Abstract
Integrin αvβ3 expression is upregulated during tumor growth and invasion in newly formed endothelial cells in tumor neovasculature and in some tumor cells. A tetrameric RGD‐based peptide, regioselectively addressable functionalized template‐(cyclo‐[RGDfK])4 (RAFT‐RGD), specifically targets integrin αvβ3 in vitro and in vivo. When labeled with indium‐111, the RAFT‐RGD is partially reabsorbed and trapped in the kidneys, limiting its use for further internal targeted radiotherapy and imaging investigations. We studied the effect of Gelofusine on RAFT‐RGD renal retention in tumor‐bearing mice. Mice were imaged using single photon emission computed tomography and optical imaging 1 and 24 h following tracer injection. Distribution of RAFT‐RGD was further investigated by tissue removal and direct counting of the tracer. Kidney sections were analyzed by confocal microscopy. Gelofusine significantly induced a >50% reduction of the renal reabsorption of 111 In‐DOTA‐RAFT‐RGD and A700‐RAFT‐RGD, without affecting tumor uptake. Injection of Gelofusine significantly reduced the renal retention of labeled RAFT‐RGD, while increasing the tumor over healthy tissue ratio. These results will lead to the development of future therapeutic approaches. (C ancer S ci 2012; 103: 1105–1110)
It has been proposed that RGD‐based peptides selectively target tumor neovasculature through αvβ3 integrin recognition.1, 2, 3, 4 In previous studies, we highlighted the specific targeting of tumors expressing αvβ3 in mice by a tetrameric RGD‐peptide named RAFT‐c[‐RGDfK‐]4, or regioselectively addressable functionalized template‐(cyclo‐[RGDfK])4 (RAFT‐RGD).5, 6 This hydrophilic peptide‐based compound (approximately 5 kDa) is highly specific, can be labeled with radionuclide7, 8, 9, 10 or fluorescence,11, 12, 13 and is mainly eliminated by the kidneys. We therefore proposed RAFT‐RGD as a potent tumor targeting agent for cancer diagnosis and therapy (internal targeted radiotherapy (ITRT) or drug‐based therapy). However, we reported that approximately 30–50% of the total dose of RAFT‐RGD labeled with technetium (99mTc) or indium‐111 (111In) remained trapped in the kidneys 24 h after injection (see Ahmadi et al. for biodistribution at 1, 4, 24, and 48 h).7, 10 Other studies also reported that the tetramerisation of RGD‐tracers enhanced renal uptake, compared with monomers.14 Such pharmacokinetic behavior could impair the optimal therapeutic dosing of the compound in patients.
Following glomerular filtration, peptides are reabsorbed and endocytosed by the tubular proximal cells into lysosomes for proteolytic digestion. Metabolized peptides are released into the bloodstream, whereas in the case of radiolabeled peptides, radiometal chelated amino acids might been retained in the tubular cells, leading to high delivered dose, radiation‐induced toxicity, and subsequent side‐effects (nausea, hyperkalemia).15 In the present case, part of the native and metabolized forms of radiolabeled 111In‐DOTA‐RAFT‐RGD, as well as fluorescent RAFT‐RGD, is reabsorbed by the proximal tubules and retained in tubular cells, for at least 4 days post‐injection. Such a long retention in patients under ITRT would leave the kidneys exposed to high energy radiations (90‐Yttrium or 177‐Lutetium), and might also generate a weak imaging contrast for tumors surrounding the kidneys.
To reduce the renal reabsorption of radiolabeled peptides or small molecules, a possible approach consists of interfering with the receptor‐mediated endocytosis pathway at the membrane of the proximal tubule cells. The megalin/cubilin system has been described to play an important role among the different receptors involved in the tubular reabsorption of peptides.16, 17 Megalin is a multiligand receptor belonging to the low density lipoprotein (LDL)‐receptor family. It is expressed on the apical border of renal proximal tubules as well as in the glomeruli. This receptor is a high‐capacity transport system implicated in the reabsorption of vitamin D binding protein, albumin, β2‐ and α1‐microglobulin, and several hormones.16, 18 Among the gelatin‐based plasma expenders, that is, Voluven (hydroxyethylamidon; Fresenius Kabi, Paris, France), Heamaccel (Behring, Marburg, Germany), and Gelofusine (B. Braun Medical, Boulogne Billancourt, France), the latter consists of succinylated bovine gelatine molecules, a mixture of collagen‐derived peptides, and is used in clinical emergency units. Infusion of Gelofusine has been reported to increase the renal excretion of megalin ligands.19, 20 For example, pre‐injection of Gelofusine was described to efficiently reduce renal retention of somatostatin analogues by approximately 40%.21 Similarly, Gelofusine infusions also lead to a decrease in the retention of radiolabeled compounds in the cortical proximal tubules. Thus, the total radiation delivered in the kidneys is reduced, through the increased urine elimination of the radioactive compound.
In the present study, we evaluated the effect of Gelofusine on renal reabsorption of fluorescent RAFT‐RGD or radiolabeled 111In‐DOTA‐RAFT‐RGD in tumor‐bearing mice using single photon emission computed tomography (SPECT) and fluorescence imaging, to maximize tumor‐to‐background ratio during diagnostic imaging and to minimize radiation‐induced nephrotoxicity during future ITRT settings with 90Y‐DOTA‐RAFT‐RGD. In this study, the effect of Gelofusine injection with radiolabeled or fluorescent RAFT‐RGD on renal reabsorption and tumor uptake was investigated in αvβ3‐positive tumor‐bearing mice.
Materials and Methods
RGD‐peptide synthesis and radioactive/fluorescent labeling
The different compounds were synthesized according to previously reported procedures.5, 22 Four copies of the c[‐RGDfK‐] peptide were grafted onto the upper face of the cyclic decapeptide RAFT backbone. On the opposite side, the radiolabeling with 111In was possible due to the presence of a DOTA group. The radiolabeling was carried out as previously described.7 For optical imaging experiments, the fluorescent AlexaFluor700 (A700) mono N‐hydroxysuccinimide ester (Amersham Biosciences, Uppsala, Sweden) was grafted at a lysine side‐chain, as previously described.22
Cell lines and culture conditions
Murine mammary carcinoma TS/A‐pc cells were cultured in RPMI medium supplemented with 10% FCS. The cell line HEK293(β3) is a stable human embryonic kidney cell line (HEK293) stably transfected with the human β3 integrin that was cultured as described in Jin et al.12 in DMEM supplemented with 1% glutamine, 10% FBS, and 700 μg/mL Geneticin (G418 sulfate; Gibco, Paisley, UK). All cell lines were cultured at 37°C in a humidified 95% air/5% CO2 atmosphere. Cells described here are integrin αvβ3 positive.
In vivo imaging
Five‐ to six‐week‐old female NMRI nude mice were obtained from Janvier (Laval Le Genest, France). The animals were housed for 1 week prior to tumor cell administration with free access to water and standard or weakly fluorescent rodent food. All the experiments described in this study were approved by the Animal Care and Use Committee of the ComEth38 (Grenoble, France) (authorization #2010_57_IAB‐U823‐SL‐05), and the experiments were carried out under the supervision of authorized individuals (L. Sancey, DDPP authorization #38 05 32 and A. Briat, DDPP authorization #38 74 23). Briefly, 106 TS/A‐pc or 107 Hek293(β3) cells (in 100 μL PBS) were injected s.c. in the right flank of mice. Once tumors reached approximately 200 mm3, the animals were anesthetized by isoflurane (CSP, Cournon, France) 4% for induction and 2% thereafter.
Nuclear imaging
Twenty‐four tumor‐bearing mice were distributed in four groups (n = 6/group). Each received a tail vein injection of 4 μg 111In‐DOTA‐RAFT‐RGD (12 MBq). Gelofusine 4% or PBS was either pre‐ or co‐injected with the tracer as follows: Group 1, co‐injection (120 μL mix) of 4 mg Gelofusine (100 μL) and 12 MBq 111In‐DOTA‐RAFT‐RGD (20 μL); Group 2, co‐injection (120 μL mix) of 12 MBq 111In‐DOTA‐RAFT‐RGD (20 μL) and PBS (100 μL); Group 3, pre‐injection of 4 mg of Gelofusine (100 μL) 3 min prior to 12 MBq 111In‐DOTA‐RAFT‐RGD (100 μL); and Group 4, pre‐injection of PBS (100 μL) 3 min prior to 12 MBq 111In‐DOTA‐RAFT‐RGD (100 μL).
At 1 and 24 h following tracer injection, animals were imaged using a small animal SPECT imaging camera (NanoSPECT/CT; Bioscan, Washington, DC, USA) and whole‐body SPECT/CT tomographic images were acquired (four detectors, nine‐pinhole settings). Briefly, mice under general anesthesia were placed into an imaging cassette allowing full control of gas anesthesia and temperature (Minerve Anaesthesia; Minerve, Esternay, France). The CT and SPECT images were acquired sequentially using Nucline software (Mediso, info:http://www.mediso.hu/). The CT parameters were: 240 projections; 500 ms per projection; and 55 kV. The SPECT parameters were: 24 projections; and 80–100 s per projection. Reconstruction of SPECT images was carried out using HiSPECT NG (Bioscan). Reconstruction of CT images (Exact Cone Beam algorithm and Shepp Logan filter) and SPECT/CT image co‐registration were carried out using InVivoScope 1.42 software (Bioscan). Animals were euthanized following the 24 h time point acquisition, and tissues were collected and weighed. Radioactivity was directly counted using a gamma counter (Berthold, Thoiry, France).
Optical imaging
Two hundred microliters of A700‐RAFT‐RGD (i.e. 10 nmol dye/mouse) was injected i.v. as described in the previous section, 2–5 min after i.v. injection of 100 μL Gelofusine 4% (n = 3/group, pre‐injection). One to 24 h after injection, anesthetized animals were imaged by the Fluobeam‐700 device (Fluoptics, Grenoble, France) as previously described.23 Twenty‐four hours after injection, mice were euthanized and organs were collected for ex vivo fluorescence imaging. Organs were illuminated by 660‐nm light‐emitting diodes equipped with interference filters. Fluorescence images as well as black and white pictures were acquired by a back‐thinned CCD camera at −80°C (ORCAII‐BT‐512G; Hamamatsu, Massy, France) fitted with a colored glass high‐pass RG 9 filter (Schott, Clichy, France).
Kidney section analysis
Confocal microscopy of thin kidney sections (7 μm) was carried out on an LSM710 LNO confocal microscope (Carl Zeiss, Jena, Germany) using a 40× oil immersion objective of 1.0 N.A. The 633‐nm laser intensity was set up at 3% of its maximum intensity.
Statistical analysis
Statistical analysis was carried out using Statview software (SAS Institute, San Francisco, CA, USA). Tissue uptakes and ratios were compared using an unpaired t‐test. A P‐value ≤0.05 was considered as significant.
Results
Influence of Gelofusine on RAFT‐RGD distribution: ex vivo biodistribution by direct gamma counting
Radiolabeled RAFT‐RGD has been used to specifically target integrin αvβ3 in vitro and in vivo. In tumor‐bearing mice, 111In‐DOTA‐RAFT‐RGD was mainly and rapidly cleared through the renal route. The amount of 111In‐DOTA‐RAFT‐RGD was <1% of injected dose per gram (%ID/g) in most of the organs, 1 h after injection, except in the tumor expressing integrin αvβ3 and in the kidneys. As shown in Figure 1, pre‐ and co‐injection of Gelofusine strongly reduced the uptake of RAFT‐RGD in the kidneys, as well as in urine; as described in other published reports, the amount of radiotracer in the kidneys was reduced, through the increased urine elimination of the radioactive compound. As the urine was collected at the end of the experiment (i.e. 24 h after injection), the remaining %ID/g of urine was very low in both conditions, with a significant difference in favor of Gelofusine, as expected. Pre‐injection and co‐injection of Gelofusine strongly reduced the activity in the kidney by 49.2% and 47.9%, respectively (P = 0.002 and P < 0.0001) (Fig. 1A). The presence of radioligand in the blood was comparable in the presence or absence of the plasma expender. Muscle uptake of 111In‐DOTA‐RAFT‐RGD was decreased by 10% in the presence of Gelofusine, whereas tumor uptake was not significantly affected (4.4% decrease with Gelofusine pre‐injection, 16.4% increase with Gelofusine co‐injection; P > 0.05, not significant [NS]). Similar results were obtained with the fluorescent A700‐RAFT‐RGD (Fig. 1B). Pre‐injection of Gelofusine reduced A700‐RAFT‐RGD kidney uptake by 63% (P < 0.01) and tumor uptake was not significantly affected (P > 0.05, NS).
Figure 1.
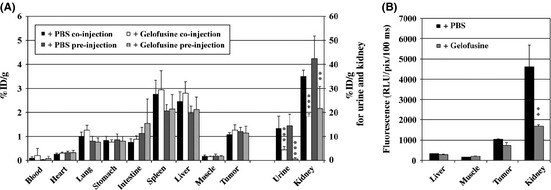
Influence of Gelofusine on RAFT‐RGD labeled with 111‐indium (A) or A700 (B) biodistribution. (A) Mice were euthanized 24 h after i.v. injection of labeled RAFT‐RGD, with preliminary injection of PBS or Gelofusine. Gelofusine significantly reduced renal and urine activities of 111 In‐DOTA‐RAFT‐RGD. The results are expressed as the percentage of injected dose per gram of organ (%ID/g). (B) Similar results were obtained with pre‐injection of Gelofusine (gray) before A700‐RAFT‐RGD. The results are expressed as the mean of the relative light unit (RLU) per pixel. **P < 0.01, ***P < 0.001, Gelofusine versus PBS.
Influence of Gelofusine on RATF‐RGD distribution: Non‐invasive in vivo imaging
Non‐invasive SPECT‐CT imaging was used to quantify the reduction of renal uptake and the effect of Gelofusine on the tumor imaging contrast, that is, tumor‐to‐contralateral muscle (T/M) and tumor‐to‐kidney (T/K) ratios (Fig. 2). In the kidneys, 111In‐DOTA‐RAFT‐RGD was mainly localized in the renal cortex, as shown in Figure 2(A). In the presence of Gelofusine, this renal capture was also localized in the cortex but was highly reduced (Fig. 2B). Optical imaging confirmed the reduction of fluorescent RAFT‐RGD into kidney after Gelofusine injection, as shown in Figure 3. In both experimental conditions, tumors expressing integrin αvβ3 were seen (Fig. 2, bottom right panels). Quantitative values were obtained from the imaging experiments (Table 1). One hour after RAFT‐RGD injection, the T/K ratio was significantly reduced in nuclear (0.06 ± 0.01 for PBS vs 0.16 ± 0.02 for Gelofusine; P < 0.05) and optical imaging (0.66 ± 0.13 for PBS vs 1.24 ± 0.26 for Gelofusine; P < 0.05, *). Similarly and in both imaging modalities, the quantifications on dissected organs confirmed that the T/K ratio was doubled 24 h after injection of Gelofusine (P < 0.05, *). Of note, the T/M ratio was usually not significantly different after PBS and Gelofusine injections.
Figure 2.
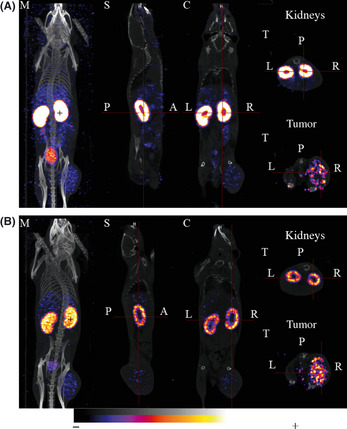
Representative single photon emission computed tomography (SPECT)/CT imaging of tumor‐bearing mice after injection of 111 In‐DOTA‐RAFT‐RGD and PBS (A) or Gelofusine (B). The SPECT/CT images were acquired 24 h after i.v. injection of PBS + 111 In‐DOTA‐RAFT‐RGD (A) or Gelofusine + 111 In‐DOTA‐RAFT‐RGD (B). Gelofusine or PBS were injected 2–5 min prior to RAFT‐RGD. Gelofusine strongly reduced renal uptake without affecting tumor uptake. From left to right, SPECT was merged with CT (M, maximum intensity projection or MIP), or representative sections, sagittal (S), coronal (C) or transversal (T), are shown. Scale bar for 111 In is 7–70% for MIP and kidney slices and 3–12% for tumor slices (minimum/maximum 0–20 Bq). Scale bar for CT is 1–70% (color set: exp; intensity 90%). A, anterior view; L, left; P, posterior view; R, right. [Correction added on 21 May 2012, after first online publication: ‘single positron emission’ in the first sentence is corrected to ‘single photon emission’.]
Figure 3.

Fluorescence imaging of tumor‐bearing mice after injection of A700‐RAFT‐RGD with PBS or Gelofusine. (A) Representative images of tumor‐bearing mice 1–24 h post‐injection (p.i.) of A700‐RAFT‐RGD with PBS or Gelofusine 4%. (B) Organs were imaged at 24 h. Gelofusine strongly reduced renal uptake, as shown in whole body and organ imaging.
Table 1.
Quantification of RAFT‐RGD after PBS or Gelofusine pre‐injection 5 min prior to labeled tracer
| PBS | Gelofusine | |||||
|---|---|---|---|---|---|---|
| 1 h | 24 h | Dissected organs | 1 h | 24 h | Dissected organs | |
| 111In | ||||||
| T/M | 4.24 ± 0.44 | 4.50 ± 0.90 | 6.42 ± 2.30 | 3.63 ± 0.66NS | 5.21 ± 0.76NS | 7.03 ± 1.62NS |
| T/K | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.16 ± 0.02* | 0.16 ± 0.01* | 0.06 ± 0.01* |
| A700 | ||||||
| T/M | 2.02 ± 0.09 | 2.18 ± 0.33 | 6.87 ± 0.34 | 1.95 ± 0.43NS | 2.03 ± 0.64NS | 3.80 ± 0.04* |
| T/K | 0.66 ± 0.13 | 1.33 ± 0.01 | 0.35 ± 0.12 | 1.24 ± 0.26* | 1.68 ± 0.37NS | 0.73 ± 0.41* |
Quantifications were obtained from analysis of non‐invasive imaging at 1 and 24 h after injection or from analysis of dissected organs at 24 h after injection. Results are expressed as the mean ± SEM. 111In, 111In‐DOTA‐RAFT‐RGD; A700, A700‐RAFT‐RGD; T/K, tumor‐to‐kidney ratio; T/M, tumor‐to‐contralateral muscle ratio. NS, P > 0.05, *P < 0.05, Gelofusine versus PBS.
Localization of labeled RAFT‐RGD in kidneys
Labeled with single photon emitter 111In (Fig. 4A) or with fluorescent A700 (Fig. 4B), RAFT‐RGD was mainly trapped in the renal cortex 24 h after injection. Table 2 summarizes the quantifications of the tracer in the kidney, from the nuclear and the optical imaging as well as on isolated organs. Twenty‐four hours after injection of Gelofusine, the presence of the tracer was reduced by 49% and 63% into the dissected kidneys compared with PBS, in nuclear and optical imaging, respectively. From the non‐invasive imaging, the renal amount of tracer in the kidney was decreased by 31% in optical imaging in the Gelofusine injected mice, and decreased to 66% in nuclear imaging. The transversal sections of mice injected with 111In‐DOTA‐RAFT‐RGD clearly illustrated the efficient decrease of the accumulated RAFT‐RGD trapped into the kidneys after pre‐injection of Gelofusine (Fig. 4A). Thin kidney sections analyzed with the confocal microscope revealed no effect of Gelofusine on RAFT‐RGD localization into the kidney or into a particular cell type. However, these analyses confirmed that Gelofusine reduced the amount of labeled peptide in the cortical region of the kidney.
Figure 4.
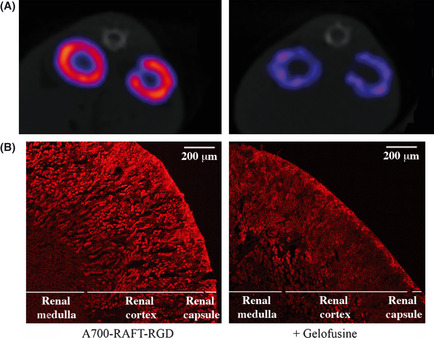
Influence of Gelofusine on renal distribution of RAFT‐RGD. (A) Transversal sections of mice crossing the two kidneys with pre‐injection of PBS (left) or Gelofusine (right) before 111 In‐DOTA‐RAFT‐RGD injection. (B) Zoom on a transversal section of kidney with pre‐injection of PBS (left) or Gelofusine (right) before A700‐RAFT‐RGD. Gelofusine strongly reduced renal uptake of RAFT‐RGD, without affecting its local distribution. Scale bar, 200 μm.
Table 2.
Quantification of RAFT‐RGD in mouse kidney after PBS or Gelofusine pre‐injection 5 min prior to labeled tracer
| PBS | Gelofusine | |||||
|---|---|---|---|---|---|---|
| 1 h | 24 h | Dissected organs | 1 h | 24 h | Dissected organs | |
| 111In | 13.35 ± 1.55 | 5.83 ± 0.52 | 42.30 ± 9.30 | 4.95 ± 0.95*** | 1.97 ± 0.23*** | 21.50 ± 9.30** |
| A700 | 10457 ± 1470 | 985 ± 213 | 4601 ± 1086 | 5040 ± 580** | 682 ± 167* | 1698 ± 61** |
Quantifications were obtained from analysis of non‐invasive imaging at 1 and 24 h after injection or from analysis of dissected organs 24 h after injection. Results are expressed as the mean ± SEM. 111In, 111In‐DOTA‐RAFT‐RGD (% injected dose [ID]/500 mm3 or%ID/g); A700, A700‐RAFT‐RGD (mean of relative light unit [RLU]/pixel/100 ms). *P < 0.05, **P < 0.01, ***P < 0.001, Gelofusine versus PBS.
Discussion
Targeting tumors expressing integrin αvβ3 is an intensive field of experimental and clinical research. Currently, RGD‐based tracers are used in nuclear medicine units, where they provide information about tumor characterization and constitute a major tool for the assessment of anti‐angiogenic treatment efficacy. RAFT‐RGD was developed to efficiently target tumors expressing integrin αvβ3, as a contrast agent for nuclear medicine and optical imaging,7, 9, 10, 12 as well as an anticancer agent for ITRT and drug delivery.22, 24 During ITRT, the delivered dose is mainly determined by toxicity induced in fragile and vital tissues such as kidneys or bone marrow. Peptides and low‐molecular‐weight proteins are easily filtered and subsequently reabsorbed in the proximal tubule of kidney: this reabsorption leads to the accumulation of those radiolabeled peptides in the kidney and thus might contribute to renal toxicity.
A decrease in RAFT‐RGD renal retention is thus crucial to minimize nephrotoxicity during treatment with β‐emitters such as 90Y‐labeled or 177Lu‐labeled RAFT‐RGD. The partial reabsorption of RAFT‐RGD was efficiently reduced through pre‐injection or co‐injection of Gelofusine, but still too high to prevent any renal damage during ITRT. Several studies, mainly focused on peptide‐receptor radionuclide therapy, reported the use of Gelofusine, alone or in combination with positively charged amino acids, to reduce renal uptake and/or enhance the amount of the therapeutic radionuclide to increase its effect.21, 25, 26, 27 Charges of ligands may play a crucial role in the binding to megalin; some ligands bind to megalin through their cationic sites and their reabsorption can be reduced by co‐administration of cationic compounds such as lysine or a mixture of positively charged amino acids.15 Therefore, co‐injection of lysine and arginine has become a standard procedure during peptide receptor radionuclide therapy with 177Lu‐labeled or 90Y‐labeled somatostatin analogues,28 and similarly reduced renal retention of the radio compound such as [111In‐DTPAo]‐octreotide by approximately 40%.28, 29 Using Gelofusine alone, renal uptake of octreotate and octreotite were reduced by 42%30 and 46%,21 respectively, in kidneys of rats, whereas a combination of the two, positively charged amino acids plus Gelofusine, might be additive.31 Surprisingly, tumor uptake was not affected by the combination of Gelofusine plus lysine in a study carried out on rats bearing sst2‐receptor‐expressing tumors.25 We previously showed that 24 h after injection, approximately 6% of the RAFT‐RGD metabolites are positively charged in mice kidneys.7 In our case, pre‐injection of positively charged amino acids did not affected RAFT‐RGD renal retention (Arnaud Briat, unpublished data, 2010). Other plasma expenders, such as Voluven, or the lipid solution Medialipid (B. Braun Medical) were also pre‐injected prior to labeled RAFT‐RGD but failed to reduce renal retention (data not shown). If the renal accumulation of labeled RAFT‐RGD was significantly reduced and would facilitate tumor imaging, further investigations are required into reducing renal accumulation more efficiently for ITRT.
The labeling of the tracer by radioactive 111In or fluorescent A700 influenced the biodistribution of the tracer in a similar fashion; the tracer's biodistributions were similar when comparing both imaging methods. The differences observed for the dark‐red organs, that is, spleen and liver, might be explained in part by the limits of optical imaging.32 The fluorescence in these red‐colored organs is always underestimated compared to the other “white” organs.
As described in Figure 4, the partial reabsorption of RAFT‐RGD was mainly observed at the renal cortex. Gelofusine reduced the total amount of RAFT‐RGD in the kidney, therefore contributing to lower the radiation dose delivered to this fragile organ. Several approaches have been developed to interfere with renal retention of radiolabeled peptides. When other compounds are reabsorbed by endocytosis or transporters, co‐injection of succinylated gelatin and/or positively charged amino acids (lysine/arginine) helps to saturate the kidney with competitive inhibitors.29 Few anaphylactoid reactions were reported using gelatin derivatives and concerned approximately 0.033% of patients.33 Other strategies have been suggested to decrease endocytosis, such as the use of colchicine, which interferes with the microtubule function preventing the megalin receptor to return to the cell membrane after endocytosis; unfortunately, its use in clinical investigations was not suitable because of potential toxicity.34 Chemical modifications of the compound itself are also an attractive way to facilitate elimination. We previously added a glucose moiety on the RAFT scaffold of RAFT‐RGD to increase its elimination.35 The affinity for integrin αvβ3 was unaffected by this modification as well as tumor uptake, but it failed to reduce renal reabsorption. When reducing renal uptake is not efficient, alternative strategies might be useful, such as the use of radioprotectors that minimize oxidative stress (i.e., aminofostine),36 dose fractionation that increases time to repair sublethal damages,37 or the use of 177Lu instead of 90Y, which lowers the radiation dose delivered to the radiosensitive glomeruli.38
In complement to the optimisation of the delivered dose in radionuclide therapy, reduction of renal uptake would also be useful during tumor imaging, increasing information during imaging due to a better T/K ratio. Small targeted tumors next to the kidneys could be disguised by a high concentration of radiolabeled or fluorescent tracer in the kidney; by enhancing tracer elimination through the urine, imaging information would be gained.
Limiting the total dose delivered to patients, as well as reducing specific renal radiation, are major clinical points during ITRT. Similarly, reduction of background (i.e., non‐specific organ uptake) is crucial during diagnostic imaging, for tumor localization and characterization. RAFT‐RGD is a promising agent, for both αvβ3‐positive tumor imaging and vectorization of radionuclides or therapeutic drugs for tumor therapy. Injection of the plasma expander Gelofusine efficiently reduced renal reabsorption of RAFT‐RGD, therefore supporting its use for further preclinical and clinical investigations.
Disclosure Statement
The authors have no conflicts of interest.
[Correction added on 21 May 2012, after first online publication: ‘single positron emission’ in the title is corrected to ‘single photon emission’.]
References
- 1. Anderson CR, Hu X, Zhang H et al Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest Radiol 2011; 46: 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beer AJ, Kessler H, Wester HJ, Schwaiger M. PET imaging of integrin alphaVbeta3 expression. Theranostics 2011; 1: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pool SE, Krenning EP, Koning GA et al Preclinical and clinical studies of peptide receptor radionuclide therapy. Semin Nucl Med 2010; 40: 209–18. [DOI] [PubMed] [Google Scholar]
- 4. Schnell O, Krebs B, Carlsen J et al Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto‐RGD positron emission tomography. Neuro Oncol 2009; 11: 861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boturyn D, Coll JL, Garanger E, Favrot MC, Dumy P. Template assembled cyclopeptides as multimeric system for integrin targeting and endocytosis. J Am Chem Soc 2004; 126: 5730–9. [DOI] [PubMed] [Google Scholar]
- 6. Garanger E, Boturyn D, Jin Z, Dumy P, Favrot MC, Coll JL. New multifunctional molecular conjugate vector for targeting, imaging, and therapy of tumors. Mol Ther 2005; 12: 1168–75. [DOI] [PubMed] [Google Scholar]
- 7. Ahmadi M, Sancey L, Briat A et al Chemical and biological evaluations of an (111)in‐labeled RGD‐peptide targeting integrin alpha(V) beta(3) in a preclinical tumor model. Cancer Biother Radiopharm 2008; 23: 691–700. [DOI] [PubMed] [Google Scholar]
- 8. Dimastromatteo J, Riou LM, Ahmadi M et al In vivo molecular imaging of myocardial angiogenesis using the alpha(v)beta3 integrin‐targeted tracer 99mTc‐RAFT‐RGD. J Nucl Cardiol 2010; 17: 435–43. [DOI] [PubMed] [Google Scholar]
- 9. Jin ZH, Furukawa T, Galibert M et al Noninvasive visualization and quantification of tumor alphaVbeta3 integrin expression using a novel positron emission tomography probe, 64Cu‐cyclam‐RAFT‐c(‐RGDfK‐)4. Nucl Med Biol 2011; 38: 529–40. [DOI] [PubMed] [Google Scholar]
- 10. Sancey L, Ardisson V, Riou LM et al In vivo imaging of tumour angiogenesis in mice with the alpha(v)beta (3) integrin‐targeted tracer 99mTc‐RAFT‐RGD. Eur J Nucl Med Mol Imaging 2007; 34: 2037–47. [DOI] [PubMed] [Google Scholar]
- 11. Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands‐design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem 2007; 7: 552–8. [DOI] [PubMed] [Google Scholar]
- 12. Jin ZH, Josserand V, Foillard S et al In vivo optical imaging of integrin alphaV‐beta3 in mice using multivalent or monovalent cRGD targeting vectors. Mol Cancer 2007; 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin ZH, Josserand V, Razkin J et al Noninvasive optical imaging of ovarian metastases using Cy5‐labeled RAFT‐c(‐RGDfK‐)4. Mol Imaging 2006; 5: 188–97. [PubMed] [Google Scholar]
- 14. Dijkgraaf I, Kruijtzer JA, Liu S et al Improved targeting of the alpha(v)beta (3) integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging 2007; 34: 267–73. [DOI] [PubMed] [Google Scholar]
- 15. Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging 2003; 30: 9–15. [DOI] [PubMed] [Google Scholar]
- 16. Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 2001; 280: F562–73. [DOI] [PubMed] [Google Scholar]
- 17. Baines RJ, Brunskill NJ. The molecular interactions between filtered proteins and proximal tubular cells in proteinuria. Nephron Exp Nephrol 2008; 110: e67–71. [DOI] [PubMed] [Google Scholar]
- 18. Christensen EI, Verroust PJ. Megalin and cubilin, role in proximal tubule function and during development. Pediatr Nephrol 2002; 17: 993–9. [DOI] [PubMed] [Google Scholar]
- 19. ten Dam MA, Branten AJ, Klasen IS, Wetzels JF. The gelatin‐derived plasma substitute Gelofusine causes low‐molecular‐weight proteinuria by decreasing tubular protein reabsorption. J Crit Care 2001; 16: 115–20. [DOI] [PubMed] [Google Scholar]
- 20. Veldman BA, Schepkens HL, Vervoort G, Klasen I, Wetzels JF. Low concentrations of intravenous polygelines promote low‐molecular weight proteinuria. Eur J Clin Invest 2003; 33: 962–8. [DOI] [PubMed] [Google Scholar]
- 21. van Eerd JE, Vegt E, Wetzels JF et al Gelatin‐based plasma expander effectively reduces renal uptake of 111In‐octreotide in mice and rats. J Nucl Med 2006; 47: 528–33. [PubMed] [Google Scholar]
- 22. Foillard S, Sancey L, Coll JL, Boturyn D, Dumy P. Targeted delivery of activatable fluorescent pro‐apoptotic peptide into live cells. Org Biomol Chem 2009; 7: 221–4. [DOI] [PubMed] [Google Scholar]
- 23. Keramidas M, Josserand V, Righini CA, Wenk C, Faure C, Coll JL. Intraoperative near‐infrared image‐guided surgery for peritoneal carcinomatosis in a preclinical experimental model. Br J Surg 2010; 97: 737–43. [DOI] [PubMed] [Google Scholar]
- 24. Dufort S, Sancey L, Hurbin A et al Targeted delivery of a proapoptotic peptide to tumors in vivo. J Drug Target 2011; 19: 582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melis M, Bijster M, de Visser M et al Dose‐response effect of Gelofusine on renal uptake and retention of radiolabelled octreotate in rats with CA20948 tumours. Eur J Nucl Med Mol Imaging 2009; 36: 1968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rolleman EJ, Melis M, Valkema R, Boerman OC, Krenning EP, de Jong M. Kidney protection during peptide receptor radionuclide therapy with somatostatin analogues. Eur J Nucl Med Mol Imaging 2010; 37: 1018–31. [DOI] [PubMed] [Google Scholar]
- 27. Vegt E, Eek A, Oyen WJ, de Jong M, Gotthardt M, Boerman OC. Albumin‐derived peptides efficiently reduce renal uptake of radiolabelled peptides. Eur J Nucl Med Mol Imaging 2010; 37: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valkema R, Pauwels SA, Kvols LK et al Long‐term follow‐up of renal function after peptide receptor radiation therapy with (90)Y‐DOTA(0),Tyr(3)‐octreotide and (177)Lu‐DOTA(0), Tyr(3)‐octreotate. J Nucl Med 2005; 46(Suppl 1): 83S–91S. [PubMed] [Google Scholar]
- 29. Vegt E, Wetzels JF, Russel FG et al Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J Nucl Med 2006; 47: 432–6. [PubMed] [Google Scholar]
- 30. Rolleman EJ, Bernard BF, Breeman WA et al Molecular imaging of reduced renal uptake of radiolabelled [DOTA0,Tyr3]octreotate by the combination of lysine and Gelofusine in rats. Nuklearmedizin 2008; 47: 110–5. [DOI] [PubMed] [Google Scholar]
- 31. Gotthardt M, van Eerd‐Vismale J, Oyen WJ et al Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nucl Med 2007; 48: 596–601. [DOI] [PubMed] [Google Scholar]
- 32. Josserand V, Texier‐Nogues I, Huber P, Favrot MC, Coll JL. Non‐invasive in vivo optical imaging of the lacZ and luc gene expression in mice. Gene Ther 2007; 14: 1587–93. [DOI] [PubMed] [Google Scholar]
- 33. Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977; 1: 466–9. [DOI] [PubMed] [Google Scholar]
- 34. Rolleman EJ, Krenning EP, Van Gameren A, Bernard BF, De Jong M. Uptake of [111In‐DTPA0]octreotide in the rat kidney is inhibited by colchicine and not by fructose. J Nucl Med 2004; 45: 709–13. [PubMed] [Google Scholar]
- 35. Galibert M, Sancey L, Renaudet O, Coll JL, Dumy P, Boturyn D. Application of click‐click chemistry to the synthesis of new multivalent RGD conjugates. Org Biomol Chem 2010; 8: 5133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vegt E, de Jong M, Wetzels JF et al Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med 2010; 51: 1049–58. [DOI] [PubMed] [Google Scholar]
- 37. Stewart FA, Lebesque JV, Hart AA. Progressive development of radiation damage in mouse kidneys and the consequences for reirradiation tolerance. Int J Radiat Biol Relat Stud Phys Chem Med 1988; 53: 405–15. [DOI] [PubMed] [Google Scholar]
- 38. Bodei L, Cremonesi M, Ferrari M et al Long‐term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y‐DOTATOC and 177Lu‐DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 2008; 35: 1847–56. [DOI] [PubMed] [Google Scholar]


