Abstract
It has been found that the composition of intestinal microbiota can indicate the risk of disease to each individual. The concepts of biodynamics as used by the Benziger Winery in California, which treats every part of an agricultural environment as a living, breathing entity, can be usefully used in the construction of a system for cancer prevention, which seeks to use the relationship of coexistence (symbiosis) shared between people and intestinal symbiosis, that is, microbiota. Changes in the incidence rate of cancer among Japanese emigrants to Hawaii demonstrate the effect of the changes in the living environment. This leads to the hypothesis that an intake of soy‐derived food products and the metabolization of the isoflavones they contain by intestinal microbiota is one of the factors for the significant difference in the incidence rate of prostate cancer among Asian and European/North American populations. It is further hypothesized that isoflavones, particularly equol, are a key factor in the difference in incidence rate between Asia and the West. It is suggested that not having equol converting bacteria in the intestine (non‐equol producers) can be a risk factor for prostate cancer and that one direction for future research will be to examine the possibility of improving the intestinal environment to enable equol production. (Cancer Sci 2012; 103: 969–975)
Prostate cancer is a disorder that attracts significant attention, not merely from a medical perspective, but also from socio‐economic and cultural perspectives. It is complicated by the fact that although there is a high overall incidence rate, there is a noticeable difference in the incidence rates in Asian and Western countries. In addition, there are many means of treating prostate cancer and it is unique in that at times, although being diagnosed as “cancer,” physicians may recommend “active surveillance” without any specific treatment. In addition a particularly sensitive tumor marker for prostate cancer, known as prostate‐specific antigen (PSA) is in wide use around the world as part of screening systems. As a result there has been a rapid increase in the probability of early diagnosis, causing widespread discussion on the use of screening from a socio‐economic perspective. In addition prostate cancer has a strong association with androgens that are a factor in causing cancer, and the relationship between prostate cancer and the living environment has also been noted, with active efforts now underway to construct a preventive strategy that takes such factors into account. Many debates on the biological properties of cancer start from discussions on genetic alteration. However, when considering means to prevent prostate cancer it is also important to take into account and discuss environmental factors as well as host factors. Currently one of the factors for reducing risk of prostate cancer that is being highlighted is the large quantity of soy bean‐derived products consumed in Asia, including Japan. Among these soy‐derived products research into the metabolic specificity of soy isoflavones is well advanced and their interaction with intestinal microbiota has gained attention. In this paper we focus on the strong relationship between the volume of soy‐derived food products consumed and the difference in incidence rate of prostate cancer, seeking to find the essence of the relationship.
Intestinal Microbiota as the “Second Human Genome”
Biodynamics in Sonoma Mountain
The Benziger Family Winery is situated in the Sonoma Mountain region, close to San Francisco in the United States. (http://www.snooth.com/articles/benziger-leading-the-way-on-sonoma-mountain/#ixzz1V3NVplkJ) This winery is renowned for its development and production of wines using “biodynamics” since the mid 1990s. In agricultural production methods, to date, it has been the received wisdom that organic produce, grown without recourse to chemical fertilizers etc., was preferable to produce grown using such industrial chemicals. However, biodynamics seeks to go even further, considering the bird, livestock and insect life in the vicinity of the winery as a means of adjusting the water, soil and even microorganisms present in the soil where grapes are grown, with the aim of producing the perfect wine from an ideal combination of these environmental factors. The composition of microorganisms in the soil is important in the cultivation of healthy grapes and efforts to adjust the balance of the soil and other environmental aspects represent an interesting and groundbreaking concept. In the sense that the environment (vineries) and the host (grapes) are dynamically adjusted and monitored, this method of viticulture has aspects in common with the ways to achieve a healthy balance in the human body.
Human intestinal microbiota
According to Flint, the human intestine is home to very large numbers of micro‐organisms, with bacterial cells exceeding 1011/mL.1 In recent years the important role of these microorganisms has been gaining attention, not only as a potential danger for infection, but also as a significant contributor to nutrient and energy supply, intestine development and immune mechanisms. In addition, there are indications of links between intestinal microbial activities and disorders such as infectious colitis, colorectal cancer, and also conditions such as metabolic syndrome. To date the identification and classification of intestinal microbiota has required these microorganisms to be cultured in a laboratory environment, which has created an obstacle to research, resulting in a situation where many types of intestinal microbiota and their functions remained unknown. However, in recent years, thanks to advances in molecular bacteriology it has become possible to identify and classify these microbiota at the genomic level, and advances in this field have been significant. Arumugam et al.2 have implemented research to uncover the species composition and functional composition of human intestinal microbiomes. By combining 22 newly sequenced fecal metagenomes of individuals from four countries with previously published datasets, Arumugam et al. succeeded in identifying three robust clusters (referred to as enterotypes) that are not nation or continent specific. In addition, by also confirming the enterotypes in two published, larger cohorts, Arumugam et al. demonstrated that intestinal microbiota variation is generally stratified, not continuous. Their research indicates further the existence of a limited number of well‐balanced host‐microbial symbiotic states that might respond differently to diet and drug intake. They noted that the enterotypes are mostly driven by species composition, but abundant molecular functions are not necessarily provided by abundant species, highlighting the importance of a functional analysis to understand microbial communities. Although individual host properties such as body mass index (BMI), age, or gender cannot explain the observed enterotypes, data‐driven marker genes or functional modules can be identified for each of these host properties. For example, Arumugam et al. argue that 12 genes significantly correlate with age and three functional modules with BMI, hinting at the diagnostic potential of microbial markers. To put it another way, it can be said that this research has highlighted the potential for intestinal microbiota to express the risk of certain diseases for individuals.
Catechins belong to the family of flavonids and are widely found in foods and plants. They are largely found in green and black tea, apples and red wine and are said to help reduce the risk of cardiovascular disease and colorectal cancer. Kutschera et al.3 isolated two catechin‐converting bacterial strains, rK3 and aK2, from an epicatechin‐converting human fecal suspension. Using 16S rRNA gene sequencing methods, the isolates were identified as Eggerthella lenta and Flavonifractor plautii. This study thus widened the potential for catechin activation and catechin applications for humans.
In addition, De Filippo et al.4 discovered that the intestinal microbiota of children living in two different locations were completely different, one group being from cities in Western Europe, where sanitation concepts are advanced, and one group being from villages in Africa, where similar concepts are still in a state of development. De Fillippo et al. hypothesized that intestinal microbiota coevolved with the polysaccharide‐rich diet of the African children, allowing them to maximize energy intake from fibers while also protecting them from the risk of infectious and noninfectious colonic diseases.
The above studies have shown that the structures of intestinal microbiota are determined by living environments and diet and that this can cause changes to the disease structure in humans, which are the hosts to these microbiota. Cancer is known to present itself over a relatively long period of time, due to gradual mutations in DNA composition. While it is certainly the case that genetic barriers are one of the main causes of cancer, it is of particular importance to consider environmental and dietary factors that could modify such genetic mutations. In recent years success has been achieved in the development of many molecular‐targeted drugs for human cancer. However, it has also been confirmed that these molecular‐targeted drugs are almost incapable of effecting a permanent cure for the disease. In other words, at the current point it is still not possible to completely restore DNA damage and therefore permanently cure cancer.
The concept of environmental development behind biodynamics at the Benziger Family Winery and the development of a system of coexistence (symbiosis) between the many hundreds of millions of intestinal microbiota and human beings can be seen as working to narrow down the limitations we currently face in cancer treatment, and could be particularly beneficial in terms of building cancer prevention structures.
NATTS Bacteria
We identified NATTS bacteria from among the intestinal microbiota of a normal Japanese male (Fig. 1). This bacteria is classified in the family Coriobacteriacea, belonging to the Slackia genus and we confirmed that it has a mechanism whereby it can degrade one of the daidzeins in soy isoflavones into equol with a high degree of efficiency.5 The NATTS strain‐specific primer reacts only with the NATTS strain and did not demonstrate any reaction with other test species or 45 bacteria strains commonly found in the human intestine. At the same time, given that the primer sequence matches 100% with the 16S rRNA base sequence of the already reported HE8 strain of equol‐producing bacterium (family Coriobacteriaceae), this suggests that using this primer it would also be possible to detect the HE8 strain in the same way as the NATTS strain. Through reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR) using the primer, it was found possible to detect a population level of 103.5–109 cells/g feces of the NATTS strain in the human intestine. Out of 40 healthy adults, the Slackia sp. NATTS strain and similar bacteria were detected in 16 subjects (40%), numbering 6.4 ± 2.4 (log10 cells/g feces).
Figure 1.
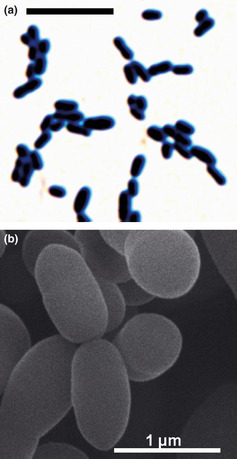
Electron microscopic imaging of newly identified NATTS bacteria. The human intestinal bacterium NATTS converts daidzein found in soy isoflavones to equol. It is a Gram‐positive bacillus and is classified in the family Coriobacteriacea, belonging to the Slackia genus. The NATTS bacillus was detected and identified in the course of our research, but other equol‐converting bacteria have also been reported. The NATTS bacillus is the most efficient in its equol‐converting capacity. (a) Gram staining. (b) Scanning electron microscopy.
It should be noted that our study is starting to make clear that NATTS bacteria have not been detected in all humans and that there is a noticeable difference in serum equol concentrations measured in so‐called equol‐producers, depending on the country (or region) (Fig. 2). In addition, it has been confirmed that the risk of prostate cancer is lowered in men capable of degrading daidzein to equol (Fig. 3). This leads us to our hypothesis that the characteristics of ingesting soy‐derived food products and the metabolization of isoflavones by intestinal microbiota are one fact in the noticeable difference in the incidence rate of prostate cancer in Asian and Western countries.6, 7, 8
Figure 2.
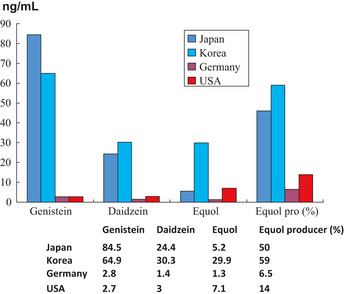
Comparisons of serum isoflavone concentrations and equol producer in the controls in each country. Comparison of serum isoflavone concentrations and ratios of equol producers among adults in Japan, Korea, the United States and Germany. The serum concentrations of genistein and daidzein are extremely high in Japanese and Korean subjects, who have a daily intake of soy‐derived food products, in comparison with US and German subjects. Of special mention is the ratio of equol products (the fourth column from left in the graph). Among control groups without prostate cancer, there was a marked difference in the proportion of equol producers in each country: Japan (50%), Korea (59%), USA (14%), Germany (6%).
Figure 3.
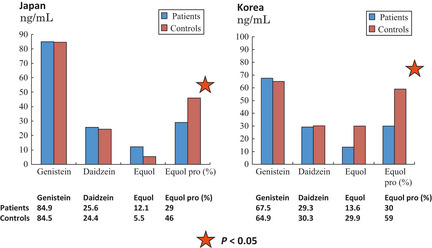
Comparisons between cases and controls in Japan and Korea. Results of case–control studies on the serum isoflavone concentrations and the proportion of equol producers for case groups with prostate cancer and control groups that tested negative for cancer. This case–control study for Japan and Korea was implemented as a joint study. The case and control groups for both countries showed high concentrations of genistein and daidzein. However, the ratio of equol producers was in statistical terms significantly lower in prostate cancer patients in the case group (χ2 test; P < 0.05). In other words, equol non‐producers can be said to be a high risk group for prostate cancer. This would further suggest that the presence or absence of equol‐producing bacteria could have an effect on the risk of protracting prostate cancer. This is possibly a new function for intestinal microbiota.
Unique Epidemiology of Prostate Cancer
We should now consolidate the relationship to prostate cancer of the points that have been made above. The epidemiology of prostate cancer has certain distinguishing features. Below is a summary of the changes in the incidence rate of prostate cancer, looking at differences in mortality depending on country and age, and the changes in the incidence rate for Japanese people who relocate to a different region where the daily living environment is different from that in Japan.
Changes in the incidence rate by country, age, and emigration/overseas relocation
An international comparison of age‐standardized incidence rates for prostate cancer around the world indicates that the incidence rate for Asian men in such countries as China, Japan, India and Singapore, is significantly lower than in Western countries such as the United States, Canada and Europe.9 If looking at the changes in the age‐adjusted prostate cancer mortality rates for Japan and the major Western countries side‐by‐side, it is evident that the prostate cancer mortality rate in the US, United Kingdom, France and Italy is high, while the mortality rate in Japan has been historically low.10 But, the issue we are currently facing is that the mortality rate in Japan is rising rapidly. In Western countries the incidence rate of prostate cancer is the highest for all cancers among adult males and the mortality rate is the second highest. In Japan, according to 2003 statistics, the incidence rate among adult males is third highest, after stomach and lung cancer (if colon and rectal cancer are counted together, prostate cancer takes fourth place). With regard to the mortality rate in Japan, although prostate cancer stood in 9th place in 1995, by 2007 it had risen to 7th place. (http://ganjoho.ncc.go.jp/professional/statistics/statistics.html) The factors behind the rapid increase in the incidence and mortality rates among Japanese males are thought to be the lengthened average lifespan of males, the permeation of PSA screening and the westernization of diets. Kolonel et al.11 demonstrated the change in the incidence rate of major cancers among Japanese migrants to Hawaii. For prostate, breast and colon (male) cancers the incidence rate showed a rapid rise for both first and second‐generation immigrants from Japan to Hawaii. Although the incidence rate of migrants does not reach the same rate as Caucasians for prostate and breast cancer, the incidence rate for colon cancer has in fact exceeded the incidence rate for Caucasians. However, the incidence rate for stomach cancer, which is prevalent in Japan, demonstrates a completely opposite tendency.
Contributing epidemiological factors and characteristics
In the abovementioned study by Kolonel et al.11 the incidence rate for prostate cancer among people between 50 and 74 years of age was almost the same for Latino, native Hawaiian and Caucasian ethnicities, with the rate for Japanese migrants falling below this rate. However, the incidence rate among African Americans was much high than for other groups. This suggests that the incidence of prostate cancer demonstrates some degree of genetic background; however, the surge in the incidence rate among Japanese migrants makes it easy to understand the significant effect of changes in the living environment. In Western countries that have a high mortality rate from prostate cancer the main energy source is from animal‐derived food products, while the intake of soy‐derived food products is zero. Conversely, in Asian countries where the incidence and mortality rates are lower, the reverse is true, with soy‐derived food products accounting for a large portion of energy intake.12
Survey of dietary habits and environmental factors in Japan showed that although there was a tendency for consumption of meat to elevate prostate cancer risk, it was not significant.13, 14 In contrast, the consumption of large quantities of fish was significantly associated with decreased risk of prostate cancer (P = 0.04). In addition, in the fourth quartile with the largest consumption of soy‐derived products (odds ratio [OR] = 0.48), tofu (OR = 0.45) and natto (OR = 0.27) there was a significant decrease in the risk of prostate cancer. In terms of soy isoflavones, in the fourth quartile with the largest consumption volume of isoflavones (OR = 0.19, P < 0.01), daidzein (OR = 0.37, P < 0.01) and genistein (OR = 0.37, P = 0.01) a significant decrease in prostate cancer risk was witnessed. Although there was a tendency for consumption of green tea to decrease prostate cancer risk, it was not significant.15 In terms of other environmental factors, there was a significant risk when subjects had a previous medical history of prostatic enlargement (OR = 5.15).
In addition, a meta‐analysis on the volume of soy‐derived food products and the risk of prostate cancer based on the results of two cohort studies and six case–controls confirmed that the intake of soy‐derived food products reduces the overall risk estimate of prostate cancer by 70% (P < 0.001).16 No publication bias was detected and it was concluded that the results of the analysis showed that consumption of soy food was associated with a lower risk of prostate cancer in men. Following the first well designed meta‐analysis, several papers have been published recently. Yan and Spitznagel17 suggested that soy foods had an association with reduction of prostate cancer risk, and that the effect was stronger in Asian populations than in Western populations (RR/OR; 0.52 vs 0.85). The other meta‐analysis by Hwang et al.18 also showed the same results indicating OR for total soy foods consumption as 0.69.
Indeed, given that the number of prostate cancer patients has been rising in Japan in recent years, Fujimoto et al.19 conducted a comparison by age of serum isoflavone concentrations. The results showed that the proportion of equol producers in their teens was extremely low and that the proportion was high in those people above 50 years of age. This suggests that changes in the dietary habits of young Japanese people could result in a future rise in prostate cancer in Japan. At the same time, however, there were no changes witnessed in Korea. These results raise concerns about the relationship between changes in dietary habits among young people in Japan and the rapid rise in the incidence rate of prostate cancer, and preventive measures are likely to be necessary in the next generation.
Taking a look at other epidemiological studies that focus on the difference in prostate cancer incidence rates between Japan/Asia and Western countries, there are no factors other than soy‐derived food products that clearly demonstrate a risk reduction in the incidence rate.
This begs the question, therefore, can the clear differences in incidence rates in prostate cancer between Asian and Western countries be put to good use in reducing the risk of prostate cancer on a global level?
If we see that soy‐derived food products are truly a contributing factor to risk reduction, would it be possible to recommend that the people of Western countries transform their dietary habits and make soy an integral part of their daily food intake? The most likely answer would be “No.” It would be too much to expect that people could change their dietary habits, which is one of the most fundamental aspects of any culture.
How then, can we adequately use the valuable information that has been gained from research?
Research into the Relationship Between Soy Isoflavones and Reducing the Risk of Prostate Cancer
Isoflavones are one of the constituents present in soy beans that are generating the greatest attention. Since it was reported that there is a relationship between soy‐derived food products that have been eaten since ancient times and a reduced risk of prostate cancer and cardiac disorders, among others, interest in soy‐derived foods has continued to grow rapidly.20
This study targeted 14 Swedish and 14 Japanese males and it was found that the concentrations of serum isoflavinoids were between 7 and 110 times greater in Japanese men. Given that the highest levels of genistein, which functions as a tyrosine kinase inhibitor, were 276 nmol/L, this would suggest a relationship with the low mortality rate from prostate cancer among Japanese men. While most elderly Japanese people have an intake of isoflavones amounting to 30–50 mg/day, in contrast the intake of isoflavones in Western countries is said to be 3 mg/day. Isoflavones are one type of organic polyphenols and include genistein, daidzein and equol, which are all glycosides. Of these, equol is produced when daidzein is broken down and modified by certain types of intestinal microbiota in the intestine like NATTS. Isoflavones have the following properties: (i) hormonal effects as a phytoestrogen; (ii) antioxidant effects; and (iii) other beneficial effects.21 In addition to demonstrating such effects more strongly than other isoflavones, equol acts as a strong anti‐androgen by binding with dihydro‐testosterone (DHT)22 (Fig. 4). Indeed, Ozasa et al.23 reported in their nested case–control study among Japanese men that high serum levels of phytoestrogens, especially equol seemed to reduce prostate cancer risk, and their effects seemed to be independent of serum total testosterone and sex hormone binding globulin (SHBG) levels. Kurahashi et al.24 have also reported a relation of plasma isoflavones and prostate cancer risk in their nested case–control study. In this report, the highest tertile for plasma equol was significantly associated with a decreased risk of localized and advanced prostate cancer (OR = 0.60). In a study outside Japan, Park et al.25 failed to show a prostate cancer risk reductive effects for equol. But not surprisingly, the study cohort is people who live in Hawaii and California and the serum concentration of equol is very low.
Figure 4.
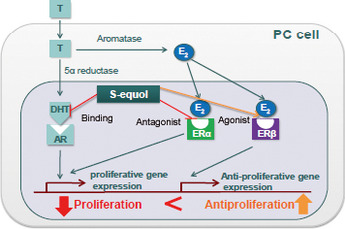
Pattern diagram showing the antiproliferation effect of equol on prostate cancer cells. Working hypothesis of growth inhibition of PC cells by equol. AR, androgen receptor; DHT, dihydrotestosterone; E2, estrogen; Erα, estrogen receptor α; Erβ, estrogen receptor β; T, testosterone.
Evaluating the Risk of Prostate Cancer and Chemoprevention
It is no exaggeration to say that the only successful preventive trials against prostate cancer implemented internationally have been two randomized placebo trials that used 5α‐reductase inhibitor.26, 27 One of these was the Prostate Cancer Prevention Trial (PCPT), which used finasteride, while the other was conducted under the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) study, which used dutasteride. The former was a 7‐year trial (1993–2004) that enrolled more than 18 000 healthy people over 55 years of age. The latter was a 4‐year trail that enrolled 8250 men between the ages of 50 and 75, who had elevated prostate‐specific antigen (PSA) levels and had had one negative prostate biopsy. In both of these studies 5α‐reductase inhibitor significantly reduced the frequency of prostate cancer. However, although there was only a very slight difference, it was the case that the histology of prostate cancer in subjects in the dutasteride/finasteride groups showed a higher degree of malignancy than in the control groups, which generated a great deal of debate. As such these reductase inhibitors have not yet been approved as a preventive drug for prostate cancer. It should also be noted that both drugs are already approved and in use in many countries around the world as treatment for prostatic hyperplasia.
Following the methodology used in the REDUCE trial, we implemented a double‐blind trial, introducing isoflavones to male subjects who had been diagnosed through screening with elevated PSA levels, but for who the results of a prostate biopsy had proven negative. We also examined blood kinetics in equol non‐producers who were provided with isoflavones, seeking to investigate the potential for improving host factors.
A Double‐Blind Trial Using Isoflavone Preparation on Subjects at High Risk of Prostate Cancer
Our comparisons of serum isoflavone concentrations showed that the proportion of prostate cancer patients who are equol producers, who possess the capability to metabolize daidzein to equol, was at a significantly low level. In international comparative studies it has been suggested that in contrast to Japan and Korea, where the incidence rate of prostate cancer is low, in the case of Western countries, where incidence rates are high, the serum isoflavone concentrations are low, and the proportion of equol producers is also markedly low. It was further found that the equol producing capabilities of healthy young Japanese subjects was very much lower than Japanese subjects over 50 years of age, raising concerns about a rapid rise in the prostate cancer incidence rate in Japan in the future.
We have made an investigative double‐blind trial using isoflavone preparation (60 mg/day) on subjects at high risk of prostate cancer for 12 months.28 The composition of isoflavones preparation is described previously.28 This trial was positioned as a large‐scale clinical research pilot study with the aim of examining the tolerance to oral administration of soy isoflavones and evaluating secretion and changes in PSA levels. The study was designed as a randomized, double‐blind, placebo‐controlled trial and the main purpose was to assess the tolerance to oral administration of soy isoflavones and examine the changes in secretion and serum PSA levels following administration. The secondary purpose was to examine the risk of biopsy‐detected prostate cancer 1 year following the trial. The effects on the isoflavone‐administered group and the control group, as well as on equol and non‐equol producers were evaluated.
The subjects of these research activities were provided with written explanations and were free to choose whether or not to participate. For subjects who agreed to participate, signed consent forms were received. A total of 158 specimens were recorded in the double‐blind trial of isoflavone preparations on subjects at high risk of prostate cancer. No problems were detected relating to tolerance to oral administration of soy isoflavones. All cases were classified into isoflavone and placebo groups and although there was no significant difference among the two groups in terms of the positive ratio of prostate cancer, a comparison of subjects over the age of 65 showed that the isoflavone group had a significantly lower positive ratio of prostate cancer. Figure 5 shows the changes in serum equol concentrations in both groups before and after the trial. In cases where equol producers were administered isoflavones, it was confirmed that serum equol concentrations were significantly elevated. Table 1 shows the results of prostate biopsies taken from subjects in both groups 1 year after the trial was implemented. In comparisons of trial subjects above the age of 65, which is the peak age of onset, the prostate cancer positive ratio among equol non‐producers in the placebo group was 66.7%. In contrast, this reduced 25% in the isoflavone group and among equol producers the positive rate was 50% in the placebo group and 30.8% isoflavone‐administered group. Although the number of overall samples made it difficult to engage in a sufficient statistical comparison of the two groups, in analysis combining both the equol producer/non‐producer groups the isoflavone‐administered group showed a significant decrease in the diagnosis rate for prostate cancer (P < 0.049).
Figure 5.
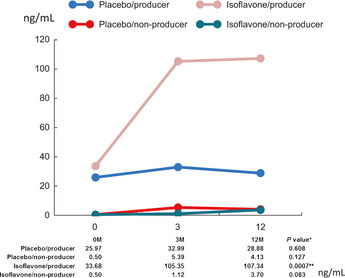
Changes in serum equol level. Double‐blind phase II trials involving isoflavone administration for high risk prostate cancer group. Changes in serum equol concentrations before and after the administration of isoflavones. In the equol‐producer group, although 25–33 ng/mL of equol was detected, in the equol non‐producer group almost no equol was detected. It was observed that serum equol concentrations only increased in equol‐producers who were provided with an isoflavone dose, thus demonstrating the validity of the trial.
Table 1.
Double‐blind trial involving isoflavone administration for high risk prostate cancer group
| Positive biopsy | Equol producer | P | Non‐producer | P | ||
|---|---|---|---|---|---|---|
| Isoflavone (22) | Placebo (22) | Isoflavone (20) | Placebo (25) | |||
| Age | ||||||
| All | 5/22 (22.7%) | 8/22 (36.4%) | 0.255 | 4/20 (20) | 8/25 (32) | 0.288 |
| −64 | 1/9 (11.1) | 0/6 (0) | 0.600 | 1/8 (12.5) | 0/13 (0) | 0.381 |
| 65< | 4/13 (30.8) | 8/16 (50) | 0.293 | 3/12 (25) | 8/12 (66.7) | 0.049* |
| Gleason score | ||||||
| 5–6 | 2 (40) | 6 (75) | 3 (75) | 6 (75) | ||
| 7–9 | 3 (60) | 2 (25) | 0.250 | 1 (25) | 2 (25) | 0.764 |
| High grade PIN | 1 (4.6) | 4 (18.2) | 0.172 | 1 (5) | 4 (16) | 0.251 |
We compiled the results of prostate biopsies for each group 1 year after the trial had been implemented. In comparisons of trial subjects above the age of 65 years, which is the peak age of onset, the prostate cancer positive ratio among equol non‐producers in the placebo group was 66.7%. In contrast, this reduced 25% in the isoflavone group and among equol producers the positive rate was 50% in the placebo group and 30.8% isoflavone‐administered group. Although the number of overall samples made it difficult to engage in a sufficient statistical comparison of the two groups, in analysis combining both the equol producer/non‐producer groups the isoflavone‐administered group showed a significant decrease in the diagnosis rate for prostate cancer (Fisher's exact test; P < 0.049).
In this double‐blind trial of isoflavone preparations on subjects at high risk of prostate cancer the preventive effect of equol was clinically indicated. We believe that this study has therefore produced valuable results that could form a primary prevention strategy for prostate cancer chemoprevention. The trial was positioned as a large‐scale clinical pilot study and on the basis of the results further international clinical trials are warranted in Japan, Korea and the United States.
Conclusion
In recent years there has been a noticeable increase in interest in prostate cancer chemoprevention. However, even with regard to 5α‐reductase inhibitors, the effectiveness of which has been proven with well‐documented evidence, given concerns about pathological results that showed a higher occurrence rate in the inhibitor‐administrated groups than for the control groups and also about specific drug side effects, and also considering the issue of additional expenses for medical systems, these inhibitors have yet to receive general support for use against high grade cancers. Furthermore, selection criteria for subjects and the establishment of more accurate biomarkers are required. An appropriate near‐term response would probably be to seek to position pharmaceutical prophylaxis as a treatment for low‐risk cancer patients. It is a fact that in use of the chemoprevention drug tamoxifen used to treat breast cancer, out of the 89 women at high risk who were provided with the drug, only one patient reported side effects.29 Conversely, intervention using food products such as soy‐derived foods is thought of as having relatively little risk. However, when dealing with human customs and culture that are closely associated with dietary habits, it will be necessary to adopt a cautious approach with regard to intervention methods. Discussion to date has focused on soy isoflavones, but it is very obviously not appropriate to simply assume that the food culture of Asia, which includes high levels of soy, could be transplanted as a model to transform dietary habits in Western countries. Our research suggests that an inability to convert daidzein to equol in the intestine, or in other words an intestinal environment in which these equol‐converting bacteria do not exist, is a risk factor for prostate cancer. Given this fact, we believe, therefore, that one direction for future study is to improve the intestinal environment, enabling it to produce equol. This is the challenge that was first discussed in this paper. In addition, using the NATTS strain bacteria that we discovered and identified in this study, another method could be to produce bioactive equol; s‐equol artificially and for this s‐equol to be administered as a supplement. In the isoflavone intervention trial that we implemented, the rate of prostate cancer diagnosis was lowered in the isoflavone group. However, given the small number of subjects and the short time‐frame of the trial, we were not able to arrive at any clear conclusions concerning the significance of equol and equol‐producers. In the future we will advance considerations on the possibility of implementing clinical trials in regions that have a high incidence rate of prostate cancer, as we continue our search for biotics using the NATTS strain bacterium.
Disclosure Statement
The author has no conflict of interest.
Acknowledgments
The manuscript was written on the basis of the results of studies supported by Ministry of Education, Culture, Sports, Science and Technology‐funded scientific research FY2000‐2000 and FY2003‐2009, Research in Priority Areas Relating to Cancer Research (17015006), Evaluating the risk of prostate cancer and chemoprevention, and Scientific support program for cancer research, Grant‐in‐Aid for Scientific Research on Innovative Areas, Ministry of Education, Culture, Sports, Science and Technology. The author deeply thanks the following co‐researchers: Taiji Tsukamoto, MD (Professor, School of Medicine, Sapporo Medical University), Seiji Naito, MD (Professor, Department of Urology, University of Kyushu), Mikio Namiki, MD (Professor, Department of Urology, Kanazawa University Graduate School of Medical Science), Yoshihiko Hirao, MD (Professor, Department of Urology, Nara Medical University), Tomoaki Fujioka, MD (Professor, Department of Urology, School of Medicine, Iwate Medical University), Shigeo Horie, MD (Professor, Department of Urology, Teikyo University School of Medicine), Satoru Takahashi MD, (Professor, Nihon University School of Medicine), Mitsuru Mori, MD (Professor, Department of Epidemiology, Sapporo Medical University), Naoto Miyanaga, MD (Department of Urology, University of Tsukuba), Shiro Hinotsu, MD (Associate Professor, Department of Pharmacoepidemiology, Graduate School of Medicine and Public Health, Kyoto University), and Hirokazu Tsuji, MD (Yakurt Central Laboratory).
References
- 1. Flint HJ, O'Toole PW, Walker AW. Special issue: the human intestinal microbiota. Microbiology 2010; 156: 3203–4. [DOI] [PubMed] [Google Scholar]
- 2. Arumugam M, Raes J, Pelletier E et al Enterotype of the human gut microbiome. Nature 2011; 473: 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin‐converting human intestinal bacteria. J Appl Microbiol 2011; 111: 165–75. [DOI] [PubMed] [Google Scholar]
- 4. De Filippo C, Cavalieri D, Di Paola M et al Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. Isolation and characterization of the equol‐producing bacterium Slackia sp. Strain NATTS. Arch Microbiol 2010; 192: 279–87. [DOI] [PubMed] [Google Scholar]
- 6. Akaza H, Miyanaga N, Takashima N et al Is daidzein non‐metabolizer a high risk for prostate cancer? A case–controlled study of serum soybean isoflavone concentration Jpn J Clin Oncol 2002; 32: 296–300. [DOI] [PubMed] [Google Scholar]
- 7. Akaza H, Miyanaga N, Takashima N et al Comparisons of percent equol producer between prostate cancer patients and controls: case–controlled studies of isoflavones in Japanese, Korean, and American residents. Jpn J Clin Oncol 2004; 34: 86–9. [DOI] [PubMed] [Google Scholar]
- 8. Akaza H, Miyanaga N, Naito S et al Prostate cancer and isoflavone. In: Tominaga S, Moore MA, Tajima K, Tsugane S, eds. Developments in Cancer Epidemiology – Prospects for Cancer Control in the Asia Pacific. Tokyo: Princess Takamatsu Cancer Research Fund, 2005; 160–6. [Google Scholar]
- 9. Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, eds. Cancer Incidence of Five Continents. Vol. III, Lyon: IARC, 2002. [Google Scholar]
- 10. World Health Organization (WHO). WHO mortality database. Geneva: WHO, 2005 [Cited 01 Oct 2005]. Available from URL:http://www.who.int/healthinfo/morttables/en/
- 11. Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer 2004; 4: 1–9. [DOI] [PubMed] [Google Scholar]
- 12. Hebert JR, Hurley TG, Olendzki BC et al Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross‐national study. J Natl Cancer Inst 1998; 90: 1637–47. [DOI] [PubMed] [Google Scholar]
- 13. Sonoda T, Nagata Y, Mori M et al A case–control study of diet and prostate cancer in Japan: possible protective effect of traditional Japanese diet. Cancer Sci 2004; 95: 238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mori M, Masumori N, Fukuta F et al Relationship between serum isoflavone concentrations and frequency of soybean products consumption in patients with prostate cancer. Tumor Res 2008; 43: 25–9. [Google Scholar]
- 15. Miyanaga N, Akaza H, Takashima N et al Higher consumption of green tea may enhance equol production. Asian Pac J Cancer Prev 2003; 4: 297–301. [PubMed] [Google Scholar]
- 16. Yan L, Spitznagel EL. Meta‐analysis of soy food and risk of prostate cancer in men. Int J Cancer 2005; 117: 667–9. [DOI] [PubMed] [Google Scholar]
- 17. Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta‐analysis. Am J Clin Nutr 2009; 89: 1155–63. [DOI] [PubMed] [Google Scholar]
- 18. Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta‐analysis of observational studies. Nutr Cancer 2009; 61: 598–606. [DOI] [PubMed] [Google Scholar]
- 19. Fujimoto K, Tanaka M, Hirao Y et al Age‐stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer Prostatic Dis 2008; 11: 252–7. [DOI] [PubMed] [Google Scholar]
- 20. Adlercreutz H, Markkanen H, Watanabe S. Plasma concentration of phyto‐oestrogens in Japanese men. Lancet 1993; 342: 1209–10. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe S, Functionality of Isoflavones. In: Kitamura K. ed. Everything about Soybeans. Abiko: Science Forum, 2010; 260–3. [Google Scholar]
- 22. Lund TD, Munson DJ, Haldy ME et al Equol is a novel anti‐androgen that inhibits prostate growth and hormone feedback. Biol Reprod 2004; 70: 1188–95. [DOI] [PubMed] [Google Scholar]
- 23. Ozasa K, Nakao M, Watanabe Y et al Serum phytoestrogens and prostate cancer risk in a nested case–control study among Japanese men. Cancer Sci 2004; 95: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurahashi N, Iwasaki M, Inoue M, Sasazuki S, Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case–control study: the Japan Public Health Center. J Clin Oncol 2008; 26: 5923–9. [DOI] [PubMed] [Google Scholar]
- 25. Park SY, Wilkens LR, Franke AA et al Urinary phytoestrogen excretion and prostate cancer risk: a nested case–control study in the Multiethnic Cohort. Br J Cancer 2009; 101: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson IM, Goodman PJ, Tangen CM et al The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349: 215–24. [DOI] [PubMed] [Google Scholar]
- 27. Andriole G, Bostwick DG, Brawley OW et al Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010; 362: 1192–202. [DOI] [PubMed] [Google Scholar]
- 28. Miyanaga N, Akaza H, Hinotsu S et al Prostate Cancer Chemoprevention Study: an investigative randomized control study using purified isoflavones in men with rising prostate‐specific antigen. Cancer Sci 2012; 103: 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waters EA, Cronin KA, Graubard BI et al Prevalence of Tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev 2010; 19: 443–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


