Abstract
Background
Infection with SARS-CoV-2 is responsible for the COVID-19 crisis affecting the whole world. This virus can provoke acute respiratory distress syndrome (ARDS) leading to overcrowed the intensive care unit (ICU). Over the last months, worldwide experience demonstrated that the ARDS in COVID-19 patients are in many ways “atypical”. The mortality rate in ventilated patients is high despite the application of the gold standard treatment (protective ventilation, curare, prone position, inhaled NO). Several studies suggested that the SARS-CoV-2 could interact negatively on red blood cell homeostasis. Furthermore, SarsCov2 creates Reactive Oxygen Species (ROS), which are toxic and generate endothelial dysfunction. Hypothesis/objective(s)
We hypothesis that HEMO2Life® administrated intravenously is safe and could help symptomatically the patient condition. It would increase arterial oxygen content despite lung failure and allow better tissue oxygenation control. The use of HEMO2Life® is also interesting due to its anti-oxidative effect preventing cytokine storm induced by the SARS-CoV-2. Evaluation of the hypothesis: Hemarina is based on the properties of the hemoglobin of the Arenicola marina sea-worm (HEMO2Life®). This extracellular hemoglobin has an oxygen capacity 40 times greater than the hemoglobin of vertebrates. Furthermore, the size of this molecule is 250 times smaller than a human red blood cell, allowing it to diffuse in all areas of the microcirculation, without diffusing outside the vascular sector. It possesses an antioxidative property du a Superoxide Dismutase Activity. This technology has been the subject of numerous publications and HEMO2Life® was found to be well-tolerated and did not induce toxicity. It was administered intravenously to hamsters and rats, and showed no acute effect on heart rate and blood pressure and did not cause microvascular vasoconstriction. In preclinical in vivo models (mice, rats, and dogs), HEMO2Life® has enabled better tissue oxygenation, especially in the brain. This molecule has already been used in humans in organ preservation solutions and the patients showed no abnormal clinical signs.
Consequences of the hypothesis
The expected benefits of HEMO2Life® for COVID-19 patients are improved survival, avoidance of tracheal intubation, shorter oxygen supplementation, and the possibility of treating a larger number of patients as molecular respirator without to use an invasive machine.
Keywords: Hypoxemia, COVID-19, Oxygen Carrier, HEMO2Life, M101, SARS-CoV-2
Background
The world has changed, suddenly and unexpectedly because of COVID-19 [1] onset. What began as a regional health crisis in late 2019 had, by June of 2020, mushroomed into a global calamity the likes of which had not been seen for a century, touching virtually every aspect of modern life and endangering countless lives, along with the entire global economy. Globally, as of 6 November 2020, there have been 48,534,508 confirmed cases of COVID-19, including 1,231,017 deaths [2], reported to the World Health Organization (WHO). These numbers continue to grow every day.
Initially, the Surviving Sepsis Campaign panel [3] recommended “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the Intensive Care Unit (ICU).” Gattinotti et al. stated that COVID-19 pneumonia [4], despite falling in most of the circumstances under the Berlin definition of Acute Respiratory Distress Syndrome (ARDS) [1], is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance [5].
Over the last 5 months, worldwide experiences demonstrated that the ARDS in COVID-19 patients are in many ways “atypical”. The mortality rate in ventilated patients is high [6] despite the applying the gold standard treatment (protective ventilation, curare, prone position, inhaled Nitric Oxide (NO)). Furthermore, Guiseppe et al., revealed a decrease of hemoglobin content in some patients [7]. Indeed, the mean hemoglobin difference of the four individual studies reporting continuous values of this parameter showed that the hemoglobin value was significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a Weighted Mean Difference (WMD) of −7.1 g/L; 95% CI, −8.3 to −5.9 g/L). Several elements suggested that the SARS-CoV-2 could interact negatively on red blood cell homeostasis. Firstly, direct signs seem to confirm this theory as the modification of O2 concentration in the bloodstream by using a hyperbaric oxygen therapy report a condition improvement in severe COVID-19 patients (“Naval Specialty Medical Center Program Team” document uploaded in the attachment section).
Indirect signs also support this interaction as acidosis, high level of lactate, anemia, a high level of ferritin [8] in severe patients are correlated with a high mortality rate.
Therefore, an important physiopathology feature is not thoroughly taken into account: “the oxygenation component”. We suggest and provide to the physicians a new therapeutic tool to help to struggle symptomatically the hypoxemia with a Natural Oxygen carrier off-shelf, HEMO2Life®, also called M101.
The hypothesis
We hypothesize that the intravenous injection of HEMO2Life® in cases of acute respiratory failure, attributable to COVID-19, would allow an improvement in oxygen transport to the tissues and that this could avoid the progression to multiorgan failure in case of persistence or worsening of hypoxemia.
This molecule has been administered on human for transplantation as an additive in conservative solution [9], but never directly intravenously (IV), this study revealed the safety of this product considered as a medical device of class III. However, several published preclinical data showed an effective way to transfer oxygen to hypoxia, in particular to the brain, in a study published in the journal of Neurotrauma by the Naval Medical Research Centers on Trauma brain injury animal model. This work was accomplished across a Cooperative Research and Development Agreement (CRADA) with the US Navy [10]. The use of HEMO2Life® is also interesting due to its anti-oxidative effect preventing cytokine storm induced by the SARS-CoV-2. Indeed, HEMO2Life® has a superoxide-dismutase activity that can address this problem and a recent Canadian study revealed an anti-IL6 action during lung transplantation [11].
Evaluation of the hypothesis
The first reason to use HEMO2Life® is because of its oxygen carrier properties with the main hypothesis that it can improve tissue oxygenation without modifying ventilation for COVID-19 patients. HEMO2Life® is composed by an extracellular hemoglobin coming from the lugworm Arenicola marina. This extracellular hemoglobin has an oxygen capacity 40 times higher than the HbA of vertebrates. Furthermore, the size of this molecule is 250 times smaller than a human red blood cell, allowing it to diffuse in all areas of the microcirculation, without diffusing outside the vascular sector. This product has been the subject of numerous publications [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. This worm is farmed in aquaculture in a very strict good manufacturer practice conditions under ISO-13485 regulation. This molecule is composed of 156 globin chains and 42 linker chains for a molecular weight of 3.6 MDa (Mega Dalton). The quaternary structure of this molecule is a hexagonal-bilayer with a dimension of 25 nm (face view) and 15 nm (profil view) [13]. Each globin chain has a heme group similar to human and the linker chains possess an anti-oxidative property due to a Superoxide Dismutase Activity (SOD) activity-like based on copper and zinc [15]. Thus, HEMO2Life® can carry up to 156 molecules of O2. Oxygen is released against a gradient in the absence of allosteric effectors, providing the environment with just the right amount of O2; is active across a wide range of temperature (from 4 °C to 37 °C) [25], [26].
We showed that this molecule does not have immunogenic effect, neither an allergenic effect. Its oxygen affinity is p50 = 7.5 mm of Hg and a cooperativity of 2.5 [14] and it does not need cofactor in order to release oxygen, these parameters are similar to the HbA inside the red blood cells [12].
O2 releasing is just done in a partial oxygen gradient, when po2 is below the p50, the oxygen is released passively to the tissues, and consumed by cells or tissues, avoiding oxidative damages. Two important points, the p50 of the myoglobin is 2.6 mm of Hg, so below the p50 of this oxygen carrier and even this molecule is high oxygen affinity the p50 is similar of the hemoglobin A (HbA) inside the red blood cell [15]. HEMO2Life® has a red color, it is sterile, is pyrogen-free and frozen at −20° C (+/3° C). HEMO2Life® is packaged in 1 g vials for a volume of 20 mL at a concentration of 50 mg/mL. The composition of a 20 mL bottle of HEMO2Life® e is 1 g of an active substance (extracellular hemoglobin from Arenicola marina), 203.3 mg of Magnesium chloride, 105.2 mg of sodium chloride, 100.3 mg of sodium gluconate, 73.5 mg of sodium acetate, 7.5 mg of potassium chloride, 7.3 mg of calcium chloride, ≤ 35.2 mg of ascorbic acid, 20 mg of water for injection. The half-life is 48–72 h, so the washout period is maximum 4 days. The evaluation of the dissociation of HEMO2Life® was carried out in vitro under human physiological conditions. HEMO2Life® was incubated at 37° C in human plasma for 96 h in a controlled CO2 atmosphere (5% CO2/95% air ambient) [12]. The structure of the active substance HEMO2Life® was followed by high-performance liquid chromatography at 414 nm and the area under the curve was calculated [14]. Two independent studies have been carried out. HEMO2Life® remained present and functional for 96 h with a half-life of 36 h [21], [22]. Analysis reveals that its dissociation is directly correlated to the formation of proteins not containing heme (apoproteins): their molecular weight (<150 kDa) and the recovery by human serum albumin (HSA) of hemin (free oxidized heme) to form metallic albumin, prevent any toxic side effect related to free hemin. The globin chain structure of HEMO2Life® is very similar to that of humans. We showed that there was no interaction between HEMO2Life® and hemopexin, an important plasma protein in the clearance of hemolyzed haemoglobin [12]. In this same article, chelation of heme by HSA has also been shown. This molecule being close to human hemoglobin, it is metabolized by the liver and the spleen. Otherwise, the SARS-CoV-2 is an enveloped positive-sense RNA virus replicating in the DNA in the nuclei of the target cells [27]. The DNA contain in the nucleus of the red blood cells progenitor’s nucleus is therefore probably one of the cells targeted by the virus, and this explains leucoerythroblastic reaction described in patient with COVID‐19 infection [28]. Few studies to date have provided detailed blood usage in COVID‐19 patients. It has been put forward that hospitalized COVID‐19 patients required fewer blood transfusions than other hospitalized patients [29]. Data from Italy showed that 39% of patients required transfusion (median duration of hospitalization of 15 days) for the main indication of anemia (non-bleeding), with very few patients requiring platelets or plasma [30]. If the hypothesis of an improvement in oxygen transport by transfusion has been put forward [31], we did not find in our literature review any comparative study specifically looking at the role of treatment by increasing oxygen transport by blood transfusion in COVID-19 patients. Only a case report from the early days of the pandemic reports improved in a patient after a blood transfusion [32]. In July 2020, a Lancet study [33] stated that there is no data available to inform whether patients with SARS-CoV-2 infection, with substantial respiratory symptoms and oxygen dependency, might benefit from red blood cell transfusion to maintain a hemoglobin concentration above 70 g/L. Convalescence plasma is a treatment strategy that has been further studied. However, the results are disappointing with no reduction in the progression of the virus to a severe form [34], [35].
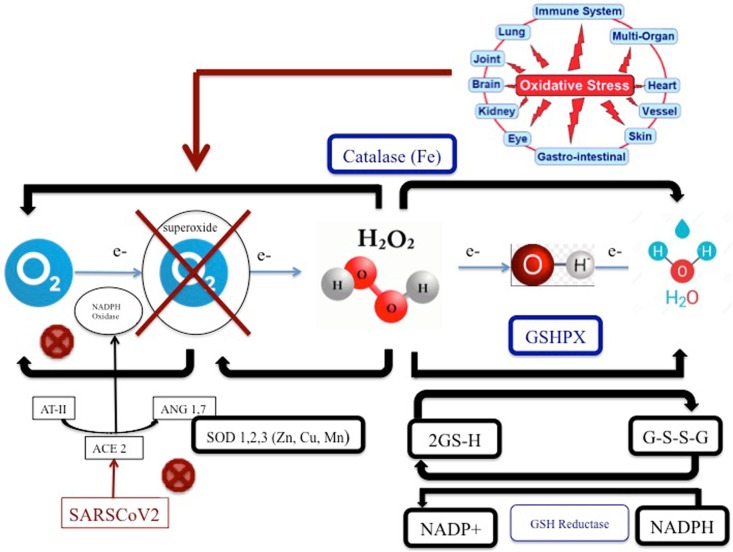
HEMO2Life® is not contained in a cell nucleus and therefore, it will not be a target since the COVID-19 will not recognized this oxygen carrier not contained in red blood cells. In the paper of Liu Wenzhong et al. [36], it seems that the virus must hang on the red blood cell with more affinity of AB blood typing which will be not possible with an extracellular hemoglobin. Consequently, this molecule seems to be well adapted to deliver oxygen in order to avoid hypoxia responsible of dyspnea while avoiding being targeted by virus. Another reason to use HEMO2Life® is related to its properties to reduce oxidative stress. Indeed, SarsCov2 has an action on Angiotensin Converting Enzyme (ACE) receptor [37], [38]. By bidding on ACE receptor, the virus inhibits the transformation of Angiotensin II to Angiotensin 1,7. This last one is fundamental to NADPH oxidase. The system is the overload with e- (oxidative) and creates Reactive Oxygen Species (ROS), which are toxic and generate endothelial dysfunction. HEMO2Life®, by its SOD properties can reverse this phenomenon by changing O2° to O2 or H2O2. HEMO2Life® has also an action on Fe and then can potentially stimulate Catalase (see Fig. 1 ). In the past months, several publications have appeared in different countries regarding the characteristics of patients who become seriously ill. COVID-19 causes prolonged and progressive hypoxia due to anemia, coagulopathy, thrombosis and multi organ failure [39]. A retrospective cohort study coming from Wuhan and published in the Lancet Journal is very instructive on a clinical panel of these patients [6]. Damage to the lungs on radiographic scans may come from the release of oxidative iron by heme groups, which overwhelms the natural defenses against pulmonary oxidative stress and causes bilateral ground glass opacities, high level of ferritin is also found in non-survivor patient from COVID-19 1435.3 (728.9–2000) against 503.2 (264.0–921.5) for the survivor. The goal of this protein is to detoxify the free heme in circulation, which could provoke high level of inflammation. Indeed, when iron ions are stripped of their hemoglobin, intubation for ventilation is futile in treating patients and does not attack the root of the disease and free iron could be responsible of cytokine storm due to its very high pro-oxidant activity [40]. Patients returning for rehospitalization days or weeks after recovery and suffering from delayed post-hypoxic leuko-encephalopathy [41] reinforce the notion that COVID-19 patients suffer from hypoxia despite the absence of signs of respiratory fatigue or exhaustion. This clinical panel is called “happy hypoxia” and patients could collapse in rapid way if nothing is done.
Fig. 1.
Pathophysiology of the oxidative stress reducing activity of HEMO2Life®. ACE: Angiotensin Converting Enzyme; ANG 1.7: Angiotensin (1–7); AT-II: alveolar Type II Epithelial Cell; e-: electron Fe: iron; GSH: Reduced glutathione; GSHPX: Glutathione; Peroxidase G-S-S-G: Glutathione disulfide; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; H2O: water; H 20 2: Hydrogen peroxide; NADP: Nicotinamide adenine dinucleotide phosphate; NADPH: Nicotinamide adenine dinucleotide phosphate carrying electrons and bonded with a hydrogen; SOD: Superoxide dismutase activity.
Tissue hypoxia, although it has been rarely evaluated in the literature, could represent an interesting complementary evaluation measure. Indeed, a recent study assesses the presence of sublingual microcirculatory and skin perfusion alterations in COVID-19 pneumonia and reveal that COVID-19 patients showed altered tissue perfusion [42]. Hypoxia of this component could be further evaluated using subcutaneous probes. Furthermore, in another Lancet study 15% of the patients will require essential care of critical illness or oxygen [43]. Red blood cells carry oxygen from the lungs to all organs and the rest of the body. Red blood cells can do this thanks to hemoglobin, a protein made up of four “hemes” [44]. Hemes have a special type of iron ion, which is usually quite toxic in its free form, enclosed in its center with a porphyrin acting as its “container”. In this way, the iron ion can be caged and transported safely by hemoglobin but used to bind oxygen when it reaches the lungs. When the red blood cells reach the alveoli where all gas exchange occurs, this small iron ion switches between the Fe2+ to Fe3+ states and binds to oxygen, then leaves to deliver the O2 elsewhere. Once the body has gotten out of control, with all the oxygen carriers circulating in a vacuum and tons of the toxic form of iron floating in the bloodstream [45], [46], other defenses are triggered. While the lungs are occupied with all of this oxidative stress that they cannot handle, the organs are starving for O2 and the liver is trying its best to remove and store iron [47]. However, this organ is overwhelmed and starving for oxygen too and releases an enzyme called alanine aminotransferase (ALT). The patient's immune system does not fight the virus in time before its oxygen saturation in the blood drops too low, ventilator or not, the organs start to stop. The only way to try to keep them alive is the maximum amount of oxygen, and perhaps the best would be with a hyperbaric chamber [48], if available, with 100% oxygen at several atmospheres of pressure, just to give functional hemoglobin a chance to transport enough oxygen to the organs and keep them alive. There is not enough hyperbaric chamber and it is currently estimated that more than 1,350 hospitals in the US offer Hyperbaric Oxygen Therapy (HBOT) services and Medicare covers HBOT for more than a dozen conditions [49]. Furthermore, HBOT could create oxidative damages since O2 needs to be delivered to the patients in a physiological way, not the case with HBOT at the opposite of HEMO2Life®.
Otherwise, HEMO2Life® may be important in addressing the microthrombosis phenomena described in CoV-2-SARS infections. In fact, skin and lung histological analyses of a report of 5 cases showed a complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection [50] and a retrospective study of 183 patients reveals abnormal coagulation results, especially markedly elevated D‐dimer and fibrin degradation product are common in COVID-19 deaths [51]. This micro thrombosis is due to a cascade of events causing the destruction of vascular endothelium by iron-derived reactive oxygen species [52]. Indeed, as discussed previously, in a hypoxemic environment as created by COVID-19, the production of reactive oxygen species [53] by the cytotoxic free haem [54] leads to endothelial cell oxidative damage [55]. This cytotoxic heam is itself released by the red blood cell destruction caused by the virus [56]. The very high ferritin levels found in the blood in patients hospitalized with COVID-19 reflect this massive release of toxic iron [57]. This micro thrombosis phenomenon cause acute respiratory failure and systemic coagulopathy, which are critical aspects of the morbidity and mortality characterizing infection with the SARS-CoV-21, [58], [59]. As HEMO2Life® is 250 times smaller than red blood cells and extracellular [13], it can cross the thrombus generated by the SARS-CoV-2. This hypothesis is also supported by the fact that we have shown in a rat model affected with traumatic brain injury [10] and therefore highly susceptible to intravascular microthrombosis[60] that our oxygen carrier could rapidly reduce acute brain hypoxia tissue, by overcoming classic, post-traumatic vascular size reduction without inducing vasoconstriction itself. Indeed, HEMO2Life® does not have a vasoconstriction effect compared to with other HBOC of first or second generation developed so far [15], [19]. Finally, the hypothesis that the COVID-19 could remove the heme on β-globin chain and removed its iron has been put forward [36] at least on red blood cell progenitors with the nucleus. If this hypothesis proves to be correct, the use of HEMO2Life® will find here an additional reason for its usefulness in the treatment of COVID-19 hypoxemia. However, this potential inactivation of the Hemoglobin by fixation of SarsCov2 on hemoglobin has been called into question by a recent study. It tends to disprove the plausibility of this last, theoretical hypothesis [61]. The biocompatibility of HEMO2Life® has been verified experimentally. The preclinical studies were selected according to International Organization for Standardization SO 10993 (Biological evaluation of medical devices, part 1), using a biological risk assessment methodology and considering the intended use of the product. The following evaluations were performed: cytotoxicity, inflammation by cytokines, platelet interaction, the effect on the complement, skin irritation, delayed hypersensitization, systemic toxicity, genotoxicity, biodegradation in human plasma, immunotoxicity and pyrogenicity. All these studies have demonstrated the safety of HEMO2Life®. HEMO2Life® was found to be well tolerated and did not induce toxicity. It is non-pyrogenic and devoid of mutagenic effects, is not cytotoxic and is not irritant [14]. When administered intravenously to hamsters and rats by Pr. Intagliatta group in San Diego, a well-known group specialist on Hemoxygen-Based Oxygen Carriers (HBOC), HEMO2Life® showed no acute effect on heart rate and blood pressure and did not cause microvascular vasoconstriction [14]. In another study [12], fluorescently labeled HEMO2Life® was administered to mice (60 mg/kg, 600 mg/kg, 1200 mg/kg) and was found to be safe, the animals showed no abnormal clinical signs and a half-life of 2.5 days was found. In these preclinical in vivo models, HEMO2Life® has enabled better tissue oxygenation, especially in the brain (direct tissue measurements) [10]. Extracellular hemoglobin HEMO2Life® was evaluated in humans’ kidney transplantation in the OXYOP study (NCT02652520) [9]. This study, the first in humans, demonstrated that adding the oxygen transporter HEMO2Life® to the kidney transplant preservation solution is safe. Although this study was not designed to show the superiority of HEMO2Life®, the analysis of the secondary efficacy criteria offers significantly less Delayed Graft Function (DFG) and better renal function in recipients of kidneys preserved with HEMO2Life®. This study calls for the use of HEMO2Life® in organ conservation, and for new investigations assessing the cost-effectiveness and long-term benefit of HEMO2Life® (OXYOP2 NCT04181710 study in progress). It also justifies new research lines and the use of HEMO2Life® in diseases linked to ischemia–reperfusion injuries and hypoxia HEMO2Life® was used in the first face re-transplantation for graft preservation [16]. Some oxygen carriers have been the subjects of studies demonstrating their effectiveness in the ARDS preclinical model.
Thus, in 2004, Henderson et al. [62] evaluate whether hemoglobin-based oxygen-carrying solution (HBOC)-201 (Biopure) is an effective alternative to donor blood for extracorporeal membrane oxygenation support in a porcine model of ARDS. HBOC-201 appears to be an effective alternative circuit-priming agent for use during extracorporeal membrane oxygenation, offering the advantages of rapid availability and diminished donor blood cell exposure. In a rodent study conducted in 2006 [63] of shock-induced PMN-mediated acute respiratory distress syndrome (ARDS), the simulated prehospital administration of an HBOC markedly attenuated lung injury.
However, more recently in 2018, Voelker et al. [64] showed that pulmonary vasoconstriction by hemoglobin glutamer-200 combined with inhaled nitric oxide did not improve arterial oxygenation in ARDS in a rodent model. HEMO2Life® has not yet been the subject of a specific preclinical study for this pathology but we can expect superior efficiency, as it did not provoke vasoconstriction as demonstrated in comparison with the HBOC of the first and second generation [15]. Patients in profound hypoxemia admitting in intensive care in the context of the COVID-19 pandemic could constitute a population likely to benefit from the intravenous administration of HEMO2Life®.
Consequences of the hypothesis
Given its oxygen carrier property and its oxidative stress reduction action, we suggest adding HEMO2Life® (i.e. M101) to the current treatment protocols of COVID-19 as it might be effective in tackling hypoxia and oxidative stress due to SARS-CoV-2.
From our previous studies, we evaluate that the intake of 5 g of HEMO2Life® for a subject of 70 kg (70 mg/kg) whose blood volume is estimated at 5 L represents an increase in arterial O2 content of 1 mL of O2 per 100 mL of blood (or 5% of “physiological” oxygen content of arterial blood (CaO2) or 7% if Partial pressure of oxygen (PAO2) is at 80 mmHg). The administration will start with a 10 mg “test dose” to check for an anaphylactic reaction. Then, each 20 mL (1 g) vial will be administered intravenously using an electric syringe (20 mL in 10 min). A tolerance assessment will be performed after administering of each 20 mL vial, checking for skin rash, bronchospasm, hypotension or sudden tachycardia for the next 5 min before proceeding to the next vial. If the administration of 70 mg/kg of HEMO2Life® (i.e. 1.4 mL/kg) does not significantly improve the tissue oxygenation parameters and if the initial dose is well tolerated, we will repeat the administration of 70 mg/kg of HEMO2Life® for a total of 140 mg/kg which will correspond to a 10% increase in CaO2. As HEMO2Life® has a transport capacity 40 times greater than HbA, it could therefore increase the arterial O2 content in a situation where the pulmonary exchanger fails as O2 binding and release occur passively in a simple O2 gradient and in the absence of allosteric effector. This molecular respirator could improve COVID-19 patient’s survival, avoid tracheal intubation and shorter an oxygen supplementation and open the possibility of treating a larger number of patients in the event of a lack of respirators. This hypothesis merits clinical trials.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu N., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int .
- 3.Alhazzani W., Møller M.H., Arabi Y.M. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Crit Care Med. 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan E., Brodie D., Slutsky A.S. Acute Respiratory Distress Syndrome. JAMA. 2018;319(7):698. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G., Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116–117. doi: 10.1016/j.htct.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Meur Y., Badet L., Essig M. First-in-human use of a marine oxygen carrier (M101) for organ preservation: A safety and proof-of-principle study. Am J Transplant. 2020;20(6):1729–1738. doi: 10.1111/ajt.15798. [DOI] [PubMed] [Google Scholar]
- 10.Moon-Massat P., Mullah S.-H.-E.-R., Abutarboush R. Cerebral Vasoactivity and Oxygenation with Oxygen Carrier M101 in Rats. J Neurotrauma. 2017;34(19):2812–2822. doi: 10.1089/neu.2015.3908. [DOI] [PubMed] [Google Scholar]
- 11.Ali A., Watanabe Y., Galasso M. An extracellular oxygen carrier during prolonged pulmonary preservation improves post-transplant lung function. J Heart Lung Transplant. 2020;39(6):595–603. doi: 10.1016/j.healun.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Rousselot M., Delpy E., Drieu La Rochelle C. Arenicola marina extracellular hemoglobin: a new promising blood substitute. Biotechnol J. 2006;1(3):333–345. doi: 10.1002/biot.200500049. [DOI] [PubMed] [Google Scholar]
- 13.Zal F., Green B.N., Lallier F.H., Vinogradov S.N., Toulmond A. Quaternary Structure of the Extracellular Haemoglobin of the Lugworm Arenicola marina. A Multi-Angle-Laser-Light-Scattering and Electrospray-Ionisation-Mass-Spectrometry Analysis. Eur J Biochem. 1997;243(1–2):85–92. doi: 10.1111/j.1432-1033.1997.85_1a.x. [DOI] [PubMed] [Google Scholar]
- 14.Le Gall T., Polard V., Rousselot M. In vivo biodistribution and oxygenation potential of a new generation of oxygen carrier. J Biotechnol. 2014;187:1–9. doi: 10.1016/j.jbiotec.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Tsai G.A., Intaglietta M., Sakai H. Microcirculation and NO-CO Studies of a Natural Extracellular Hemoglobin Developed for an Oxygen Therapeutic Carrier. Curr Drug Discov Technol. 2012;9(3):166–172. doi: 10.2174/157016312802650814. [DOI] [PubMed] [Google Scholar]
- 16.Lellouch AG, Taveau CB, Cetrulo CL LL. No Title. In: Management Of Ischemia Duration In The First FVCA Re-Transplantation: The Use Of A Novel Marine Oxygen Carrier M101. 65thAnnual Meeting of the Research Council, May 28-31, 2020 at the Omni King Edward Hotel in Toronto, Canada; 2020.
- 17.Glorion M., Polard V., Favereau F. Prevention of ischemia-reperfusion lung injury during static cold preservation by supplementation of standard preservation solution with HEMO2Life® in pig lung transplantation model. Artif cells, nanomedicine. Biotechnol. 2017:1–8. doi: 10.1080/21691401.2017.1392315. [DOI] [PubMed] [Google Scholar]
- 18.Teh E.S., Zal F., Polard V., Menasché P., Chambers D.J. HEMO2Life® as a protective additive to Celsior solution for static storage of donor hearts prior to transplantation. Artif cells, nanomedicine, Biotechnol. 2017;45(4):717–722. doi: 10.1080/21691401.2016.1265974. [DOI] [PubMed] [Google Scholar]
- 19.Alayash A.I. Mechanisms of Toxicity and Modulation of Hemoglobin-based Oxygen Carriers. Shock. 2019;52(1S Suppl 1):41–49. doi: 10.1097/SHK.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire F., Sigrist S., Delpy E. Beneficial effects of the novel marine oxygen carrier M101 during cold preservation of rat and human pancreas. J Cell Mol Med. 2019 Dec;23(12):8025–8034. doi: 10.1111/jcmm.14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuillier R., Dutheil D., Trieu M.T., Mallet V., Allain G., Rousselot M. Supplementation with a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation. Am J Transplant. 2011 Sep;11(9):1845–1860. doi: 10.1111/j.1600-6143.2011.03614.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski J., Hannaert P., Kasil A. Efficacy of the natural oxygen transporter HEMO 2 life® in cold preservation in a preclinical porcine model of donation after cardiac death. Transpl Int. 2019;32(9):985–996. doi: 10.1111/tri.13434. [DOI] [PubMed] [Google Scholar]
- 23.Mallet V., Dutheil D., Polard V. Dose-ranging study of the performance of the natural oxygen transporter HEMO2 Life in organ preservation. Artif Organs. 2014;38(8):691–701. doi: 10.1111/aor.12307. [DOI] [PubMed] [Google Scholar]
- 24.Batool F, Stutz C, Petit C, Benkirane-Jessel N, Delpy E, Zal F, Leize-Zal E, Huck O. A therapeutic oxygen carrier isolated from Arenicola marina decreased P. gingivalis induced inflammation and tissue destruction. Sci Rep. 2020 Sep 8;10(1):14745. [DOI] [PMC free article] [PubMed]
- 25.Toulmond A. Blood oxygen transport and metabolism of the confined lugworm Arenicola marina (L.) J Exp Biol. 1975;63(647–660):22. doi: 10.1242/jeb.63.3.647. [DOI] [PubMed] [Google Scholar]
- 26.Toulmond A., Tchernigovtzeff C. Ventilation and respiratory gas exchanges of the lugworm Arenicola marina (L.) as functions of ambient PO2 (20–700 torr) Respir Physiol. 1984;57:349–363. doi: 10.1016/0034-5687(84)90083-5. [DOI] [PubMed] [Google Scholar]
- 27.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra A., Dwyre D.M., Schivo M. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95(8):999–1000. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barriteau CM, Bochey P, Lindholm PF, Hartman K, Sumugod R, Ramsey G. Blood transfusion utilization in hospitalized COVID-19 patients. Transfusion. 2020 Jun 24:10.1111/trf.15947. [DOI] [PMC free article] [PubMed]
- 30.Berzuini A., Bianco C., Paccapelo C. Red cell bound antibodies and transfusion requirements in hospitalized patients with COVID-19. Blood. 2020 doi: 10.1182/blood.2020006695. published online June 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geier M.R., Geier D.A. Respiratory conditions in coronavirus disease 2019 (COVID-19): Important considerations regarding novel treatment strategies to reduce mortality. Med Hypotheses. 2020 Jul;140 doi: 10.1016/j.mehy.2020.109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ejigu T., Patel N., Sharma A., Vanjarapu J.M.R., Nookala V. Packed Red Blood Cell Transfusion as a Potential Treatment Option in COVID-19 Patients With Hypoxemic Respiratory Failure: A Case Report. Cureus. 2020 Jun 1;12(6) doi: 10.7759/cureus.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanworth S.J., New H.V., Apelseth T.O., Brunskill S., Cardigan R., Doree C. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7(10):e756–e764. doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;22(371) doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ChemRxiv. 2020 [Google Scholar]
- 37.Gan R., Rosoman N.P., Henshaw D.J.E., Noble E.P., Georgius P., Sommerfeld N. COVID-19 as a viral functional ACE2 deficiency disorder with ACE2 related multi-organ disease. Med Hypotheses. 2020;23(144) doi: 10.1016/j.mehy.2020.110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edeas M., Saleh J., Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? [published online ahead of print, 2020 Jun 1] Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.05.110. S1201-9712(20)30417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A. Radmanesh A. Derman K. Ishida COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy [published online ahead of print, 2020 May 27] J Neurol Sci. 2020 415:116945. [DOI] [PMC free article] [PubMed]
- 42.Kanoore Edul V.S., Caminos Eguillor J.F., Ferrara G., Estenssoro E., Siles D.S.P., Cesio C.E. Microcirculation alterations in severe COVID-19 pneumonia. J Crit Care. 2020;17(61):73–75. doi: 10.1016/j.jcrc.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker T., Schell C.O., Petersen D.B. Essential care of critical illness must not be forgotten in the COVID-19 pandemic. Lancet. 2020;395(10232):1253–1254. doi: 10.1016/S0140-6736(20)30793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gell D.A. Structure and function of haemoglobins. Blood Cells Mol Dis. 2018;70:13–42. doi: 10.1016/j.bcmd.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. doi: 10.4081/cp.2020.1271. Published 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eid R., Arab N.T., Greenwood M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim Biophys Acta Mol Cell Res. 2017;1864(2):399–430. doi: 10.1016/j.bbamcr.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Feng G., Zheng K.I., Yan Q.Q. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8(1):18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thibodeaux K., Speyrer M., Raza A., Yaakov R., Serena T.E. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: a retrospective case series. J Wound Care. 2020;29(Sup5a):S4–S8. doi: 10.12968/jowc.2020.29.Sup5a.S4. [DOI] [PubMed] [Google Scholar]
- 49.Kaiser Health News. Available at: https://khn.org/news/hospitals-put-more-stock-in-hyperbaric-therapy-for-diabetics-despite-concerns/. Accessed on 8 April 2020.
- 50.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arachchillage D.R.J., Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(5):1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong B.W., Marsch E., Treps L., Baes M., Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36(15):2187–2203. doi: 10.15252/embj.201696150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 54.Balla J., Vercellotti G.M., Nath K. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant. 2003;18(Suppl 5):v8–v12. doi: 10.1093/ndt/gfg1034. [DOI] [PubMed] [Google Scholar]
- 55.Craige S.M., Kant S., Keaney J.F., Jr. Reactive oxygen species in endothelial function - from disease to adaptation. Circ J. 2015;79(6):1145–1155. doi: 10.1253/circj.CJ-15-0464. [DOI] [PubMed] [Google Scholar]
- 56.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published online ahead of print, 2020 Feb 7] JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein S.C., Graham D.I., Chen X.H., Smith D.H. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery. 2004;54(3):687–691. doi: 10.1227/01.neu.0000108641.98845.88. [DOI] [PubMed] [Google Scholar]
- 60.Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care. 2016;4(29) doi: 10.1186/s40560-016-0138-3. Published 2016 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daniel Y., Hunt B.J., Retter A. Haemoglobin Oxygen Affinity in Patients with Severe COVID-19 Infection [published online ahead of print, 2020 May 26] Br J Haematol. 2020 doi: 10.1111/bjh.16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henderson C.L., Anderson C.M., Sorrells D.L., Wilson B.J., Dick E.J., DiGeronimo R.J. The use of a hemoglobin-based oxygen-carrying solution (HBOC-201) for extracorporeal membrane oxygenation in a porcine model with acute respiratory distress syndrome. Pediatr Crit Care Med. 2004;5(4):384–390. doi: 10.1097/01.pcc.0000123544.46047.ba. [DOI] [PubMed] [Google Scholar]
- 63.Moore E.E., Cheng A.M., Moore H.B., Masuno T., Johnson J.L. Hemoglobin-based oxygen carriers in trauma care: scientific rationale for the US multicenter prehosptial trial. World J Surg. 2006;30(7):1247–1257. doi: 10.1007/s00268-005-0499-6. [DOI] [PubMed] [Google Scholar]
- 64.Voelker M.T., Bergmann A., Busch T., Jahn N., Laudi S., Noreikat K. The effects of hemoglobin glutamer-200 and iNO on pulmonary vascular tone and arterial oxygenation in an experimental acute respiratory distress syndrome. Pulm Pharmacol Ther. 2018;49:130–133. doi: 10.1016/j.pupt.2018.01.009. [DOI] [PubMed] [Google Scholar]