Abstract
Previous reports have shown that circulating endothelial progenitor cells (CEPs) are released in response to cytotoxic chemotherapy. We investigate the relationship between the kinetics of CEPs during one cycle of chemotherapy and the response to cytotoxic chemotherapy and prognostic impacts. Previously untreated patients (n = 38) receiving cytotoxic chemotherapy for non‐small‐cell lung cancer were included. Blood sampling was carried out on day 1, day 8, and just before the second cycle of chemotherapy. The mononuclear cell fraction was analyzed for CEPs by FACS analysis. We evaluated the relationship between the kinetics of CEPs, each independent clinicopathological variable, the response to chemotherapy, and the risk factors associated with prognosis. On the eighth day after chemotherapy, a significant decrease in CEPs was observed. In contrast, CEP counts before the second cycle of chemotherapy were significantly increased. The high percentage change in CEPs between day 1 and before the second cycle of chemotherapy is an independent predictive factor for response to chemotherapy. However, the change in CEP levels did not predict progression‐free survival. These findings indicate that the late release of CEPs is a common phenomenon after chemotherapeutic treatment. The correlation with clinical response to chemotherapy provides further support for the biologic relevance of these cells in patients' prognosis and highlights the potential use of CEPs as therapeutic targets. (Cancer Sci 2012; 103: 1065–1070)
Lung cancer is responsible for more cancer‐related deaths than any other tumor type. Despite extensive efforts to improve early diagnosis and treatment of lung cancer patients, the overall survival rate is dismal.1 Developments in surgical technique, radiation, and new chemotherapy regimens have not improved tumor progression in cases of small‐cell lung cancer and non‐small‐cell lung cancer (NSCLC).1 Response rates to various combinations of chemotherapy regimens in patients with NSCLC vary between 20% and 50% with slight prolongation of survival.1 Treatment for NSCLC is currently moving beyond conventional chemotherapy with the advent of molecular‐targeted therapies, and a key therapeutic strategy is inhibition of specific cytokines essential for tumor vascularization.2 Recently, the concept of angiogenesis has evolved from a simple model of new blood vessel formation from the pre‐existing vasculature to a multifaceted process in which bone marrow‐derived endothelial progenitor cells contribute to neovascularization. It is postulated that circulating endothelial progenitor cells (CEPs) are mobilized from the bone marrow into the circulation by tumor‐ or ischemia‐induced signals, such as stromal cell‐derived factor‐1α (SDF‐1α), MMP‐9, vascular endothelial growth factor (VEGF), placental growth factor, and granulocyte colony‐stimulating factor (G‐CSF). Circulating endothelial progenitor cells subsequently home to sites of tumor neovascularization, where they differentiate into endothelial cells and contribute to angiogenesis.3, 4, 5 In animal models, these angiogenic processes are considered essential for tumor growth.6 In the clinical setting, two studies have previously shown that CEPs are significantly increased in patients with NSCLC, correlating with poor clinical outcome.7, 8
A recent study in an animal model showed that CEPs exit the bone marrow and home to the tumor immediately after certain types of chemotherapy, predominantly paclitaxel.9 Several clinical studies also showed that CEPs increase after cytotoxic chemotherapy in cancer patients with advanced stage cancers.10, 11, 12 These findings have provided new insight into the mechanism of tumor regrowth, resistance to chemotherapy, recurrence, and metastasis formation during chemotherapy. Despite several reports about the relationship between CEPs and the efficacy of anti‐angiogenic drugs such as bevacizumab, little is known about CEP kinetics after treatment with cytotoxic anticancer drugs and the effect on clinical outcome during chemotherapy. Here we investigated the kinetics and clinical significance of changes in CEP number after the first cycle of cytotoxic chemotherapy in patients with advanced NSCLC.
Materials and Methods
Patients and data collection
Patients with histologically or cytologically proven stage III or IV NSCLC who had not previously received chemotherapy or thoracic radiotherapy were eligible for this study. All patients who had pneumonia, pleural empyema, or any other sign of infection were excluded. Patients who had received supportive therapy with G‐CSF during the first cycle of chemotherapy were also excluded from analysis. Patients were recruited between April 2010 and June 2011 at Kyoto University Hospital (Kyoto, Japan) and followed until August 30, 2011. The study was approved by the institutional ethics committee, and written informed consent was obtained from all patients. Staging was carried out according to the seventh edition of the TNM Classification.13
Evaluation of treatment efficacy
Treatment efficacy for each patient in the study was assessed using computed tomography after every two courses of chemotherapy. In accordance with the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1),14 at every assessment, patients were separated into three groups based on the variation in the sum of the largest diameters (SLD): below 30%, partial response (PR); between 30% and −20%, stable disease (SD); and above −20%, progressive disease (PD). The treatment response was defined as the best response recorded during the period from the beginning of treatment to the time of disease progression or discontinuation of treatment. The best percentage of tumor reduction corresponded to the largest reduction in SLD observed during the course of treatment compared with baseline SLD.
Information on survival was obtained through active follow‐up based on the verification of patients' vital status until August 30, 2011. Progression‐free survival (PFS) was defined as the time from commencement of chemotherapy to disease progression or death from any cause. It was determined as the date of the last follow‐up visit for patients who were still alive and who had no disease progression.
Flow cytometry analysis
Blood samples were collected before chemotherapy in tubes containing EDTA, 8 days after chemotherapy began (day 8), and immediately before the second cycle of chemotherapy (days 22–29). We enumerated CEPs by four‐color, rare event, flow cytometry analysis (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA), following the procedure of Mancuso et al.,15 using optimized concentrations of a panel of mAbs. The antibodies used were PerCP‐conjugated anti‐CD45 (BD Pharmingen, San Diego, CA, USA), FITC‐conjugated anti‐CD31 (BD Pharmingen), APC‐conjugated anti‐CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany), and PE‐conjugated anti‐CD34 (BD Pharmingen). Fluorochrome‐ and isotype‐matched controls, as well as unstained cell samples, were measured and processed as negative controls to normalize the appropriate regions. The gating strategy described previously16 was used to identify CEP subtypes while excluding interfering red blood cells, platelets, dead cells, cell debris, and neutrophils. Reference fluorescent beads (Flow Count beads; Beckman‐Coulter, Fullerton, CA, USA) were used to obtain absolute cell count, subsequently excluding hematopoietic cells expressing the CD45 antigen. Endothelial progenitors were defined as negative for the hematopoietic marker CD45, positive for the endothelial cell markers CD34 and CD31, and positive for the CEP marker CD133. A direct lyse–no‐wash procedure was used to avoid cell and bead loss. Each sample was analyzed for a minimum of 300 000 total events by flow cytometry. Data were analyzed in duplicate by the same investigator using Expo 32 software (Beckman‐Coulter).
Absolute CEP numbers (cell/μL) were calculated using the following formula: number of measured CEPs/number of fluorescent beads counted × number of beads/μL.
Statistical analysis
Statistical analysis was carried out using the (paired) t‐test and Pearson's correlation when data were normally distributed. Non‐parametric analysis of the Wilcoxon signed rank test was carried out for other distributions. The univariable relationship between two independent categorical variables was examined using either the chi‐square‐test or Fisher's exact test. A receiver operating characteristic (ROC) analysis applied to response data was used to detect the best cut‐off value for the percent change in CEP number between day 1 and just before the second cycle of therapy. The patients were divided into two groups according to the best cut‐off values of the percent changes in CEP number. For analysis of responses to chemotherapy, a multivariable logistic regression model was applied to estimate odds ratios and 95% confidence intervals. The variables were selected through backward procedures with a cut‐off P‐value < 0.10. To evaluate risk factors associated with PFS, a Cox proportional hazards regression model was used. A PFS curve was estimated using the Kaplan–Meier method and evaluated using the log–rank test. All statistical analyses were carried out using JMP 8.0.2 software (SAS Institute, Cary, NC, USA). Error bars shown are one SD. A P‐value (two‐sided) <0.05 was considered significant.
Results
Patient background
From April 2009 to June 2010, 38 patients with NSCLC received cytotoxic chemotherapy. The clinical characteristics of the patients are summarized in Table 1. All patients were Japanese and included 23 (60.5%) men and 15 (39.5%) women, with a median age of 65.5 years (range, 41–77). Twenty‐four (63.2%) patients were former or current smokers, and 14 (36.8%) patients have never smoked. The Eastern Cooperative Oncology Group performance status was 0–1 for 35 patients and 2–3 for three patients. Each patient (2.6%) was determined stage IIIA and IIIB, respectively. There were 36 patients (94.7%) classified as stage IV. Thirty‐three patients (86.8%) were diagnosed with adenocarcinoma. Another patient group (13.2%) was diagnosed with squamous cell carcinoma. Thirty‐one patients (81.6%) received platinum‐based chemotherapy: CBDCA + PEM (carboplatin, area under the curve [AUC] 6 (mg/L)h on day 1; pemetrexed, 500 mg/m2 on day 1); CBDCA + PAC (carboplatin, AUC 6 (mg/L)h on day 1; paclitaxel, 210 mg/m2 on day 1); CBDCA + GEM (carboplatin, AUC 6 (mg/L)h on day 1; gemcitabine, 1000 mg/m2 on days 1 and 8); CDGP + GEM (nedaplatin, 70 mg/m2 on day 8; gemcitabine, 800–1000 mg/m2 on days 1 and 8). Seven patients (18.4%) received pemetrexed (500 mg/m2 on day 1) or docetaxel monotherapy (60 mg/m2 on day 1). Each course lasted 3 weeks.
Table 1.
Characteristics of patients with non‐small cell lung cancer (n = 38)
| Variables | Total N = 38 |
|---|---|
| Gender, n (%) | |
| Male (%) | 23 (60.5) |
| Female (%) | 15 (39.5) |
| Age, years | |
| Median | 65.5 |
| Range | 41–77 |
| ECOG PS, n (%) | |
| 0–1 (%) | 35 (92.1) |
| ≥2 (%) | 3 (7.9) |
| Smoking history, n (%) | |
| Never (%) | 14 (36.8) |
| Former + current (%) | 24 (63.2) |
| Disease stage at commencement of therapy, n (%) | |
| IIIA | 1 (2.6) |
| IIIB | 1 (2.6) |
| IV (%) | 36 (94.7) |
| Histology, n (%) | |
| Adenocarcinoma | 33 (86.8) |
| Squamous cell carcinoma | 5 (13.2) |
| EGFR status, n (%) | |
| Mutation | 11 (28.9) |
| Unknown or wild type | 27 (71.1) |
| Treatment regimen, n (%) | |
| CBDCA + PEM | 23 (60.5) |
| CBDCA + PAC | 3 (7.9) |
| CBDCA + GEM | 1 (2.6) |
| CDGP + GEM | 4 (10.5) |
| PEM | 6 (15.8) |
| DOC | 1 (2.6) |
| Best response to chemotherapy | |
| PR | 16 (42.1) |
| SD | 15 (39.5) |
| PD | 7 (18.4) |
CBDCA, carboplatin; CDGP, nedaplatin; DOC, docetaxel; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; GEM, gemcitabine; PAC, paclitaxel; PD, progressive disease; PEM, pemetrexed; PR, partial response; SD, stable disease.
Sixteen (42.1%) of 38 patients had a PR, whereas seven patients (18.4%) had PD.
Kinetics of CEPs during the first cycle of chemotherapy and association between changes in CEPs and chemotherapy regimen
Overall, the number of CEPs significantly decreased at day 8 after chemotherapy compared with the number of CEPs at day 1 of chemotherapy (Fig. 1A) (P < 0.0001, Wilcoxon test). However, the number of CEPs significantly increased before the second cycle of treatment relative to the number of CEPs at day 1 (Fig. 1A) (P = 0.004, Wilcoxon test).
Figure 1.
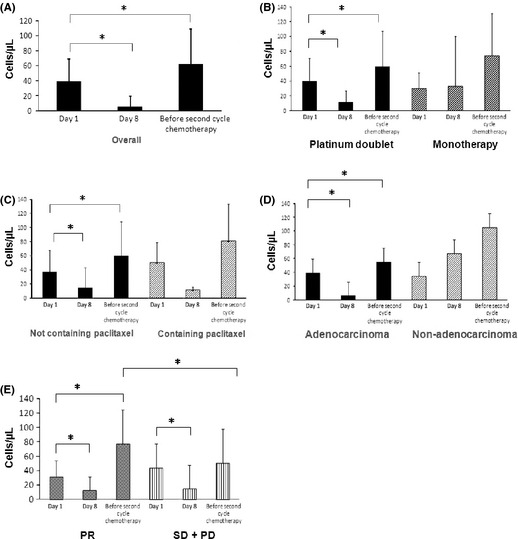
(A) Circulating endothelial progenitor cell (CEP) kinetics during the first cycle of chemotherapy in patients with non‐small‐cell lung cancer. A significant decrease was seen at day 8 (n = 37) and before the second cycle of chemotherapy (n = 38). (B) Number of CEPs (cells/μL) in patients treated with the platinum doublet regimen (n = 32, P < 0.0001 and n = 33, P = 0.0276). Number of CEPs (cells/μL) in patients treated with cytotoxic monotherapy. (C) Number of CEPs (cells/μL) in patients treated with regimens not containing PAC (cisplatin, doxorubicin, cyclophosphamide) (n = 34, P < 0.0001 and n = 35, P = 0.0131). (D) Number of CEPs (cells/μL) in patients with adenocarcinoma and non‐adenocarcinoma patients (P < 0.0001 and P = 0.0466, respectively). (E) Number of CEPs (cells/μL) in patients with partial response (PR) and stable disease (SD) + progressive disease (PD) patients (P = 0.0264). *P < 0.05, Wilcoxon test, paired t‐test, or t‐test.
These changes were also seen when stratified by platinum doublet therapy and monotherapy (Fig. 1B). There were significant changes at day 8 and before the second cycle of chemotherapy compared with day 1 CEPs in the group receiving platinum doublet therapy (Fig. 1B) (P < 0.0001 and P = 0.0276, respectively, Wilcoxon test) but not in the monotherapy regimen group.
In the group not treated with the PAC regimen (cisplatin, doxorubicin, cyclophosphamide), there were significant changes in the number of CEPs at day 8 and before the second cycle of chemotherapy compared with day 1 (Fig. 1C) (P < 0.0001 and P = 0.0131, respectively, Wilcoxon test).
In the group diagnosed with adenocarcinoma, there were significant changes in the number of CEPs at day 8 and before the second cycle of chemotherapy compared with day 1 (Fig. 1D) (P < 0.0001 and P = 0.0466, respectively, Wilcoxon test).
Changes in CEP levels after chemotherapy associated with response and PFS
The CEP counts at day 1 did not correlate with either the percent tumor shrinkage after two cycles of chemotherapy (according to RECIST) or the best reduction rate in tumor volume. There was no significant correlation between the logarithm of changes in CEP number during one cycle of chemotherapy and tumor shrinkage after two cycles of chemotherapy or the best reduction rate in tumor volume (Fig. 2). Changes in CEP number at day 8 did not correlate with response to chemotherapy.
Figure 2.
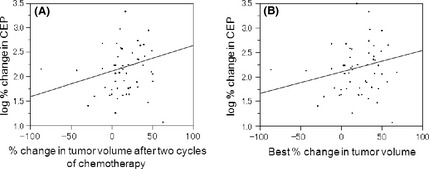
Correlation between log% change in circulating endothelial progenitor cells (CEPs) and the percent tumor shrinkage according to RECIST after two cycles of chemotherapy (A) or the best percent tumor shrinkage during chemotherapy (B) in patients with non‐small‐cell lung cancer (n = 38). Pearson R = 0.005 and 0.004, respectively.
In a stratified analysis by best response to chemotherapy (Fig. 1E), there was a significant change in CEP number at day 8 and before the second cycle of chemotherapy compared with CEP counts at day 1 in PR patients (P = 0.0103 and P = 0.0021, respectively, Wilcoxon test). However, in the group containing both SD and PD patients (SD + PD), the level of CEPs was significantly decreased only at day 8 (P = 0.0003, Wilcoxon test). The level of CEPs before the second cycle of chemotherapy and at day 1 differed significantly between patients with PR and SD + PD patients (P = 0.0264, Wilcoxon test). In PR patients, the percentage change in CEPs before the second cycle of chemotherapy was higher than that in SD + PD patients (Table 2, 439.0% vs 143.0%; P = 0.0130, Wilcoxon test).
Table 2.
Relationship between characteristics of patients with non‐small‐cell lung carcinoma and response to chemotherapy (n = 38)
| Variables |
PR n = 16 |
SD + PD n = 22 |
P‐value |
|---|---|---|---|
| Gender | |||
| Male/female | 13/3 | 10/12 | 0.0258a |
| Age, years | |||
| Median | 64 | 67 | 0.9777 |
| Range | 42–77 | 41–77 | |
| <70 years old/≥70 years old | 12/4 | 15/7 | 0.6473 |
| ECOG PS | |||
| 0–1/≥2 | 15/1 | 20/2 | 0.7485 |
| Smoking history | |||
| Never/former + current | 3/13 | 11/11 | 0.0486a |
| Histology | |||
| Adenocarcinoma/squamous cell carcinoma | 13/3 | 20/2 | 0.6318 |
| EGFR status | |||
| Mutation/unknown or wild type | 4/12 | 7/15 | 0.7292 |
| Treatment regimen | |||
| Platinum doublet/monotherapy | 13/3 | 20/2 | 0.6318 |
| Not PAC regimen/PAC regimen | 0/16 | 3/19 | 0.2489 |
| CEP day 1 (cells/μL) | |||
| Mean, standard deviation | 31.7 (22.4) | 43.6 (33.7) | 0.2270 |
| CEP before 2nd chemotherapy (cells/μL) | |||
| Mean, standard deviation | 77.3 (11.7) | 50.2 (10.1) | 0.0264a |
| % change in CEPb | |||
| Mean, standard deviation | 439.0 (514.3) | 143.0 (121.6) | 0.0130a |
| High/lowc | 12/4 | 4/17 | 0.0014a |
ECOG PS, Eastern Cooperative Oncology Group performance status; PAC, paclitaxel; PD, progressive disease; PR, partial response; SD, stable disease.
P < 0.05 in chi‐square‐test, Fisher's exact test, paired t‐test, or t‐test.
Percentage change in circulating endothelial progenitor cells (CEPs) is the ratio of CEP numbers just before the second cycle of therapy to CEP numbers on day 1.
Cut‐off value between high and low CEP% changes was determined from a receiver operating characteristic curve.
A ROC curve was generated to determine a cut‐off value (168.7%) between the higher and lower percentage changes in CEP number before the second cycle of chemotherapy (Fig. 3). In terms of the percent changes in CEPs, there were 17 patients above the cut‐off point and 21 patients below it. The response rate was higher in patients with high percentage changes in CEP number (>168.7%), which was statistically significant (70.6% vs 19.1%; P = 0.0014, χ2‐test). The number of male patients (P = 0.0258, χ2‐test) in PR cases was significantly higher than in SD + PD cases (Table 2). Among the clinical characteristics studied, including percentage changes in CEP number before the second cycle of chemotherapy, three factors were selected through backward procedures using a cut‐off P‐value < 0.10. Using a multivariable logistic regression model analysis, it was found that males with large percentage changes in CEP number before the second cycle of chemotherapy (odds ratio, 10.200; 95% confidence interval, 2.447–52.06; P = 0.0011) had a favorable response to chemotherapy (Table 3).
Figure 3.
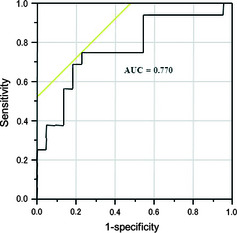
Receiver operating characteristic curve generated to define the cut‐off value between the higher and lower percentage changes in circulating endothelial progenitor cell numbers before the second cycle of chemotherapy. The cut‐off value is calculated to yield the optimal sensitivity and specificity to distinguish partial response + stable disease from progressive disease. At this threshold, the cut‐off value is 168.6%, the sensitivity is 75.0%, and the specificity is 77.3%. AUC, area under the curve.
Table 3.
Multivariate analysis for best response to therapy in patients with non‐small‐cell lung carcinoma (n = 38)
| Variables | OR (95% CI) | P‐value |
|---|---|---|
| % change in CEP (high/low) | 10.200 (2.447–52.06) | 0.0011a |
CEP, circulating endothelial progenitor cell; CI, confidence interval; OR, odds ratio.
P < 0.05, logistic regression model analysis.
Subsequently, using the univariate Cox proportional hazard model, we examined whether CEP levels, at baseline and/or consecutive time points after chemotherapy, could predict PFS. At baseline, day 8, and before the second cycle of chemotherapy, no association between PFS and CEP status was observed (P > 0.05; data not shown). Furthermore, clinical characteristics, including gender, performance status, smoking history, histology, epidermal growth factor receptor mutation status, or chemotherapy regimen, did not predict PFS in a univariate analysis (P > 0.05; data not shown). Patients with high percentage changes in CEP number before the second cycle of chemotherapy relative to day 1 levels did not have a significantly longer median PFS than those with low percentage changes in CEP number (P = 0.2951, log–rank test) (Fig. 4).
Figure 4.
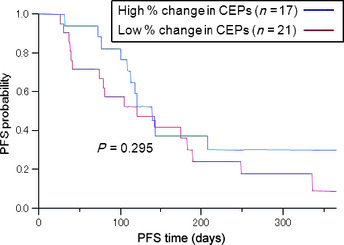
Progression‐free survival (PFS) for patients with non‐small‐cell lung cancer with high (n = 17) and low (n = 21) percentage changes in circulating endothelial progenitor cell (CEP) number before the second cycle of chemotherapy. Patients with high percentage changes in CEPs did not have a significantly longer PFS than those with low percentage changes, as determined by a log–rank test (high versus low, 139 vs 120 days, P = 0.295).
Discussion
We showed that CEPs were significantly decreased at day 8 after chemotherapy and increased just before the second course of chemotherapy in NSCLC patients. Significant CEP changes were also observed when we analyzed patients receiving the platinum and non‐platinum regimens and patients diagnosed with adenocarcinoma and squamous cell carcinoma, as well as those who did not receive the CBDCA + PAC regimen. In contrast, no significant changes in CEP levels were observed in patients who received the PAC regimen, mainly because of a small sample size (n = 3). In previous reports, it was shown that CEPs home to the tumor immediately after taxane‐containing chemotherapy.9, 11 However, our data showed that CEPs decreased on day 8 regardless of paclitaxel‐containing regimen. This may be due to the small sample size of patients receiving the paclitaxel‐containing regimen, and the myelosuppressive effects of conventional chemotherapy. Stoelting et al.10 reported that CEP counts were significantly decreased at day 10 compared with those of baseline in 24 patients receiving conventional chemotherapy (maximum tolerated dose), who were diagnosed with breast cancer or lymphoma. However, increased levels of CEP were observed just before the second cycle of chemotherapy regardless of chemotherapy regimen. In preclinical settings, we have shown that mobilization of CEPs was induced by the upregulation of various cytokines such as SDF‐1 after chemotherapy, including certain drugs such as paclitaxel and 5‐fluorouracil.9 However, in the clinical setting, numbers of CEPs, and G‐CSF and VEGF levels were increased after other chemotherapy regimens not including paclitaxel.10, 11 This suggests that various types of cytotoxic drugs can induce CEP mobilization by release of cytokine and growth factor. There are few reports about the kinetics of CEPs after cytotoxic chemotherapy. Our study is the first to identify the implications of CEP kinetics specifically in patients with NSCLC receiving cytotoxic chemotherapy.
Recent studies have shown that tumor vasculature can arise through the recruitment and differentiation of bone marrow‐derived endothelial progenitor cells into mature endothelial cells,17 suggesting that the CEP response may be important in predicting patient outcome. In this study, we showed that the degree of increase in CEP number between day 1 and before the second cycle of chemotherapy was significantly associated with the response to chemotherapy. Furthermore, the high percentage change in CEP number after one cycle of chemotherapy was an independent predictive factor for the response to chemotherapy. Several studies on the relationship between CEPs and the response to cytotoxic chemotherapy in a variety of tumor types have been reported.8, 11, 12, 18 Dome et al.8 showed that during anticancer treatment (e.g. cytotoxic chemotherapy, chemoradiotherapy, and surgery) CEP numbers decreased in the responder population, but increased in non‐responders. In other studies that included patients with different types of cancer, no significant correlation was observed between CEP number and the response to chemotherapy alone.12 The discrepancy between our results and those of previous reports may be due to the other studies' inclusion of a variety of cancer types, treatment regimens, and disease stages. We found that the increase in CEP number after chemotherapy without anti‐angiogenic agents was significantly associated with tumor shrinkage and was deemed an independent predictive factor for response.
However, our data did not show any association between the high percentage change in CEP number during the first cycle of chemotherapy and PFS. The production and release of cytokines, chemokines, and proteolytic enzymes such as VEGF, SDF‐1, and MMP by lung cancer tissues mobilize CEPs in the bone marrow. Mobilized CEPs may home to tumor tissue and play important roles in tumor neovascularization, metastasis, and progression.18, 19 In a recent study of cancer patients receiving cytotoxic chemotherapy, increased levels of CEPs at day 7 after chemotherapy predicted PFS and overall survival, regardless of the tumor type or chemotherapy regimen. This result suggests that CEPs, which form vessels in tumor tissues, may alter both the delivery of anticancer drugs and subsequent cytotoxicity at an early stage after chemotherapy, but still contribute to progression or metastasis over time. Preclinical evidence shows that anti‐angiogenic therapy could blunt the release of CEPs by (chemo)therapy.9, 20 Perhaps this inhibition of CEP release provides an additional explanation for the synergistic efficacy of anti‐angiogenic agents and chemotherapeutic regimens. These findings suggest that combining chemotherapy with agents capable of inhibiting the release of progenitor cells may be a beneficial therapeutic strategy.
A limitation of this study is the small sample size and the heterogeneous chemotherapy regimens included. There were significant changes in the number of CEPs at day 8 and before the second cycle of chemotherapy compared with day 1 in the regimen with PAC. Figure 1(C) seems to indicate the limitation of sample size in this pooled analysis of various patients with different clinical backgrounds. However, we did find that the host bone marrow response was independent of the type of chemotherapy. Furthermore, certain types of chemotherapy that are associated with high response rates in adenocarcinoma could be confounders in the analysis.21 Clinical characteristics, including tumor histology and chemotherapy regimen (platinum doublet or monotherapy), were not predictive of response in univariate and multivariate analyses. This conclusion suggests that our results were not influenced by specific subgroups. There is still some controversy over the definition of CEPs. Unique markers have not yet been reported, and functional characterization of rare putative populations by FACS analysis is difficult to undertake for a large dataset.
In conclusion, we show that cytotoxic chemotherapy without anti‐angiogenic agents evokes CEP release. A large increase in the number of CEPs following such a therapy positively correlates with the response to therapy. These findings identify a potential predictive marker for response to chemotherapy and highlight new opportunities to enhance chemotherapeutic efficacy through the inhibition of released progenitor cells. To confirm that CEPs can be used as early predictors of response to therapy, prospective studies should have more stringent inclusion criteria and a larger cohort of patients.
Disclosure Statement
The authors have no conflicts of interest.
References
- 1. Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med 2005; 172: 523–9. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol 2005; 23: 3243–56. [DOI] [PubMed] [Google Scholar]
- 3. Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 2008; 319: 195–8. [DOI] [PubMed] [Google Scholar]
- 4. Asahara T, Murohara T, Sullivan A et al Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–7. [DOI] [PubMed] [Google Scholar]
- 5. Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti‐angiogenesis therapy? Nat Rev Cancer 2002; 2: 826–35. [DOI] [PubMed] [Google Scholar]
- 6. Lyden D, Hattori K, Dias S et al Impaired recruitment of bone‐marrow‐derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001; 7: 1194–201. [DOI] [PubMed] [Google Scholar]
- 7. Nowak K, Rafat N, Belle S et al Circulating endothelial progenitor cells are increased in human lung cancer and correlate with stage of disease. Eur J Cardiothorac Surg 2010; 37: 758–63. [DOI] [PubMed] [Google Scholar]
- 8. Dome B, Timar J, Dobos J et al Identification and clinical significance of circulating endothelial progenitor cells in human non‐small cell lung cancer. Cancer Res 2006; 66: 7341–7. [DOI] [PubMed] [Google Scholar]
- 9. Shaked Y, Henke E, Roodhart JM et al Rapid chemotherapy‐induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 2008; 14: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stoelting S, Trefzer T, Kisro J, Steinke A, Wagner T, Peters SO. Low‐dose oral metronomic chemotherapy prevents mobilization of endothelial progenitor cells into the blood of cancer patients. In Vivo 2008; 22: 831–6. [PubMed] [Google Scholar]
- 11. Roodhart JM, Langenberg MH, Vermaat JS et al Late release of circulating endothelial cells and endothelial progenitor cells after chemotherapy predicts response and survival in cancer patients. Neoplasia 2010; 12: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vizio B, Novarino A, Giacobino A et al Pilot study to relate clinical outcome in pancreatic carcinoma and angiogenic plasma factors/circulating mature/progenitor endothelial cells: preliminary results. Cancer Sci 2010; 101: 2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstraw P, Crowley J, Chansky K et al The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 14. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 15. Mancuso P, Calleri A, Cassi C et al Circulating endothelial cells as a novel marker of angiogenesis. Adv Exp Med Biol 2003; 522: 83–97. [DOI] [PubMed] [Google Scholar]
- 16. Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc 2007; 2: 805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davidoff AM, Ng CY, Brown P et al Bone marrow‐derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. Clin Cancer Res 2001; 7: 2870–9. [PubMed] [Google Scholar]
- 18. Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti‐angiogenic therapies. Drug Resist Updat 2008; 11: 219–30. [DOI] [PubMed] [Google Scholar]
- 19. Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358: 2039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaked Y, Ciarrocchi A, Franco M et al Therapy‐induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 2006; 313: 1785–7. [DOI] [PubMed] [Google Scholar]
- 21. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]


