all patients prior to their participation in the study.
The presence of EBV was assessed by in situ hybridization for EBV-encoded RNA (EBER) [18]. EBER expression was detected by an automated method in alcohol-fixed paraffin-embedded tumors prepared as tissue microarrays, with inclusion of known EBER-positive and -negative tumors as controls. EBER-positive tumors had positive EBER staining localized in the nuclei of malignant tumor cells, and EBER-negative tumors had either no staining or staining present only in benign-appearing lymphoid cells. A control for RNA preservation was done to assure that EBV-negative tumors were truly negative.
Cases were compared first based on their anatomic localization and then by EBV status. The Mann-Whitney U Test was used to compare age between groups. Chi-squared analyses were performed for categorical variables. Age- and sex-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated with NCGA and EBV-negative cases as the reference. Survival was estimated by the Kaplan-Meier method for EBV-positive and EBV-negative cases and compared statistically by the log-rank test. A Cox proportional hazards regression model with adjustment for age, sex, tumor differentiation, and the presence of distant metastases was used to estimate hazard ratios (HRs) and 95% CIs for mortality comparing EBV-positive and EBV-negative cases. Follow-up time was censored on the date of death or date the patient was last known to be alive. Statistical significance was defined as p<0.05. Statistical analyses were performed and figures were generated using SAS version 9.4 (SAS Inc, Cary, NC).
Results
Overall, 661 (64%) of the 1035 GAC cases were CGA, and the remainder were NCGA. CGA patients were older and more commonly male compared to NCGA patients (Table 1). CGA (versus NCGA) localization was positively associated with intestinal subtype, better differentiation, and more advanced primary tumor stage (Table 1). There were no significant associations between anatomic subsite and lymph node positivity, metastatic spread, or family history of cancer (Table 1), nor with tobacco or alcohol use, pickled food consumption or the receipt of chemoradiotherapy (not tabulated). Patients with EBV-positive tumors were younger compared to those with EBV-negative tumors (Table 2). There were no associations between EBV-positivity and tobacco use or the consumption of alcohol, pickled vegetables, or pickled vegetable juice (Table 2). Additionally, EBV-positivity was associated with poorer differentiation and the absence of distant metastases (Table 2).
Table 1.
Clinical characteristics of gastric adenocarcinoma by anatomic subsite
| Cardia (n=661) | Non-cardia (n=374) | aOR (95% CI) | |
|---|---|---|---|
| Patient Characteristics | |||
| Male | 574 (83.2)b | 297 (74.1) | 1.67 (1.23-2.27) |
| cAge (IQR) | 61 (55-65) | 58 (50-63) | p < 0.001 |
| Family History | |||
| Any Cancer | 230 (34.8) | 122 (32.6) | 1.15 (0.88-1.52) |
| Upper GI Cancer | 147 (22.2) | 72 (19.3) | 1.23 (0.89-1.70) |
| Esophageal Cancer | 91 (13.8) | 40 (10.7) | 1.36 (0.90-2.03) |
| Gastric Cardia Cancer | 27 (4.1) | 10 (2.7) | 1.55 (0.73-3.29) |
| Gastric Non-cardia Cancer | 50 (7.6) | 29 (7.8) | 0.98 (0.60-1.59) |
| Tumor Characteristics | |||
| EBV Status | |||
| Positive | 6 (0.9) | 16 (4.3) | 0.22 (0.08-0.59) |
| Differentiation | |||
| Well | 265 (40.1) | 99 (26.5) | 1.00 |
| Moderate | 112 (16.9) | 42 (11.2) | 1.10 (0.71-1.69) |
| Poor | 256 (38.7) | 198 (52.9) | 0.52 (0.38-0.70) |
| Missing | 28 (4.2) | 35 (9.4) | |
| Lauren Classification | |||
| Intestinal-type | 384 (58.1) | 138 (36.9) | 1.00 |
| Diffuse-type | 249 (37.7) | 201 (53.7) | 0.47 (0.35-0.61) |
| Missing | 28 (4.2) | 35 (9.4) | |
| Primary Tumor Stage | |||
| Early (T0, Tis, T1, T2) | 32 (4.8) | 47 (12.6) | 1.00 |
| Late (T3, T4) | 628 (95.0) | 325 (86.9) | 2.95 (1.82-4.77) |
| Missing | 1 (0.2) | 2 (0.5) | |
| Regional Lymph Node Status | |||
| Negative | 96 (14.5) | 53 (14.2) | 1.00 |
| Positive | 497 (75.2) | 270 (72.2) | 1.02 (0.70-1.48) |
| Missing | 68 (10.3) | 51 (13.6) | |
| Distant Metastasis | |||
| Negative | 164 (24.8) | 101 (27.0) | 1.00 |
| Positive | 495 (74.9) | 270 (72.2) | 1.14 (0.85-1.53) |
| Missing | 2 (0.3) | 3 (0.8) | |
Age- and sex-adjusted odds ratios (ORs) with 95% confidence intervals for comparison between gastric adenocarcinoma originating from cardia and non-cardia (reference) anatomic subsites, when applicable. Unadjusted odds ratio shown for sex, and p-value shown for age.
Number of patients (column percent)
Median age (interquartile range) noted in years
Table 2.
Clinical characteristics of gastric adenocarcinoma by EBV status
| EBV-positive (n=22) | EBV-negative (n=1013) | aOR (95% CI) | |
|---|---|---|---|
| Patient Characteristics | |||
| Male | 19 (86.4)b | 809 (79.9) | 1.60 (0.47-5.45) |
| cAge (IQR) | 51 (44-62) | 60 (53-65) | p = 0.01 |
| Lifestyle Factors | |||
| Ever alcohol | 13 (59.1) | 581 (57.4) | 0.79 (0.30-2.10) |
| Ever tobacco | 17 (77.3) | 714 (70.5) | 1.18 (0.32-4.31) |
| Ever pickled vegetable consumption | 19 (86.4) | 875 (86.4) | 0.98 (0.28-3.36) |
| Ever pickled vegetable juice consumption | 14 (63.6) | 573 (56.6) | 1.43 (0.59-3.45) |
| Family History | |||
| Any Cancer | 4 (18.2) | 348 (34.4) | 0.40 (0.13-1.18) |
| Upper GI Cancer | 2 (9.1) | 217 (21.4) | 0.35 (0.08-1.52) |
| Esophageal Cancer | 2 (9.1) | 129 (12.7) | 0.70 (0.16-3.02) |
| Gastric Cardia Cancer | 0 (0) | 37 (3.7) | N/A |
| Gastric Non-cardia Cancer | 0 (0) | 79 (7.8) | N/A |
| Tumor Characteristics | |||
| Anatomic Subsite | |||
| Cardia | 6 (27.3) | 655 (64.7) | 0.22 (0.08-0.59) |
| Differentiation | |||
| Well | 2 (9.1) | 362 (35.7) | 1.00 |
| Moderate | 6 (27.3) | 148 (14.6) | 6.63 (1.31-33.45) |
| Poor | 14 (63.6) | 440 (43.4) | 5.61 (1.26-24.98) |
| Missing | 0 | 63 (6.2) | |
| Lauren Classification | |||
| Intestinal-type | 8 (36.4) | 514 (50.7) | 1.00 |
| Diffuse-type | 14 (63.6) | 436 (43.0) | 2.06 (0.85-5.01) |
| Missing | 0 (0) | 63 (6.2) | |
| Primary Tumor Stage | |||
| Early (T0, Tis, T1, T2) | 4 (18.2) | 75 (7.4) | 1.00 |
| Late (T3, T4) | 18 (81.8) | 935 (92.3) | 0.39 (0.13-1.19) |
| Missing | 0 (0) | 3 (0.3) | |
| Regional Lymph Node Status | |||
| Negative | 6 (27.3) | 143 (14.1) | 1.00 |
| Positive | 12 (54.6) | 755 (74.5) | 0.39 (0.14-1.07) |
| Missing | 4 (18.2) | 115 (11.4) | |
| Distant Metastasis | |||
| Negative | 10 (45.5) | 255 (25.2) | 1.00 |
| Positive | 12 (54.6) | 753 (74.3) | 0.42 (0.18-0.99) |
| Missing | 0 (0) | 5 (0.5) | |
| Chemotherapy | |||
| Yes | 2 (9.1) | 76 (7.5) | 1.00 |
| No | 18 (81.8) | 866 (85.5) | 1.22 (0.28-5.38) |
| Missing | 2 (9.1) | 71 (7.0) | |
| Radiotherapy | |||
| Yes | 11 (50.0) | 461 (45.5) | 1.00 |
| No | 9 (40.9) | 481 (47.5) | 1.34 (0.55-3.29) |
| Missing | 2 (9.1) | 71 (7.0) | |
Age- and sex-adjusted odds ratios (ORs) with 95% confidence intervals for comparison between gastric adenocarcinoma originating from cardia and non-cardia (reference) anatomic subsites, when applicable. Unadjusted odds ratio shown for sex, and p-value shown for age.
Number of patients (column percent)
Median age (interquartile range) noted in years
Four percent of NCGA and only 0.9% of CGA were EBV-positive (OR 0.22, 95% CI 0.08-0.59; Table 1). In the adjusted model, EBV-positivity (HR 0.30, 95% CI 0.14-0.63) was associated with better overall survival, while the presence of distant metastases (HR 2.78, 95% CI 2.27-3.41) and poorer differentiation (moderately-differentiated HR 1.58, 95% CI 1.27-1.98; poorly-differentiated HR 1.54, 95% CI 1.30-1.83) were associated with worse overall survival. The median duration of survival for EBV-negative cases was 1.8 years. Median survival time of EBV-positive cases was not reached, with median follow-up time of 3.6 years (range 0.1-8.6 years). Overall survival among EBV-positive cases was better than that of EBV-negative cases in an unadjusted model (Figure 1, log-rank p = 0.001).
Fig. 1. Survival Curves for Gastric Adenocarcinoma by EBV status.
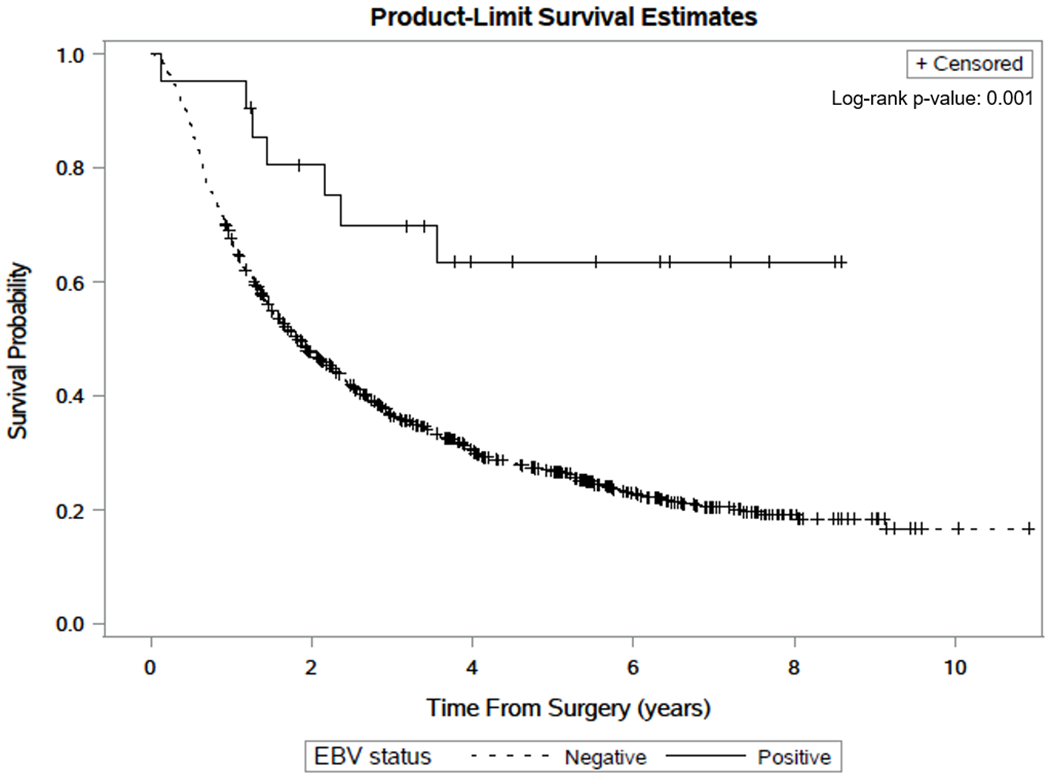
Kaplan-Meier plots estimating survival time after surgery for Epstein-Barr virus (EBV)-positive and EBV-negative gastric adenocarcinoma cases from Shanxi province, China, with log-rank p-value noted. + denotes censored cases along the respective curves
Discussion
Shanxi Province has an extremely high rate of gastric cancer, with incidence dwarfing the worldwide average at double the rates from most other high-incidence regions [8]. Cardia tumors predominated in our case series, whereas NCGA incidence is higher than that of CGA among most populations globally [9]. Even when compared with other high-incidence populations, this relative abundance of CGA is unusual [9].
While Shanxi’s GAC distribution by anatomic subsite is noteworthy, further support for this region’s unique carcinogenic profile is linked to its low EBV prevalence as compared to tumors from other parts of the world. In a prior meta-analysis, most populations had between 8-10% EBV-positive tumors despite marked international variation in absolute incidence [16]. This relatively fixed fraction of EBV-associated cases worldwide suggests that a consistent set of risk factors is usually responsible for GAC regardless of the overall burden [16, 8].
Here we report low EBV prevalence among GAC cases from a very high-incidence population. While the EBV prevalence was moderately low in the NCGA tumors, it was extraordinarily low among CGA cases. The lower EBV prevalence in CGA is unusual, as prior studies have found that EBV-positive tumors more commonly affect the cardia subsite of the stomach [17]. A notable exception to this trend comes from a high-incidence GAC Iranian cohort, in which tumor EBV prevalence was 3% in GAC overall but only 1% in CGA [19]. These extreme deviations from the generally constant worldwide EBV prevalence and the usual higher prevalence of EBV in cardia cancers suggest that excess GAC in Shanxi and Iran are potentially attributable to a unique combination of etiologic factors not operative elsewhere [12].
Patients in our series with EBV-positive GAC were less likely to have distant metastases, suggesting that these cases may have followed a more indolent clinical course relative to EBV-negative GAC. Additionally, there was a survival advantage for EBV-positive as compared to EBV-negative GAC in our adjusted model, consistent with two prior analyses which both incorporated the current cases [17, 19]. Those studies associated EBV-positivity with reduced mortality (HR 0.72, 95% CI 0.61-0.86; HR 0.67, 95% CI 0.55-0.79) in a pooled analysis [17] and a meta-analysis [19], respectively.
Our population’s relative homogeneity may limit generalizability of the findings to other dissimilar populations. Also, our study included only a modest sample size with a small number of EBV-positive cases. Prolonged and more standardized long-term follow-up would have allowed for a more robust survival analysis. Additionally, we recognize the substantial impact of Helicobacter pylori (H. pylori) infection on gastric cancer. While no serologic testing for H. pylori is available for this group, 16S microbiome testing of 80 biopsies from visually normal parts of the stomach in GC cases from this series showed that 94% of the samples were colonized with H. pylori, indicating that the infection was ubiquitous in this population [20].
We found a very low EBV prevalence among GAC tumors from Shanxi Province, China, especially among CGA tumors. Excess cases of GAC in Shanxi may all be EBV-negative, superimposed on a background incidence of GAC that is attributable to the common risk factors in all populations, thus diluting the overall EBV prevalence. These findings reinforce the concept that GAC in Shanxi may be etiologically different from GAC in other high-incidence areas worldwide. Future studies should investigate the etiology of this uniquely low-EBV GAC, which may potentially inform our understanding of carcinogenic pathways and eventually provide insight for cancer prevention.
Acknowledgements
The authors would like to acknowledge the assistance of staff members of the Shanxi Cancer Hospital.
Funding
This study was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, United States National Cancer Institute.
Footnotes
Compliance with Ethical Standards
All procedures performed were in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the institutional review boards of the Shanxi Cancer Hospital, the National Cancer Institute, and the University of North Carolina. Informed consent to be included in the study was obtained from all individual participants included in the study.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no personal or financial conflict of interest.
References
- 1.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Matsuda T, Di Carlo V et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. doi: 10.1016/s0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Coggon D, Osmond C, Wickham C. Poor housing in childhood and high rates of stomach cancer in England and Wales. Br J Cancer. 1990;61(4):575–8. doi: 10.1038/bjc.1990.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P, Zhou Y, Chen B et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45(16):2867–73. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. 2001;49(3):347–53. doi: 10.1136/gut.49.3.347 %J Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Tao L, Murphy JD et al. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology. 2019;156(1):59–62.e4. doi: 10.1053/j.gastro.2018.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman DBF, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay J, editors. Cancer Incidence in Five Continents, Vol. X IARC Scientific Publication No 164 Lyon: International Agency for Research on Cancer Lyon: International Agency for Research on Cancer. 2014;X. [Google Scholar]
- 9.Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64(12):1881–8. doi: 10.1136/gutjnl-2014-308915. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Zheng R, Wang N et al. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30(3):291–8. doi: 10.21147/j.issn.1000-9604.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Hu N, Han X et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC cancer. 2009;9:269. doi: 10.1186/1471-2407-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Hu N, Han XY et al. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer epidemiology. 2011;35(6):e91–9. doi: 10.1016/j.canep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JI. Epstein-Barr virus infection. The New England journal of medicine. 2000;343(7):481–92. doi: 10.1056/nejm200008173430707. [DOI] [PubMed] [Google Scholar]
- 14.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nature reviews Cancer. 2004;4(10):757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 15.Imai S, Koizumi S, Sugiura M et al. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(19):9131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137(3):824–33. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camargo MC, Kim WH, Chiaravalli AM et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63(2):236–43. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. The Journal of molecular diagnostics : JMD. 2008;10(4):279–92. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Liu J, Qiu H et al. Prognostic significance of Epstein-Barr virus infection in gastric cancer: a meta-analysis. BMC cancer. 2015;15:782. doi: 10.1186/s12885-015-1813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu G, Torres J, Hu N et al. Molecular Characterization of the Human Stomach Microbiota in Gastric Cancer Patients. Frontiers in cellular and infection microbiology. 2017;7:302. doi: 10.3389/fcimb.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]


