Abstract
We conducted a retrospective Japan–Korea multicenter study to better elucidate the clinicopathologic features and therapeutic modalities for aggressive natural killer cell leukemia (ANKL). A total of 34 patients were analyzed. The median age of the patients was 40 years. Among the patients in the study, four had a history of Epstein–Barr virus‐related disorders. Three types of ANKL cells were categorized according to their morphological features. Leukemic cells were below 20% in both peripheral blood and bone marrow of 11 patients. The clinical characteristics and prognoses of these 11 patients did not differ significantly from those of the others. As an initial therapy, l‐asparaginase chemotherapy resulted in a better response. A total of six patients received allogeneic hematopoietic stem cell transplantation (HSCT) and two received autologous HSCT, with all in non‐complete remission (CR). After HSCT, four with allogeneic and one with autologous HSCT reached CR. Median survival of all patients was 51 days. Median survival for the patients with and without HSCT were 266 and 36 days, respectively. A total of two patients with allogeneic HSCT were alive and in CR. All patients without HSCT died of ANKL. The use of l‐asparaginase was indicated as a factor for longer survival (HR 0.33, 95% confidence interval; 0.13–0.83, P = 0.02). Early diagnosis of ANKL, l‐asparaginase‐based chemotherapy and allogeneic HSCT might lead to improved patient outcomes. (Cancer Sci 2012; 103: 1079–1083)
Aggressive natural killer cell leukemia (ANKL) is a malignant disorder of mature natural killer (NK) cells that is relatively common in East Asia and is closely associated with Epstein–Barr virus (EBV).1, 2, 3 The prognosis of ANKL is dismal and the survival rate is one of the worst among the lymphoid neoplasms. Previous studies on ANKL are mostly limited to the anecdotal, except for a few single‐institution case series and two multicenter studies.4, 5, 6, 7, 8 Therefore, the characteristics and optimal management of this disease are still unclear. Genetic alternations for ANKL, downregulation of PRDM1, overexpression of micro‐RNA (miR) 21 and miR 155, and high expression of survivin have been shown as molecular background factors,9, 10, 11, 12 although most studies have been based on concomitant analyses with other mature NK‐cell neoplasms. Because of its rarity and dismal prognosis, it is a challenge to diagnose ANKL, especially at initial presentation, and to optimally manage it. To clarify the pathological and clinical profiles and the effects of therapeutic modalities on survival of ANKL, we conducted a retrospective multicenter study (ANKL07) in Japan and Korea.
Patients and Methods
Patient selection criteria
Patients diagnosed with ANKL between 1985 and 2007 were selected from the medical records at each institution. The diagnosis of ANKL was based on the 2001 World Health Organization (WHO) classification.13 In ANKL, granular lymphocytes with immunological phenotypes of NK cells increase in peripheral blood (PB) or bone marrow (BM). The diagnosis in the medical charts, such as granular lymphocyte leukemia, NK‐cell type, was also included. In typical cases, ANKL has a rapidly progressive clinical course as is associated with multi‐organ disorders. Although ANKL is highly associated with EBV, the positivity of EBV is not necessary for the diagnosis of ANKL.
Methods
A survey form inquiring about the existence of records of patients meeting the selection criteria was distributed to the 16 institutes participating in this study (primary survey). Using the form, the presence of eligible patients was reported to the study coordinator (FI) and, if the patients were eligible, the secondary form was completed with their data and returned to the data analyzer (RS). The form includes clinical data at diagnosis, information on chemotherapy and hematopoietic stem cell transplantation (HSCT), and outcome. Histopathological specimens at diagnosis were also collected. Responses were assessed by each institution based on WHO response criteria with modifications.14, 15
Central reviews were performed first in each country; then, a combined review was performed. Three hematopathologists (JS, YHK and SN) reviewed the specimens and three hematologists (RS, WSK, and FI) reviewed the clinical data. Discrepancies were discussed until consensus was reached. The present study was conducted by the NK‐cell Tumor Study Group and approved by the institutional review board of each participating institute.
Statistical analysis
Differences in characteristics between two or more groups were examined using the chi‐square test, the Fisher exact test or the Mann–Whitney U‐test. Patient survival data were analyzed using the Kaplan–Meier method and differences in survival were tested using the log‐rank test. Factors affecting prognosis were analyzed using the Cox proportional hazard model. These analyses were performed using Statistical Analysis version 1.5 (Esumi, Tokyo, Japan) or STATA software (version 11; STATA, College Station, TX, USA).
Results
Patient registration
A total of 16 institutes from Japan and Korea participated in this study, with 41 patients registered. Some of the patients are included in previous reports.6, 7, 16, 17 After central reviews, seven patients were excluded due to potential T‐cell origin, existence of nasal lesions or insufficient data, with the data of 34 patients further analyzed. All patients were of East Asian origin.
Morphological findings
The fundamental feature of ANKL cells is the appearance of mature lymphoid cells with cytoplasmic granules. Substantial heterogeneities were recognized in the details of the structures. We categorized ANKL cells into three types depending on the nuclear and cytoplasmic ratio, the nuclear shape, the color of the cytoplasm, the existence of nucleoli and the size of the cells on May–Giemsa staining of PB or BM smear specimens (Fig. 1). Type I has large granular lymphocyte (LGL) appearance, in which the cytoplasm is slightly basophilic and has larger cytoplasmic granules than in normal LGL. On some occasions, granules are hardly recognized. In type III, the cells exhibit pleomorphic‐like appearance, together with basophilic cytoplasm and a bizarre nucleus containing one or two nucleoli. Type II is a mixture of type I and type III in each patient, or intermediate characteristics, which include monocyte‐like features. For these types, there were 13, 10 and 11 patients recognized, respectively. We compared clinical characteristics, including survival time, among these three types. Differences were not recognized except for the incidence of hemophagocytosis (HPS). HPS was recognized in 3, 8 and 8 patients, respectively (P = 0.04).
Figure 1.
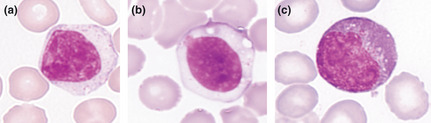
Morphology of aggressive NK‐cell leukemia cells. Three types of leukemic cells were classified. Type I (a) are large granular lymphocytes. Type III (c) are pleomorphic and atypical cells with prominent nucleoli. Type II (b) shows intermediate morphology between types I and III.
Clinical characteristics and laboratory data
Demographics for the 34 patients are presented in Table 1. Median age was 40 years and distributed in a pattern with two peaks of younger and elderly populations (Fig. 2). Male patients were predominant. Four patients were suspected to have had previous EBV‐related disorders, such as mosquito bite hypersensitivity, chronic active EBV infection or liver dysfunction. It was noteworthy that three female patients developed ANKL during pregnancy. All patients suffered from fever and some showed other B symptoms. Performance status (PS) was generally poor. Most of the patients presented hepatomegaly and/or splenomegaly and occasionally lymphadenopathy or serous effusions. Skin lesions were rare. A total of 19 patients (56%) also showed HPS.
Table 1.
Demographics
| Age (median, years) | 40 (16–76) |
| Sex: male/female | 26/8 |
| Fever | 34 (100%) |
| Hepatomegaly | 24 (71%) |
| Splenomegaly | 24 (71%) |
| Lymphadenopathy | 9 (26%) |
| Skin lesion | 3 (9%) |
| Pleural effusion/ascites | 6 (18%) |
| Performance status ≥ 2 | 14 (41%) |
Figure 2.
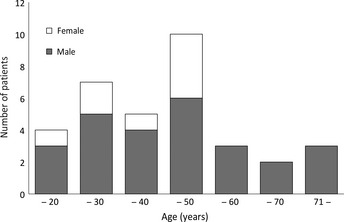
Age and sex distribution of the patients. Age range is indicated on the abscissa; the number of the patients is shown on the ordinate.
In terms of the laboratory data (Table 2), thrombocytopenia and serum lactose dehydrogenase elevation were recognized in the vast majority of patients. In 85% of patients, the tumor cells were positive for EBV using the EBV‐encoded small RNA (EBER) in situ hybridization method or the PCR method. Chromosomal abnormalities by G‐banding method were found in 57% of the patients analyzed. There were four patients with chromosome 6q‐related changes. Immunophenotypes of the tumor cells by flow cytometric analyses were typically positive for CD2, CD16, CD56 and HLA‐DR, and were negative for CD3, CD4, CD5, CD8 and CD57. In 72% of the patients examined, CD7 was also positive.
Table 2.
Laboratory data
| WBC; median (range) (×109/L) | 3.2 (0.38–26.95) |
| Platelets < 150 × 109 (/L) | 31 (91%) |
| LDH > normal | 32 (94%) |
| EBV‐positive | 28 (85%) |
| Chromosomal analysisa | |
| Abnormal karyotype | 17 (57%) |
| Normal karyotype | 13 (43%) |
With G‐banding method. EBV, Epstein–Barr virus; LDH, lactose dehydrogenase; WBC, white blood cell count.
In terms of leukemia, the proportion of tumor cells in PB and BM is of concern. The median proportions of leukemic cells in PB and BM were 8 (range: 0–91) and 22% (range: 1–72), respectively. Of patients, 11 presented with <20% leukemic cells in both PB and BM. When these patients were compared with 23 other patients with more than 20% leukemic cells, characteristics of the patients with fewer leukemic cells in PB and BM did not differ, except for the frequency of HPS (Table 3). The Kaplan–Meier survival estimate of all patients is shown in Figure 3 and the median survival time is 51 days (range: 1–1630).
Table 3.
Characteristics in relation to aggressive natural killer leukemia cells in peripheral blood and bone marrow
| <20% (N = 11)a | ≥20% (N = 23)b | P‐value | |
|---|---|---|---|
| Age (median, years) | 48 | 36 | 0.28 |
| Male/female | 7/4 | 18/5 | 0.72 |
| Splenomegaly | 7 | 21 | 0.07 |
| Performance status ≥ 2 | 6 | 8 | 1 |
| EBV‐positive/negative | 8/11 | 20/22 | 0.3 |
| Hemophagocytic syndrome | 9/9 | 10/20 | 0.01c |
| WBC count (median; ×109/L) | 2.42 | 3.59 | 0.27 |
| CD16‐positive | 7/8 | 21/22 | 0.47 |
| Survival (median; days) | 35 | 76 | 0.09 |
Tumor cells <20% in both peripheral blood (PB) and bone marrow (BM).
Tumor cells more than 20% in either PB or BM.
Statistically significant.
EBV, Epstein–Barr virus; WBC, white blood cell count.
Figure 3.
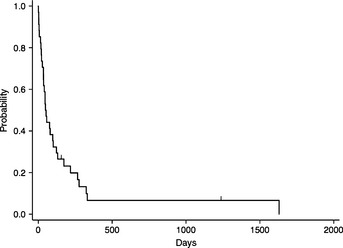
Survival curve of all patients with aggressive natural killer cell leukemia.
Chemotherapy
As initial chemotherapies, 18 patients received multi‐agent chemotherapy, while the remaining 16 patients had no chemotherapy, single agent or corticosteroid, mainly because of poor general conditions or earlier study periods. Anthracycline‐based regimens, mainly the CHOP regimen, were used in 13 patients. l‐asparaginase (l‐asp)‐containing chemotherapies were administered in five patients. One patient each reached CR with anthracycline‐based and l‐asp‐containing regimen. Three patients each with different medications showed partial responses to the therapy. There was no difference in overall survival (OS) (P = 0.64) with or without anthracyclines (Fig. 4a). OS was extended significantly with the l‐asp‐containing regimen (P = 0.03, log‐rank test) (Fig. 4b). The regimens including l‐asp varied and were generally combined with multiple drugs, such as etoposide, methotrexate, ifosfamide, cytosine arabinoside or corticosteroid, without anthracyclines.18, 19
Figure 4.
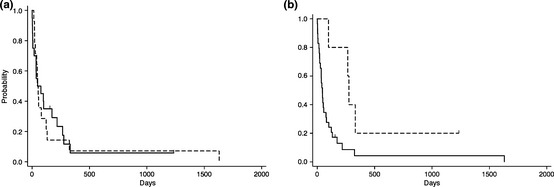
Initial chemotherapy for aggressive natural killer cell leukemia. (a) Survival curve with (dotted line) or without (solid line) anthracycline‐based regimens. (b) Survival curve with (dotted line) or without (solid line) l‐asparaginase‐combined chemotherapy.
Hematopoietic stem cell transplantation
A total of nine HSCT, two autologous (auto) and seven allogeneic (allo), were performed in eight patients; all were not in complete remission (CR) at the time of transplant. For allo‐HSCT, the donor source was human leukocyte antigen (HLA)‐matched related bone marrow in 2, cord blood in 2, peripheral blood stem cell (PBSC) from HLA‐matched unrelated donor in 1, and HLA‐mismatched related PBSC in 2. The preconditioning regimen was a standard cyclophosphamide and total body irradiation in 4, and a fludarabine‐based regimen with melphalan in 2. After HSCT, one patient with auto‐HSCT and four with allo‐HSCT reached CR.
All 26 patients without HSCT died within 12 months, and median survival was 36 days (range: 1–324). In contrast, the median survival of the patients who received HSCT was 266 days (range: 80–1630) (Fig. 5). Of these patients, two were alive and in CR and one died of sepsis while in CR. The other five died of disease. The living patients had received l‐asp‐containing chemotherapy as initial or secondary treatment and then proceeded to allo‐HSCT with a myeloablative‐conditioning regimen within 80 days of the initial diagnosis of ANKL.
Figure 5.
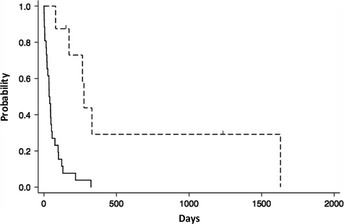
Hematopoietic stem cell transplantation (HSCT) for aggressive natural killer cell leukemia. Survival curve of the patients without HSCT (solid line, N = 26) and the patients who received HSCT (dotted line, N = 8).
Factors affecting the prognosis
In univariate analysis, l‐asparaginase administration was the only clinical factor associated with better survival (hazard ratio [HR]: 0.29, 95% confidence interval [CI]: 0.12–0.70, P = 0.008). Age (HR: 1.02, 95% CI: 0.998–1.08, P = 0.07) and the use of etoposide (HR: 0.48, 95% CI: 0.22–1.07, P = 0.07) were marginally significant (Table 4). Multivariate Cox analysis indicated that the use of l‐asparaginase was an independently significant factor for better survival (HR: 0.33, 95% CI: 0.13–0.83, P = 0.02) (Table 5).
Table 4.
Univariate analysis of factors contributing to survival
| Characteristic | Hazard ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Age | 1.02 | 0.998–1.06 | 0.07 |
| Sex | 0.98 | 0.40–2.43 | 0.97 |
| Log (WBC count) | 1.01 | 0.49–2.05 | 0.99 |
| IPI | 1.29 | 0.83–2.01 | 0.25 |
| EBV positivity | 1.31 | 0.52–3.27 | 0.56 |
| Hemophagocytosis | 1.41 | 0.67–2.96 | 0.35 |
| Cell morphology | 1.1 | 0.67–2.96 | 0.35 |
| Anthracycline | 1.08 | 0.51–2.28 | 0.84 |
| Corticosteroid | 0.47 | 0.11–1.97 | 0.29 |
| Etoposide | 0.48 | 0.22–1.07 | 0.07 |
| l‐asparaginase | 0.29 | 0.12–0.70 | 0.008a |
Statistically significant.
EBV, Epstein–Barr virus; IPI, international prognostic index; WBC, white blood cell count.
Table 5.
Multivariate analysis of factors contributing to survival
| Characteristic | Hazard ratio | 95% confidence interval | P‐value |
|---|---|---|---|
| Age | 1.02 | (0.99–1.04) | 0.15 |
| Etoposide | 0.86 | (0.35–2.10) | 0.74 |
| l‐asparaginase | 0.33 | (0.13–0.83) | 0.02a |
Statistically significant.
Discussion
This study is the largest case series of ANKL in the literature with central reviews. From this study, ANKL could be divided into several groups in terms of the age at onset and background. ANKL in adolescents and young adults is a major group, with the peak age in the twenties. Men and women are affected almost equally and a small population has an associated history of EBV‐related disorders. Some female patients develop ANKL during pregnancy. The second population with ANKL is an elderly group aged from their fifties to their seventies, with male predominance. However, age was not a significant factor for survival in this study. Further investigation is necessary concerning the clinical relevance of age in ANKL.
It is of interest to compare the biological behavior of ANKL from the perspective of EBV. A literature review of ANKL show that <20% of ANKL cases were negative for EBV.5, 6 There were no differences in clinical characteristics between EBV‐positive and EBV‐negative ANKL in the present study (data not shown). Still, in the case of EBV‐negative ANKL, caution must be taken in diagnosis. In our series, leukemic cells were essentially positive for EBV according to the EBER in situ hybridization method and/or the Southern blot method, and there were six patients positive for EBV DNA, detected using the PCR method. It remains uncertain whether they all had EBV‐positive tumor cells or whether some were positive by chance, although most of the patients had significantly high values of EBV DNA copies.
Relationships between ANKL and ENKL have been discussed in the published literature, especially in advanced stage lymphoma.20 The differences between stage IV ENKL and ANKL are clarified by Suzuki et al. (21).21 According to Suzuki et al., ANKL is associated with higher rates of liver, BM and PB involvement. There are no nasal lesions, in contrast to ENKL. In addition, ANKL is immunophenotypically CD16‐positive. These findings are consistent with those in the present study. In addition, nodular lesions are frequent as hepatic lesions in ENKL, although diffuse involvements are recognized in ANKL, at least with imaging studies.
Aggressive NK cell leukemia has been recognized as a leukemia of mature NK cells,1 although the diagnostic criteria for ANKL remain unclear. In our series, 32% of the patients showed leukemic cells at <20% in PB and BM, which is considered a standardized limit for diagnosis of acute leukemia. No clinical characteristics, including prognosis, were different between patients with or without high leukemic cell percentage in PB and/or BM. Therefore, the percentage of tumor cells in PB and BM is not critical for the diagnosis of ANKL. In some patients, only a limited number of leukemic cells were recognized at initial presentation and hesitation to diagnose as ANKL might have led to a delay in early therapeutic intervention and also poor prognosis.
The prognosis of ANKL has been reported to be <3 months.4, 5, 16 With anthracycline/anthraquenone‐containing regimens, three of 13 patients reached CR. In the current study, patients treated with l‐asp‐containing regimens had a 60% response rate and were able to proceed to HSCT, although the regimens varied. Etoposide is also a candidate therapeutic agent of ANKL. These results indicate that intensive chemotherapy, particularly that containing MDR‐independent drugs, will improve patient outcome. It has been shown by in vitro experiment that ANKL cells are sensitive to l‐asp.22 The SMILE regimen, a combination of corticosteroid, methotrexate, ifosfamide, l‐asp and etoposide, is a candidate protocol for ANKL based on these concepts.23 Myelotoxic adverse reaction of SMILE is a concern for ANKL patients because the PS is often poor at initial presentation. Pretreatment with l‐asp as a single agent is a strategy for patients with limited capacity, and a prospective study is needed to identify the optimal treatment regimen for ANKL.
Early studies suggested the effectiveness of HSCT for ANKL or NK‐LGL leukemia with an aggressive course.4, 15 Allo‐HSCT from a related donor or cord blood under non‐CR conditions was selected as a main approach in the present study. This strategy is justified by the rapidly progressive clinical course and absence of optimal initial chemotherapy, together with the limited number of previous reports with successful outcomes. With allo‐HSCT, two patients have survived and are in CR after 2 years. Other patients reached CR after HSCT, and subsequently relapsed or died of transplantation‐related causes. From these observations, allo‐HSCT at an earlier setting after bridging therapy with an l‐asp‐containing regimen is worthy of examination in a clinical trial.
The diagnosis of ANKL should be made depending on three factors: cellular characteristics, involved sites and clinical features.24 In brief, a diagnosis of ANKL needs to be considered when tumor cells are granular lymphocytes, sometimes pleomorphic and large, the immunophenotypes are those of NK cells, including the expression of CD16, and there is a lack of myeloid and B‐cell markers. T‐cell receptor genes should be in the germline configuration, and EBV is generally positive. The main proliferation sites are BM, PB, liver and spleen, in which the cells infiltrate diffusely. Clinical features consist of B symptoms, HPS, liver dysfunction and rapidly progressive course.
To improve the poor prognosis of ANKL, we would like to emphasize three issues from our results: early diagnosis with high suspicion based on the unique characteristics mentioned above, chemotherapy with l‐asp‐containing regimens and allogeneic HSCT. This strategy needs to be examined in further studies.
Disclosure Statement
The authors declare that they have no potential conflicts of interest.
Acknowledgments
The authors thank collaborators from the following institutions: Dr N. Tsukamoto of the Gumma University School of Medicine, Dr T. Sekine from Matsuzaka City General Hospital, Dr Y. Aoyama from Seichokai Fuchu Hospital and Dr K. Kitano from the Matsumoto Medical Center Matsumoto Hospital. This study was supported in part by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor, and Welfare of Japan.
References
- 1. Chan JKC, Jaffe ES, Raffeld E, Ko Y‐H. Aggressive NK‐cell leukaemia In: Swerdlow SH, Campo E, Harris NL. et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoic Tissues. Lyon: IARC, 2008; 276–7. [Google Scholar]
- 2. Ishida F, Kwong YL. Diagnosis and management of natural killer‐cell malignancies. Expert Rev Hematol 2010; 3: 593–602. [DOI] [PubMed] [Google Scholar]
- 3. Ham MF, Ko YH. Natural killer cell neoplasm: biology and pathology. Int J Hematol 2010; 92: 681–9. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki R, Suzumiya J, Nakamura S et al Aggressive natural killer‐cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia 2004; 18: 763–70. [DOI] [PubMed] [Google Scholar]
- 5. Ryder J, Wang X, Bao L, Gross SA, Hua F, Irons RD. Aggressive natural killer cell leukemia: report of a Chinese series and review of the literature. Int J Hematol 2007; 85: 18–25. [DOI] [PubMed] [Google Scholar]
- 6. Ko YH, Park S, Kim K, Kim SJ, Kim WS. Aggressive natural killer cell leukemia: is Epstein–Barr virus negativity an indicator of a favorable prognosis? Acta Haematol 2009; 120: 199–206. [DOI] [PubMed] [Google Scholar]
- 7. Makishima H, Ito T, Asano N et al Significance of chemokine receptor expression in aggressive NK cell leukemia. Leukemia 2005; 19: 1169–74. [DOI] [PubMed] [Google Scholar]
- 8. Ruskova A, Thula R, Chan G. Aggressive natural killer‐cell leukemia: report of five cases and review of the literature. Leuk Lymphoma 2004; 45: 2427–38. [DOI] [PubMed] [Google Scholar]
- 9. Nakashima Y, Tagawa H, Suzuki R et al Genome‐wide array‐based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK‐cell leukemia and extranodal Nk/T‐cell lymphoma, nasal type. Genes Chromosom Cancer 2005; 44: 247–55. [DOI] [PubMed] [Google Scholar]
- 10. Iqbal J, Kucuk C, Deleeuw RJ et al Genomic analyses reveal global functional alterations that promote tumor growth and novel tumor suppressor genes in natural killer‐cell malignancies. Leukemia 2009; 23: 1139–51. [DOI] [PubMed] [Google Scholar]
- 11. Yamanaka Y, Tagawa H, Takahashi N et al Aberrant overexpression of microRNAs activate AKT signaling via downregulation of tumor suppressors in NK‐cell lymphoma/leukemia. Blood 2009; 114: 3265–75. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Ryland L, Yang J et al Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK‐LGL leukemia. Blood 2010; 116: 4192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan JKC, Wong KF, Jaffe ES, Ralfkiaer E. Aggressive NK‐cell leukaemia In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. WHO Classification of Tumours: Pathology & Genetics of Tumours of Haematopoietic and Lymphoic Tissues. Lyon: IARC, 2001; 198–200. [Google Scholar]
- 14. World Health Organization . WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization, 1979. [Google Scholar]
- 15. Ito T, Makishima H, Nakazawa H et al Promising approach for aggressive NK cell leukaemia with allogeneic haematopoietic cell transplantation. Eur J Haematol 2008; 81: 107–11. [DOI] [PubMed] [Google Scholar]
- 16. Song SY, Kim WS, Ko YH, Kim K, Lee MH, Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica 2002; 87: 1343–5. [PubMed] [Google Scholar]
- 17. Ino K, Masuya M, Nakamori Y et al [Aggressive NK‐cell leukemia with sustained relapse‐free survival after allogeneic peripheral blood stem cell transplantation]. Jan J Clin Hematol [Rinsho Ketsueki] 2010; 51: 258–63. [PubMed] [Google Scholar]
- 18. Yamaguchi M, Suzuki R, Kwong YL et al Phase I study of dexamethasone, methotrexate, ifosfamide, L‐asparaginase, and etoposide (SMILE) chemotherapy for advanced‐stage, relapsed or refractory extranodal natural killer (NK)/T‐cell lymphoma and leukemia. Cancer Sci 2008; 99: 1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito T, Makishima H, Nakazawa H, Senoo Y, Senoo N, Ishida F. Donor lymphocyte infusion for extranodal NK/T cell lymphoma, nasal type, relapsed after allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46: 1270–1. [DOI] [PubMed] [Google Scholar]
- 20. Sokol L, Loughran TP, Jr. Large granular lymphocyte leukemia. Oncologist 2006; 11: 263–73. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki R, Suzumiya J, Yamaguchi M et al Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol 2010; 21: 1032–40. [DOI] [PubMed] [Google Scholar]
- 22. Ando M, Sugimoto K, Kitoh T et al Selective apoptosis of natural killer‐cell tumours by l‐asparaginase. Br J Haematol 2005; 130: 860–8. [DOI] [PubMed] [Google Scholar]
- 23. Yamaguchi M, Kwong YL, Kim WS et al Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T‐cell lymphoma, nasal type: the NK‐Cell Tumor Study Group study. J Clin Oncol 2011; 29: 4410–6. [DOI] [PubMed] [Google Scholar]
- 24. Oshimi K. Progress in understanding and managing natural killer‐cell malignancies. Br J Haematol 2007; 139: 532–44. [DOI] [PubMed] [Google Scholar]


