Abstract
Expression cloning was used to initially isolate the reversion‐inducing cysteine‐rich protein with Kazal motifs (RECK) gene as a suppressor of transformation. The gene was found to encode a membrane‐anchored regulator of MMPs. Experimental studies showed that RECK can suppress tumor invasion, metastasis, and angiogenesis. However, the clinical impact of RECK remains unclear. To assess the clinical significance of RECK expression in invasive breast cancer, a total of 119 patients with invasive breast cancer were retrospectively examined. Expression of RECK in tumor tissues was assessed by immunohistochemical staining. A significant correlation between RECK expression and 5‐year survival rate was documented. The 5‐year survival rate for patients with strong RECK expression was significantly higher than that for patients with weakly expressing tumors. Univariate and multivariate analyses confirmed that reduced RECK expression was an independent and significant factor in predicting a poor prognosis. In conclusion, RECK expression is a significant prognostic factor correlated with long‐term survival for patients with invasive breast cancer. RECK expression is therefore a potentially useful prognostic marker for breast cancer. (Cancer Sci 2012; 103: 1084–1089)
Metastatic disease, rather than the primary tumor itself, is responsible for death in most solid tumors, including breast cancer.1, 2, 3 The metastatic process involves a complex cascade of events, including the organized breakdown of the ECM by MMPs.4, 5 The MMPs are able to degrade all ECM components. Each ECM element is cleaved by a specific MMP or MMP group.6 Recently, a novel MMP inhibitor, reversion‐inducing cysteine‐rich protein with Kazal motifs (RECK), was identified by screening a fibroblast expression library for cDNAs that changed the round morphology of v‐Ki‐ras‐transformed NIH3T3 cells into a non‐transformed flat morphology.7, 8, 9 RECK is a membrane‐anchored glycoprotein of approximately 110 kDa that contains multiple epidermal growth factor‐like repeats and serine protease inhibitor‐like domains.10 However, RECK has no structural homology with tissue inhibitors of matrix metalloproteinase, the major endogenous regulators of MMPs. RECK is expressed in various normal human tissues but was described as undetectable in tumor‐derived cell lines and oncogenically transformed cells.10 Previous studies revealed that RECK could inhibit MMP‐2, MMP‐9, and membrane type 1 MMP (MMP‐14) secretion and activity by an as yet unknown mechanism.11, 12 Due to these functions, RECK is able to inhibit tumor angiogenesis, invasion, and metastasis.10, 11, 12, 13 Furthermore, increased transcriptional expression of RECK in tumor cells compared with concomitant normal tissue cells is correlated with prolonged survival in patients with hepatocellular carcinoma,14 pancreatic cancer,15 colorectal cancer,16 non‐small‐cell lung cancer,17 and gastric cancer.18 In this study, using immunohistochemical staining, we analysed RECK expression levels in patients with breast cancer and compared these data with the clinicopathological features of these patients. To our knowledge, the current study is the first to investigate the expression of RECK immunohistochemically in relation to clinicopathological features and survival in a large number of patients with breast adenocarcinoma.
Materials and Methods
Patients and tissue specimens
This study used archival material from the Department of Pathology at the Third Affiliated Hospital of Harbin Medical University (Harbin, China). Breast cancer tissue specimens were obtained from patients undergoing primary mastectomies at the institution from January 1, 2003 to December 31, 2004. Pathologists diagnostically examined tumor and benign breast tissues removed from these patients. The benign tissues were confirmed to be free from tumor deposits. The most important inclusion criterion for the patients was presence of primary, unilateral, and operable infiltrating ductal carcinomas. Among exclusion criteria were distant metastasis at the time of diagnosis, locally advanced disease, inflammatory carcinoma, and synchronous bilateral breast cancer. The pathologist measured the tumors in millimeters at the largest diameter of the invasive carcinoma. All archival tumor blocks of each tumor were initially assessed by H&E staining to select a tumor block with an invasive carcinoma and to include the tumor border and a cross‐sectional area as large as possible. Sections from the paraffin blocks (4 μm thick) were mounted on ChenMate slides (ZSGB‐Bio, Beijing, China). Morphology and protein expression were evaluated in consecutive sections. Histological classification of tumors was based on World Health Organization criteria (Table 1). All protocols were reviewed and approved by the Ethical Committee of Harbin Medical University. Written consent was obtained from all participating patients.
Table 1.
Clinical characteristics of patients with breast carcinoma
| Variables | No. of cases |
|---|---|
| Age (years) | |
| Median | 49 |
| Range | 29–73 |
| Lymph node metastasis | |
| Negative | 54 |
| Positive | 65 |
| TNM stage | |
| I | 22 |
| II | 48 |
| III | 47 |
| IV | 2 |
| Histologic grade | |
| G1 | 13 |
| G2 | 69 |
| G3 | 37 |
| Tumor size (cm) | |
| Mean | 3 |
| Median | 3 |
| Range | 1–7 |
| Estrogen receptor | |
| Negative | 46 |
| Positive | 73 |
| Progesterone receptor | |
| Negative | 52 |
| Positive | 67 |
| HER‐2 | |
| IHC 0–1+ | 92 |
| IHC 2+ | 9 |
| IHC 3+ | 18 |
| NPI | |
| 1 | 25 |
| 2 | 51 |
| 3 | 43 |
NPI, Nottingham prognostic index.
Follow‐up
Clinical and pathological records of all patients in the study were reviewed periodically. Patients were followed regularly for 5 years at the Third Affiliated Hospital of Harbin Medical University. Clinical records were obtained from the follow‐up department of the hospital. All patients were followed until death or the study closing date (October 10, 2010). Disease‐free survival (DFS), which measured the first recurrence at any site, and overall survival (OS), which measured death from any case, were the two assessments used for prognostic analyses.
Patients were seen (history, physical examination, routine laboratory investigations) once every 3 months during the first 2 years, once every 6 months for 5 years, and once every year after that. An X‐ray mammography was carried out once a year or when disease recurrence was suspected. Magnetic resonance imaging was also used when disease recurrence was suspected. During the follow‐up period, 76 patients had disease recurrence and 55 patients died. Contralateral breast carcinoma or second malignancies were not considered to be cases of recurrent disease.
Determination of RECK
Immunohistochemical staining was carried out using the two‐step plus poly‐HRP method as described previously.9 Briefly, one representative section of the tissue was cut at 4 μm and placed on poly‐l‐lysine coated slides. The slides were deparaffinized, dehydrated, immersed in 10 mM sodium citrate buffer (pH 6.0) and pretreated in a microwave oven for 10 min. This was followed by a 10‐min rinse with PBS. After blocking with 3% hydrogen peroxide for 10 min at room temperature, the slides were incubated at 4°C overnight with primary anti‐RECK antibody (1:50 goat mAb; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Afterwards, the slides were stained with the two‐step plus poly‐HRP anti‐goat IgG detection system (ZSGB‐Bio, Beijing, China). After visualization of the reaction with DAB chromogen, the slides were counterstained with hematoxylin and covered with a glycerin gel. For negative controls, the primary antibody was substituted with PBS in order to confirm the specificity of the primary antibody.
Evaluation of immunohistochemical staining
Two blinded, experienced investigators, who provided a consensus opinion of stain patterns by light microscopy, evaluated sections. RECK expression was estimated from the staining intensity and graded as follows: Grade 0, no staining; Grade 1, faint staining; Grade 2, moderate staining; and Grade 3, strong staining. The positively stained area (distribution) was expressed as the percentage of the whole area under evaluation and scored as follows: 0, no staining; 1, 1–25% positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells; and 4, 76–100% positive cells. Overall expression was then graded as low expression (score 0–2), intermediate expression (score 3–5), and high expression (score 6–7). Patients were classified into two groups according to RECK expression. Patients showing high expression were classified as RECK positive and the remainder as RECK negative.
Statistical analysis
All analyses were carried out using the statistical software spss 13.0 (SPSS, Chicago, IL, USA). The correlation of RECK immunoreactivity with patients' clinicopathological variables was analysed by the chi‐square test or Fisher's exact test. The Kaplan–Meier method was used to estimate OS. Survival differences according to RECK expression were analysed by the log–rank test. The influence of variables on survival was assessed using Cox univariate and multivariate regression analyses. The risk ratio and its 95% confidence interval were recorded for each marker. P‐values < 0.05 were considered statistically significant in all of the analyses.
Results
Clinical results
A total of 119 patients with a mean age of 51 years (range, 29–73 years; median, 49 years) were enrolled in the study. The mean follow‐up period was 59 months (range, 3–169 months; median, 60 months). Fifty‐five patients (46.2%) died, and 64 patients (53.8%) were alive at the time of study completion. Twenty‐two patients (18.5%) were at stage I, 48 patients (40.3%) were at stage II, 47 patients (39.5%) were at stage III, and two patients (1.7%) were at stage IV. Thirteen patients were classified as grade I, 69 were grade II, and the remaining patients were grade III. Lymph node metastases were present in 65 patients (54.6%).
Immunohistochemical pattern of RECK expression in normal tissue and carcinoma
RECK was detected in the cytoplasm of normal acinar and ductal cells (Fig. 1a). Of the 119 breast cancer specimens, 56 patients were classified as RECK positive (Fig. 1b,c) and the remainder (n = 63) as RECK negative (Fig. 1d). We also showed positive RECK staining in 18 (81%) of the 22 stage I patients, 28 (58.3%) of the 48 stage II patients, 10 (21.2%) of the 47 stage III patients, and in none of the stage IV patients. In contrast, RECK was positive in 11 (84.6%) of the 13 grade I tumors, 30 (43.4%) of the 69 grade II tumors, and 15 (45.9%) of the 37 grade III tumors. These data indicate that the frequency of RECK expression was much higher in high‐grade than in low‐grade breast cancer.
Figure 1.
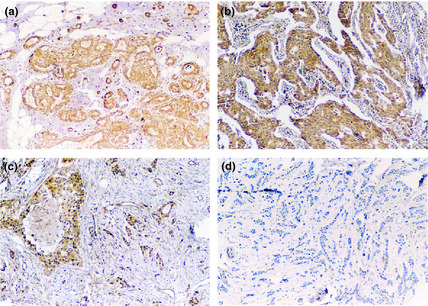
Immunohistochemical pattern of RECK expression in normal tissue and breast carcinoma. (a) RECK expression in benign breast disease. (b) Strong RECK‐positive cells in an invasive ductal breast cancer specimen. (c) RECK‐positive cells in an invasive ductal breast cancer specimen. (d) RECK‐negative specimen.
RECK expression in relation to prognosis
We used the Nottingham prognostic index (NPI)19 as an indicator to evaluate the patient's prognosis. The NPI range was 2.3–7.4, with a median survival value of 4.6. Patients with a good prognosis formed the NPI‐1 group (n = 25), with an NPI of 3.4. Patients with a moderate prognosis formed the NPI‐2 group (n = 51), with an NPI of 3.4–5.4, and patients with a poor prognosis formed the NPI‐3 group (n = 43), with an NPI of 5.4. We found a significant association between RECK expression levels and NPI status using the Kruskal–Wallis test (P = 0.010) (Table 2).
Table 2.
Correlation of RECK expression with breast cancer prognosis
| Variables | No. of cases | RECK | ||||
|---|---|---|---|---|---|---|
| Negative | Positive | Score range | Median | P‐value | ||
| NPI | ||||||
| 1 | 25 | 5 | 20 | 2.30–3.36 | 3.30 | 0.010 |
| 2 | 51 | 25 | 26 | 3.60–5.30 | 4.40 | |
| 3 | 43 | 33 | 10 | 5.60–7.40 | 6.20 | |
| Survival status | ||||||
| Good | 43 | 15 | 28 | nd | nd | 0.004 |
| Poor | 76 | 48 | 28 | nd | nd | |
nd, not done; NPI, Nottingham prognostic index.
RECK expression and survival status
We used an average 5‐year follow‐up period to assess the survival of breast cancer patients in the context of RECK expression levels. Patients were divided into two groups on the basis of their prognosis. The good prognosis group (n = 43) comprised patients who remained disease‐free, and the poor prognosis group (n = 76) comprised patients who had recurrence, metastasis to a distant site, or had died as a result of breast cancer. Our results indicated that patients with a poor prognosis had low levels of RECK expression (P = 0.004) (Table 2).
Correlations between RECK expression and various clinicopathological features
Correlations between RECK expression and various clinicopathological features are summarized in Table 3. We did not find significant correlations between RECK expression and age, menopausal status, adjuvant treatment, tumor size, estrogen receptor (ER) expression, progesterone receptor (PR) expression, or HER‐2 expression. RECK expression, however, was significantly associated with lymph node metastasis (P = 0.002) and histopathological grading (P = 0.006). Lymph node negative patients had higher RECK expression (34/54, 62.9%) than lymph node positive patients (22/65, 33.8%). We also found that low histopathological grade (G1) breast cancer patients had higher RECK expression (11/13, 84.6%) than high histopathological grade (G2–G3) breast cancer patients (45/106, 42.5%).
Table 3.
Correlation between RECK expression and various clinicopathological features in breast cancer patients
| Variables | No. of cases | RECK | ||
|---|---|---|---|---|
| Negative | Positive | P‐value | ||
| Age (years) | ||||
| <50 | 60 | 31 | 29 | 0.855 |
| ≥50 | 59 | 32 | 27 | |
| Tumor size | ||||
| pT1 | 40 | 16 | 24 | 0.080 |
| pT2–T4 | 79 | 46 | 33 | |
| Menopausal status | ||||
| Premenopausal | 67 | 35 | 32 | 1.000 |
| Postmenopausal | 52 | 28 | 24 | |
| Histologic grade | ||||
| G1 | 13 | 2 | 11 | 0.006 |
| G2–G3 | 106 | 61 | 45 | |
| Lymph node status | ||||
| N0 | 54 | 20 | 34 | 0.002 |
| N1–N3 | 65 | 43 | 22 | |
| ER status | ||||
| Positive | 73 | 38 | 35 | 0.852 |
| Negative | 46 | 25 | 21 | |
| PR status | ||||
| Positive | 67 | 36 | 31 | 0.855 |
| Negative | 52 | 27 | 25 | |
| HER‐2 status | ||||
| Positive (IHC 3+) | 18 | 7 | 11 | 0.573 |
| Negative (IHC 1+) | 92 | 35 | 57 | |
| Adjuvant systemic therapy | ||||
| Yes | 80 | 42 | 38 | 1.000 |
| No | 39 | 21 | 18 | |
ER, estrogen receptor; NPI, Nottingham prognostic index; PR, progesterone receptor.
Univariate and multivariate analyses of RECK expression and clinicopathological variables
Univariate analysis of OS using Cox regression analysis identified Reck‐positive expression (P = 0.001), tumor diameter (P = 0.015), lymph node metastasis (P = 0.007), pathological TNM stage (P = 0.018), and ER (P = 0.016) and PR status (P = 0.010) as significant prognostic predicators. Age, menopausal status, and histopathological grade had no prognostic value. Multivariate analysis was carried out on the same set of patients for RECK expression and pathological predictors for survival time using the Cox regression model. The results indicated that RECK status (risk ratio, 2.304; P = 0.012), ER status (risk ratio, 1.914; P = 0.023), and PR status (risk ratio, 1.994; P = 0.017) were independent favorable prognostic factors. Lymph node metastasis was an independent unfavorable prognostic factor (risk ratio, 2.035; P = 0.028) (Table 4).
Table 4.
Prognostic factors in Cox proportional hazards model
| Variables | Risk ratio | Univariate 95% CI | P‐value | Risk ratio | Multivariate 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| ≥50 /<50 | 0.976 | 0.560–1.699 | 0.931 | nd | nd | 0.994 |
| Menopausal status | ||||||
| Premenopausal/postmenopausal | 1.219 | 0.700–2.123 | 0.485 | nd | nd | 0.405 |
| Histologic grade | ||||||
| G1/G2–G3 | 3.536 | 0.859–14.556 | 0.080 | nd | nd | 0.478 |
| Tumor size | ||||||
| pT1/pT2–T4 | 2.217 | 1.171–4.197 | 0.015 | nd | nd | 0.238 |
| Lymph node metastasis | ||||||
| N0/N1–N3 | 2.288 | 1.248–4.196 | 0.007 | 2.035 | 1.079–3.837 | 0.028 |
| Pathologic stage (TNM) | ||||||
| I / II–IV | 3.428 | 1.233–9.532 | 0.018 | nd | nd | 0.614 |
| Estrogen receptor | ||||||
| Negative/positive | 1.979 | 1.136–3.447 | 0.016 | 1.914 | 1.094–3.349 | 0.023 |
| Progesterone receptor | ||||||
| Negative/positive | 2.089 | 1.194–3.653 | 0.010 | 1.994 | 1.134–3.506 | 0.017 |
| RECK | ||||||
| Negative/positive | 2.906 | 1.564–5.399 | 0.001 | 2.304 | 1.204–4.408 | 0.012 |
nd, not done.
Kaplan–Meier survival analysis
Kaplan–Meier survival curves are shown in Figure 2. Among the 119 study patients, RECK‐positive patients showed higher DFS rates when compared with RECK‐negative patients (P = 0.002, log–rank test; Fig. 2a). RECK‐positive patients also had significantly higher OS rates than RECK‐negative patients (P < 0.001, log–rank test; Fig. 2b).
Figure 2.
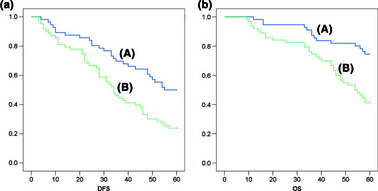
Kaplan–Meier analysis for disease‐free survival (DFS) and overall survival (OS) based on RECK expression in breast cancer patients. (a) Kaplan–Meier analysis for DFS based on RECK expression in patients with breast cancer (P = 0.002, log–rank test). (b) Kaplan–Meier analysis for OS based on RECK expression in patients with breast cancer (P < 0.001, log–rank test). (A) RECK‐positive patients (n = 56); (B) RECK‐negative patients (n = 63).
Discussion
In this study, we showed, using immunohistochemical staining, that RECK expression is suppressed in breast cancer tissue when compared to normal tissue. Low RECK expression was closely correlated with lymph node metastasis. Furthermore, low RECK expression was a negative survival factor for breast cancer.
A decrease in expression of RECK in various cancers was reported. Our observations are consistent with reports on cancers of the liver, pancreas, breast, colon, and lung.11, 12, 13, 14, 15, 16 Previous studies11, 12, 13, 14, 15 reported that RECK expression was lower in cancer tissues than in normal tissues and that lower RECK expression was correlated with a poor prognosis. In a previous study of breast cancer, Span et al.20 analyzed the RECK expression by quantitative real‐time RT‐PCR. They showed that reduced expression of RECK is a predictor of poor prognosis in resected breast cancer. As the mRNA levels are not always consistent with the protein expression, it is necessary to evaluate the significance of RECK protein expression in patients with breast cancer. In the present study, we used immunohistochemical staining to examine RECK expression. We confirmed that RECK protein levels were dramatically lower in breast cancer tissue compared with normal breast tissue. We also analyzed relationships between RECK expression and survival. RECK‐positive patients showed higher 5‐year survival rates and DFS rates when compared with RECK‐negative patients. These data, consistent with a previous study, indicate that RECK was a marginally significant prognostic factor in breast cancer. Furthermore, we evaluated the correlation between RECK expression with various clinicopathological features. We found that low histopathological grade breast cancer patients had higher frequency of RECK expression (11/13, 84.6%) than high histopathological grade breast cancer patients (45/106, 42.5%) (P < 0.01). Additionally, RECK expression was significantly associated with lymph node metastasis (P = 0.002). Lymph node negative patients had higher RECK expression (34/54, 62.9%) than lymph node positive patients (22/65, 33.8%). To the best of our knowledge, our study is the first to show a strong correlation between decreased RECK expression with lymph node metastasis in breast carcinoma. In a previous study, Hsu et al.21 showed that the HER‐2/neu oncogene inhibits the expression of RECK to promote cell invasion. Therefore, we discussed the relationship between HER‐2 and RECK expression. Among the 18 cases that were HER‐2 positive, RECK expression was reduced in 38.9% (7/18). However, no significant correlations between RECK expression and HER‐2 expression were observed in our study (P = 0.573) (Table 3). Of note, we found that approximately 15% of patients were HER‐2 positive, which was much lower than the 20–25% found in countries such as North America and Europe Zhang et al.22 examined HER‐2 expression in 5000 Chinese women with breast cancer. In their study, HER‐2 positive cases were observed in 11.1% of patients. Our data are consistent with previous studies that indicate there may be ethnic differences in levels of HER‐2 expression.
Cancer cell invasion comprises steps in the destruction of the basement membrane and migration of cells into the connective tissue. These cells further migrate into lymph ducts and small vessels to create metastasis. The ECM provides a microenvironment for cells, and its destruction is associated with cancer cell invasion. Matrix metalloproteinases are a family of Zn‐dependent endopeptidases that, collectively, are capable of cleaving virtually all ECM substrates, and play an important role in physiological and pathological processes, including cancer cell metastasis.23, 24, 25 RECK, as a gene which suppresses transformation, can inhibit the process of tumor invasion and metastasis though regulating the expression of several MMPs. Many studies suggest that RECK inhibits the activity of at least three MMP members, including MMP‐2, MMP‐9, and MMP‐14.26, 27, 28, 29 Both MMP‐2 and MMP‐9 have the capacity to degrade native collagen type IV, a major component of the basement membrane. Furthermore, MMP‐2 and MMP‐9 are also involved in the processes of cell differentiation, apoptosis, angiogenesis, the immune response, and growth of tumor cells.30, 31 These two proteins also play an important role in tumor development and progression. MMP‐14 plays a dual role in pathophysiological digestion of several ECM components (including collagen I) through direct cleavage of the substrates in vivo and activation of MMP‐2.32, 33 To explore the relationship between MMP‐2/9 and RECK, we collected 30 fresh breast cancer tissues to examine the expression of MMP‐2/9 and RECK by real‐time RT‐PCR (Data S1). We found that RECK mRNA expression levels were directly correlated with expression of MMP‐2, but not MMP‐9 (Fig. S1). The induction of RECK gene expression could be a cellular response to enhanced expression and activity of MMP‐2. Our report is consistent with a previous study that showed elevated levels of RECK were associated with better prognosis. Therefore, tumors that present overexpression of RECK could display a better clinical course, depending on the balance of RECK/MMPs.
In addition to previous studies, our data strongly indicate that RECK is an important molecule in preventing tumor cell metastasis in breast carcinoma. In conclusion, RECK expression was reduced in breast carcinoma. Low levels of RECK protein correlated with shorter survival in patients with breast carcinoma. Low RECK expression was associated with lymph node metastasis. Our results suggest that RECK is a negative predictor of breast carcinoma and RECK could emerge as an attractive new drug target in the treatment of breast carcinoma.
Disclosure Statement
The authors have no conflicts of interest.
Supporting information
Data S1. Materials and methods in detail.
Fig. S1. Correlation between expression of MMP‐2/9 and RECK.
Acknowledgments
The study was supported by the Foundation of Educational Commission of Heilongjiang Province for Returned Overseas Chinese Scholars (Grant No. 1154h23). This experiment was completed in the Oncobiology Key Laboratory of Heilongjiang Common Institution of Higher Learning.
References
- 1. Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA‐MB‐435 cells after transfection with the metastasis suppressor gene, KiSS‐1. Cancer Res 1997; 57: 2384–7. [PubMed] [Google Scholar]
- 2. Welch DR, Steeg PS, Rinker‐Schaeffer CW. Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res 2000; 2: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang J, Mani SA, Donaher JL et al Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004; 117: 927–39. [DOI] [PubMed] [Google Scholar]
- 4. Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial‐ mesenchymal transitions. Curr Biol 2004; 14: R719–21. [DOI] [PubMed] [Google Scholar]
- 5. Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem 2002; 38: 21–36. [DOI] [PubMed] [Google Scholar]
- 6. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827–39. [DOI] [PubMed] [Google Scholar]
- 7. Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras‐related gene with transformation suppressor activity. Cell 1989; 56: 77–84. [DOI] [PubMed] [Google Scholar]
- 8. Noda M, Kitayama H, Matsuzaki T et al Detection of genes with a potential for suppressingthe transformed phenotype associated with activated ras genes. Proc Natl Acad Sci USA 1989; 86: 162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi C, Akiyama N, Matsuzaki T, Takai S, Kitayama H, Noda M. Characterization of a human MSX‐2 cDNA and its fragment isolated as a transformation suppressor gene against v‐Ki‐ras oncogene. Oncogene 1996; 12: 2137–46. [PubMed] [Google Scholar]
- 10. Takahashi C, Sheng Z, Horan TP et al Regulation of matrix metalloproteinase‐9 and inhibition of tumor invasion by the membrane‐anchored glycoprotein RECK. Proc Natl Acad Sci USA 1998; 95: 13221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oh J, Takahashi R, Kondo S et al The membrane‐anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 2001; 107: 789–800. [DOI] [PubMed] [Google Scholar]
- 12. Rhee JS, Coussens LM. RECKing MMP function: implications for cancer development. Trends Cell Biol 2002; 12: 209–11. [DOI] [PubMed] [Google Scholar]
- 13. Sasahara RM, Takahashi C, Sogayar MC, Noda M. Oncogenemediated downregulation of RECK, a novel transformation suppressor gene. Braz J Med Biol Res 1999; 32: 891–5. [DOI] [PubMed] [Google Scholar]
- 14. Furumoto K, Arii S, Mori A et al RECK gene expression in hepatocellular carcinoma: correlation with invasion‐related clinicopathological factors and its clinical significance. Reverse‐inducing cysteinerich protein with Kazal motifs. Hepatology 2001; 33: 189–95. [DOI] [PubMed] [Google Scholar]
- 15. Masui T, Doi R, Koshiba T et al RECK expression in pancreatic cancer: its correlation with lower invasiveness and better prognosis. Clin Cancer Res 2003; 9: 1779–84. [PubMed] [Google Scholar]
- 16. van der Jagt MF, Sweep FC, Waas ET et al Correlation of reversion‐inducing cysteinerich protein with Kazal motifs (RECK) and extracellular matrix metalloproteinase inducer (EMMPRIN), with MMP‐2, MMP‐9, and survival in colorectal cancer. Cancer Lett 2006; 237: 289–97. [DOI] [PubMed] [Google Scholar]
- 17. Takenaka K, Ishikawa S, Kawano Y et al Expression of a novel matrix metalloproteinase regulator, RECK, and its clinical significance in resected nonsmall cell lung cancer. Eur J Cancer 2004; 40: 1617–23. [DOI] [PubMed] [Google Scholar]
- 18. Song SY, Son HJ, Nam E, Rhee JC, Park C. Expression of reversion‐inducing‐cysteine‐rich protein with Kazal motifs(RECK) as a prognostic indicator in gastric cancer. Eur J Cancer 2006; 42: 101–8. [DOI] [PubMed] [Google Scholar]
- 19. Haybittle JL, Blamey RW, Elston CW et al A prognostic index in primary breast cancer. Br J Cancer 1982; 45: 361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Span PN, Sweep CG, Manders P, Beex LV, Leppert D, Lindberg RL. Matrix metalloproteinase inhibitor reversion‐inducing cysteine‐rich protein with Kazal motifs: a prognostic marker for good clinical outcome in human breast carcinoma. Cancer 2003; 97: 2710–5. [DOI] [PubMed] [Google Scholar]
- 21. Hsu MC, Chang HC, Hung WC. HER‐2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion. J Biol Chem 2006; 281: 4718–25. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Hui R, Liu P, Yu Y, Liu Y, Hao X. Clinical pathologic status and expression of tumor markers in 5,000 Chinese breast cancers. J Clin Oncol 2006 ASCO Annual Meeting Proceedings 2006; 24: 10644. [Google Scholar]
- 23. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011; 278: 16–27. [DOI] [PubMed] [Google Scholar]
- 24. Szarvas T, vom Dorp F, Ergün S, Rübben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol 2011; 8: 241–54. [DOI] [PubMed] [Google Scholar]
- 25. Chabottaux V, Noel A. Breast cancer progression: insights into multifaceted matrix metalloproteinases. Clin Exp Metastasis 2007; 24: 647–56. [DOI] [PubMed] [Google Scholar]
- 26. Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev 2003; 22: 167–75. [DOI] [PubMed] [Google Scholar]
- 27. Takagi S, Simizu S, Osada H. RECK negatively regulates matrix metalloproteinase‐9 transcription. Cancer Res 2009; 69: 1502–8. [DOI] [PubMed] [Google Scholar]
- 28. Figueira RC, Gomes LR, Neto JS, Silva FC, Silva ID, Sogayar MC. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer 2009; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vagima Y, Avigdor A, Goichberg P et al MT1‐MMP and RECK are involved in human CD34+ progenitor cell retention, egress, and mobilization. J Clin Invest 2009; 119: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 2004; 1705: 69–89. [DOI] [PubMed] [Google Scholar]
- 31. Planagumà J, Liljeström M, Alameda F et al Matrix metalloproteinase‐2 and matrix metalloproteinase‐9 codistribute with transcription factors RUNX1/AML1 and ETV5/ERM at the invasive front of endometrial and ovarian carcinoma. Hum Pathol 2011; 42: 57–67. [DOI] [PubMed] [Google Scholar]
- 32. Shen Q, Lee ES, Pitts RL, Wu MH, Yuan SY. Tissue inhibitor of metalloproteinase‐2 regulates matrix metalloproteinase‐2‐mediated endothelial barrier dysfunction and breast cancer cell transmigration through lung microvascular endothelial cells. Mol Cancer Res 2010; 8: 939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holmbeck K, Bianco P, Yamada S, Birkedal‐Hansen H. MT1‐MMP: a tethered collagenase. J Cell Physiol 2004; 200: 11–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and methods in detail.
Fig. S1. Correlation between expression of MMP‐2/9 and RECK.


