Abstract
Molecules highly expressed in tumor endothelial cells (TEC) are important for specific targeting of these cells. Previously, using DNA microarray analysis, we found that the prostacyclin receptor (IP receptor) gene was upregulated in TEC compared with normal endothelial cells (NEC). Although prostacyclin is implicated in re‐endothelialization and angiogenesis, its role remains largely unknown in TEC. Moreover, the effect of the IP receptor on TEC has not been reported. In the present study we investigated the function of the IP receptor in TEC. The TEC were isolated from two types of human tumor xenografts in nude mice, while NEC were isolated from normal counterparts. Prostacyclin secretion levels in TEC were significantly higher than those in NEC, as shown using ELISA. Real‐time RT‐PCR showed that the IP receptor was upregulated in TEC compared with NEC. Furthermore, migration and tube formation of TEC were suppressed by the IP receptor antagonist RO1138452. Immunohistostaining showed that the IP receptor was specifically expressed in blood vessels of renal cell carcinoma specimens, but not in glomerular vessels of normal renal tissue. These findings suggest that the IP receptor is a TEC‐specific marker and might be a useful therapeutic target. (Cancer Sci 2012; 103: 1038–1044)
Angiogenesis is essential for tumor growth and metastasis and is an important component of cancer progression. Its inhibition is a valuable new approach to cancer therapy.1, 2, 3, 4 Tumor blood vessels deliver oxygen, nutrients and growth factors to cancer cells and permit their dissemination into the systemic circulation, resulting in metastasis.5, 6 Increased tumor vascularity is associated with poor clinical outcome, and the extent of angiogenesis correlates inversely with patient survival.6 The inhibition of angiogenesis therefore offers an attractive approach to cancer therapy.
The pharmacological targeting of vascular endothelial cells suppresses tumor angiogenesis and growth, and the efficacy of anti‐angiogenic therapy has been validated in the clinic.7 Although a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) prolonged survival in patients with certain types of cancer, some types of tumors appear to be less responsive. The results have been more modest than predicted by most preclinical examinations and improvements in progression‐free survival are frequently not accompanied by improvements in overall survival. Furthermore, some side‐effects have been reported since VEGF is essential for the survival of normal endothelial cells (NEC).7, 8, 9, 10, 11 A target that is more specific for tumor endothelial cells (TEC) is needed to improve the outcome of anti‐angiogenic therapy.
We previously reported that TEC differ from NEC in gene profile12, 13 and behavior, including enhanced cell survival14, 15 and motility.16, 17, 18 Using DNA microarray analysis, we previously identified several molecules that were preferentially highly expressed in mouse TEC derived from three different types of human tumor xenografts. We found that prostacyclin receptor (IP receptor) mRNA expression levels were significantly upregulated in TEC compared with NEC.
Prostacyclin (PGI2), the ligand of the IP receptor, is synthesized by PGI2 synthase (PGIS). PGI2 plays an important role as a potent inhibitor of platelet aggregation and an endothelium‐derived vasodilator.19, 20 PGI2 mainly signals through the IP receptor, a member of the seven‐transmembrane G‐protein‐coupled receptor superfamily.19, 20 PGI2 acts through the IP receptor to inhibit thromboxane A2 activity and modulate vascular pathological change.21 It also modulates the peroxisome proliferator‐activated receptor signaling pathways, also with important clinical implications for angiogenesis.22, 23, 24, 25, 26 A pro‐angiogenic function of PGI2 was also suggested on the basis of two other observations. Perfusion of rat lung tissue with PGI2 induces VEGF synthesis and antisense‐mediated inhibition of PGIS interferes with capillary‐like tube formation in HUVEC cultures.27, 28 The IP receptor signaling upregulates angiogenic gene expression in human endometrium through crosstalk with the epidermal growth factor (EGF) receptor and the extracellular signaling receptor kinase 1/2 pathway.29 During angiogenesis, PGI2 regulates endothelial sprouting and VEGF‐induced vascular permeability.30, 31, 32 These previous studies were concerned with physiological angiogenesis for reproduction or repair of tissue. However, there is no study of IP receptor function in pathological angiogenesis, such as tumor angiogenesis. We reported that COX‐2, which increases the synthesis of PGI2, was upregulated in TEC and that TEC were more sensitive to COX‐2 inhibitor than NEC were. Furthermore, COX‐2 inhibition suppressed tumor angiogenesis and growth in vivo by inhibiting migration of TEC.33 Thus, COX‐2 is a key molecule in tumor angiogenesis. However, no study has examined IP receptor function or expression in tumor blood vessels or the effect of PGI2/IP receptor on TEC. The present study was designed to analyze IP receptor expression and function in TEC, both in vitro and in vivo.
Materials and Methods
Cell line and culture conditions
The human renal clear cell carcinoma cell line OS‐RC‐2 was purchased from the RIKEN Cell Bank (Tsukuba, Japan). The cells were cultured in RPMI 1640 medium (Sigma–Aldrich, St. Louis, MO, USA) containing 10% FBS. The super‐metastatic human malignant melanoma cell line A375SM was a kind gift from Dr Isaiah J. Fidler (MD Anderson Cancer Center, Houston, TX, USA). The cells were cultured in minimum essential medium (Gibco, Grand Island, NY, USA) containing 10% FBS in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
TEC and NEC isolation
Mouse TEC (mTEC) and mouse NEC (mNEC) were isolated as previously described.16, 34 mTEC were isolated from human tumor xenografts (A375SM and OS‐RC‐2) in nude mice. mNEC were isolated from the dermis and kidney and used as controls. All procedures for animal experiments were approved by the local animal research authority, and animal care was in accordance with the institutional guidelines of Hokkaido University. mTEC and mNEC were isolated using a magnetic cell sorting system (Miltenyi Biotec, Tokyo, Japan) with anti‐CD31 antibody (eBioscience, San Diego, CA, USA). CD31‐positive cells were sorted and plated on 1.5% gelatin‐coated culture plates and grown in EGM‐2MV medium containing 20% FBS. Diphtheria toxin (DT; 500 ng/mL; Calbiochem, San Diego, CA, USA) was added to mTEC subcultures to kill any remaining human tumor cells, and to mNEC subcultures for technical consistency. Human cells express a heparin‐binding EGF‐like growth factor (HB‐EGF), which is a DT receptor. However, DT does not interact with mouse HB‐EGF, and hence, mouse endothelial cells survive this treatment. Isolated mouse endothelial cells were purified by a second round of purification using FITC–BS1‐B4 lectin. Human TEC (hTEC) and NEC (hNEC) were isolated from human renal cell carcinoma (RCC) and normal renal parenchyma dissected apart from cancerous tissue, respectively. The methods used for human endothelial cells isolation were as described above except for the DT technique.
RT‐PCR and real‐time RT‐PCR
Total RNA was extracted from each type of endothelial cell (EC) using the RNeasy Micro kit (Qiagen, Valencia, CA, USA). Total RNA was used for first‐strand cDNA synthesis in ReverTra‐Plus (Toyobo Co., Osaka, Japan). cDNA was amplified using PCR. Real‐time RT‐PCR was performed using SsoFast EvaGreen Supermix (Bio‐Rad, Hercules, CA, USA). Cycling conditions followed the manufacturer's instructions and the CFX Manager was used for analyses (Bio‐Rad). Relative expression levels were normalized to GAPDH. The primers used were as follows: mouse GAPDH, forward 5′‐TCTGACGTGCCGCCTGGAG‐3′, reverse 5′‐TCGCAGGAGACAACCTGGTC‐3′; mouse IP receptor, forward 5′‐TCTGACGTGCCGCCTGGAG‐3′, reverse 5′‐TCGCAGGAGACAACCTGGTC‐3′; human GAPDH, forward 5′‐ACAGTCAGCCGCATCTTCTT‐3′, reverse 5′‐GCCCAATACGACCAAATCC‐3′; and human IP receptor, forward 5′‐AGGAGAGCAGACACTCTAACC‐3′, reverse 5′‐GGATGCCGAAGGTTCTATGG‐3′.
Measurement of 6‐keto‐PGF1α
Because PGI2 is non‐enzymatically hydrated to 6‐keto‐PGF1α (t 1/2 = 2–3 min), PGI2 was measured using a 6‐keto‐PGF1α enzyme immunoassay kit (Cayman, Ann Arbor, MI, USA). The EC (4 × 103 cells/well) were seeded in 96‐well culture dishes containing EGM‐2 medium for 24 h.
Supernatants were collected and 6‐keto‐PGF1α levels were measured according to the manufacturer's instructions. To inhibit endogenous PGI2 in mTEC, the mTEC were treated with the COX‐2 inhibitor, NS‐398 (0, 10, 50, 100 μM). NS‐398 was purchased from Cayman Chemical. Results are expressed as picogram per milliliter and calculated according to the 6‐keto‐PGF1α standard curve. The experiment was repeated three times and similar results were obtained.
Flow cytometry
After human TEC and NEC were isolated from human samples, these cells were incubated with fluorescein Ulex europaeus agglutinin I (UEA‐1 lectin; Vector Laboratories, Burlingame, CA, USA) and primary antibodies against CD31, CD105 and CD45 for 20 min at 4°C. The primary antibodies used were anti‐human CD31 antibody (BioLegend, San Diego, CA, USA), anti‐human CD105 antibody (BD Biosciences, San Jose, CA, USA) and phycoerythrin‐conjugated anti‐human CD45 antibody (BD Biosciences). Alexa Fluor 488 goat anti‐mouse IgG (Invitrogen, Carlsbad, CA, USA) was used as the secondary antibody. Expressions of the EC markers were analyzed using FACSAria II (Becton Dickinson, San Jose, CA, USA). Representative data were analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Cell migration assay
Cell migration was measured using the Boyden chamber. In the upper chamber, 1.5 × 104 cells in endothelial basal medium (EBM)‐2 without FBS were seeded and then DMSO or the IP receptor antagonist RO1138452 (0, 5, 10, 20 μM) was added. RO1138452 was purchased from Cayman Chemical. EBM‐2 containing 5% FBS was placed in the lower chamber. After 4 h at 37°C, the cells migrating through the 0.5% gelatin‐coated polycarbonate filter (8‐μm pores; Corning Costar, Nagog Park, MA, USA) were fixed in 10% formaldehyde and stained with hematoxylin. To see the inhibitory effect of RO1138452 under inhibition of endogenous PGI2, mTEC were seeded on the upper chamber in the presence of NS‐398 (100 μM) or indomethacin (0.5 mM). Indomethacin was purchased from Sigma–Aldrich. The mTEC were preincubated with or without RO1138452 (10 μM) for 10 min prior to exposure to NS‐398 and beraprost (Cayman Chemical). The number of cells that migrated through the vitronectin‐coated (3 μg/mL) polycarbonate filter to the lower side of the filter was counted in five fields at a magnification of ×20. The experiment was repeated three times and similar results were obtained.
Tube formation assay
Matrigel tube formation assays were performed to assess in vitro angiogenesis. Growth factor‐reduced Matrigel (BD Biosciences) was transferred to each well of a 24‐well dish and incubated at 37°C for 30 min to allow the matrix solution to solidify. The mTEC were harvested and resuspended in EBM‐2 containing 5% FBS and then seeded at a density of 1 × 105 cells per well, followed by incubation at 37°C for 18 h with vehicle or RO1138452 (10 μM).35 Similarly, the mTEC were preincubated with or without RO1138452 (10 μM) for 10 min prior to exposure to NS‐398 (100 μM) and beraprost (10 μM). Tube formation was observed using an inverted microscope (CKX41; Olympus, Tokyo, Japan) and the experimental results were recorded at three different times with similar results. Tube length was measured at a magnification of ×4 in three random fields with ImageJ software (National Institute of Health, Rockville, MD, USA) and expressed as a percentage of the control in micrometers.
Immunohistochemistry
Human tissue samples were obtained from excised RCC and normal renal parenchyma dissected apart from the cancerous tissue of six patients at Hokkaido University Hospital, Hokkaido, Japan. Informed consent was obtained from all patients before samples were used. The protocols were approved by the Ethics Committee of Hokkaido University, and written informed consent was obtained from each patient before surgery. Frozen sections were double stained using anti‐CD31 antibody, Alexa Fluor 594 goat anti‐mouse IgG, and anti‐IP receptor antibody, Alexa Fluor 488 goat anti‐rabbit IgG, to show co‐localization of the IP receptor in tumor vessels. The IP receptor antibody was purchased from GenWay Biotech (San Diego, CA, USA). All samples used for immunohistochemistry were counterstained with DAPI (Roche Diagnostics, Mannheim, Germany) and visualized using the FluoView FV1000 confocal microscope (Olympus).
Statistical analysis
Differences between groups were evaluated using the Mann–Whitney U‐test. P < 0.05 was considered significant and P < 0.01 was considered highly significant.
Results
IP receptor mRNA expression was upregulated in mouse TEC
We isolated two different types of mTEC (melanoma and RCC EC) from tumor xenografts in nude mice and two types of mNEC (skin and kidney EC). They expressed typical EC markers, such as CD31, CD105 and CD144.36 Using these EC isolated from primary cultures, we reported previously that TEC differ from NEC in gene expression and aspects of biological activity, such as survival and motility.16, 17 Our recent microarray analysis showed that several genes, such as VEGF receptor 2, and TEC markers, including TEM‐837 and CD13,38 were strongly expressed in TEC. We selected IP receptor mRNA to examine its expression in TEC. Real‐time RT‐PCR analyses showed that IP receptor mRNA levels in mTEC were higher than those in mNEC (Fig. 1A).
Figure 1.
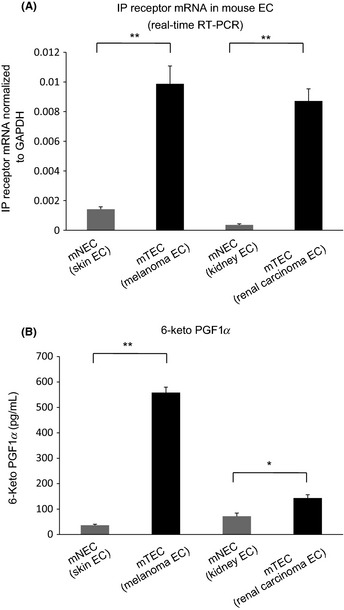
The prostacyclin receptor (IP receptor) mRNA expression levels were upregulated and 6‐keto‐PGF1α secretion levels in mouse tumor endothelial cells (mTEC) were higher than those in mouse normal endothelial cells (mNEC). (A) Relative expression of the IP receptor mRNA in mTEC (melanoma and renal cell carcinoma endothelial cells [EC]) and mNEC (skin and kidney EC) was measured using real‐time RT‐PCR. *P < 0.01; Mann–Whitney U‐test (mean ± SD, n = 3). (B) 6‐Keto‐PGF1α secretion levels in mTEC were significantly higher than those in mNEC, suggesting that mTEC released significantly higher PGI 2 levels. *P < 0.05, **P < 0.01 vs control; Mann–Whitney U‐test (mean ± SD, n = 3).
6‐Keto‐PGF1α secretion levels in TEC were higher than those in mouse NEC
Next we analyzed 6‐keto‐PGF1α (the main metabolite of PGI2) levels in the supernatant of cultured mTEC and mNEC using ELISA. 6‐Keto‐PGF1α levels in mTEC were significantly higher than those in mNEC (melanoma EC, 558.1 ± 21.5 pg/mL [P < 0.01 vs skin EC 36.7 ± 3.6 pg/mL]; RCC EC, 143.6 ± 12.9 pg/mL [P < 0.05 vs kidney EC 71.9 ± 12.2 pg/mL]) (Fig. 1B).
Mouse TEC migration was inhibited by an IP receptor antagonist
Endothelial cell migration in the presence or absence of the IP receptor antagonist RO1138452 was analyzed on gelatin‐coated filters in the Boyden chamber. RO1138452 significantly inhibited migration of mTEC, whereas that of mNEC was little affected (Fig. 2). However, proliferation of mTEC and mNEC was not suppressed by equivalent concentrations of RO1138452 (data not shown). To analyze the effect of inhibiting endogenous PGI2 in mTEC, NS‐398 and indomethacin were used in a migration assay. In previous literature, indomethacin is reported to inhibit the production of PGI2 significantly.39, 40 The migration of mTEC was significantly inhibited by indomethacin but it was not restored by the IP agonist, beraprost. This observation is consistent with a previous study39 (Supporting Information Fig. S1). Next, a COX‐2 inhibitor, NS‐398, was used to inhibit endogenous PGI2 in a migration assay. Using ELISA, it was demonstrated that NS‐398 inhibited PGI2 synthesis in mTEC in a dose‐dependent manner (Fig. S2). NS‐398 significantly inhibited the migration of mTEC. The migration was restored by the IP agonist, beraprost. RO1138452 inhibited beraprost‐induced cell migration of mTEC in the presence of NS‐398 (Fig. S3). Therefore, it was confirmed that RO1138452 is specific for the IP receptor.
Figure 2.
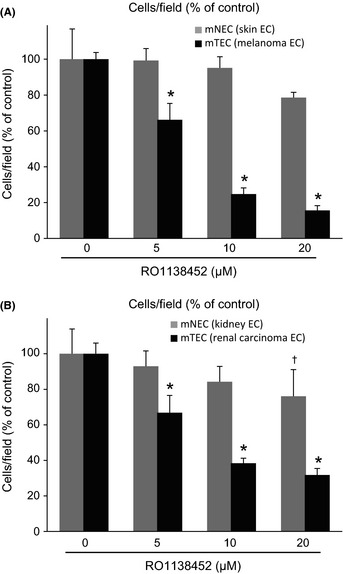
Migration of mouse tumor endothelial cells (mTEC) was inhibited by the prostacyclin receptor (IP receptor) antagonist RO1138452. Cell migration was analyzed on gelatin‐coated filters using the Boyden chamber. The filter was fixed and stained with hematoxylin after 4 h of incubation in the presence or absence of RO1138452 (0, 5, 10, 20 μM). Cells that migrated to the lower side of the filter were counted in five fields at a magnification of ×20. RO11384528 significantly inhibited migration of mTEC but had little effect on mouse normal endothelial cell (mNEC) migration. (A) Skin endothelial cells (EC) and melanoma EC, and (B) kidney EC and renal cell carcinoma EC. *P < 0.05 vs control; Mann–Whitney U‐test (mean ± SD, n = 5).
IP receptor antagonist suppressed tube formation in mTEC
Proliferation, migration, invasion and matrix remodeling are necessary for the angiogenic effects of EC. Morphogenesis in the Matrigel assay provides an indication of the ability of EC to reorganize capillary tube‐like structures. The involvement of PGI2 in the angiogenic properties of mTEC was investigated using the tube formation assay (Fig. 3). The ability to form capillary‐like structures was impaired by RO1138452 in mTEC. In contrast, RO1138452 had little effect on tube formation in mNEC. RO1138452 suppressed the angiogenic phenotype of mTEC but had little effect in this regard on mNEC. These data suggest that the IP receptor has an important role in tumor angiogenesis. To see the inhibitory effect of RO1138452 on mTEC under inhibition of endogenous PGI2, NS‐398 or indomethacin were used in a tube formation assay. Tube formation was not restored by beraprost when treated with indomethacin (Fig. S4). Under inhibition of endogenous PGI2 by NS398, beraprost could recover tube formation and it was blocked by RO1138452, suggesting that this drug specifically inhibited the IP receptor (Fig. S5).
Figure 3.
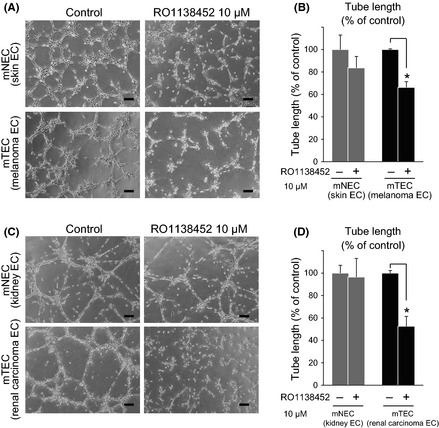
Tube formation of mouse tumor endothelial cells (mTEC) was inhibited by the prostacyclin receptor (IP receptor) antagonist RO1138452. The mTEC were seeded on Matrigel in basal medium. Tube formation was observed after 18 h of incubation and tube length was measured. Tube formation was significantly inhibited by RO1138452 in mTEC (melanoma and renal cell carcinoma [RCC] endothelial cells [EC]) but not in mouse normal endothelial cells (mNEC) (skin and kidney EC). Representative figures are shown. (A,B) Skin EC and melanoma EC, and (C,D) kidney EC and RCC EC. Bar, 100 μm. *P < 0.05 vs control; Mann–Whitney U‐test (mean ± SD, n = 3).
TEC and NEC isolation from human RCC and normal renal parenchyma
We isolated hTEC and hNEC from surgically resected RCC and normal renal parenchyma from six patients, respectively, as previously described.41 UEA‐1 lectin binding and CD31 and CD105 expression showed that isolated human EC are highly pure. The isolated EC were negative for the hematopoietic marker CD45 using flow cytometry (Fig. 4A). Endothelial marker expression levels were examined from the first to fourth passages and similar results were confirmed during cell culture.
Figure 4.
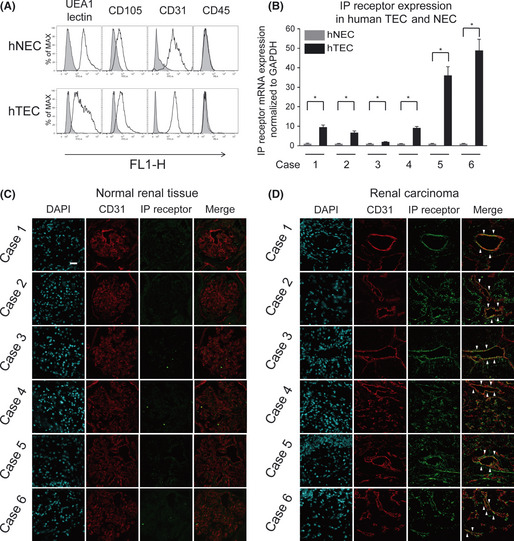
The prostacyclin receptor (IP receptor) was upregulated in human tumor endothelial cells (hTEC) in vitro and in vivo. (A) Verification of endothelial cells (EC) from a human sample. Representative flow cytometry of human normal endothelial cells (hNEC) and hTEC showing the expression area (unshaded) of the endothelial markers CD105, CD31 and UEA‐1 lectin. Isolated EC were negative for the monocyte marker CD45. Gray areas show the control levels with normal‐isotype IgG. (B) The IP receptor mRNA upregulation in hTEC isolated from human renal cell carcinoma (RCC). RT‐PCR analysis confirmed that the IP receptor mRNA was upregulated in hTEC from all six cases compared with hNEC. (C) In immunofluorescent staining, tumor vessels were double stained with anti‐CD31 antibody and anti‐IP receptor antibody in human RCC. The IP receptor was specifically expressed in blood vessels of RCC specimens, but not in the glomerular vessels of normal renal tissue. Bar, 20 μm.
IP receptor was upregulated in human TEC in vitro and in vivo
Using cultured hTEC and hNEC isolated from human RCC, we investigated IP receptor mRNA expression levels. Six samples of hTEC and hNEC were obtained from six patients (Fig. 4B). The clinical background of the RCC specimens is shown in Table 1. The IP receptor mRNA expression levels were higher in hTEC than those in hNEC in all the six cases. Double immunofluorescence staining with anti‐IP receptor and anti‐CD31 antibody was performed in tumor tissues dissected from the six patients with RCC. Immunohistochemical staining with the IP receptor antibody revealed that the IP receptor was specifically expressed in the blood vessels of RCC specimens, but not in the glomerular vessels of normal renal tissues (Fig. 4C,D). These results suggest that the IP receptor was strongly expressed in hTEC compared with hNEC.
Table 1.
Clinical background of the renal cell carcinoma specimens
| Sample no. | M/F | Age (years) | TNMa | Subtype | Gradeb, INF, v |
|---|---|---|---|---|---|
| 1 | M | 66 | T1a, Nx, M0 | Clear cell | G2, INFa, v(−) |
| 2 | M | 49 | T1b, Nx, M0 | Clear cell | G2, INFa, v(+) |
| 3 | F | 48 | T1b, Nx, M0 | Clear cell | G2, INFa, v(−) |
| 4 | F | 50 | T1b, Nx, M0 | Clear cell | G2, INFa, v(−) |
| 5 | M | 60 | T1a, Nx, M0 | Clear cell | G2, INFa, v(−) |
| 6 | M | 54 | T2b, Nx, M0 | Clear cell | G2, INFa, v(+) |
Discussion
The main results of the present study are as follows: (i) the IP receptor mRNA was upregulated in mouse and human TEC; (ii) the IP receptor was expressed in TEC of human RCC in vivo; (iii) PGI2 secretion levels in mTEC were significantly higher than those in mNEC; and (iv) an IP receptor antagonist significantly inhibited random motility and tube formation in mTEC.
PGI2 is a member of the prostanoid family and is downstream of COX‐1 and COX‐2. COX‐2 metabolizes arachidonic acid into prostanoids such as PGE2, PGF2 and PGI2.42 PGI2 mediates cellular activity through the IP receptor, which increases cyclic AMP levels and modulates target molecules related to vasodilation and angiogenesis. Human IP receptor mRNA is expressed in the kidneys, lungs, aorta and brain sites where PGI2 modulates vascular tone and regulates blood flow.43, 44, 45
We demonstrated that the IP receptor was significantly upregulated in TEC. Furthermore, a specific antagonist of the IP receptor significantly inhibited motility and tube formation in TEC but not in NEC. The TEC are more dependent on PGI2 and the IP receptor pathway than NEC. These results suggest that the IP receptor maintains the angiogenic phenotype of TEC and that it has key roles in tumor angiogenesis. Because PGI2 was secreted at much higher levels from TEC than from NEC, it is suggested that PGI2 acts on TEC in an autocrine manner.
The COX‐2 enzyme was reported to be a key regulator of tumor angiogenesis and an important target for anti‐angiogenic therapy.46 COX‐2 is a key regulatory enzyme that leads PGI2 synthesis from PGH2 in vascular EC.47, 48 COX‐2 expression and PGI2 secretion are correlated.49, 50 Recently, we demonstrated that TEC expressed higher levels of COX‐2 than NEC, and a COX‐2 inhibitor, NS398, specifically impaired migration of TEC. COX‐2 is suggested to be involved in the high migration potential of TEC. In our previous study, tumor growth was suppressed on inhibition of tumor angiogenesis in vivo by a COX‐2 inhibitor.33
Taken together, our findings suggest that the IP receptor might maintain an angiogenic switch in the “on” state in TEC. The IP receptor might be a TEC‐specific marker and a useful anti‐angiogenic target in cancer therapy. Selective IP receptor antagonists might have an advantage over conventional COX‐2 inhibitors in suppressing tumor angiogenesis.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Migration of mouse tumor endothelial cells (melanoma endothelial cells [EC] and renal cell carcinoma EC) was inhibited by indomethacin.
Fig. S2. PGI 2 synthesis in mouse tumor endothelial cells was significantly inhibited by NS‐398.
Fig. S3. RO1138452 suppressed mTEC migration by an inhibitory effect on the prostacyclin receptor (IP receptor).
Fig. S4. Tube formation of mouse tumor endothelial cells was inhibited by indomethacin.
Fig. S5. RO1138452 suppressed mouse tumor endothelial cell tube formation by an inhibitory effect on the prostacyclin receptor (IP receptor).
Acknowledgments
The authors thank Dr Aya Yanagawa, Ms Tomomi Takahashi, Mrs Midori Muranaka, Dr Miyako Kondoh, Dr Nako Maishi, Dr Kazuyuki Yamamoto, Mr Taisuke Kawamoto, Ms Hitomi Omura and Ms Yuko Suzuki for technical assistance.
References
- 1. Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2002; 2: 727–39. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 3. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 4. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 5. Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the ‘angiogenesis progression’ hypothesis. Eur J Cancer 1996; 32A: 2438–50. [DOI] [PubMed] [Google Scholar]
- 6. Weidner N. Angiogenesis as a predictor of clinical outcome in cancer patients. Hum Pathol 2000; 31: 403–5. [DOI] [PubMed] [Google Scholar]
- 7. McCarthy M. Antiangiogenesis drug promising for metastatic colorectal cancer. Lancet 2003; 361: 1959. [DOI] [PubMed] [Google Scholar]
- 8. Yang JC, Haworth L, Sherry RM et al A randomized trial of bevacizumab, an anti‐vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller KD. E2100: a phase III trial of paclitaxel versus paclitaxel/bevacizumab for metastatic breast cancer. Clin Breast Cancer 2003; 3: 421–2. [DOI] [PubMed] [Google Scholar]
- 10. Trail PA, King HD, Dubowchik GM. Monoclonal antibody drug immunoconjugates for targeted treatment of cancer. Cancer Immunol Immunother 2003; 52: 328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garber K. Angiogenesis inhibitors suffer new setback. Nat Biotechnol 2002; 20: 1067–8. [DOI] [PubMed] [Google Scholar]
- 12. Nako M, Ohga N, Hida Y et al CXCR7: a novel tumor endothelial marker in renal cell carcinoma. Pathol Int 2012. (In press). [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto K, Ohga N, Hida Y et al Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells. Br J Cancer 2012; 106: 1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akiyama K, Ohga N, Hida Y et al Tumor endothelial cells acquire drug resistance by MDR1 upregulation via VEGF signaling in tumor microenvironment. Am J Pathol 2012; 180: 1283–93. [DOI] [PubMed] [Google Scholar]
- 15. Ohga N, Ishikawa S, Maishi N et al Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from highly metastatic and low metastatic tumors. Am J Pathol 2012; 180: 1294–307. [DOI] [PubMed] [Google Scholar]
- 16. Hida K, Hida Y, Amin DN et al Tumor‐associated endothelial cells with cytogenetic abnormalities. Cancer Res 2004; 64: 8249–55. [DOI] [PubMed] [Google Scholar]
- 17. Hida K, Klagsbrun M. A new perspective on tumor endothelial cells: unexpected chromosome and centrosome abnormalities. Cancer Res 2005; 65: 2507–10. [DOI] [PubMed] [Google Scholar]
- 18. Ohga N, Hida K, Hida Y et al Inhibitory effects of epigallocatechin‐3 gallate, a polyphenol in green tea, on tumor‐associated endothelial cells and endothelial progenitor cells. Cancer Sci 2009; 100: 1963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep 2008; 60: 3–11. [PubMed] [Google Scholar]
- 20. Kawabe J, Ushikubi F, Hasebe N. Prostacyclin in vascular diseases: recent insights and future perspectives. Circ J 2010; 74: 836–43. [DOI] [PubMed] [Google Scholar]
- 21. Dogne JM, Hanson J, Pratico D. Thromboxane, prostacyclin and isoprostanes: therapeutic targets in atherogenesis. Trends Pharmacol Sci 2005; 26: 639–44. [DOI] [PubMed] [Google Scholar]
- 22. Pola R, Gaetani E, Flex A et al Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: a possible role for peroxisome proliferator‐activated receptors. J Mol Cell Cardiol 2004; 36: 363–70. [DOI] [PubMed] [Google Scholar]
- 23. Biscetti F, Pola R. Endothelial progenitor cells and angiogenesis join the PPARty. Circ Res 2008; 103: 7–9. [DOI] [PubMed] [Google Scholar]
- 24. Biscetti F, Gaetani E, Flex A et al Selective activation of peroxisome proliferator‐activated receptor (PPAR)alpha and PPAR gamma induces neoangiogenesis through a vascular endothelial growth factor‐dependent mechanism. Diabetes 2008; 57: 1394–404. [DOI] [PubMed] [Google Scholar]
- 25. He T, Lu T, d'Uscio LV, Lam CF, Lee HC, Katusic ZS. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res 2008; 103: 80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biscetti F, Gaetani E, Flex A et al Peroxisome proliferator‐activated receptor alpha is crucial for iloprost‐induced in vivo angiogenesis and vascular endothelial growth factor upregulation. J Vasc Res 2009; 46: 103–8. [DOI] [PubMed] [Google Scholar]
- 27. Hoper MM, Voelkel NF, Bates TO et al Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol 1997; 17: 748–56. [DOI] [PubMed] [Google Scholar]
- 28. Spisni E, Griffoni C, Santi S et al Colocalization prostacyclin (PGI2) synthase–caveolin‐1 in endothelial cells and new roles for PGI2 in angiogenesis. Exp Cell Res 2001; 266: 31–43. [DOI] [PubMed] [Google Scholar]
- 29. Smith OP, Battersby S, Sales KJ, Critchley HO, Jabbour HN. Prostacyclin receptor up‐regulates the expression of angiogenic genes in human endometrium via cross talk with epidermal growth factor receptor and the extracellular signaling receptor kinase 1/2 pathway. Endocrinology 2006; 147: 1697–705. [DOI] [PubMed] [Google Scholar]
- 30. Kuwashima L, Graeber J, Glaser BM. Stimulation of endothelial cell prostacyclin release by retina‐derived factors. Invest Ophthalmol Vis Sci 1988; 29: 1213–20. [PubMed] [Google Scholar]
- 31. Hahn GL, Polgar PR. Prostaglandin production in phenotypically distinct cultured bovine pulmonary artery endothelium. Atherosclerosis 1984; 51: 143–50. [DOI] [PubMed] [Google Scholar]
- 32. Murohara T, Horowitz JR, Silver M et al Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation 1998; 97: 99–107. [DOI] [PubMed] [Google Scholar]
- 33. Muraki C, Ohga N, Hida Y et al Cyclooxygenase‐2 inhibition causes antiangiogenic effects on tumor endothelial and vascular progenitor cells. Int J Cancer 2012; 130: 59–70. [DOI] [PubMed] [Google Scholar]
- 34. Kurosu T, Ohga N, Hida Y et al HuR keeps an angiogenic switch on by stabilising mRNA of VEGF and COX‐2 in tumour endothelium. Br J Cancer 2011; 104: 819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turner EC, Mulvaney EP, Reid HM, Kinsella BT. Interaction of the human prostacyclin receptor with the PDZ adapter protein PDZK1: role in endothelial cell migration and angiogenesis. Mol Biol Cell 2011; 22: 2664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuda K, Ohga N, Hida Y et al Isolated tumor endothelial cells maintain specific character during long‐term culture. Biochem Biophys Res Commun 2010; 394: 947–54. [DOI] [PubMed] [Google Scholar]
- 37. St Croix B, Rago C, Velculescu V et al Genes expressed in human tumor endothelium. Science 2000; 289: 1197–202. [DOI] [PubMed] [Google Scholar]
- 38. Pasqualini R, Koivunen E, Kain R et al Aminopeptidase N is a receptor for tumor‐homing peptides and a target for inhibiting angiogenesis. Cancer Res 2000; 60: 722–7. [PMC free article] [PubMed] [Google Scholar]
- 39. Dormond O, Foletti A, Paroz C, Ruegg C. NSAIDs inhibit alpha V beta 3 integrin‐mediated and Cdc42/Rac‐dependent endothelial‐cell spreading, migration and angiogenesis. Nat Med 2001; 7: 1041–7. [DOI] [PubMed] [Google Scholar]
- 40. Kawabe J, Yuhki K, Okada M et al Prostaglandin I2 promotes recruitment of endothelial progenitor cells and limits vascular remodeling. Arterioscler Thromb Vasc Biol 2010a; 30: 464–70. [DOI] [PubMed] [Google Scholar]
- 41. Akino T, Hida K, Hida Y et al Cytogenetic abnormalities of tumor‐associated endothelial cells in human malignant tumors. Am J Pathol 2009; 175: 2657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moncada S. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B Biol Sci 2006; 361: 735–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boie Y, Rushmore TH, Darmon‐Goodwin A et al Cloning and expression of a cDNA for the human prostanoid IP receptor. J Biol Chem 1994; 269: 12173–8. [PubMed] [Google Scholar]
- 44. McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long‐term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med 1998; 338: 273–7. [DOI] [PubMed] [Google Scholar]
- 45. Nakagawa O, Tanaka I, Usui T et al Molecular cloning of human prostacyclin receptor cDNA and its gene expression in the cardiovascular system. Circulation 1994; 90: 1643–7. [DOI] [PubMed] [Google Scholar]
- 46. Gately S. The contributions of cyclooxygenase‐2 to tumor angiogenesis. Cancer Metastasis Rev 2000; 19: 19–27. [DOI] [PubMed] [Google Scholar]
- 47. Dubois RN, Abramson SB, Crofford L et al Cyclooxygenase in biology and disease. FASEB J 1998; 12: 1063–73. [PubMed] [Google Scholar]
- 48. Herschman HR, Xie W, Reddy S. Inflammation, reproduction, cancer and all that… The regulation and role of the inducible prostaglandin synthase. BioEssays 1995; 17: 1031–7. [DOI] [PubMed] [Google Scholar]
- 49. Norata GD, Callegari E, Inoue H, Catapano AL. HDL3 induces cyclooxygenase‐2 expression and prostacyclin release in human endothelial cells via a p38 MAPK/CRE‐dependent pathway: effects on COX‐2/PGI‐synthase coupling. Arterioscler Thromb Vasc Biol 2004; 24: 871–7. [DOI] [PubMed] [Google Scholar]
- 50. Nussmeier NA, Whelton AA, Brown MT et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005; 352: 1081–91. [DOI] [PubMed] [Google Scholar]
- 51. Sobin LH, Wittekind Ch, editions. International Union Against Cancer (UICC). TNM classification of malignant tumors. 5th ed. New York: 1997: 180–2.
- 52. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Migration of mouse tumor endothelial cells (melanoma endothelial cells [EC] and renal cell carcinoma EC) was inhibited by indomethacin.
Fig. S2. PGI 2 synthesis in mouse tumor endothelial cells was significantly inhibited by NS‐398.
Fig. S3. RO1138452 suppressed mTEC migration by an inhibitory effect on the prostacyclin receptor (IP receptor).
Fig. S4. Tube formation of mouse tumor endothelial cells was inhibited by indomethacin.
Fig. S5. RO1138452 suppressed mouse tumor endothelial cell tube formation by an inhibitory effect on the prostacyclin receptor (IP receptor).


