Abstract
Cancer survivors are at excess risk of developing second primary cancers, but the precise level of risk in Japanese patients is not known. To investigate the risk of survivors developing second primary cancers, we conducted a retrospective cohort study using data from the Osaka Cancer Registry. The study subjects comprised all reported patients aged 0–79 years who were first diagnosed with cancer between 1985 and 2004 in Osaka and who survived for at least 3 months, followed‐up through to December 2005. A metachronous second primary cancer was defined as any invasive second cancer that was diagnosed between 3 months and 10 years after the first cancer diagnosis. The main outcome measures were incidence rates per 100 000 person‐years, cumulative risk and standardized incidence ratios (SIR) of second primary cancer. Metachronous second primary cancers developed in 13 385 of 355 966 survivors (3.8%) after a median follow‐up of 2.5 years. Sex‐specific incidence rates of metachronous second primary cancer per 100 000 person‐years increased with age, and were higher among men than women (except for the 0–49 years age group), but these rates did not differ over the study period. The 10‐year cumulative risk was estimated as 13.0% for those who first developed cancer at 60–69 years of age (16.2% for men, 8.6% for women). The SIR among those with first cancer diagnosed at 0–39 and 40–49 years of age were 2.13 and 1.52, respectively, in both sexes, whereas the SIR among cancers of the mouth/pharynx, esophagus and larynx were much higher than one as for site relationships. We showed that cancer survivors in Osaka, Japan, were at higher risk of second primary cancers compared with the general population. Our findings indicate that second primary cancers should be considered as a commonly encountered major medical problem. Further investigations are required to advance our understanding to enable the development of effective measures against multiple primary cancers. (Cancer Sci 2012; 103: 1111–1120)
Approximately 50% of men and 40% of women will develop a cancer during their lifetime,1 and half of all cancer patients in Japan will survive for at least 5 years.2 Because of the longer survival times for several forms of cancer and the aging of the population, it is estimated that 5–10% of all cancer patients develop a further, independent primary cancer.3, 4 A better understanding of multiple primary cancers should yield greater insights into the shared etiological factors and basic mechanisms of carcinogenesis and could thus provide a more sound basis for the management of cancer patients, including the development of protective measures.5
In a previous study using data from the Osaka Cancer Registry (2000 Census population; 8.8 million), one of the largest population‐based cancer registries in the world, we reported that 2.0% of cancer patients developed metachronous second primary cancer between 1966 and 1986.4 We also calculated the 10‐year cumulative risk for metachronous second primaries to be approximately 10% for those who developed their first cancer at 60–69 years of age between 1978 and 1983.4 However, investigations into trends or site combinations could not be completed owing to the short cancer registration period. In the present study, we updated the data for the incidence of metachronous second primary cancers in Osaka, Japan, according to sex, age groups, calendar year at diagnosis, primary cancer sites, and follow‐up interval. This was done not only to provide an insight into the etiology of cancer, but also to provide information for effective medical care by clinical oncologists.
Materials and Methods
Study subjects and definition of metachronous second primary cancer
The present study was designed as a retrospective cohort study. Individual case records were obtained from the Osaka Cancer Registry, which was founded in 1962 for the purpose of registering all malignant tumors and benign intracranial tumors arising in Osaka Prefecture.6 The study subjects were all reported patients aged 0–79 years in Osaka who were initially diagnosed as having a first primary cancer between 1985 and 2004 and had survived for at least 3 months. The incidence of second primary cancers among the study subjects was examined through to the end of 2005 for a maximum of 10 years after the first cancer diagnosis.
Metachronous second primary cancer was defined as any invasive second cancer that was diagnosed between 3 months and 10 years after diagnosis of the first cancer. In situ carcinomas, benign intracranial tumors, and any third or fourth (or more) primaries were excluded. Each cancer site was categorized into 16 selected major groups according to International Classification of Diseases Tenth Revision (ICD‐10)7, to analyze the cancer site relationships between first and second cancer. The ICD‐10 codes used in the present study are given as mouth/pharynx (C00‐14), esophagus (C15), stomach (C16), colorectum (C18‐20), liver (C22), gallbladder (C23, C24), pancreas (C25), larynx (C32), lung (C33, C34), breast (female) (C50), uterus (C53‐55), ovary (C56), prostate (C61), kidney/urinary tract/bladder (C64‐68), thyroid (C73) and blood (C81‐85, C88, C90, C91‐96).
Statistical analysis
To estimate the risk for second primary cancer, person‐years at risk were calculated as the time from 3 months after diagnosis of the first cancer until whichever of the following came first: (i) December 31, 2005; (ii) the date of diagnosis of the metachronous second primary cancer; (iii) the date of death; (iv) the date when a patient reached 80 years of age; or (v) the date 10 years after the diagnosis of the first cancer.8
The incidence rate per 100 000 person‐years and cumulative risk9 for metachronous second primary cancer were estimated according to sex, age group and calendar year at the time of diagnosis of the first cancer.
The observed number of metachronous second primary cancers was compared with the expected number according to sex, age group, selected site of the first and second cancer, and follow‐up interval. A standardized incidence ratio (SIR) was then obtained by dividing the observed number of cases of a second primary cancer by the expected number. Thus, the SIR is used to estimate the risk of a cancer patient developing a second primary malignancy compared with the incidence of cancer among the general population. In the analyses for site relationship between the first and second cancer, we report only the cancer site combinations where more than 10 eligible metachronous second primary cancers were obtained. The significance and 95% confidence intervals (CI) for the SIR were tested by Poisson distribution analysis.
In Osaka, we used the rules suggested by the International Agency for Research on Cancer (IARC)10 and the third edition of the ICD‐O11 to define the circumstance under which an individual is considered to have more than one cancer. The IARC's definition does not accept any tumors in the same site as a second primary cancer unless their major histological type differs from that of the first primary cancer. Therefore, the SIR for all sites will be underestimated, particularly in the case of first cancers with high person‐years, such as cancer of the stomach, colorectum, liver, lung, and breast. To avoid such underestimations, we excluded both the observed and expected numbers of second primary cancer in the same site as the first cancer from the SIR calculations for all sites. Therefore, in the present study a second primary cancer of the same site (as defined by the three‐digit rubric of the ICD with some exceptions according to the rules) was excluded even if its histological type differed from that of the first primary cancer when we used the variable of cancer site in the analyses.8
Probability values for statistical tests were two‐tailed and P < 0.05 was considered significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
The total number of study subjects was 355 966, of whom 79.7% were histologically verified. During the follow‐up period (median follow‐up duration 2.5 years; mean 3.9 years), metachronous second primary cancers developed in 13 385 subjects (3.8%). Figure 1 shows age‐ and sex‐specific incidence rates of metachronous second primary cancers per 100 000 person‐years, in which study subjects were classified into four groups according to calendar year at diagnosis of the first cancer. The incidence rates increased remarkably with an increase in age and were higher among men than women, except in the 0–39 and 40–49 years age groups. In terms of calendar year at the time of diagnosis of the first cancer, age‐specific incidence rates did not differ within either sex.
Figure 1.
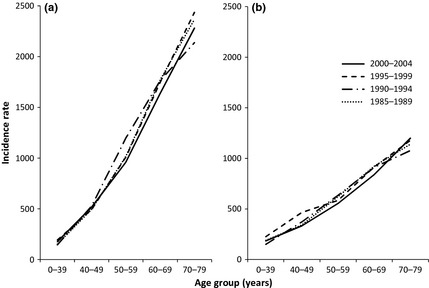
Age‐specific incidence rates of metachronous second primary cancer per 100 000 person‐years in (a) men and (b) women according to the calendar year at diagnosis of the first cancer, Osaka, 1985–2004.
Table 1 shows the cumulative risks of metachronous second primary cancers according to age and calendar year at diagnosis of the first cancer. The 10‐year cumulative risk was estimated as 13.0% for those who developed their first cancer at 60–69 years of age (16.2% for men, 8.6% for women). No difference or increasing trend was observed during the study period.
Table 1.
Cumulative risk of metachronous second primary cancer (%), according to sex, age and calendar year at the time of diagnosis of the first cancer
| Sex/age (years) | Duration | Year of diagnosis of first cancer | ||||
|---|---|---|---|---|---|---|
| 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | Total | ||
| Male | ||||||
| 0–39 | 3 months–5 years | 0.4 | 0.9 | 1.0 | 0.9 | 0.8 |
| 3 months–10 years | 1.5 | 1.4 | 1.8 | – | 1.6 | |
| 40–49 | 3 months–5 years | 2.2 | 2.0 | 2.1 | 2.3 | 2.1 |
| 3 months–10 years | 5.1 | 4.8 | 4.9 | – | 5.0 | |
| 50–59 | 3 months–5 years | 4.9 | 4.1 | 4.6 | 4.5 | 4.5 |
| 3 months–10 years | 11.4 | 9.8 | 9.5 | – | 10.2 | |
| 60–69 | 3 months–5 years | 7.2 | 7.3 | 7.9 | 7.5 | 7.5 |
| 3 months–10 years | 16.4 | 16.5 | 15.7 | – | 16.2 | |
| 70–79 | 3 months–5 years | 9.2 | 10.1 | 10.8 | 10.2 | 10.2 |
| 3 months–10 years | 20.8 | 22.5 | 21.7 | – | 21.8 | |
| Female | ||||||
| 0–39 | 3 months–5 years | 0.7 | 0.6 | 0.9 | 0.8 | 0.7 |
| 3 months–10 years | 1.9 | 1.5 | 2.3 | – | 1.9 | |
| 40–49 | 3 months–5 years | 1.5 | 1.9 | 1.9 | 1.6 | 1.7 |
| 3 months–10 years | 3.3 | 3.5 | 4.6 | – | 3.7 | |
| 50–59 | 3 months–5 years | 2.7 | 2.7 | 2.8 | 2.6 | 2.7 |
| 3 months–10 years | 6.0 | 6.1 | 5.5 | – | 5.8 | |
| 60–69 | 3 months–5 years | 4.0 | 3.9 | 4.3 | 3.9 | 4.1 |
| 3 months–10 years | 8.7 | 8.6 | 8.4 | – | 8.6 | |
| 70–79 | 3 months–5 years | 4.9 | 4.7 | 5.6 | 5.5 | 5.2 |
| 3 months–10 years | 11.7 | 11.0 | 10.1 | – | 11.0 | |
| All patients | ||||||
| 0–39 | 3 months–5 years | 0.6 | 0.7 | 1.0 | 0.8 | 0.8 |
| 3 months–10 years | 1.7 | 1.5 | 2.1 | – | 1.8 | |
| 40–49 | 3 months–5 years | 1.7 | 1.9 | 2.0 | 1.8 | 1.9 |
| 3 months–10 years | 3.9 | 4.0 | 4.7 | – | 4.2 | |
| 50–59 | 3 months–5 years | 3.9 | 3.5 | 3.7 | 3.5 | 3.7 |
| 3 months–10 years | 8.8 | 8.1 | 7.5 | – | 8.1 | |
| 60–69 | 3 months–5 years | 5.8 | 6.0 | 6.6 | 6.1 | 6.2 |
| 3 months–10 years | 12.8 | 13.3 | 12.9 | – | 13.0 | |
| 70–79 | 3 months–5 years | 7.3 | 7.7 | 8.6 | 8.4 | 8.1 |
| 3 months–10 years | 16.4 | 17.2 | 16.8 | – | 17.0 | |
Table 2 shows the SIR according to sex, age at diagnosis of the first cancer, and follow‐up interval. The SIR and 95% CI among those (of both sexes) who developed their first cancer at 0–39 and 40–49 years of age were 2.54 (1.43–3.66) and 1.75 (1.41–2.10), respectively, for the first year; 2.34 (1.86–2.82) and 1.61 (1.46–1.77), respectively, for the next 4 years; 1.90 (1.51–2.29) and 1.39 (1.25–1.53), respectively, for the next 5–10‐year period; and 2.13 (1.84–2.43) and 1.52 (1.42–1.62), respectively, for all 10 years after diagnosis of the first cancer. These ratios were higher than those in the total and other age groups. During the period 1–5 years after the diagnosis of the first cancer, 38% and 48% excess risk of metachronous second primary cancers was observed among men and women, respectively, who developed their first cancer at 50–59 years of age. Women aged 50–79 years had a tendency for a higher SIR of metachronous second primaries than men aged 50–79 years, whereas women aged 0–49 years had a tendency for a lower SIR than men aged 0–49 years.
Table 2.
Observed numbers and standardized incidence ratios of metachronous second primary cancer according to sex, age at diagnosis of the first cancer and years after diagnosis of the first cancer, 1985–2004
| Age at diagnosis of the first cancer (years) | Years after diagnosis of the first cancer | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months–1 year | 1–5 years | 5–10 years* | Total (3 months–10 years) | |||||||||||||
| No. second primary cancers | Person‐years | SIR | 95% CI | No. second primary cancers | Person‐years | SIR | 95% CI | No. second primary cancers | Person‐years | SIR | 95% CI | No. second primary cancers | Person‐years | SIR | 95% CI | |
| Male patients | ||||||||||||||||
| 0–39 | 9 | 5 739 | 4.59 | 1.59–7.59 | 34 | 20 737 | 3.88 | 2.58–5.19 | 27 | 16 937 | 2.34 | 1.46–3.23 | 70 | 43 414 | 3.15 | 2.41–3.88 |
| 40–49 | 37 | 10 557 | 1.81 | 1.23–2.39 | 170 | 35 274 | 1.89 | 1.61–2.18 | 164 | 27 466 | 1.50 | 1.27–1.73 | 371 | 73 298 | 1.69 | 1.52–1.86 |
| 50–59 | 282 | 31 382 | 1.53 | 1.35–1.70 | 927 | 93 355 | 1.38 | 1.29–1.47 | 731 | 59 066 | 1.22 | 1.13–1.30 | 1940 | 183 805 | 1.33 | 1.27–1.39 |
| 60–69 | 655 | 47 644 | 1.05 | 0.97–1.13 | 2279 | 130 211 | 1.19 | 1.14–1.23 | 1311 | 66 678 | 1.07 | 1.01–1.13 | 4245 | 244 535 | 1.13 | 1.09–1.16 |
| 70–79 | 638 | 32 168 | 0.93 | 0.86–1.01 | 1581 | 65 729 | 1.10 | 1.05–1.16 | 346 | 12 526 | 1.19 | 1.06–1.31 | 2565 | 110 423 | 1.07 | 1.03–1.11 |
| Total (0–79) | 1621 | 127 490 | 1.07 | 1.02–1.12 | 4991 | 345 305 | 1.21 | 1.18–1.24 | 2579 | 182 673 | 1.15 | 1.11–1.20 | 9191 | 655 475 | 1.17 | 1.14–1.19 |
| Female patients | ||||||||||||||||
| 0–39 | 11 | 8 811 | 1.86 | 0.76–2.97 | 57 | 34 455 | 1.89 | 1.40–2.38 | 65 | 28 115 | 1.76 | 1.33–2.19 | 133 | 71 383 | 1.82 | 1.51–2.13 |
| 40–49 | 64 | 16 943 | 1.72 | 1.30–2.15 | 247 | 67 177 | 1.47 | 1.28–1.65 | 224 | 55 039 | 1.32 | 1.15–1.49 | 535 | 139 162 | 1.43 | 1.30–1.55 |
| 50–59 | 144 | 25 098 | 1.64 | 1.37–1.90 | 503 | 86 858 | 1.48 | 1.35–1.61 | 389 | 59 469 | 1.34 | 1.21–1.48 | 1036 | 171 427 | 1.45 | 1.36–1.53 |
| 60–69 | 211 | 27 930 | 1.24 | 1.07–1.41 | 799 | 88 050 | 1.35 | 1.25–1.44 | 524 | 54 128 | 1.17 | 1.07–1.27 | 1534 | 170 110 | 1.27 | 1.20–1.33 |
| 70–79 | 240 | 21 790 | 1.14 | 0.99–1.28 | 568 | 49 613 | 1.16 | 1.06–1.25 | 148 | 11 723 | 1.20 | 1.01–1.39 | 956 | 83 127 | 1.16 | 1.08–1.23 |
| Total (0–79) | 670 | 100 573 | 1.31 | 1.21–1.41 | 2174 | 326 153 | 1.34 | 1.28–1.4 | 1350 | 208 474 | 1.27 | 1.20–1.33 | 4194 | 635 209 | 1.31 | 1.27–1.35 |
| All patients (both sexes) | ||||||||||||||||
| 0–39 | 20 | 14 550 | 2.54 | 1.43–3.66 | 91 | 55 192 | 2.34 | 1.86–2.82 | 92 | 45 052 | 1.90 | 1.51–2.29 | 203 | 114 796 | 2.13 | 1.84–2.43 |
| 40–49 | 101 | 27 500 | 1.75 | 1.41–2.10 | 417 | 102 451 | 1.61 | 1.46–1.77 | 388 | 82 505 | 1.39 | 1.25–1.53 | 906 | 212 460 | 1.52 | 1.42–1.62 |
| 50–59 | 426 | 56 480 | 1.56 | 1.41–1.71 | 1430 | 180 213 | 1.42 | 1.34–1.49 | 1120 | 118 535 | 1.26 | 1.18–1.33 | 2976 | 355 232 | 1.37 | 1.32–1.42 |
| 60–69 | 866 | 75 575 | 1.09 | 1.02–1.16 | 3078 | 218 260 | 1.22 | 1.18–1.27 | 1835 | 120 805 | 1.10 | 1.05–1.15 | 5779 | 414 645 | 1.16 | 1.13–1.19 |
| 70–79 | 878 | 53 958 | 0.98 | 0.92–1.05 | 2149 | 115 342 | 1.12 | 1.07–1.16 | 494 | 24 249 | 1.19 | 1.09–1.30 | 3521 | 193 550 | 1.09 | 1.05–1.13 |
| Total (0–79) | 2291 | 228 063 | 1.13 | 1.08–1.18 | 7165 | 671 459 | 1.25 | 1.22–1.28 | 3929 | 391 147 | 1.19 | 1.15–1.23 | 13385 | 1 290 684 | 1.21 | 1.19–1.23 |
CI, confidence interval. *Because of the high proportion of censored data, the low reliability of the standardized incidence ratios (SIR) among the population aged 70–79 years for the period 5–10 years after diagnosis of the first cancer should be kept in mind.
Table 3 lists the SIR according to selected sites of the first cancer and the follow‐up interval. There were no clear increasing or decreasing trends of SIR for any site of the first cancer for the duration of follow‐up. The highest SIR (~2.0–2.5) were observed for cancers of the mouth/pharynx, esophagus, and larynx, followed by cancers of the lung, breast, uterus, ovary, thyroid, and blood (~1.4–1.7). The ratios for the remaining cancer sites were approximately 1.1–1.3 and usually > 1.0 regardless of the significance.
Table 3.
Observed numbers and standardized incidence ratios of second primary cancer according to site of the first cancer and follow‐up interval after diagnosis of the first cancer, 1985–2004, for both sexes combined
| Site of the first cancer | ICD‐10 | Follow‐up interval after diagnosis of the first cancer | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months–1 year | 1–5 years | 5–10 years | Total (3 months–10 years) | ||||||||||||||
| No. second primary cancers | Person‐years | SIR | 95% CI | No. second primary cancers | Person‐years | SIR | 95% CI | No. second primary cancers | Person‐years | SIR | 95% CI | No. second primary cancers | Person‐years | SIR | 95% CI | ||
| Mouth/pharynx | C00–14 | 107 | 5 578 | 2.27 | 1.84–2.70 | 368 | 16 439 | 2.66 | 2.39–2.93 | 187 | 9 621 | 2.26 | 1.93–2.58 | 662 | 31 638 | 2.47 | 2.28–2.65 |
| Esophagus | C15 | 109 | 5 460 | 1.96 | 1.59–2.32 | 205 | 9 576 | 2.04 | 1.76–2.32 | 86 | 3 570 | 2.10 | 1.66–2.55 | 400 | 18 606 | 2.03 | 1.83–2.23 |
| Stomach | C16 | 432 | 47 535 | 1.17 | 1.06–1.28 | 1522 | 145 980 | 1.33 | 1.26–1.39 | 1013 | 96 018 | 1.27 | 1.19–1.34 | 2967 | 289 536 | 1.28 | 1.24–1.33 |
| Colorectum | C18–20 | 376 | 36 192 | 1.29 | 1.16–1.42 | 1301 | 114 495 | 1.36 | 1.29–1.43 | 793 | 66 397 | 1.34 | 1.25–1.43 | 2470 | 217 085 | 1.34 | 1.29–1.39 |
| Liver | C22 | 239 | 22 872 | 1.14 | 1.00–1.29 | 619 | 53 731 | 1.19 | 1.10–1.29 | 138 | 13 654 | 1.00 | 0.84–1.17 | 996 | 90 258 | 1.15 | 1.08–1.22 |
| Gallbladder | C23, C24 | 34 | 3 613 | 0.98 | 0.65–1.31 | 81 | 6 205 | 1.40 | 1.10–1.71 | 34 | 2 703 | 1.35 | 0.90–1.81 | 149 | 12 522 | 1.27 | 1.06–1.47 |
| Pancreas | C25 | 36 | 3 824 | 1.02 | 0.68–1.35 | 45 | 3 730 | 1.37 | 0.97–1.77 | 13 | 1 339 | 1.14 | 0.52–1.76 | 94 | 8 893 | 1.18 | 0.94–1.42 |
| Larynx | C32 | 63 | 2 269 | 2.32 | 1.75–2.90 | 259 | 8 158 | 2.52 | 2.22–2.83 | 148 | 5 220 | 2.03 | 1.70–2.35 | 470 | 15 647 | 2.32 | 2.11–2.53 |
| Lung | C33, C34 | 284 | 22 705 | 1.32 | 1.16–1.47 | 533 | 39 087 | 1.47 | 1.34–1.59 | 162 | 13 571 | 1.24 | 1.05–1.43 | 979 | 75 364 | 1.38 | 1.29–1.47 |
| Breast (females) | C50 | 115 | 25 252 | 1.44 | 1.18–1.71 | 509 | 102 282 | 1.49 | 1.36–1.62 | 383 | 70 034 | 1.47 | 1.32–1.61 | 1007 | 197 571 | 1.48 | 1.38–1.57 |
| Uterus | C53–55 | 46 | 9 606 | 1.25 | 0.89–1.61 | 232 | 36 008 | 1.63 | 1.42–1.84 | 204 | 28 203 | 1.66 | 1.43–1.88 | 482 | 73 819 | 1.59 | 1.45–1.74 |
| Ovary | C56 | 24 | 3 592 | 1.78 | 1.07–2.50 | 67 | 10 212 | 1.81 | 1.38–2.24 | 33 | 5 840 | 1.54 | 1.01–2.07 | 124 | 19 644 | 1.72 | 1.42–2.03 |
| Prostate | C61 | 88 | 5 436 | 1.03 | 0.82–1.25 | 302 | 15 868 | 1.18 | 1.05–1.31 | 95 | 4 756 | 1.16 | 0.92–1.39 | 485 | 26 061 | 1.15 | 1.04–1.25 |
| Kidney/urinary tract/bladder | C64–68 | 128 | 9 816 | 1.26 | 1.04–1.48 | 493 | 33 330 | 1.42 | 1.30–1.55 | 272 | 20 392 | 1.24 | 1.09–1.39 | 893 | 63 539 | 1.34 | 1.25–1.43 |
| Thyroid | C73 | 30 | 3 432 | 1.81 | 1.16–2.46 | 97 | 14 893 | 1.36 | 1.09–1.63 | 83 | 11 953 | 1.43 | 1.12–1.74 | 210 | 30 278 | 1.44 | 1.25–1.64 |
| Blood |
C81–85, C88, C90, C91–96 |
93 | 10 121 | 1.45 | 1.15–1.74 | 258 | 28 528 | 1.56 | 1.37–1.75 | 126 | 15 685 | 1.54 | 1.27–1.80 | 477 | 54 334 | 1.53 | 1.39–1.67 |
CI, confidence interval; SIR, standardized incidence ratios.
Table 4 lists the SIR according to selected sites of the first and second primary cancers. Some specific associations were observed between the sites of the first and second primary cancers; specifically, the SIR for cancers of the mouth/pharynx, esophagus, and larynx were much higher. That is, the SIR between these three sites ranged between 4 and 22; however, the SIR for lung and the three sites was estimated to be approximately 2–3. The site relationships between breast, uterus, and ovary were relatively high, especially after first breast cancer (i.e. the SIR [95% CI] was 2.07 [1.71–2.43] for first breast to second uterus; 2.16 [1.62–2.70] for first breast to second ovary; 1.40 [1.10–1.71] for first uterus to second breast; and 1.43 [0.82–2.04] for first ovary to second breast). In addition, a high SIR for second thyroid cancer, but not first thyroid cancer, was observed.
Table 4.
Observed numbers and standardized incidence ratios of second primary cancer according to the site of first and second primary cancer, 1985–2004, in both sexes
| First cancer site | Second cancer site | No. second primary cancers | Person‐years | SIR | 95% CI |
|---|---|---|---|---|---|
| Mouth/pharynx | Esophagus | 137 | 32 483 | 13.62 | 11.34–15.90 |
| Mouth/pharynx | Stomach | 81 | 32 571 | 1.38 | 1.08–1.68 |
| Mouth/pharynx | Colorectum | 53 | 32 558 | 1.35 | 0.99–1.71 |
| Mouth/pharynx | Liver | 66 | 32 599 | 1.49 | 1.13–1.85 |
| Mouth/pharynx | Gallbladder | 11 | 32 684 | 1.52 | 0.62–2.41 |
| Mouth/pharynx | Pancreas | 17 | 32 684 | 1.57 | 0.82–2.31 |
| Mouth/pharynx | Larynx | 12 | 32 674 | 4.35 | 1.89–6.81 |
| Mouth/pharynx | Lung | 113 | 32 562 | 2.45 | 2.00–2.90 |
| Mouth/pharynx | Prostate | 18 | 32 657 | 1.73 | 0.93–2.53 |
| Mouth/pharynx | Kidney/urinary tract/bladder | 16 | 32 656 | 1.30 | 0.66–1.94 |
| Mouth/pharynx | Blood | 28 | 32 661 | 2.39 | 1.50–3.27 |
| Esophagus | Mouth/pharynx | 94 | 19 043 | 21.63 | 17.26–26.00 |
| Esophagus | Stomach | 58 | 19 128 | 1.32 | 0.98–1.66 |
| Esophagus | Colorectum | 33 | 19 117 | 1.15 | 0.76–1.54 |
| Esophagus | Liver | 40 | 19 154 | 1.21 | 0.83–1.58 |
| Esophagus | Pancreas | 16 | 19 193 | 2.00 | 1.02–2.97 |
| Esophagus | Larynx | 14 | 19 181 | 6.38 | 3.04–9.72 |
| Esophagus | Lung | 62 | 19 129 | 1.71 | 1.29–2.14 |
| Esophagus | Prostate | 14 | 19 181 | 1.51 | 0.72–2.30 |
| Esophagus | Kidney/urinary tract/bladder | 19 | 19 173 | 2.00 | 1.10–2.90 |
| Esophagus | Blood | 16 | 19 174 | 1.89 | 0.97–2.82 |
| Stomach | Mouth/pharynx | 89 | 294 635 | 1.54 | 1.22–1.86 |
| Stomach | Esophagus | 171 | 294 528 | 1.68 | 1.42–1.93 |
| Stomach | Colorectum | 563 | 293 399 | 1.40 | 1.28–1.51 |
| Stomach | Liver | 486 | 293 978 | 1.07 | 0.97–1.16 |
| Stomach | Gallbladder | 91 | 294 693 | 1.17 | 0.93–1.41 |
| Stomach | Pancreas | 134 | 294 709 | 1.18 | 0.98–1.38 |
| Stomach | Larynx | 39 | 294 685 | 1.36 | 0.94–1.79 |
| Stomach | Lung | 632 | 294 047 | 1.26 | 1.16–1.36 |
| Stomach | Breast (female) | 126 | 294 397 | 1.63 | 1.34–1.91 |
| Stomach | Uterus | 35 | 294 742 | 1.09 | 0.73–1.45 |
| Stomach | Ovary | 15 | 294 779 | 1.04 | 0.51–1.56 |
| Stomach | Prostate | 157 | 294 497 | 1.36 | 1.15–1.57 |
| Stomach | Kidney/urinary tract/bladder | 163 | 294 433 | 1.26 | 1.07–1.45 |
| Stomach | Thyroid | 31 | 294 695 | 1.86 | 1.20–2.51 |
| Stomach | Blood | 137 | 294 653 | 1.14 | 0.95–1.33 |
| Colorectum | Mouth/pharynx | 47 | 222 016 | 1.13 | 0.81–1.45 |
| Colorectum | Esophagus | 95 | 221 949 | 1.31 | 1.05–1.57 |
| Colorectum | Stomach | 558 | 220 824 | 1.28 | 1.17–1.39 |
| Colorectum | Liver | 352 | 221 454 | 1.07 | 0.96–1.19 |
| Colorectum | Gallbladder | 58 | 222 035 | 0.97 | 0.72–1.22 |
| Colorectum | Pancreas | 109 | 222 020 | 1.28 | 1.04–1.52 |
| Colorectum | Larynx | 29 | 222 010 | 1.50 | 0.96–2.05 |
| Colorectum | Lung | 410 | 221 554 | 1.14 | 1.03–1.25 |
| Colorectum | Breast (female) | 91 | 221 774 | 1.22 | 0.97–1.47 |
| Colorectum | Uterus | 50 | 221 961 | 1.64 | 1.19–2.10 |
| Colorectum | Ovary | 34 | 222 048 | 2.43 | 1.61–3.24 |
| Colorectum | Prostate | 107 | 221 886 | 1.31 | 1.06–1.56 |
| Colorectum | Kidney/urinary tract/bladder | 120 | 221 805 | 1.30 | 1.07–1.53 |
| Colorectum | Thyroid | 42 | 221 956 | 3.00 | 2.09–3.91 |
| Colorectum | Blood | 108 | 221 970 | 1.20 | 0.97–1.43 |
| Liver | Mouth/pharynx | 31 | 91 628 | 1.51 | 0.98–2.04 |
| Liver | Esophagus | 58 | 91 598 | 1.56 | 1.16–1.96 |
| Liver | Stomach | 264 | 91 223 | 1.22 | 1.08–1.37 |
| Liver | Colorectum | 170 | 91 367 | 1.19 | 1.01–1.37 |
| Liver | Gallbladder | 22 | 91 655 | 0.82 | 0.48–1.17 |
| Liver | Pancreas | 34 | 91 647 | 0.85 | 0.57–1.14 |
| Liver | Lung | 146 | 91 519 | 0.82 | 0.69–0.95 |
| Liver | Breast (female) | 24 | 91 633 | 1.26 | 0.75–1.76 |
| Liver | Ovary | 12 | 91 658 | 3.23 | 1.40–5.06 |
| Liver | Prostate | 47 | 91 597 | 1.09 | 0.78–1.41 |
| Liver | Kidney/urinary tract/bladder | 52 | 91 585 | 1.13 | 0.82–1.44 |
| Liver | Thyroid | 11 | 91 647 | 2.14 | 0.88–3.41 |
| Liver | Blood | 67 | 91 605 | 1.60 | 1.21–1.98 |
| Gallbladder | Stomach | 27 | 12 699 | 1.11 | 0.69–1.53 |
| Gallbladder | Colorectum | 33 | 12 669 | 1.92 | 1.26–2.57 |
| Gallbladder | Liver | 13 | 12 696 | 0.74 | 0.34–1.14 |
| Gallbladder | Lung | 27 | 12 705 | 1.37 | 0.85–1.89 |
| Pancreas | Stomach | 14 | 8 994 | 0.83 | 0.39–1.26 |
| Pancreas | Colorectum | 18 | 8 991 | 1.55 | 0.83–2.26 |
| Pancreas | Lung | 18 | 8 998 | 1.33 | 0.72–1.94 |
| Larynx | Mouth/pharynx | 26 | 16 492 | 6.03 | 3.71–8.35 |
| Larynx | Esophagus | 45 | 16 471 | 5.56 | 3.94–7.19 |
| Larynx | Stomach | 84 | 16 291 | 1.81 | 1.43–2.20 |
| Larynx | Colorectum | 41 | 16 431 | 1.44 | 1.00–1.88 |
| Larynx | Liver | 47 | 16 472 | 1.32 | 0.94–1.70 |
| Larynx | Gallbladder | 10 | 16 527 | 1.92 | 0.73–3.11 |
| Larynx | Pancreas | 12 | 16 534 | 1.50 | 0.65–2.35 |
| Larynx | Lung | 136 | 16 336 | 3.48 | 2.90–4.07 |
| Larynx | Prostate | 11 | 16 517 | 1.10 | 0.45–1.75 |
| Larynx | Kidney/urinary tract/bladder | 20 | 16 490 | 1.98 | 1.11–2.85 |
| Larynx | Blood | 13 | 16 525 | 1.54 | 0.70–2.38 |
| Lung | Mouth/pharynx | 35 | 76 773 | 2.14 | 1.43–2.84 |
| Lung | Esophagus | 60 | 76 748 | 2.04 | 1.52–2.55 |
| Lung | Stomach | 250 | 76 462 | 1.39 | 1.22–1.57 |
| Lung | Colorectum | 146 | 76 533 | 1.24 | 1.04–1.44 |
| Lung | Liver | 107 | 76 694 | 0.82 | 0.66–0.97 |
| Lung | Gallbladder | 30 | 76 798 | 1.27 | 0.82–1.73 |
| Lung | Pancreas | 46 | 76 789 | 1.36 | 0.96–1.75 |
| Lung | Larynx | 21 | 76 778 | 2.51 | 1.44–3.58 |
| Lung | Breast (female) | 34 | 76 724 | 1.66 | 1.10–2.21 |
| Lung | Uterus | 11 | 76 806 | 1.30 | 0.53–2.07 |
| Lung | Prostate | 62 | 76 713 | 1.67 | 1.26–2.09 |
| Lung | Kidney/urinary tract/bladder | 64 | 76 730 | 1.65 | 1.25–2.06 |
| Lung | Thyroid | 20 | 76 780 | 4.29 | 2.41–6.17 |
| Lung | Blood | 43 | 76 777 | 1.22 | 0.86–1.59 |
| Breast (female) | Mouth/pharynx | 16 | 200 696 | 1.40 | 0.71–2.08 |
| Breast (female) | Esophagus | 20 | 200 702 | 1.92 | 1.08–2.76 |
| Breast (female) | Stomach | 180 | 200 292 | 1.40 | 1.20–1.61 |
| Breast (female) | Colorectum | 152 | 200 362 | 1.20 | 1.01–1.40 |
| Breast (female) | Liver | 88 | 200 620 | 1.18 | 0.93–1.43 |
| Breast (female) | Gallbladder | 36 | 200 686 | 1.28 | 0.86–1.69 |
| Breast (female) | Pancreas | 42 | 200 705 | 1.27 | 0.88–1.65 |
| Breast (female) | Lung | 103 | 200 563 | 1.24 | 1.00–1.48 |
| Breast (female) | Uterus | 126 | 200 389 | 2.07 | 1.71–2.43 |
| Breast (female) | Ovary | 61 | 200 574 | 2.16 | 1.62–2.70 |
| Breast (female) | Kidney/urinary tract/bladder | 26 | 200 631 | 1.24 | 0.77–1.72 |
| Breast (female) | Thyroid | 83 | 200 359 | 5.06 | 3.97–6.15 |
| Breast (female) | Blood | 43 | 200 682 | 1.08 | 0.76–1.41 |
| Uterus | Stomach | 54 | 74 675 | 1.06 | 0.78–1.35 |
| Uterus | Colorectum | 75 | 74 568 | 1.56 | 1.21–1.92 |
| Uterus | Liver | 29 | 74 741 | 0.98 | 0.62–1.33 |
| Uterus | Gallbladder | 19 | 74 751 | 1.62 | 0.89–2.35 |
| Uterus | Pancreas | 21 | 74 753 | 1.63 | 0.93–2.32 |
| Uterus | Lung | 82 | 74 668 | 2.60 | 2.03–3.16 |
| Uterus | Breast (female) | 82 | 74 497 | 1.40 | 1.10–1.71 |
| Uterus | Kidney/urinary tract/bladder | 15 | 74 735 | 1.86 | 0.92–2.80 |
| Uterus | Thyroid | 13 | 74 711 | 2.12 | 0.97–3.27 |
| Uterus | Blood | 35 | 74 733 | 2.32 | 1.55–3.10 |
| Ovary | Stomach | 11 | 19 858 | 0.99 | 0.40–1.57 |
| Ovary | Colorectum | 33 | 19 802 | 3.04 | 2.00–4.07 |
| Ovary | Lung | 12 | 19 876 | 1.73 | 0.75–2.71 |
| Ovary | Breast (female) | 21 | 19 844 | 1.43 | 0.82–2.04 |
| Ovary | Blood | 13 | 19 876 | 3.74 | 1.71–5.78 |
| Prostate | Mouth/pharynx | 21 | 26 800 | 2.47 | 1.41–3.52 |
| Prostate | Esophagus | 14 | 26 833 | 0.82 | 0.39–1.25 |
| Prostate | Stomach | 119 | 26 628 | 1.23 | 1.01–1.46 |
| Prostate | Colorectum | 80 | 26 698 | 1.35 | 1.05–1.65 |
| Prostate | Liver | 59 | 26 741 | 0.87 | 0.65–1.09 |
| Prostate | Gallbladder | 16 | 26 825 | 1.41 | 0.72–2.09 |
| Prostate | Pancreas | 21 | 26 824 | 1.21 | 0.69–1.73 |
| Prostate | Lung | 62 | 26 777 | 0.67 | 0.50–0.83 |
| Prostate | Kidney/urinary tract/bladder | 50 | 26 763 | 2.21 | 1.60–2.83 |
| Prostate | Blood | 22 | 26 822 | 1.24 | 0.72–1.75 |
| Kidney/urinary tract/bladder | Mouth/pharynx | 17 | 65 125 | 1.21 | 0.64–1.79 |
| Kidney/urinary tract/bladder | Esophagus | 36 | 65 118 | 1.41 | 0.95–1.88 |
| Kidney/urinary tract/bladder | Stomach | 167 | 64 815 | 1.12 | 0.95–1.29 |
| Kidney/urinary tract/bladder | Colorectum | 122 | 64 847 | 1.26 | 1.04–1.49 |
| Kidney/urinary tract/bladder | Liver | 111 | 64 975 | 1.00 | 0.81–1.19 |
| Kidney/urinary tract/bladder | Gallbladder | 25 | 65 137 | 1.35 | 0.82–1.88 |
| Kidney/urinary tract/bladder | Pancreas | 33 | 65 147 | 1.21 | 0.80–1.62 |
| Kidney/urinary tract/bladder | Lung | 177 | 64 960 | 1.41 | 1.20–1.61 |
| Kidney/urinary tract/bladder | Breast (female) | 12 | 65 136 | 0.97 | 0.42–1.52 |
| Kidney/urinary tract/bladder | Uterus | 12 | 65 116 | 2.32 | 1.01–3.64 |
| Kidney/urinary tract/bladder | Prostate | 64 | 65 005 | 2.08 | 1.57–2.59 |
| Kidney/urinary tract/bladder | Blood | 34 | 65 108 | 1.18 | 0.78–1.58 |
| Thyroid | Stomach | 32 | 30 711 | 1.24 | 0.81–1.67 |
| Thyroid | Colorectum | 27 | 30 689 | 1.25 | 0.78–1.72 |
| Thyroid | Liver | 12 | 30 753 | 0.70 | 0.30–1.10 |
| Thyroid | Lung | 26 | 30 716 | 1.43 | 0.88–1.98 |
| Thyroid | Breast (female) | 37 | 30 666 | 1.97 | 1.34–2.61 |
| Thyroid | Blood | 13 | 30 746 | 1.93 | 0.88–2.97 |
| Blood | Mouth/pharynx | 14 | 55 012 | 2.18 | 1.04–3.32 |
| Blood | Esophagus | 17 | 55 027 | 1.61 | 0.84–2.37 |
| Blood | Stomach | 79 | 54 898 | 1.20 | 0.94–1.47 |
| Blood | Colorectum | 55 | 54 931 | 1.20 | 0.88–1.51 |
| Blood | Liver | 82 | 54 916 | 1.71 | 1.34–2.07 |
| Blood | Lung | 71 | 54 957 | 1.38 | 1.06–1.70 |
| Blood | Breast (female) | 10 | 55 005 | 0.65 | 0.25–1.04 |
| Blood | Prostate | 19 | 55 015 | 1.77 | 0.98–2.57 |
| Blood | Kidney/urinary tract/bladder | 24 | 54 990 | 1.77 | 1.06–2.47 |
| Blood | Thyroid | 14 | 55 017 | 5.54 | 2.64–8.43 |
CI, confidence interval; SIR, standardized incidence ratios.
Discussion
The present study shows that in Osaka, Japan, 3.8% of study subjects (of both sexes) developed metachronous second primary cancers within 10 years of the first primary cancer between 1985 and 2005. Compared with our previous study, in which we reported that 2.0% of cancer patients developed metachronous second primary cancers between 1966 and 1986,4 the proportion of multiple primary cancers has approximately doubled in the past 20 years. Although constantly elevated SIR was not observed in the first decade in our previous study,4 elevated SIR for metachronous second primary cancers was observed among almost all sex and age groups over the study period in the present study. Almost all cancer patients were more likely to develop metachronous second primary cancers than the general population, as also found in another recent study in Australia.12 A possible explanation for this finding could be the accumulation or concentration of cancer risk factors, such as smoking, alcohol drinking, radiotherapy treatment, and other potential factors, including genetic factors, within an individual. It may be difficult to improve cancer patients' lifestyle. For example, smoking cessation is challenging even for patients recovering from lung cancer with curative treatment because a significant proportion of smokers with cancer do not receive formal assistance to quit.13 However, detection or surveillance bias may also play a part in the results, as evidenced in the elevated SIR of second thyroid cancer.14
We also found that the 10‐year cumulative risk for second primaries was 13.0% for those who developed their first cancer at 60–69 years of age. Although this finding was slightly higher than our previous results, with an approximate 10% 10‐year cumulative risk for second primaries for those who developed their first cancer at 60–69 years of age in 1978–1983,4 we added sex‐stratified information on cumulative risk for second primaries. Men with their first cancer diagnosed when they were at 60–69 years of age had a high cumulative risk of 16.2%, compared with an 8.6% risk in women. These figures clearly show that second primary cancer should be regarded as a problem commonly encountered in routine medical practice rather than a rare and unusual event to be described in case reports.
The SIR for metachronous second primary cancers from the first cancer of the mouth/pharynx, esophagus, and larynx, generally accepted as smoking and alcohol related, was higher than those from the other first cancers, including lung cancer. Robust site relationships of first and second primary cancers within these three cancer sites (mouth/pharynx to esophagus, mouth/pharynx to larynx, esophagus to larynx and each others) were found (see Table 4). Tobacco smoking is clearly one of the major causes of second primary cancers, as it is for first cancers.15 Generally, cigarette smokers had an approximate 15–30‐fold higher risk of lung cancer than non‐smokers, whereas an approximate 2–10‐fold higher risk of cancer of the mouth/pharynx, esophagus, or larynx has been reported for cigarette smokers.16 In the present study, a lower SIR between smoking‐related lung cancer and cancers of the mouth/pharynx, esophagus, or larynx was obtained than the SIR between these three sites. This may be due to combined effects of not only tobacco smoking, but also alcohol drinking, radiotherapy, or other potential factors,17 as well as to the relatively lower relative risk of cigarette smoking for lung cancer in Japan than in Western countries (i.e. 4.4 for men and 2.8 for women in Japan).18 The risk of developing a tobacco‐ or alcohol‐related second cancer has been linked mainly to patients' habits before the onset of the initial cancer, although continued smoking and drinking may enhance the risk.5, 19
Breast, uterine, and ovarian cancers had the next highest SIR for second primaries. Furthermore, we found that women aged 50–79 years had a higher SIR for second primaries than men aged 50–79 years. The site relationships between breast, uterus, and ovary were relatively high, especially after first breast cancer, whereas a robust site relationship between colorectal cancer and ovarian cancer was observed (see Table 4). These findings are consistent with previous evidence that women have slightly higher risk for second cancer compared with men because of the good survival rates for common female cancers.3 Female‐specific causes, such as hormonal environment due to menopause, may affect these relationships.17 Although the constellation of multiple cancers of the breast, uterine corpus, ovary, and colon has long intrigued investigators, nutritional and hormonal interactions, such as dietary habits (e.g. high fat intake) and reproductive factors (e.g. nulliparity), may contribute to the development of multiple primaries at these sites.5
The risk of second primary cancer can be modified ostensibly by improving medical scrutiny and notification of cancer patients.5 In our previous study, underestimation of the risk of second primary cancer may have been possible, with the incidence of second primaries differing substantially between 1966 and 1986. This may be due to the reliability of the registration indices because the number of cases registered by death certificate only and the number of cases verified histologically were not so favorable in Osaka during in the 1960s–1970s.4 The present study shows that the incidence rates per 100 000 person‐years for metachronous second primary cancers were approximately the same across all study periods (1985–2005) among both sexes in Osaka. This finding seems partly attributable to soundness of medical scrutiny and notification of cancer patients, as well as to stability in the registration indices.
One of the interesting relationships that emerged from the present analysis was that between hematological tumors and cancer of the mouth/pharynx, esophagus, uterus, ovary, and liver. Although there is evidence that hepatocellular carcinoma and lymphoma share a common risk factor, namely the hepatitis virus,20 the relationships between hematological tumors and cancer of the mouth/pharynx, esophagus, uterus, and ovary have been relatively unclear. Because of the age distribution or potential etiologies of hematological tumors, analyses using disease types (lymphoma, leukemia, and myeloma) and specific age categories, such as childhood, adolescence, and young adults, should be considered in future research.21 Although multiple primary cancers in some individuals provide a clue to understanding cancer etiology, including genetics,5, 22 a number of associations remain without apparent explanation.5 Because it is possible that other factors, such as socioeconomic status, genetics, lifestyle, or social network, may vary between people who have been diagnosed with cancer and those who have not,23 further investigations are required to identify the mechanisms underlying these relationships in the incidence of multiple primary cancers for specific interventions.
There are several limitations to the present study. First, we should keep in mind the changes in the completeness of cancer registration in Osaka when we evaluate incidence. The percentage of cases registered by death certificate only, which is often regarded as an index of completeness, was approximately 10–15% and registration has been stable in the Osaka Cancer Registry for the most recent two decades.24 Therefore, we considered that the effects of changes in completeness on incidence rates over the study periods are likely to be small. Second, some misclassifications for second primaries will be unavoidable in cancer registries, especially in cases registered by death certificate only. The IARC rules for second primaries are rather conservative compared with clinical practice; therefore, the risk for second primaries may be underestimated. Third, patients with second primary cancers may be followed‐up in hospitals where the completeness of registration of cancer cases is higher than average compared with other hospitals in Osaka. This could result in an overestimation for second primaries to some degree. Fourth, patients with cancer differ in many respects from the general population, and these characteristics may affect the risk of subsequent cancer. For example, women with cervical cancer tend to smoke more, bear children at an earlier age, and are of lower socioeconomic status than women in the general population.5 Because of the nature of cancer registries, these potential risk factors were not included in the data collection.
In conclusion, we have shown that cancer survivors in Osaka, Japan, are at higher risk of second primary cancer compared with the general population, in accordance with previous studies.11, 25, 26, 27 More than one‐tenth of cancer survivors aged over 60 years (specifically men over 50 years of age) will develop metachronous second primary cancer within 10 years of diagnosis of the first cancer. This clearly shows that second primary cancer should be considered as a commonly encountered major medical problem. The evaluation of second primary cancer identifies groups of cancer patients in need of increased surveillance for early cancer detection and management. Preventive measures are needed to reduce the occurrence of subsequent cancer and mortality.5 Because we obtained acceptably stable statistics for second primary cancer in Osaka, further studies are required to advance our understanding of effective measures against multiple primary cancers.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported by a Grant‐in‐Aid for Clinical Cancer Research from the Japanese Ministry of Health, Labour and Welfare (H22‐011). The authors are grateful to all the medical institutions in Osaka and the Osaka Medical Association that provided us with cancer incidence data.
References
- 1. Kamo K, Katanoda K, Matsuda T, Marugame T, Ajiki W, Sobue T. Lifetime and age‐conditional probabilities of developing or dying of cancer in Japan. Jpn J Clin Oncol 2008; 38: 571–6. [DOI] [PubMed] [Google Scholar]
- 2. Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T. Population‐based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011; 41: 40–51. [DOI] [PubMed] [Google Scholar]
- 3. Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. Methods Mol Biol 2009; 471: 85–105. [DOI] [PubMed] [Google Scholar]
- 4. Tsukuma H, Fujimoto I, Hanai A, Hiyama T, Kitagawa T, Kinoshita N. Incidence of second primary cancers in Osaka residents, Japan, with special reference to cumulative and relative risks. Jpn J Cancer Res 1994; 85: 339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boice JD Jr, Storm HH, Curtis RE et al Introduction to the study of multiple primary cancers. Natl Cancer Inst Monogr 1985; 68: 3–9. [PubMed] [Google Scholar]
- 6. Fujimoto I, Hanai A, Hiyama T, Tsukuma H, Takasugi Y, Sugaya T. Cancer Incidence and Mortality in Osaka 1963–1989. Tokyo: Shinohara Publishers, 1993. (in Japanese). [Google Scholar]
- 7. World Health Organization . International Statistical Classification of Diseases and Health Related Problems (The) ICD‐10 Second Edition. Geneva: World Health Organization, 2004. [Google Scholar]
- 8. Ajiki W, Koyama Y, Kinoshita N, Tsukuma H, Oshima A. The comprehensive study for multiple primary cancers based on the hospital cancer registry data of the Osaka Medical Centre for Cancer and Cardiovascular Diseases In: Tsukuma H, ed. The Report for Grant‐in‐Aid for Cancer Research (12–4) from the Ministry of Health, Labour and Welfare. Osaka: Suehiro Publishers, 2000; 111–26 (in Japanese). [Google Scholar]
- 9. Day NE. Cumurative rate and cumulative risk. In: Parkin DM, Muir CS, Whelan SL, Gao YT, Ferlay J, Powell J, eds. Cancer Incidence In Five Continents Volume VI. Lyon: IARC, 1992; 862–4. [PubMed] [Google Scholar]
- 10. International Agency for Research on Cancer (IARC) . International Rules for Multiple Primary Cancers (ICD‐O 3rd edn). Lyon: IARC, 2004. [Google Scholar]
- 11. World Health Organization (WHO) . International Classification of Diseases for Oncology (ICD‐O), 3rd edn Geneva: WHO, 2000. [Google Scholar]
- 12. Youlden DR, Baade PD. The relative risk of second primary cancers in Queensland, Australia: a retrospective cohort study. BMC Cancer 2011; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooley ME, Sarna L, Kotlerman J et al Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent. Lung Cancer 2009; 66: 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandeep TC, Strachan MW, Reynolds RM et al Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab 2006; 91: 1819–25. [DOI] [PubMed] [Google Scholar]
- 15. Engeland A, Bjorge T, Haldorsen T, Tretli S. Use of multiple primary cancers to indicate associations between smoking and cancer incidence: an analysis of 500 000 cancer cases diagnosed in Norway during 1953–93. Int J Cancer 1997; 70: 401–7. [DOI] [PubMed] [Google Scholar]
- 16. Nasca P, Pastides H. Fundamentals of Cancer Epidemiology, 2nd edn Ontario: Jones and Bartlett Publishers, 2008. [Google Scholar]
- 17. Adami HO, Hunter D, Trichopoulos D. Textbook of Cancer Epidemiology, 2nd edn New York: Oxford University Press, 2008. [Google Scholar]
- 18. Wakai K, Inoue M, Mizoue T et al Tobacco smoking and lung cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 2006; 36: 309–24. [DOI] [PubMed] [Google Scholar]
- 19. Wynder EL, Mushinski MH, Spivak JC. Tobacco and alcohol consumption in relation to the development of multiple primary cancers. Cancer 1977; 40: 1872–8. [DOI] [PubMed] [Google Scholar]
- 20. Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non‐Hodgkin's lymphoma: a meta‐analysis of epidemiological studies. Cancer Sci 2004; 95: 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reulen RC, Frobisher C, Winter DL et al Long‐term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 2011; 305: 2311–9. [DOI] [PubMed] [Google Scholar]
- 22. Kuligina E, Reiner A, Imyanitov EN, Begg CB. Evaluating cancer epidemiologic risk factors using multiple primary malignancies. Epidemiology 2010; 21: 366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong C, Hemminki K. Second primary neoplasms in 633 964 cancer patients in Sweden, 1958–1996. Int J Cancer 2001; 93: 155–61. [DOI] [PubMed] [Google Scholar]
- 24. Curado M, Edwards B, Shin H et al Cancer Incidence in Five Continents, Vol. IX. Lyon: International Agency for Research on Cancer, 2007. [Google Scholar]
- 25. Curtis RE, Boice JD Jr, Kleinerman RA, Flannery JT, Fraumeni JF Jr. Summary: multiple primary cancers in Connecticut, 1935–82. Natl Cancer Inst Monogr 1985; 68: 219–42. [PubMed] [Google Scholar]
- 26. Meadows AT, Friedman DL, Neglia JP et al Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol 2009; 27: 2356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curtis RE, Freedman D, Ron E et al New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. NIH Publication no. 05–5302. Bethesda: National Cancer Institute, 2006. [Google Scholar]


