Abstract
Diets based on high levels of corn protein have elevated concentrations of Leu, which may negatively affect N retention in pigs. An experiment was, therefore, conducted to test the hypothesis that Ile and Val supplementation may overcome the detrimental effects of excess dietary Leu on N balance and metabolism of branched-chain amino acids (BCAA) in growing pigs. A total of 144 barrows (initial body weight: 28.5 kg) were housed in metabolism crates and randomly assigned to 1 of 18 dietary treatments. The basal diet contained 0.98% standardized ileal digestible (SID) Lys and had SID Leu, Val, and Ile ratios to SID Lys of 100%, 60%, and 43%, respectively. Crystalline l-Leu (0% or 2.0%), l-Ile (0%, 0.1%, or 0.2%), and l-Val (0%, 0.1%, or 0.2%) were added to the basal diet resulting in a total of 18 dietary treatments that were arranged in a 2 × 3 × 3 factorial. Urine and fecal samples were collected for 5 d after 7 d of adaptation. Blood, skeletal muscle, and liver samples were collected at the conclusion of the experiment. There were no three-way interactions among the main effects. Excess Leu in diets reduced (P < 0.05) N retention and biological value of protein and increased (P < 0.001) plasma urea N (PUN), but PUN was reduced (P < 0.05) as dietary Val increased. Concentrations of Leu in the liver were greater (P < 0.001) in pigs fed excess Leu diets than in pigs fed adequate Leu diets, but concentrations of BCAA in muscle were greater (P < 0.05) in pigs fed low-Leu diets. Increasing dietary Ile increased (P < 0.001) plasma-free Ile and plasma concentration of the Ile metabolite, α-keto-β-methylvalerate, but the increase was greater in diets without excess Leu than in diets with excess Leu (interaction, P < 0.001). Plasma concentrations of Val and the Val metabolite α-keto isovalerate increased (P < 0.001) with increasing dietary Val in diets with adequate Leu, but not in diets with excess Leu (interaction, P < 0.001). Increasing dietary Leu increased (P < 0.001) plasma-free Leu and plasma concentration of the Leu metabolite, α-keto isocaproate (KIC). In contrast, increased dietary Val reduced (P < 0.05) the plasma concentration of KIC. In conclusion, excess dietary Leu reduced N retention and increased PUN in growing pigs, but Val supplementation to excess Leu diets may increase the efficiency of amino acid utilization for protein synthesis as indicated by reduced PUN.
Keywords: branched-chain amino acids, isoleucine, leucine, nitrogen balance, pigs, valine
Introduction
Leucine is a key regulator that stimulates catabolism of branched-chain amino acids (BCAA; i.e., Leu, Ile, and Val) in skeletal muscle and liver (Harper et al., 1984; Cemin et al., 2019a). If diets fed to pigs contain excess Leu, catabolism of all three BCAA may increase because of the stimulating effect of the Leu metabolite, α-keto isocaproate (KIC) on the branched-chain α-keto acid dehydrogenase (BCKDH) enzyme complex. The BCKDH complex is used in the metabolism of the three branched-chain α-keto acids (BCKA) that are generated after deamination of the three BCAA (Wiltafsky et al., 2010). Serum Ile and Val concentrations in growing pigs were reduced by excess dietary Leu (Duan et al., 2016; Wessels et al., 2016). Likewise, excess Leu reduced feed intake and growth performance of pigs (Gatnau et al., 1995; Wiltafsky et al., 2010), which may be a result of the imbalanced supply of BCAA that result from increased metabolism of Val and Ile. Recent data confirmed that excess dietary Leu reduced growth performance and biological value of protein and tended to reduce N balance of growing pigs, which is likely a result of reduced availability of Val and Ile (Kwon et al., 2019).
Leucine may also promote increased protein synthesis by stimulating the mechanistic target of rapamycin (mTOR) in the brain, and increased mTOR activity is associated with an inhibitory effect on feed intake (Cota et al., 2006). In neonatal pigs, Leu infusion can stimulate protein synthesis in cardiac and skeletal muscle, whereas there is no effect on Val or Ile infusion (Escobar et al., 2006). Likewise, Wilson et al. (2010) indicated that if no AA are limiting, added Leu may result in increased protein synthesis in neonatal pigs.
Supplementation of Ile and Val above the requirements in diets with excess Leu ameliorates the negative effect of excess Leu on absorption and degradation of BCAA (Morales et al., 2016). This indicates that excess dietary Leu reduces the availability of Ile and Val, and as a consequence, the addition of extra Ile and Val may prevent the negative effects of excess Leu. Therefore, the objective of this experiment was to test the hypothesis that inclusion of dietary Ile and Val above the requirement may overcome the detrimental effects of excess Leu on N balance and metabolism of BCAA in growing pigs.
Materials and Methods
Animal care procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois.
Animals, diets, and experimental design
A total of 144 growing barrows (initial body weight [BW]: 28.5 ± 2.5 kg) that were the offspring of Line 359 boars and Camborough sows (Pig Improvement Company, Henderson, TN) were assigned to 18 dietary treatments with 8 replicate pigs per treatment in a randomized complete block design. There were eight blocks with one pig per diet in each block. A basal diet was formulated to contain 0.98% standardized ileal digestible (SID) Lys and had SID Leu, Val, and Ile ratios to SID Lys of 100%, 60%, and 43%, respectively. Two levels of crystalline l-Leu (0% or 2.0%), three levels of crystalline l-Ile (0%, 0.1%, or 0.2%), and three levels of crystalline l-Val (0%, 0.1%, or 0.2%) were added to the basal diet—for a total of 18 diets that were used in a 2 × 3 × 3 factorial arrangement of treatments.
Diets were based on corn, wheat, barley, soy protein concentrate, and spray-dried blood cells (Tables 1 and 2) and formulated to be isoenergetic (3,350 kcal ME/kg) and to contain 0.98% SID Lys, which was assumed to be slightly above the SID Lys requirement for 25 to 50 kg pigs (NRC, 2012). Other indispensable amino acids (AA), except the three BCAA, were included in all diets in excess of the requirements (NRC, 2012). Glycine was included to maintain a constant concentration of dietary crude protein at 15.50%.
Table 1.
Ingredient composition of experimental diets containing 100% SID Leu:Lys ratio, as-fed basis1
| SID Ile:Lys, % | 43 | 53 | 63 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item, % | SID Val:Lys, % | 60 | 70 | 80 | 60 | 70 | 80 | 60 | 70 | 80 |
| Ground corn | 32.35 | 32.35 | 32.35 | 32.35 | 32.35 | 32.35 | 32.35 | 32.35 | 32.35 | |
| Ground wheat | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | 22.00 | |
| Ground barley | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | |
| Soy protein concentrate | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | 7.60 | |
| Spray-dried blood cells | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | |
| Soybean oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | |
| l-Lys∙HCl | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | |
| dl-Met | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | |
| l-Thr | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | |
| l-Trp | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | |
| l-Leu | — | — | — | — | — | — | — | — | — | |
| l-Ile | — | — | — | 0.10 | 0.10 | 0.10 | 0.20 | 0.20 | 0.20 | |
| l-Val | — | 0.10 | 0.20 | — | 0.10 | 0.20 | — | 0.10 | 0.20 | |
| Gly | 1.35 | 1.30 | 1.25 | 1.30 | 1.25 | 1.20 | 1.25 | 1.20 | 1.15 | |
| Cornstarch | 1.05 | 1.00 | 0.95 | 1.00 | 0.95 | 0.90 | 0.95 | 0.90 | 0.85 | |
| Limestone | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | |
| Monocalcium phosphate | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | |
| Vitamin–mineral premix2 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | |
1Nine additional diets that were similar to the above diets with the exception that the SID Leu:Lys ratio was 300% instead of 100% were also formulated. This was accomplished by including 2.0% l-Leu in all diets and reducing both Gly and cornstarch inclusion to 0.20%.
2The vitamin–micromineral premix provided the following quantities of vitamins and microminerals per kilogram of complete diet: Vitamin A as retinyl acetate, 11,136 IU; vitamin D3 as cholecalciferol, 2,208 IU; vitamin E as dl-alpha tocopheryl acetate, 66 IU; vitamin K as menadione dimethylprimidinol bisulfite, 1.42 mg; thiamin as thiamine mononitrate, 0.24 mg; riboflavin, 6.59 mg; pyridoxine as pyridoxine hydrochloride, 0.24 mg; vitamin B12, 0.03 mg; d-pantothenic acid as d-calcium pantothenate, 23.5 mg; niacin, 44.1 mg; folic acid, 1.59 mg; biotin, 0.44 mg; Cu, 20 mg as copper sulfate and copper chloride; Fe, 126 mg as ferrous sulfate; I, 1.26 mg as ethylenediamine dihydriodide; Mn, 60.2 mg as manganese sulfate; Se, 0.3 mg as sodium selenite and selenium yeast; and Zn, 125.1 mg as zinc sulfate.
Table 2.
Chemical composition of ingredients used in experimental diets, as-fed basis1
| Item | Corn | Wheat | Barley | Soy protein concentrate | Spray-dried blood cells |
|---|---|---|---|---|---|
| Crude protein, % | 7.09 | 11.74 | 10.98 | 64.48 | 93.81 |
| Dry matter, % | 87.14 | 88.64 | 89.24 | 94.82 | 91.78 |
| Indispensable amino acids, % | |||||
| Arg | 0.32 | 0.52 | 0.55 | 4.63 | 3.65 |
| His | 0.21 | 0.24 | 0.24 | 1.68 | 6.79 |
| Ile | 0.26 | 0.39 | 0.38 | 3.01 | 0.35 |
| Leu | 0.78 | 0.69 | 0.72 | 4.97 | 12.81 |
| Lys | 0.26 | 0.36 | 0.48 | 4.10 | 8.62 |
| Met | 0.14 | 0.19 | 0.19 | 0.92 | 0.98 |
| Phe | 0.30 | 0.45 | 0.43 | 1.85 | 6.92 |
| Thr | 0.32 | 0.45 | 0.49 | 3.21 | 3.54 |
| Trp | 0.06 | 0.13 | 0.12 | 0.87 | 1.60 |
| Val | 0.26 | 0.32 | 0.38 | 2.51 | 8.52 |
| Dispensable amino acids, % | |||||
| Ala | 0.50 | 0.41 | 0.47 | 2.75 | 7.93 |
| Asx2 | 0.46 | 0.58 | 0.72 | 7.08 | 10.21 |
| Cys | 0.16 | 0.26 | 0.24 | 0.93 | 0.54 |
| Glx2 | 1.21 | 2.70 | 2.19 | 11.37 | 7.52 |
| Gly | 0.29 | 0.45 | 0.45 | 2.66 | 4.12 |
| Pro | 0.62 | 0.90 | 0.93 | 3.20 | 3.27 |
| Ser | 0.33 | 0.44 | 0.42 | 2.72 | 4.12 |
| Tyr | 0.19 | 0.27 | 0.27 | 2.23 | 2.21 |
| BCAA:crude protein ratio, % | |||||
| Ile:crude protein | 3.67 | 3.32 | 3.46 | 4.67 | 0.37 |
| Leu:crude protein | 11.00 | 5.88 | 6.56 | 7.71 | 13.66 |
| Val:crude protein | 3.67 | 2.73 | 3.46 | 3.89 | 9.08 |
1Ingredients were analyzed in duplicate.
2Asx, Asp, and Asn; Glx, Glu, and Gln.
Housing and feeding
Pigs were individually housed in metabolism crates that were equipped with a feeder and a nipple drinker. Pigs were fed experimental diets for 13 d and the BW of pigs was recorded at the start and at the conclusion of the feeding period. Pigs were fed 2.3 times the maintenance requirement for metabolizable energy (i.e., 197 kcal/kg × BW0.60; NRC, 2012), which was provided in two daily meals at 0800 and 1600 hours. Water was provided on an ad libitum basis.
Sample collection
After 5 d of adaptation to experimental diets, urine and fecal samples were collected during the following 5 d according to the marker-to-marker method (Adeola, 2001). Urine was collected in buckets containing 50 mL 3 N HCl as a preservative. Fecal samples and 20% of the collected urine were stored at −20 °C immediately after collection. At the conclusion of the experiment, urine samples were thawed and mixed within animal and diet.
On day 13, pigs were fed 400 g of their experimental diets 2.5 h prior to blood sampling. Blood samples were collected from the jugular vein of all pigs using vacutainers containing heparin (BD, Franklin Lakes, NJ). All samples were centrifuged at 1,500 × g at 4 °C for 15 min to collect plasma, which was frozen at −80 °C until analyzed. After blood sampling, all pigs were euthanized. The liver and skeletal muscle (longissimus dorsi) were collected and immediately frozen in liquid N.
Sample analyses
Samples of corn, wheat, barley, soy protein concentrate, and blood cells, which were the main ingredients in the diets, and all experimental diets were analyzed for AA (method 982.30 E (a, b, c); AOAC Int., 2007) using an Amino Acid Analyzer (model L-8800; Hitachi High Technologies America Inc., Pleasanton, CA). Ingredient samples were also analyzed for dry matter (method 930.15; AOAC Int., 2007). The frozen fecal samples were dried in a forced-air drying oven at 55 °C until constant weight and ground for analysis. Ingredients, diets, fecal samples, and thawed urine samples were analyzed for crude protein (method 984.13; AOAC Int., 2007) using a Kjeltec 8400 apparatus (FOSS Inc., Eden Prairie, MN). Plasma from blood in heparinized tubes was analyzed for plasma urea N (PUN) using a Beckman Coulter Clinical Chemistry AU analyzer (Beckman Coulter Inc., Brea, CA). Lyophilized and homogenized liver and skeletal muscle samples were analyzed by Ajinomoto Animal Nutrition North America Inc. Laboratory (Eddyville, IA) for BCAA (method 999.13; AOAC Int., 2007) using an Amino Acid Analyzer (model L-8900; Hitachi High Technologies America Inc., Pleasanton, CA). Concentrations of BCAA and BCKA in plasma were analyzed by liquid chromatography-mass spectrometry (LC/MS) using a Sciex 5500 QTrap with Agilent 1200 LC (AB Sciex, Framingham, MA) according to the protocol described by Beals et al. (2016).
Calculations and statistical analyses
The apparent total tract digestibility (ATTD) of N in each experimental diet and retention of N for each pig were calculated based on the method described by Pedersen et al. (2007). The biological value of protein in the diets was calculated by expressing the retention of N as a percentage of the difference between N intake and N output in feces (Mitchell, 1924).
The normality of data was verified, and outliers were identified using the UNIVARIATE procedure (SAS Inst. Inc., Cary, NC). Data were analyzed using the PROC MIXED of SAS (SAS Institute Inc., Cary, NC) as a 2 × 3 × 3 factorial arrangement of treatments. The experimental unit was the pig and the model included SID Leu:Lys ratio, SID Ile:Lys ratio, SID Val:Lys ratio, and the interactions among SID Leu:Lys, SID Ile:Lys, and SID Val:Lys as fixed effects and block as a random effect. Treatment means were separated by using the LSMEANS statement. Statistical significance and tendency were considered as P < 0.05 and 0.05 ≤ P < 0.10, respectively.
Results
All animals remained healthy throughout the experiment. Crude protein and AA concentrations in corn, wheat, barley, soy protein concentrate, and spray-dried blood cells were in agreement with expected values (NRC, 2012; Table 2). Analyzed values for Lys and BCAA were in agreement with formulated values in all experimental diets (Tables 3 and 4).
Table 3.
Analyzed nutrient composition of experimental diets containing 100% SID Leu:Lys ratio, as-fed basis1
| SID Ile:Lys, % | 43 | 53 | 63 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | SID Val:Lys, % | 60 | 70 | 80 | 60 | 70 | 80 | 60 | 70 | 80 |
| Crude protein, % | 15.48 | 15.55 | 15.41 | 15.46 | 15.43 | 15.56 | 15.51 | 15.39 | 15.52 | |
| Indispensable amino acids, % | ||||||||||
| Arg | 0.77 | 0.76 | 0.70 | 0.77 | 0.80 | 0.76 | 0.76 | 0.76 | 0.75 | |
| His | 0.38 | 0.38 | 0.36 | 0.38 | 0.39 | 0.37 | 0.37 | 0.37 | 0.37 | |
| Ile | 0.49 | 0.51 | 0.50 | 0.63 | 0.64 | 0.62 | 0.70 | 0.70 | 0.69 | |
| Leu | 1.16 | 1.13 | 1.09 | 1.15 | 1.19 | 1.15 | 1.14 | 1.13 | 1.13 | |
| Lys | 1.12 | 1.05 | 1.04 | 1.06 | 1.11 | 1.03 | 1.06 | 1.06 | 1.09 | |
| Met | 0.27 | 0.28 | 0.27 | 0.28 | 0.29 | 0.27 | 0.26 | 0.27 | 0.26 | |
| Phe | 0.71 | 0.70 | 0.67 | 0.71 | 0.72 | 0.70 | 0.69 | 0.69 | 0.69 | |
| Thr | 0.67 | 0.65 | 0.66 | 0.68 | 0.67 | 0.65 | 0.66 | 0.68 | 0.69 | |
| Trp | 0.15 | 0.15 | 0.16 | 0.16 | 0.16 | 0.15 | 0.16 | 0.16 | 0.15 | |
| Val | 0.74 | 0.82 | 0.89 | 0.73 | 0.84 | 0.90 | 0.71 | 0.81 | 0.89 | |
| Dispensable amino acids, % | ||||||||||
| Ala | 0.71 | 0.69 | 0.66 | 0.69 | 0.72 | 0.69 | 0.69 | 0.70 | 0.68 | |
| Asx2 | 1.16 | 1.13 | 1.05 | 1.14 | 1.18 | 1.13 | 1.12 | 1.12 | 1.10 | |
| Cys | 0.23 | 0.24 | 0.23 | 0.26 | 0.25 | 0.24 | 0.24 | 0.24 | 0.24 | |
| Glx2 | 2.59 | 2.55 | 2.39 | 2.54 | 2.62 | 2.56 | 2.53 | 2.53 | 2.49 | |
| Gly | 1.90 | 1.86 | 2.04 | 1.89 | 1.77 | 1.79 | 1.85 | 1.76 | 1.69 | |
| Pro | 1.00 | 0.97 | 0.96 | 0.94 | 1.01 | 0.93 | 1.00 | 0.98 | 0.98 | |
| Ser | 0.55 | 0.54 | 0.51 | 0.54 | 0.57 | 0.55 | 0.55 | 0.56 | 0.55 | |
| Tyr | 0.40 | 0.40 | 0.38 | 0.41 | 0.42 | 0.41 | 0.41 | 0.41 | 0.40 | |
1Experimental diets were analyzed in duplicate.
2Asx, Asp, and Asn; Glx, Glu, and Gln.
Table 4.
Analyzed nutrient composition of experimental diets containing 300% SID Leu:Lys ratio, as-fed basis1
| SID Ile:Lys, % | 43 | 53 | 63 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | SID Val:Lys, % | 60 | 70 | 80 | 60 | 70 | 80 | 60 | 70 | 80 |
| Crude protein, % | 15.45 | 15.53 | 15.49 | 15.52 | 15.49 | 15.45 | 15.52 | 15.43 | 15.56 | |
| Indispensable amino acids, % | ||||||||||
| Arg | 0.71 | 0.69 | 0.69 | 0.79 | 0.76 | 0.75 | 0.76 | 0.76 | 0.76 | |
| His | 0.36 | 0.35 | 0.35 | 0.38 | 0.38 | 0.37 | 0.38 | 0.37 | 0.37 | |
| Ile | 0.49 | 0.49 | 0.49 | 0.62 | 0.62 | 0.58 | 0.69 | 0.68 | 0.67 | |
| Leu | 3.10 | 3.10 | 3.16 | 3.11 | 3.14 | 3.06 | 3.09 | 3.05 | 3.18 | |
| Lys | 1.17 | 1.11 | 1.10 | 1.13 | 1.06 | 0.97 | 1.03 | 1.00 | 1.12 | |
| Met | 0.26 | 0.29 | 0.30 | 0.28 | 0.30 | 0.28 | 0.32 | 0.31 | 0.25 | |
| Phe | 0.67 | 0.65 | 0.65 | 0.71 | 0.70 | 0.69 | 0.69 | 0.69 | 0.69 | |
| Thr | 0.69 | 0.63 | 0.63 | 0.67 | 0.66 | 0.64 | 0.66 | 0.61 | 0.72 | |
| Trp | 0.15 | 0.16 | 0.16 | 0.15 | 0.16 | 0.16 | 0.16 | 0.16 | 0.15 | |
| Val | 0.67 | 0.76 | 0.85 | 0.74 | 0.81 | 0.89 | 0.71 | 0.78 | 0.94 | |
| Dispensable amino acids, % | ||||||||||
| Ala | 0.67 | 0.65 | 0.66 | 0.71 | 0.69 | 0.69 | 0.69 | 0.69 | 0.70 | |
| Asx2 | 1.07 | 1.02 | 1.03 | 1.17 | 1.14 | 1.10 | 1.13 | 1.12 | 1.12 | |
| Cys | 0.22 | 0.23 | 0.23 | 0.27 | 0.26 | 0.25 | 0.26 | 0.24 | 0.24 | |
| Glx2 | 2.44 | 2.36 | 2.39 | 2.61 | 2.52 | 2.54 | 2.53 | 2.53 | 2.51 | |
| Gly | 0.74 | 0.68 | 0.63 | 0.70 | 0.67 | 0.62 | 0.66 | 0.62 | 0.58 | |
| Pro | 0.93 | 0.99 | 0.96 | 1.02 | 0.98 | 1.02 | 1.02 | 0.97 | 1.01 | |
| Ser | 0.56 | 0.49 | 0.51 | 0.56 | 0.55 | 0.55 | 0.56 | 0.55 | 0.57 | |
| Tyr | 0.39 | 0.38 | 0.38 | 0.42 | 0.41 | 0.40 | 0.41 | 0.41 | 0.41 | |
1Experimental diets were analyzed in duplicate.
2Asx, Asp, and Asn; Glx, Glu, and Gln.
There were no three-way interactions among the main effects. No effects of adding dietary Ile, Leu, or Val were observed for feed intake and N intake of pigs, but excess dietary Leu in diets increased (P < 0.05) urinary N excretion (Table 5). Fecal N excretion and ATTD of N were not affected by dietary Ile, Leu, or Val supplementations, but N retention (g/d and % of intake) and the biological value of protein were reduced (P < 0.05) as dietary Leu increased.
Table 5.
Main effects of dietary Leu, Ile, and Val concentrations on N balance of growing pigs, as-fed basis
| Main effect | SID Leu:Lys, % | SID Ile:Lys, % | SID Val:Lys, % | P-values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 100 | 300 | 43 | 53 | 63 | 60 | 70 | 80 | Pooled SEM | Leu | Ile | Val |
| Feed intake, g/d | 968 | 962 | 962 | 966 | 967 | 965 | 963 | 967 | 12 | 0.140 | 0.516 | 0.807 |
| N intake, g/d | 24.0 | 23.8 | 23.8 | 23.9 | 24.0 | 23.9 | 23.8 | 24.0 | 0.3 | 0.212 | 0.498 | 0.593 |
| N output in feces, g/d | 4.01 | 3.93 | 4.02 | 3.92 | 3.97 | 4.06 | 3.90 | 3.95 | 0.26 | 0.425 | 0.684 | 0.361 |
| N output in urine, g/d | 3.87 | 4.32 | 4.19 | 4.07 | 4.03 | 4.25 | 4.05 | 3.98 | 0.45 | 0.009 | 0.750 | 0.403 |
| ATTD of N, % | 83.2 | 83.5 | 83.1 | 83.6 | 83.4 | 83.0 | 83.6 | 83.5 | 1.1 | 0.535 | 0.565 | 0.374 |
| N retention, g/d | 16.1 | 15.6 | 15.6 | 16.0 | 16.0 | 15.6 | 15.9 | 16.0 | 0.6 | 0.024 | 0.356 | 0.236 |
| N retention, % | 67.1 | 65.3 | 65.5 | 66.6 | 66.6 | 65.2 | 66.6 | 66.9 | 2.1 | 0.041 | 0.488 | 0.212 |
| Biological value1, % | 80.6 | 78.2 | 78.8 | 79.7 | 79.8 | 78.6 | 79.6 | 80.1 | 2.2 | 0.008 | 0.620 | 0.346 |
1Biological value was calculated as [N retained/(N intake – N output in feces)] × 100 (Mitchell, 1924).
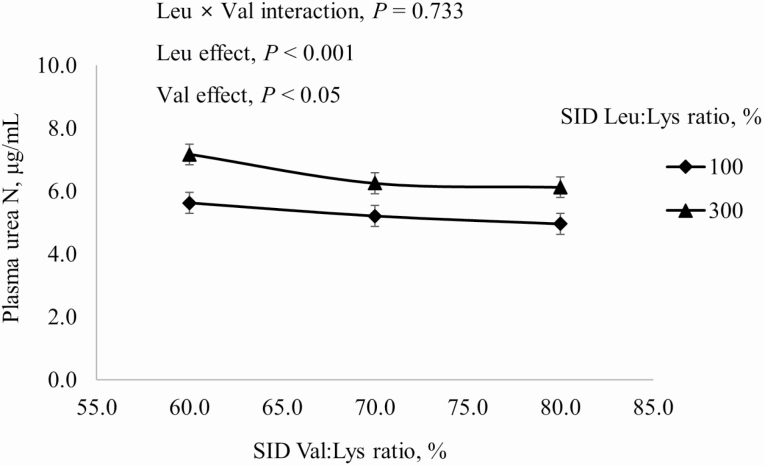
There were no three-way or two-way interactions among main effects on PUN and BCAA concentrations in the liver and skeletal muscle. Adding Leu to diets increased (P < 0.05) PUN, but PUN was reduced (P < 0.05) as dietary Val increased (Figure 1). Concentrations of Ile, Leu, and Val in the liver were greater (P < 0.05) in pigs fed excess Leu diets than in pigs fed diets where Leu was at the requirement, but concentrations of Ile, Leu, and Val in skeletal muscle were greater (P < 0.05) in pigs fed diets with Leu at the requirement than in pigs fed excess Leu diets (Table 6).
Figure 1.
Effects of dietary Leu and Val concentrations on PUN of growing pigs fed diets containing from 60% to 80% SID Val:Lys and from 100% to 300% SID Leu:Lys.
Table 6.
Main effects of dietary Ile, Leu, and Val concentrations on tissue BCAA concentrations of growing pigs, as-fed basis
| Main effect | SID Leu:Lys, % | SID Ile:Lys, % | SID Val:Lys, % | P-values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 100 | 300 | 43 | 53 | 63 | 60 | 70 | 80 | Pooled SEM | Leu | Ile | Val |
| Liver BCAA | ||||||||||||
| Ile, % | 2.72 | 2.92 | 2.86 | 2.81 | 2.80 | 2.81 | 2.82 | 2.83 | 0.08 | <0.001 | 0.195 | 0.905 |
| Leu, % | 5.32 | 5.60 | 5.51 | 5.45 | 5.42 | 5.77 | 5.47 | 5.47 | 0.13 | <0.001 | 0.208 | 0.853 |
| Val, % | 3.19 | 3.36 | 3.31 | 3.27 | 3.26 | 3.26 | 3.29 | 3.28 | 0.08 | <0.001 | 0.287 | 0.705 |
| Muscle BCAA | ||||||||||||
| Ile, % | 3.57 | 3.47 | 3.50 | 3.52 | 3.55 | 3.54 | 3.50 | 3.52 | 0.08 | <0.001 | 0.255 | 0.321 |
| Leu, % | 5.99 | 5.90 | 5.92 | 5.94 | 5.98 | 5.97 | 5.91 | 5.96 | 0.09 | 0.003 | 0.223 | 0.199 |
| Val, % | 3.54 | 3.29 | 3.35 | 3.50 | 3.39 | 3.46 | 3.34 | 3.44 | 0.28 | 0.006 | 0.405 | 0.540 |
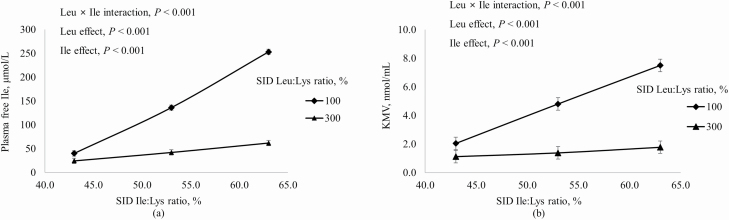
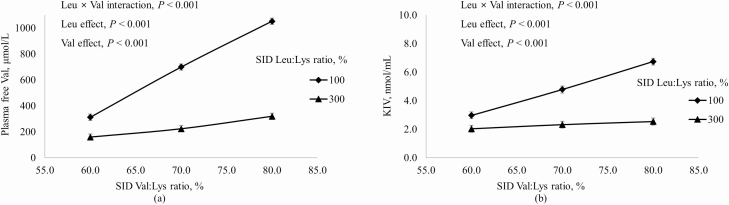
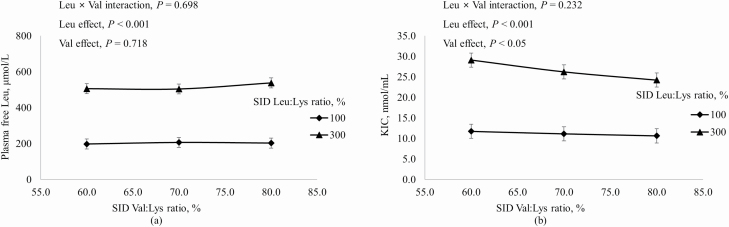
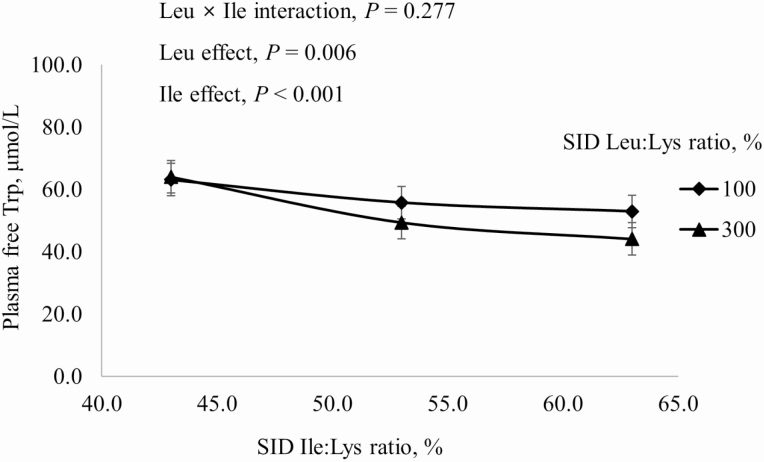
Increasing dietary Ile increased concentrations in plasma of free Ile and α-keto β-methylvalerate (KMV) more if dietary Leu was at the requirement than at 300% of the requirement (interaction, P < 0.001; Figure 2). Likewise, plasma-free Val and α-keto isovalerate (KIV) increased as dietary Val increased, but the magnitude of this increase was greater when dietary Leu was at the requirement than in excess of the requirement (interaction, P < 0.001; Figure 3). Increasing dietary Leu increased (P < 0.001) plasma-free Leu and plasma KIC, and increasing dietary Val increased (P < 0.001) plasma KIC, but not plasma-free Leu (Figure 4). Plasma-free Trp decreased (P < 0.05) as dietary Leu increased and plasma-free Trp also decreased (P < 0.001) as dietary Ile increased (Figure 5). However, plasma concentrations of all other AA were not influenced by dietary treatments.
Figure 2.
Effects of dietary Leu and Ile concentrations on (a) plasma-free Ile and (b) KMV (Ile metabolite) in plasma of growing pigs fed diets containing from 43% to 63% SID Ile:Lys and from 100% to 300% SID Leu:Lys.
Figure 3.
Effects of dietary Leu and Val concentrations on (a) plasma-free Val and (b) KIV (Val metabolite) in plasma of growing pigs fed diets containing from 60% to 80% SID Ile:Lys and from 100% to 300% SID Leu:Lys.
Figure 4.
Effects of dietary Leu and Val concentrations on (a) plasma-free Leu and (b) KIC (Leu metabolite) in plasma of growing pigs fed diets containing from 60% to 80% SID Ile:Lys and from 100% to 300% SID Leu:Lys.
Figure 5.
Effects of dietary Leu and Ile concentrations on plasma-free Trp of growing pigs fed diets containing from 43% to 63% SID Ile:Lys and from 100% to 300% SID Leu:Lys.
Discussion
The objective of the experiment was to test the hypothesis that increasing dietary Ile and Val may overcome the detrimental effects of excess Leu on N balance and metabolism of BCAA in growing pigs. Therefore, it was necessary to formulate basal diets that were co-limiting in Ile and Val to generate responses to increasing dietary Ile and Val supplementation. Spray-dried blood cells were used to formulate diets containing SID Ile:Lys ratio of 43%. Blood byproducts such as spray-dried blood cells are sometimes used in diets for nursery pigs because of their high protein quality (van Dijk et al., 2001; DeRouchey et al., 2002). However, most of the blood byproducts have imbalanced AA patterns because of very low Ile concentration (van Milgen et al., 2012), and blood byproducts have, therefore, been extensively used in dose–response experiments to determine the requirements for Ile (Parr et al., 2004; Wiltafsky et al., 2009; Htoo et al., 2014). In addition, soy protein concentrate was used because of its low Val concentration (NRC, 2012) and diets with a SID Val:Lys ratio of 60% could, therefore, be formulated.
Among the BCAA, only Leu stimulates the catabolism of BCAA in the liver (Harper et al., 1984). When diets for pigs contain excess Leu, metabolism of all three BCAA may increase by the stimulating effects of Leu or KIC on BCAA-degrading enzymes (Wiltafsky et al., 2010). Specifically, excess dietary Leu reduces pig feed intake and growth performance (Gatnau et al., 1995; Wiltafsky et al., 2010), which may be a consequence of the imbalanced supply of BCAA that results from increased metabolism of Val and Ile by excess Leu (Langer and Fuller, 2000). Feed intake may also be reduced by excess dietary Leu due to the reduced uptake of Trp in the brain and subsequently reduced synthesis of serotonin (Kwon et al., 2019). Likewise, the majority of the reduced growth performance that is caused by excess dietary Leu in diets fed to broiler chickens is a result of reduced feed intake (Calvert et al., 1982). In contrast, excess Leu and its metabolites KIC and β-hydroxy-β-methyl butyrate have the potential to promote protein synthesis by stimulating the mTOR signaling pathway in the brain (Columbus et al., 2015). Among BCAA, only Leu can stimulate protein synthesis in the cardiac and skeletal muscle by activation of mTOR followed by the upregulation of messenger ribonucleic acid (Escobar et al., 2006). However, a stimulating effect on the protein synthesis of prolonged infusion of Leu is dependent on the availability of other AA as substrates for protein synthesis (Wilson et al., 2010). Therefore, it is necessary that sufficient quantities of all AA needed for protein synthesis are provided for Leu to increase protein synthesis. Specifically, supplying Val and Ile is critical because plasma Val and Ile concentrations may be reduced by excess Leu supplementation (Yin et al., 2010; Suryawan et al., 2012). However, in most of the experiments, where the stimulation of muscle protein synthesis by Leu supplementation was observed, neonatal pigs (Escobar et al., 2006; Wilson et al., 2010; Suryawan et al., 2012) or weaned pigs at the age of 21 d (Yin et al., 2010) were used. Additional research, therefore, is needed to confirm the stimulating effect of excess dietary Leu on muscle protein synthesis in growing and finishing pigs.
The observation that excess dietary Leu reduced retention of N and the biological value of diets, which is indicative of reduced N utilization for protein deposition, is in agreement with previous data (Kwon et al., 2019). The negative effect of excess dietary Leu on protein deposition may be due to increased metabolism of the three BCAA because of increased expression of the two shared enzymes, BCAA aminotransferase and BCKDH (Kwon et al., 2019). Excess Leu, therefore, may result in a deficiency of Ile and Val for protein synthesis (Wiltafsky et al., 2010; Kwon et al., 2019). However, there was no impact of adding more Ile or Val on N retention even though the basal diets contained Ile and Val below requirements indicating that the capacity for N retention in the pigs was not affected by dietary concentrations of Ile and Val (Lordelo et al., 2008). Dietary concentrations of Ile or Val do not affect the AA composition of retained protein in pigs (Barea et al., 2009), but it is possible that the restricted feed allowance in the experiment limited N retention. Feed consumption was recorded for each pig during the adaptation period, and among the 18 dietary treatments, pigs fed the diets with 300% SID Leu:Lys ratio had the lowest feed consumption (data not shown). To minimize the confounding effects of feed intake reduction on N retention among treatments, all pigs were fed a restricted amount of feed during the collection period, which may have limited the ability of pigs to retain N (Dourmad and Etienne, 2002). The observed N retention, therefore, may be a result of the restricted feeding regime that was used in the experiment.
Results confirmed that excess dietary Leu reduced plasma-free Ile and Val concentrations, whereas increased plasma-free Leu concentration was observed. Thus, excess dietary Leu creates an imbalanced supply of Ile and Val for protein synthesis if dietary Ile and Val are close to the requirements. However, plasma-free Val concentrations were greater than in the experiment by Gloaguen et al. (2012) and do not indicate a Val deficiency. It is, however, possible that the plasma concentration of Val is related to the blood sampling time after feeding. Postprandial plasma AA profile largely depends on AA contents of test diets and rates of digestion, absorption, and first metabolism in splanchnic tissues (Gloaguen et al., 2012). It is, therefore, possible that the younger pigs used by Gloaguen et al. (2012) resulted in reduced absorption of AA compared with the pigs used in this experiment because pigs less than 20 kg have reduced digestibility of AA compared with older pigs (Pedersen et al., 2016).
The increased PUN that was observed as dietary Leu increased is most likely a result of increased catabolism of Ile and Val, which in turn reduced the availability of these AA for protein synthesis and caused an imbalance among other indispensable AA (Gatnau et al., 1995). A deficiency in Ile and Val may also have contributed to reduced protein synthesis as indicated by the reduced N retention, which may have resulted in increased deamination of other AA and a subsequent increase in PUN (Kwon et al., 2019). Whereas no effect of Ile supplementation on PUN was observed, the reduced PUN that was observed as dietary Val increased indicates that Val addition impacted the efficiency of AA utilization to a greater degree than increased Ile addition. The reason for the lack of a beneficial effect of Ile supplementation on PUN may be that the requirement for SID Ile is less than the 53% relative to SID Lys that was used in the formulation of the control diet. It is also possible that the reduced PUN was a result of different rates of transamination from BCAA to BCKA by BCAA aminotransferase (Harper et al., 1984). The Michaelis constant (Km) of BCAA aminotransferase is greater for Val than for Leu and Ile, which indicates Val has the lowest clearance rate from the plasma pool, whereas transamination of Leu and Ile proceeds more rapidly (Staten et al., 1984). In addition, transportation of Leu to the brain may be inhibited by Val and Trp, but is less affected by Ile (Hjelle et al., 1978; Hargreaves and Pardridge, 1988). It is also possible that Ile supplementation above the requirement impairs growth performance of pigs (Soumeh et al., 2014), but it is likely that greater excesses of Ile are needed to result in such a response. However, the addition of Val to a diet with excess Leu, but deficient in Ile, reduced the negative impact of excess dietary Leu on N utilization, whereas there was no effect of adding Ile to this diet (Langer and Fuller, 2000). In contrast, Wiltafsky et al. (2010) indicated that dietary Val did not influence the growth performance of pigs fed diets with excess Leu. It is, therefore, not clear exactly how Val affects interactions among BCAA.
Results of a recent meta-analysis indicated that a possible negative impact of Ile supplementation on growth performance may depend on BW or the growth rate of pigs, and increasing concentrations of dietary Val and Ile, alone or in combination, have the potential to alleviate the negative effects of excess dietary Leu on growth performance (Cemin et al., 2019b).
Tryptophan is a precursor for serotonin, which is a cerebral neurotransmitter that is important for appetite regulation (Zhang et al., 2007). Excess dietary Leu may reduce available Trp for serotonin synthesis in the brain due to competition for the shared transporter with BCAA, resulting in reduced voluntary feed intake in pigs (Henry et al., 1992). Therefore, it is possible that if dietary Leu is in excess of the requirement, extra dietary Trp may be needed to avoid a reduction in feed intake. In the present experiment, all diets were formulated to have a Trp-to-Lys ratio of 18%, which was supposed to exceed the requirement (NRC, 2012).
The observation that the liver Leu concentration increased as Leu was added to the diets likely reflects increased absorption of Leu that resulted in increased transportation of Leu from the small intestine to the liver via the hepatic portal vein. Concentrations of free AA in the liver were not analyzed and it is, therefore, not possible to determine if the extra Leu was in the form of free Leu or protein-bound Leu. However, because concentrations of Ile and Val did not increase as dietary Leu increased, it is unlikely that protein-bound Leu increased. Instead, the excess dietary Leu from the diets with 300% SID Leu:Lys was likely exported to the muscle for deamination and conversion to KIC. However, the Leu concentration in the muscle decreased as dietary Leu increased, but this is likely because of reduced protein synthesis in pigs fed the diets with excess Leu as indicated by the reduction in N retention.
The first step in the catabolism of BCAA, which is catalyzed by BCAA aminotransferase, results in the synthesis of the three corresponding BCKA, KIV, KIC, and KMV, from Ile, Leu, and Val, respectively (Harris et al., 2005). If excess Leu is included in diets for pigs, the catabolism of all three BCAA may increase because of increased activities of BCAA aminotransferase, but the greater activity of the transamination enzyme may produce more KIC, which activates BCKDH, and, therefore, changes the concentrations of BCKA in plasma. The changes in BCAA and BCKA that were observed as dietary Leu increased are in agreement with Langer et al. (2000) who reported that excess dietary Leu reduced the plasma concentrations of Ile and Val, as well as of KIV, KIC, and KMV. In addition, whole-body Val oxidation was increased due to increasing BCKDH activity. In rats, dietary supplementation of KIC to a low-protein diet resulted in increased KIC in plasma, whereas the concentrations of KIV and KMV were reduced (Crowell et al., 1990). However, the current data indicated that adding more Val to the diets reduced KIC in plasma regardless of dietary Leu concentration, which may be due to reduced activation of BCKDH, which in turn prevented the metabolism of KIV and KMV. Because KIV and KMV can be reaminated to form Ile and Val, a reduced metabolism of KIV and KMV may negate some of the negative effects of excess Leu. These observations are in agreement with data indicating that excess dietary Leu greatly decreased the overall growth performance of pigs, whereas increasing Val in diets with excess Leu alleviated the reductions of average daily gain and gain-to-feed ratio (Gloaguen et al., 2011; Millet et al., 2015). Thus, the current data indicating that dietary Val can partly ameliorate the effects of excess Leu are in agreement with data from previous research. It is, therefore, possible that excess dietary Leu may change the Val requirement of growing pigs.
Conclusions
Decreased N retention and biological value of diets and the increased PUN that were observed as dietary Leu increased indicate that excess dietary Leu increased the catabolism of Val and Ile and created an AA imbalance with reduced protein synthesis as a consequence. However, Val supplementation may increase the efficiency of AA utilization for protein synthesis in pigs fed diets with excess Leu as indicated by the reduced PUN that was observed as dietary Val increased. Concentrations of BCAA in the skeletal muscle and liver, and profiles of BCAA and their metabolites in plasma, were changed because of excess dietary Leu, but Val supplementation decreased the concentrations of KIC, which is considered the key regulator of the BCAA catabolic process. Overall, it appears that excess dietary Leu may have negative impacts on N balance and metabolism of BCAA, but Val supplementation may partially overcome the negative impacts of excess dietary Leu.
Acknowledgment
The financial support from Ajinomoto Animal Nutrition North America, Inc., Chicago, IL is greatly appreciated.
Glossary
Abbreviations
- AA
amino acids
- ATTD
apparent total tract digestibility
- BCAA
branched-chain amino acids
- BCKA
branched-chain α-keto acids
- BCKDH
branched-chain α-keto acid dehydrogenase
- BW
body weight
- KIC
α-keto isocaproate
- KIV
α-keto isovalerate
- KMV
α-keto β-methylvalerate
- mTOR
mechanistic target of rapamycin
- PUN
plasma urea N
- SID
standardized ileal digestible
Conflict of interest statement
J.A.S. is an employee of Ajinomoto Animal Nutrition North America, Inc., Chicago, IL. W.B.K. and H.H.S. have no conflicts of interest.
Literature Cited
- Adeola O. 2001. Digestion and balance techniques in pigs. In: Lewis A. J., and Southern L. L., editors. Swine nutrition. Washington (DC): CRC Press; p. 903–916. [Google Scholar]
- AOAC Int. 2007. Official methods of analysis. 18th ed. Rev. 2nd ed. In: Howitz W., and Latimer G. W. Jr, editors. Gaithersburg: (MD: ): AOAC International. [Google Scholar]
- Barea R., Brossard L., Le Floc’h N., Primot Y., Melchior D., and van Milgen J.. . 2009. The standardized ileal digestible valine-to-lysine requirement ratio is at least seventy percent in postweaned piglets. J. Anim. Sci. 87:935–947. doi: 10.2527/jas.2008-1006 [DOI] [PubMed] [Google Scholar]
- Beals J. W., Sukiennik R. A., Nallabelli J., Russell S. E., van Vliet S., Young J. R., Ulanov A. V., Li Z., Paluska S. A., De Lisio M., . et al. 2016. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am. J. Clin. Nutr. 104:1014–1022. doi: 10.3945/ajcn.116.130385 [DOI] [PubMed] [Google Scholar]
- Calvert C. C., Klasing K. C., and Austic R. E.. . 1982. Involvement of food intake and amino acid catabolism in the branched-chain amino acid antagonism in chicks. J. Nutr. 112:627–635. doi: 10.1093/jn/112.4.627 [DOI] [PubMed] [Google Scholar]
- Cemin H. S., Tokach M. D., Dritz S. S., Woodworth J. C., DeRouchey J. M., and Goodband R. D.. . 2019b. Meta-regression analysis to predict the influence of branched-chain and large neutral amino acids on growth performance of pigs. J. Anim. Sci. 97:2505–2514. doi: 10.1093/jas/skz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemin H. S., Tokach M. D., Woodworth J. C., Dritz S. S., DeRouchey J. M., and Goodband R. D.. . 2019a. Branched-chain amino acid interactions in growing pigs. Transl. Anim. Sci. 3:1246–1253. doi: 10.1093/tas/txz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus D. A., Fiorotto M. L., and Davis T. A.. . 2015. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 47:259–270. doi: 10.1007/s00726-014-1866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D., Proulx K., Smith K. A. B., Kozma S. C., Thomas G., Woods S. C., and Seeley R. J.. . 2006. Hypothalamic mTOR signaling regulates food intake. Science. 312:927–930. doi: 10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- Crowell P. L., Block K. P., Repa J. J., Torres N., Nawabi M. D., Buse M. G., and Harper A. E.. . 1990. High branched-chain alpha-keto acid intake, branched-chain alpha-keto acid dehydrogenase activity, and plasma and brain amino acid and plasma keto acid concentrations in rats. Am. J. Clin. Nutr. 52:313–319. doi: 10.1093/ajcn/52.2.313 [DOI] [PubMed] [Google Scholar]
- DeRouchey J. M., Tokach M. D., Nelssen J. L., Goodband R. D., Dritz S. S., Woodworth J. C., and James B. W.. . 2002. Comparison of spray-dried blood meal and blood cells in diets for nursery pigs. J. Anim. Sci. 80:2879–2886. doi: 10.2527/2002.80112879x [DOI] [PubMed] [Google Scholar]
- van Dijk A. J., Everts H., Nabuurs M. J. A., Margry R. J. C. F., and Beynen A. C.. . 2001. Growth performance of weanling pigs fed spray-dried animal plasma: a review. Livest. Prod. Sci. 68:263–274. doi: 10.1016/S0301-6226(00)00229-3 [DOI] [Google Scholar]
- Dourmad J. Y., and Etienne M.. . 2002. Dietary lysine and threonine requirements of the pregnant sow estimated by nitrogen balance. J. Anim. Sci. 80:2144–2150. doi: 10.1093/ansci/80.8.2144 [DOI] [PubMed] [Google Scholar]
- Duan Y. H., Zeng L. M., Li F. N., Li Y. H., Tan B. E., Ji Y. J., Kong X. F., Tang Y. L., Zhang Y. Z., and Yin Y. L.. . 2016. Effects of dietary branched-chain amino acid ratio on growth performance and serum amino acid pool of growing pigs. J. Anim. Sci. 94:129–134. doi: 10.2527/jas2015-9527 [DOI] [Google Scholar]
- Escobar J., Frank J. W., Suryawan A., Nguyen H. V., Kimball S. R., Jefferson L. S., and Davis T. A.. . 2006. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 290:E612–E621. doi: 10.1152/ajpendo.00402.2005 [DOI] [PubMed] [Google Scholar]
- Gatnau R., Zimmerman D. R., Nissen S. L., Wannemuehler M., and Ewan R. C.. . 1995. Effects of excess dietary leucine and leucine catabolites on growth and immune responses in weanling pigs. J. Anim. Sci. 73:159–165. doi: 10.2527/1995.731159x [DOI] [PubMed] [Google Scholar]
- Gloaguen M., Le Floc’h N., Brossard L., Barea R., Primot Y., Corrent E., and van Milgen J.. . 2011. Response of piglets to the valine content in diet in combination with the supply of other branched-chain amino acids. Animal 5:1734–1742. doi: 10.1017/S1751731111000760 [DOI] [PubMed] [Google Scholar]
- Gloaguen M., Le Floc’h N., Corrent E., Primot Y., and van Milgen J.. . 2012. Providing a diet deficient in valine but with excess leucine results in a rapid decrease in feed intake and modifies the postprandial plasma amino acid and α-keto acid concentrations in pigs. J. Anim. Sci. 90:3135–3142. doi: 10.2527/jas2011-4956 [DOI] [PubMed] [Google Scholar]
- Hargreaves K. M., and Pardridge W. M.. . 1988. Neutral amino-acid transport at the human blood-brain barrier. J. Biol. Chem. 263:19392–19397. [PubMed] [Google Scholar]
- Harper A. E., Miller R. H., and Block K. P.. . 1984. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 4:409–454. doi: 10.1146/annurev.nu.04.070184.002205 [DOI] [PubMed] [Google Scholar]
- Harris R. A., Joshi M., Jeoung N. H., and Obayashi M.. . 2005. Overview of the molecular and biochemical basis of branched chain amino acid catabolism. J. Nutr. 135:1527S–1530S. doi: 10.1093/jn/135.6.1527S [DOI] [PubMed] [Google Scholar]
- Henry Y., Sève B., Colléaux Y., Ganier P., Saligaut C., and Jégo P.. . 1992. Interactive effects of dietary levels of tryptophan and protein on voluntary feed intake and growth performance in pigs, in relation to plasma free amino acids and hypothalamic serotonin. J. Anim. Sci. 70:1873–1887. doi: 10.2527/1992.7061873x [DOI] [PubMed] [Google Scholar]
- Hjelle J. T., Baird-Lambert J., Cardinale G., Specor S., and Udenfriend S.. . 1978. Isolated microvessels: the blood-brain barrier in vitro. Proc. Natl. Acad. Sci. U. S. A. 75:4544–4548. doi: 10.1073/pnas.75.9.4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htoo J. K., Zhu C. L., Huber L., de Lange C. F., Quant A. D., Kerr B. J., Cromwell G. L., and Lindemann M. D.. . 2014. Determining the optimal isoleucine:lysine ratio for ten- to twenty-two-kilogram and twenty-four- to thirty-nine-kilogram pigs fed diets containing nonexcess levels of leucine. J. Anim. Sci. 92:3482–3490. doi: 10.2527/jas.2013-6934 [DOI] [PubMed] [Google Scholar]
- Kwon W. B., Touchette K. J., Simongiovanni A., Syriopoulos K., Wessels A., and Stein H. H.. . 2019. Excess dietary leucine in diets for growing pigs reduces growth performance, biological value of protein, protein retention, and serotonin synthesis. J. Anim. Sci. 97:4282−4292. doi: 10.1093/jas/skz259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S., and Fuller M. F.. . 2000. Interactions among the branched-chain amino acids and their effects on methionine utilization in growing pigs: effects on nitrogen retention and amino acid utilization. Br. J. Nutr. 83:43–48. doi: 10.1017/S0007114500000076 [DOI] [PubMed] [Google Scholar]
- Langer S., Scislowski P. W., Brown D. S., Dewey P., and Fuller M. F.. . 2000. Interactions among the branched-chain amino acids and their effects on methionine utilization in growing pigs: effects on plasma amino- and keto-acid concentrations and branched-chain keto-acid dehydrogenase activity. Br. J. Nutr. 83:49–58. doi: 10.1017/S0007114500000088 [DOI] [PubMed] [Google Scholar]
- Lordelo M. M., Gaspar A. M., Le Bellego L., and Freire J. P.. . 2008. Isoleucine and valine supplementation of a low-protein corn-wheat-soybean meal-based diet for piglets: growth performance and nitrogen balance. J. Anim. Sci. 86:2936–2941. doi: 10.2527/jas.2007-0222 [DOI] [PubMed] [Google Scholar]
- van Milgen J., Gloaguen M., Le Floc’h N., Brossard L., Primot Y., and Corrent E.. . 2012. Meta-analysis of the response of growing pigs to the isoleucine concentration in the diet. Animal 6:1601–1608. doi: 10.1017/S1751731112000420 [DOI] [PubMed] [Google Scholar]
- Millet S., Aluwé M., Ampe B., and de Campeneere S.. . 2015. Interaction between amino acids on the performance of individually housed piglets. J. Anim. Physiol. Anim. Nutr. 99:230236. doi: 10.1111/jpn.12227 [DOI] [PubMed] [Google Scholar]
- Mitchell H. H. 1924. A method of determining the biological value of protein. J. Biol. Chem. 58:873–903. [Google Scholar]
- Morales A., Arce N., Cota M., Buenabad L., Avelar E., Htoo J. K., and Cervantes M.. . 2016. Effect of dietary excess of branched-chain amino acids on performance and serum concentrations of amino acids in growing pigs. J. Anim. Physiol. Anim. Nutr. 100:39–45. doi: 10.1111/jpn.12327 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academies Press. [Google Scholar]
- Parr T. M., Kerr B. J., and Baker D. H.. . 2004. Isoleucine requirement for late-finishing (87 to 100 kg) pigs. J. Anim. Sci. 82:1334–1338. doi: 10.2527/2004.8251334x [DOI] [PubMed] [Google Scholar]
- Pedersen C., Almeida J. S., and Stein H. H.. . 2016. Analysis of published data for standardized ileal digestibility of protein and amino acids in soy proteins fed to pigs. J. Anim. Sci. 94:340–343. doi: 10.2527/jas2015-9864 [DOI] [Google Scholar]
- Pedersen C., Boersma M. G., and Stein H. H.. . 2007. Energy and nutrient digestibility in NutriDense corn and other cereal grains fed to growing pigs. J. Anim. Sci. 85:2473–2483. doi: 10.2527/jas.2006-620. [DOI] [PubMed] [Google Scholar]
- Soumeh E. A., van Milgen J., Sloth N. M., Corrent E., Poulsen H. D., and Nørgaard J. V.. . 2014. The optimum ratio of standardized ileal digestible isoleucine to lysine for 8–15 kg pigs. Anim. Feed Sci. Technol. 198:158–165. doi: 10.1016/j.anifeedsci.2014.09.013 [DOI] [Google Scholar]
- Staten M. A., Bier D. M., and Matthews D. E.. . 1984. Regulation of valine metabolism in man: a stable isotope study. Am. J. Clin. Nutr. 40:1224–1234. doi: 10.1093/ajcn/40.6.1224 [DOI] [PubMed] [Google Scholar]
- Suryawan A., Torrazza R. M., Gazzaneo M. C., Orellana R. A., Fiorotto M. L., El-Kadi S. W., Srivastava N., Nguyen H. V., and Davis T. A.. . 2012. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr. Res. 71:324–331. doi: 10.1038/pr.2011.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A. G., Kluge H., Hirche F., Kiowski A., Schutkowski A., Corrent E., Bartelt J., König B., and Stangl G. I.. . 2016. High leucine diets stimulate cerebral branched-chain amino acid degradation and modify serotonin and ketone body concentrations in a pig model. PLoS One 11:e0150376. doi: 10.1371/journal.pone.0150376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F. A., Suryawan A., Gazzaneo M. C., Orellana R. A., Nguyen H. V., and Davis T. A.. . 2010. Stimulation of muscle proteinsynthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J. Nutr. 140:264–270. doi: 10.3945/jn.109.113621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltafsky M. K., Bartelt J., Relandeau C., and Roth F. X.. . 2009. Estimation of the optimum ratio of standardized ileal digestible isoleucine to lysine for eight- to twenty-five-kilogram pigs in diets containing spray-dried blood cells or corn gluten feed as a protein source. J. Anim. Sci. 87:2554–2564. doi: 10.2527/jas.2008-1320 [DOI] [PubMed] [Google Scholar]
- Wiltafsky M. K., Pfaffl M. W., and Roth F. X.. . 2010. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 103:964–976. doi: 10.1017/S0007114509992212 [DOI] [PubMed] [Google Scholar]
- Yin Y., Yao K., Liu Z., Gong M., Ruan Z., Deng D., Tan B., Liu Z., and Wu G.. . 2010. Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486. doi: 10.1007/s00726-010-0612-5 [DOI] [PubMed] [Google Scholar]
- Zhang H., Yin J., Li D., Zhou X., and Li X.. . 2007. Tryptophan enhances ghrelin expression and secretion associated with increased food intake and weight gain in weanling pigs. Domest. Anim. Endocrinol. 33:47–61. doi: 10.1016/j.domaniend.2006.04.005 [DOI] [PubMed] [Google Scholar]