Abstract
Radiation therapy (XRT) for treatment of localized prostate cancer (PCA) has outcomes similar to surgery and medical therapy. Toxicities of XRT and the relative radioresistance of PCA limit the effectiveness of this treatment method. Safe and effective radiosensitizing agents are lacking to enhance the effectiveness for XRT for PCA. In this study, the effect of XRT in combination with the radiosensitizing agent resveratrol (RSV) was investigated in a radioresistant PCA cell line, PC‐3. Our results show the addition of RSV to XRT (XRT/RSV) synergistically enhanced XRT‐induced apoptosis and inhibition of PC‐3 proliferation. The antiproliferative effect of XRT/RSV treatment correlated with increased expression of p15, p21, and mutant p53 and decreased expression of cyclin B, cyclin D, and cdk2. Increased apoptosis correlated with increased expression of Fas and TRAILR1. Furthermore, XRT/RSV had little effect on the expression of p‐AKT, whereas it increased the expression level of p‐H2A.X, a marker for senescence. These data highlight the potential of RSV as a radiation sensitizer for PCA treatment and warrant further investigation. (Cancer Sci 2012; 103: 1090–1098)
Prostate cancer (PCA) is the most common non‐cutaneous malignancy and the second leading cause of cancer mortality in elderly men in the USA.1 Approximately 240 890 new cases of PCA and 33 720 deaths were projected to occur in the USA in 2011.1 In addition to surgery, chemotherapy, and hormonal therapy, radiation therapy (XRT) is an established therapeutic method for PCA treatment. Radiation therapy is used to treat localized PCA to decrease tumor burden and ameliorate tumor‐related symptoms. The efficacy of XRT largely depends on the radiosensitivity of the tumor. Unfortunately, PCA is among the more radioresistant malignant tumors.2 The high radiation dose associated with XRT for PCA may have severe side‐effects, such as impotence, urinary dysfunction, and rectal symptoms; low dose XRT has little effect on PCA. A safe and effective radiosensitising agent is needed to allow a decrease in the radiation dose and side‐effects associated with XRT for PCA.
Resveratrol (trans‐3,4′,5‐trihydroxystilbene, RSV) is a polyphenolic compound that occurs naturally in grapes (such as in red wine) and peanuts, as well as in other plant species such as Polygonum cuspidatum and Yucca schidigera.3, 4, 5, 6, 7, 8, 9 The biological function of RSV is very complex. Multiple studies have shown neuroprotective, immunomodulatory, anti‐inflammatory, antioxidant, and antitumor functions. In recent years, RSV has been recognized as a promising anticancer agent.3 Its antitumor functions have been investigated in breast cancer, thyroid cancer, squamous cell carcinoma, HL‐60 leukemia, colon cancer, ovarian carcinoma, and PCA cell lines.10, 11 Incubation of the PCA cell line, DU145, with RSV resulted in decreased growth12 and increased apoptosis of cancer cells.12
Several studies implicate RSV as a chemotherapy sensitizer, thus, it is reasonable to hypothesize that the combination of RSV with radiation might potentiate the destruction of cancer cells.13, 14, 15, 16 Until now, there was only one study suggesting that RSV can sensitize the DU145 PCA cell line to radiation. Confirmation of the radiosensitizing effects of RSV in other PCA cell lines and the detailed molecular mechanisms of this phenomenon have not been investigated. The current study was designed to test the hypothesis that RSV enhances radiation sensitivity in the PC‐3 PCA cell line by altering cell proliferation and apoptosis. Additionally, we report the mechanisms underlying the cellular changes observed when RSV is used in combination with XRT.
Materials and Methods
Tumor cell line
PC‐3 cells, derived from human PCA, were provided by Jessica R. Newton (University of Missouri, Columbia, MO, USA). PC‐3 cells (passage 3) were maintained in DMEM supplemented with 10% heat‐inactivated FBS and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA). Cultures were incubated at 37°C in a humidified 5% CO2 incubator (Fisher Scientific, Pittsburgh, PA, USA). Cells were grown until they reached 70–80% confluence, at which time they were subjected to the designed experimental treatment regimens.
Treatment with RSV and XRT of PC‐3 cells
PC‐3 cells at 70–80% confluence were treated with RSV at varying concentrations (0–50 μM) for 24 h, followed by XRT at 2, 4, or 8 Gy, or mock treatment. The dosage of RSV and XRT was based on previously published data.11, 17, 18 All XRT was carried out using an XRAD 320 Biological Irradiator (Precision X‐ray, North Branford, CT, USA) at 320 Kv, 12.5 mA, and 50 cm FSD with filter 1 (280 cGy/min). Cells were irradiated at room temperature in 75 cm2 culture flasks. After XRT, cells were cultured for 24 h for most experiments and for 72 h for apoptosis studies.
Clonogenic survival assay
Twenty‐four hours after XRT, cells were counted in a hemocytometer. Clonogenic survival assay was carried out by plating 1000 cells into a Petri dish (Corning, Lowell, MA, USA) in triplicate. Fresh media was added at day 5. Nine days after incubation, cells were fixed and stained with 0.05% crystal violet. The number of colonies was counted and expressed as a percentage of total colonies in controls. Combination index (CI) was used to evaluate the combination treatment effect of RSV and XRT. The formula for CI is: CI = CA,X/ICX,A + CB,X/ICX,B.19 In our system, CA,x and CB,x are the concentration of RSV and dosage of radiation used in combination to achieve x% combination effect, respectively. ICx,A and ICx,B are the concentration and dosage for single treatment (RSV or RXT) to achieve the same effect. In this manner, CI = 1, CI < 1, and CI > 1 indicate additive effect, synergism, and antagonism, respectively.19
Immunohistochemistry (IHC)
Twenty‐four hours after XRT, cells were spun into slides by a Cytopro cytocentrifuge (Wescor, Logan, Utah, USA). The IHC staining for cell proliferation marker PCNA, as well as p21, p27, p53, p‐AKT, and p‐H2A.X was described previously.20, 21 The concentration used was 1 μg/mL for the primary antibody and 2 μg/mL for the secondary antibody. Staining intensity was measured using MetaMorph image analysis software (MDS Analytical Technologies, Sunnyvale, CA, USA). Results are expressed as the average integrated immunostaining intensity of three slides ± SEM relative to that in control cells.
Determination of proliferation
In addition to IHC for PCNA, proliferation was also determined using the Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA, USA) according to the manufacturer's instructions. Expansion of the number of viable cells results in an increase in the activity of the mitochondrial dehydrogenases,22 leading to an increase in the amount of formazan dye, which can be detected by spectrometry.
Reverse transcription‐PCR
PC‐3 cells were treated with or without RSV (50 μM) for 24 h, then irradiated by XRT at 8 Gy or a mock treatment. Twenty‐four hours after XRT, cells were harvested. Cells were washed with PBS, centrifuged, and homogenized in TRIzol (Invitrogen). RNA was extracted and its concentration was determined. RNA (1 μg) was reverse transcribed as previously described.20, 21 GAPDH was used as a housekeeping gene to verify that the same amount of RNA was amplified. Primer sequences for GAPDH, pro‐ and anti‐proliferative molecules, and pro‐ and anti‐apoptotic molecules are listed in Table 1.
Table 1.
Primer sequences used in RT‐PCR
| Name | Sense | Antisense |
|---|---|---|
| GAPDH | TGCCGTCTAGAAAAACCTGC | ACCCTGTTGCTGTAGCCAAA |
| p15 | TGGGGGCGGCAGCGATGAG | AGGTGGGTGGGGGTGGGAAAT |
| p18 | CCTGATCGTCAGGACCCTAA | TTATTGAAGATTTGTGGCTCC |
| p21 | CACCCTAGTTCTACCTCAGGCA | ACTCCCCCATCATATACCCCT |
| p27 | ACGGGAGCCCTAGCCTGGAGC | TGCCCTTCTCCACCTCTTGCC |
| p53 | TGGCCATCTACAAGCAGTCACA | GCAAATTTCCTTCCACTCGGAT |
| Cyclin B | CCATTATTGATCGGTTCATGCAGA | CTAGTGGAGAATTCAGCTGTGGTA |
| Cyclin D | GGATGCTGGAGGTCTGCGAGGAAC | GAGAGGAAGCGTGTGAGGCGGTAG |
| Cyclin E | GGAAGGCAAACGTGACCGTT | GGGACTTAAACGCCACTTAA |
| Cdk2 | TTTCTGCCATTCTCATCGG | CTTGGCTTGTAATCAGGCATAGA |
| Fas | ACTTGGGGTGGCTTTGTCTT | GGATGATAGTCTGAATTTTCTCTG |
| FasL | GCCTGTGTCTCCTTGTGA | GCCACCCTTCTTATACTT |
| TRAILR1 | AGAGGGATGGTCAAGGTCAA | GAGTCAAAGGGCACGATGTT |
| TRAIL | AGTCTCTCTGTGTGGCTGTA | TGTCTATCAAGTGCTCATTT |
| Bax | AAGAAGCTGAGCGAGTGT | GGAGGAAGTCCAATGTC |
| FLIP | AATTCAAGGCTCAGAAGCGA | GGCAGAAACTCTGCTGTTCC |
| Bcl‐2 | GTGGAGGAGCTCTTCAGGGA | AGGCACCCAGGGTGATGCAA |
Western blot analysis
PC‐3 cells were treated with or without RSV (50 μM) for 24 h, then irradiated by XRT at 8 Gy or a mock treatment. Twenty‐four hours after XRT, cells were harvested. Expression of p21, p27, p53, and p‐H2A.X was quantified by Western blot with antibodies described above by adding 30 μg protein to a 10% SDS‐PAGE gel as prescribed previously.20 The concentrations used for Western blot analysis were 0.2 μg/mL for the primary antibody and 20 ng/mL for the secondary antibody.
Apoptosis determined by TUNEL staining
Apoptosis was determined by TUNEL assay using an Apoptag kit (Chemicon, El Segundo, CA, USA) as previously described.21 To quantify the number of apoptotic cells, all cells in five or six randomly selected high power fields (magnification, ×400) were manually counted using MetaMorph image analysis software. TUNEL positive cells were expressed as a percentage of total cells.
Measurement of caspase‐3 activity
Cellular caspase‐3 activity of PC‐3 cells recognizing the sequence DEVD (Asp‐Glu‐Val‐Asp) was measured using a caspase‐3/CPP32 colorimetric assay kit (BioVision) as described before.22
Statistics
All experiments were repeated at least two to three times. Statistical analysis of data was carried out using an unpaired two‐tailed Student's t‐test or the Mann–Whitney rank sum test. A P‐value <0.05 was considered significant.
Results
Resveratrol potentiates XRT‐induced inhibition of proliferation
To investigate the effect of RSV on PC‐3 cell radiosensitivity, 70–80% confluent PC‐3 cells were treated with RSV at varying concentrations (0–50 μM) for 24 h, followed by XRT at different doses, as described in “Materials and Methods”. Twenty‐four hours after XRT, cell survival was evaluated by clonogenic survival assay. The results are shown in Figure 1(a). In the absence of RSV, the percentage of colonies of PC‐3 proliferating after XRT was 91 ± 4% (2 Gy), 51 ± 4% (4 Gy) and 34 ± 5% (8 Gy), respectively. When PC‐3 cells were treated with RSV alone, the percentage of colonies of PC‐3 was 77 ± 8% (2 μM), 62 ± 5% (10 μM), and 41 ± 6% (50 μM). Both XRT and RSV decreased cell proliferation in a dose‐dependent manner. The percentage of colonies of PC‐3 after XRT at the dosage of 2 Gy was comparable to that of controls without XRT. At 4 Gy, more than half, and at 8 Gy more than one‐third of PC‐3 cells survived after XRT, indicating the radioresistance of PC‐3 cells. The percentage of colonies of PC‐3 after XRT and RSV (50 μM) treatment decreased to 15 ± 4% (2 Gy), 8 ± 3% (4 Gy) and 5 ± 2% (8 Gy), respectively, suggesting that RSV sensitized PC‐3 to XRT.
Figure 1.
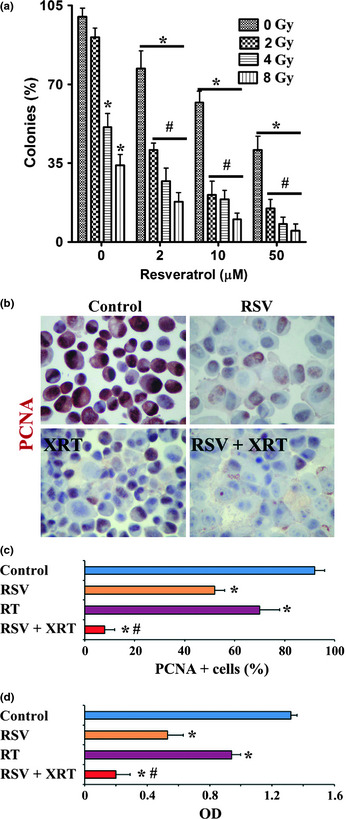
Resveratrol (RSV) potentiates radiation therapy (XRT)‐induced inhibition of proliferation in prostate cancer cells. (a) Clonogenic survival assay of PC‐3 cells with or without radiation in the presence or absence of RSV. The number of colonies was counted and expressed as a percentage of total colonies in controls (without XRT or RSV). *P < 0.05, significant difference in the percentage of colonies in each group versus controls. #P < 0.05, significant difference in the percentage of colonies in groups treated with XRT/RSV versus groups treated with the same dosage of XRT alone. (b) Representative immunohistochemistry results for cell proliferation marker PCNA in PC‐3 cells treated with or without XRT (8 Gy) in the presence or absence of RSV (50 μM). (c) Cells positive for PCNA (red) in five to six randomly selected high power fields of three slides were counted and summarized. Original magnification, ×400. (d) PC‐3 cell proliferation evaluated by a proliferation kit. Results are expressed as the mean optical density (OD) + SEM of PC‐3 cells in each group, and are representative of three independent experiments. *P < 0.05, significant difference in the percentage of PCNA+ cells or OD in each group versus controls. #P < 0.05, significant difference in the percentage of PCNA+ cells or OD between the group treated with XRT/RSV versus the group treated with XRT alone.
These differences were also evident by IHC staining for PCNA (Fig. 1b). The PCNA+ cells (red) in five to six randomly selected high‐power fields of three slides from each group were counted and the results are summarized in Figure 1(c). As shown in Figure 1(c), 92 ± 4% PC‐3 cells are PCNA+ in controls, and 52 ± 4% and 70 ± 8% are PCNA+ cells when treated with RSV (50 μM) and XRT (8 Gy) alone, respectively. Only 8 ± 4% PC‐3 cells were PCNA+ when treated with XRT (8 Gy)/RSV (50 μM). Similar results were obtained when a Quick Cell Proliferation Assay Kit was used to analyze cell proliferation (Fig. 1d). These results strongly indicate that RSV synergizes with XRT to inhibit cell proliferation and decrease the survival of PC‐3 cells.
Effect of XRT/RSV on the expression of pro‐ and antiproliferative molecules in PC‐3 cells
The normal balance between pro‐ and antiproliferative molecules plays an important role in cell proliferation and survival.23, 24, 25, 26, 27 The important antiproliferative molecules are p15, p18, p21, p27, and p53, and cyclin B, cyclin D, cyclin E, and cyclin‐dependent kinase (cdk)2 are important pro‐proliferative molecules.23, 24, 25, 26, 27 To determine if pro‐ and antiproliferative molecules are involved in the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells, PC‐3 cells were treated with or without RSV (50 μM) for 24 h, then irradiated by XRT at 8 Gy or a mock treatment. Twenty‐four hours after XRT, mRNA expression of major pro‐ and antiproliferative molecules in PC‐3 cells with or without XRT (8 Gy) in the presence or absence of RSV (50 μM) was determined by RT‐PCR. The mRNA expression of the antiproliferative molecule p15 was significantly higher and the mRNA expression of the pro‐proliferative molecules cyclin B and cdk2 was significantly lower in cells treated with XRT than in controls. PC‐3 cells differed further in their expression of these molecules when treated with XRT/RSV, suggesting their additive or synergistic effect on mRNA expression of these molecules. Of particular interest, XRT or RSV alone had little effect on mRNA expression of the antiproliferative molecules p21 and p53, whereas, XRT/RSV significantly increased the mRNA expression of p21 and p53, suggesting their potent effect in combination on the upregulation of p21 and p53. Radiation therapy increased the mRNA expression of antiproliferative molecule p27 and decreased the mRNA expression of pro‐proliferative molecule cyclin E. PC‐3 cells did not differ further in their expression of these two molecules when treated with XRT/RSV, suggesting that the upregulation effect on the mRNA expression of p27 and the downregulation effect on that of cyclin E were mainly attributed to radiation. The mRNA expression of antiproliferative molecule p18 was increased in cells treated with XRT compared to that in control cells; unexpectedly, its expression was decreased in cells treated with XRT/RSV compared to that in cells treated with XRT alone. Surprisingly, the mRNA expression of pro‐proliferative molecule cyclin D was increased in cells treated with RSV or XRT alone, whereas its expression decreased significantly in cells treated with XRT/RSV. Consistent with the mRNA expression patterns of p21 and p53, shown in Figure 2, the relative immunostaining intensity for p21 and p53 was similar in cells treated with RSV or XRT alone compared to that in controls, whereas the relative immunostaining intensity for p21 and p53 was stronger after XRT/RSV than that in controls or cells treated with XRT alone. Consistent with the mRNA expression of patterns of p27, shown in Figure 2, the relative immunostaining intensity for p27 was stronger in cells treated with XRT alone or with XRT/RSV compared to that in controls, whereas the relative immunostaining intensity for p27 was similar in cells treated with XRT/RSV compared to that treated with XRT alone (Fig. 3a,b). These results were further confirmed by Western blot for p21, p27, and p53 (Fig. 3c). Taken together, these results indicate that increased expression of antiproliferative molecules p15, p21, and p53 and decreased expression of pro‐proliferative molecules cyclin B, cyclin D, and cdk2 correlated with the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells.
Figure 2.
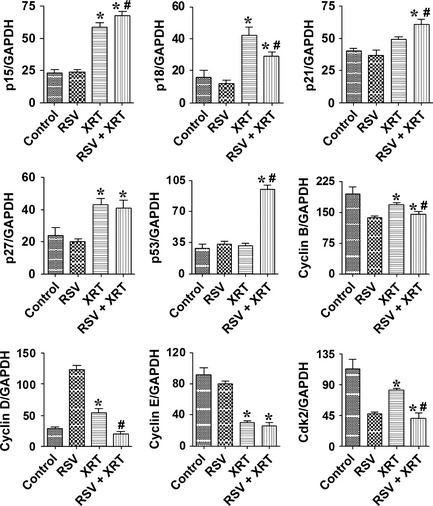
Effect of radiation therapy/resveratrol (XRT/RSV) on the expression of pro‐ and antiproliferative molecules evaluated by RT‐PCR. Results are expressed as the mean ratio of pro‐ and antiproliferative molecule densitometric units/GAPDH + SEM (×100), and are representative of three independent experiments. *P < 0.05, significant difference in groups treated with XRT or XRT/RSV versus controls. #P < 0.05, significant difference between the group treated with XRT/RSV versus the group treated with XRT alone.
Figure 3.
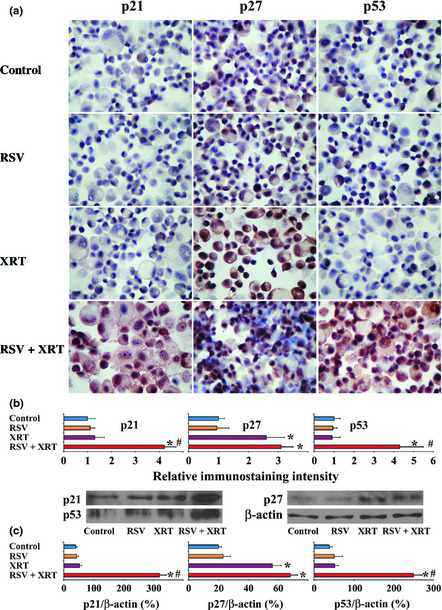
Effect of radiation therapy/resveratrol (XRT/RSV) on the expression of pro‐ and antiproliferative molecules evaluated by immunohistochemistry and Western blot. (a) Immunohistochemistry for p21, p27, and p53 in PC‐3 cells treated with or without XRT (8 Gy) in the presence or absence of RSV (50 μM). Original magnification, ×400. (b) Relative immunostaining intensity for p21, p27, and p53 in five to six randomly selected high power fields of three slides from each group analyzed by MetaMorph software. Results are expressed as the average integrated immunostaining intensity of three slides + SEM relative to that in control cells. (c) Protein expression levels of p21, p27, and p53 by Western blot analysis; 30 μg protein was loaded in each lane. *P < 0.05, significant difference in immunostaining intensity or protein expression level in the groups treated with XRT or XRT/RSV versus controls. #P < 0.05, significant difference in immunostaining intensity or protein expression level between the group treated with XRT/RSV versus the group treated with XRT alone. Figures shown are representative of two or three independent experiments.
Combined XRT/RSV increases apoptosis of PC‐3 cells
We examined the effect of XRT/RSV on apoptosis of PC‐3 cells. PC‐3 cells at 70–80% confluence were treated with RSV (50 μM) for 24 h, followed by XRT (8 Gy). Twenty‐four hours after XRT, apoptosis was evaluated by TUNEL staining. There were few TUNEL+ cells in the control, RSV, or XRT groups. The number of TUNEL+ cells was also low in the XRT/RSV group, constituting only 6 ± 3% PC‐3 cells. There was no significant difference between groups (data not shown). Similar results were obtained when caspase‐3 activity of PC‐3 cells was assayed semiquantitatively using the colorometric detection method (data not shown).
To exclude the possibility that low TUNEL positivity might be due to insufficient time for the effect to occur, apoptosis was re‐evaluated by TUNEL staining 72 h rather than 24 h after XRT (Fig. 4a,b). As shown in Figure 4(b), there were few TUNEL+ cells in the XRT group, compared to 67 ± 5% PC‐3 cells were TUNEL+ in the XRT/RSV group. This difference was significant (P < 0.05). Similar results were obtained when caspase‐3 activity of PC‐3 cells was assayed (Fig. 4c). These results suggest that increased apoptosis evaluated 72 h after XRT correlated with the synergistic effect of RSV with XRT on inhibition of survival of PC‐3 cells.
Figure 4.
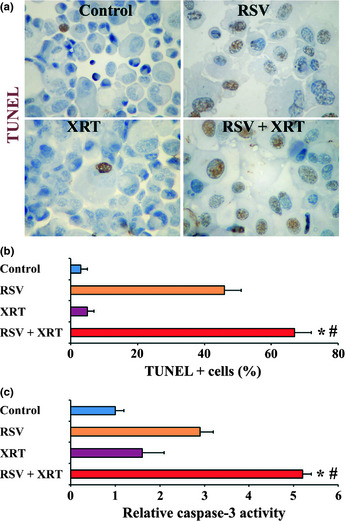
Effect of radiation therapy/resveratrol (XRT/RSV) on apoptosis of PC‐3 cells evaluated 72 h after XRT. (a) Representative TUNEL staining of PC‐3 cells. Original magnification, ×400. (b) TUNEL+ cells (brown) in five to six randomly selected high power fields of three slides were counted. (c) Cellular caspase‐3 activity was measured using a caspase‐3/CPP32 colorimetric assay kit. Results are expressed as mean caspase‐3 activity relative to controls + SEM. Assays were done in triplicate. *P < 0.05, significant difference in the percentage of TUNEL+ cells in the groups treated with XRT or XRT/RSV versus controls. #P < 0.05, significant difference in the percentage of TUNEL+ cells between the group treated with XRT/RSV versus the group treated with XRT alone. Figures are representative of two or three independent experiments.
Combined XRT/RSV alters expression of pro‐ and anti‐apoptotic molecules in PC‐3 cells
Similar to the critical role played by the balance between pro‐ and antiproliferative molecules, the balance between pro‐ and anti‐apoptotic molecules is also pivotal in cell proliferation and survival.20, 21, 28, 29, 30, 31 Fas, FasL, TRAILR1, TRAIL, and Bax are important pro‐apoptotic molecules, and FLIP and Bcl‐2 are important anti‐apoptotic molecules.20, 21, 28, 29, 30, 31 To address the role of pro‐ and anti‐apoptotic molecules in the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells, the mRNA expression of major pro‐ and anti‐apoptotic molecules in PC‐3 cells was determined by RT‐PCR. Cells were treated with or without XRT (8 Gy) in the presence or absence of RSV (50 μM) (Fig. 5). The mRNA expression of the pro‐apoptotic molecules Fas and TRAILR1 was lower in cells treated with XRT than that in controls, whereas their expression was significantly higher in cells treated with XRT/RSV than in cells treated with XRT alone. Compared to that in controls, although the mRNA expression of the pro‐apoptotic molecules was similar (for FasL), increased (for TRAIL), or decreased (for Bax) in cells treated with XRT alone, their mRNA expression was all similar when compared to that in cells treated with XRT/RSV. Compared to that in controls, although the mRNA expression of the anti‐apoptotic molecules FLIP and Bcl‐2 was decreased in cells treated with XRT alone, interestingly, the mRNA expression of FLIP was similar, but the mRNA expression of Bcl‐2 was decreased, when compared to cells treated with XRT/RSV. These results indicate that increased expression of pro‐apoptotic molecules Fas and TRAILR1 correlated with the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells.
Figure 5.
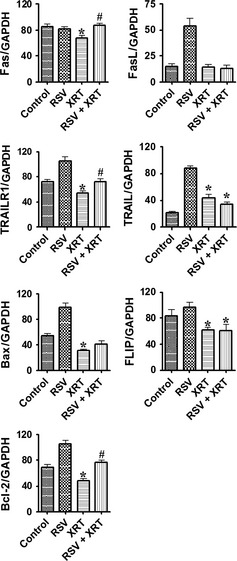
Effect of radiation therapy/resveratrol (XRT/RSV) on the expression of pro‐ and anti‐apoptotic molecules evaluated by RT‐PCR. Experiments were done in triplicate and are representative of three independent experiments. Results are expressed as the mean ratio of pro‐ and anti‐apoptotic molecule densitometric units/GAPDH + SEM (×100). *P < 0.05, significant difference in groups treated with XRT or XRT/RSV versus controls. #P < 0.05, significant difference between the group treated with XRT/RSV versus the group treated with XRT alone.
Effect of XRT/RSV on expression of p‐AKT and p‐H2A.X in PC‐3 cells
The AKT pathway is important for cell proliferation and survival in many tumors including PCA.23, 32, 33, 34 Thus, it was of interest to determine if XRT/RSV has any effect on the expression of p‐AKT. Immunohistochemistry for p‐AKT was used to evaluate the expression of p‐AKT in PC‐3 cells (Fig. 6a). As shown in Figure 6(b), although the relative immunostaining intensity for p‐AKT was weaker in cells treated with XRT alone compared to that in controls, the relative immunostaining intensity for p‐AKT was similar in cells treated with XRT/RSV compared to that treated with XRT alone. These results indicate that the expression level of p‐AKT does not correlate with the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells.
Figure 6.
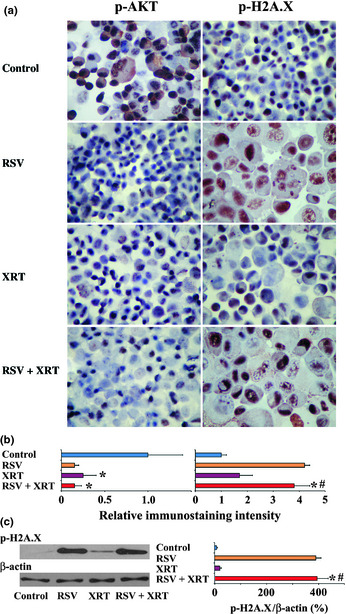
Effect of radiation therapy/resveratrol (XRT/RSV) on the expression of p‐AKT and p‐H2A.X evaluated by immunohistochemistry and Western blot analysis. (a) Immunohistochemistry for p‐AKT and p‐H2A.X in PC‐3 cells treated with or without XRT (8 Gy) in the presence or absence of RSV (50 μM). Original magnification, ×400. (b) Relative immunostaining intensity for p‐AKT and p‐H2A.X was analyzed by MetaMorph software. Results are expressed as the average integrated immunostaining intensity of three slides + SEM relative to that in control cells. (c) Shown are Western blot results for the protein expression levels of p‐AKT and p‐H2A.X; 30 μg protein was loaded in each lane. *P < 0.05, significant difference in immunostaining intensity or protein expression level in the groups treated with XRT or XRT/RSV versus controls. #P < 0.05, significant difference in immunostaining intensity or protein expression level between the group treated with XRT/RSV versus the group treated with XRT alone. Figures are representative of two or three independent experiments.
Similar to apoptosis, cellular senescence has been shown to function as a potent mechanism to inhibit tumor cell proliferation and survival, and p‐H2A.X has been suggested to be a marker for cell senescence.33, 35, 36, 37 To investigate if XRT/RSV has any effect on senescence of PC‐3 cells, IHC was first used to evaluate the expression of p‐H2A.X in PC‐3 cells (Fig. 6a). Although the relative immunostaining intensity for p‐H2A.X was similar in cells treated with XRT alone compared to that in controls, the relative immunostaining intensity for p‐H2A.X was much stronger in cells treated with XRT/RSV compared to that treated with XRT alone. These results were further confirmed by Western blot for p‐H2A.X (Fig. 6c). These results indicate that the expression level of p‐H2A.X, but not p‐AKT, correlated with the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells, suggesting the role of senescence in the synergistic effect of RSV with XRT to inhibit cell proliferation and decrease cell survival of PC‐3 cells.
Discussion
In this study, we show that RSV synergizes with XRT to inhibit the proliferation of a PCA cell line by promoting apoptosis and senescence. The antiproliferative effect of XRT/RSV correlated with increased expression of antiproliferative molecules p15, p21, and mutant p53 (mp53) and decreased expression of pro‐proliferative molecules cyclin B, cyclin D, and cdk2. Increases in apoptosis correlated with increased expression of pro‐apoptotic molecules Fas and TRAILR1. Furthermore, we showed that XRT/RSV promoted senescence as evidenced by increased expression of p‐H2A.X, but had little effect on the expression level of p‐AKT. To our knowledge, this is the first study to indicate the synergistic effect of XRT and RSV on proliferation and survival of PC‐3 PCA cells. This is also the first study to investigate the detailed molecular mechanisms by which XRT/RSV inhibits the survival of PCA cells.
Consistent with previous studies, our study further confirmed that PC‐3 cells are relatively radioresistant.2, 12, 18, 38, 39 The percentage of surviving PC‐3 colonies after radiation at 2 Gy was comparable to that of non‐radiated controls. Even at 8 Gy, proliferation was reduced only by half, revealing the resistance of PC‐3 cells to radiation. Interestingly, the percentage of PC‐3 colonies after XRT/RSV decreased to 5 ± 2% at the dose of 8 Gy. At the dose of 2 Gy, the percentage of colonies of PC‐3 cells decreased from 91 ± 4% (without RSV) to 15 ± 4% (with RSV). These results strongly suggest a radiosensitizing role for RSV. Radiosensitization of PCA may allow for reduction of effective radiation dose and side‐effects associated with XRT. Whether RSV functions in the same capacity in the in vivo setting awaits the results of further animal studies and clinical trials.
The eukaryotic cell cycle is tightly regulated.23, 24, 25, 26, 27 The balance between pro‐ and antiproliferative molecules plays an important role in cell proliferation. Cyclin B, D, and cyclin E, as well as cdk2, play major roles in proliferation. Downregulation of cyclin D delays or inhibits entry to the S phase and overexpression of cyclin D shortens the G1 phase.23, 24, 25, 26, 27 Cyclin E is active in late G1 phase and is maximal at the G1–S transition. The important antiproliferative molecules p15, p18, p21, p27, and p53 exert their effect through inhibition of cyclin‐dependent kinases.23, 24, 25, 26, 27 In this study, we found that the antiproliferative effect of XRT/RSV correlated with increased expression of p15, p21, and p53 and decreased expression of cyclin B, cyclin D, and cdk2. Decreased expression of cyclin D and cdk2 is consistent with studies using DU145 PCA cells treated with RSV. Our data indicated that XRT/RSV disrupted the pro‐proliferative mechanism and induced antiproliferative regulatory molecules in PC‐3 to inhibit cell proliferation in treated cells. Previous reports showed variable expression of p53 and/or mp53 in PC‐3 cells40, 41, 42, 43, 44 and mp53 upregulated 15‐lipoxygenase‐1 in murine and human PCA.43, 45 In our study, we detected mp53 mRNA and protein expression in PC‐3 cells.40, 43 Although the detailed functionality of mp53 protein in PC‐3 is unknown, the upregulation of mp53 by the XRT/RSV correlated with an antiproliferative effect on PC‐3 cells.
Apoptosis is mediated through the sequential activation of a series of caspases induced either through a receptor‐mediated or mitochondrial‐mediated pathway.28, 29 Fas, FasL, TRAILR1, and TRAIL belong to tumor necrosis factor receptor family and have a pro‐apoptotic function in the receptor‐mediated pathway of apoptosis.30 FLIP inhibits death receptor‐mediated apoptosis by blocking activation of caspase‐8.31 Bcl‐2 inhibits apoptosis, and Bcl‐2 family protein Bax promotes apoptosis through regulation of mitochondrial voltage‐dependent anion channels.29, 31 As early as 1 day after XRT, mRNA expression of Fas and TRAILR1 was increased in PC‐3 cells treated with XRT/RSV as compared to cells treated with XRT alone. In this same time period, few TUNEL+ apoptotic cells were detected in any treatment group, suggesting that XRT/RSV induction of apoptosis occurs more slowly than changes in the mechanisms governing inhibition of proliferation. When apoptosis was evaluated by TUNEL staining 3 days after XRT, there were still few TUNEL+ cells in the XRT group, whereas more than two‐thirds of the XRT/RSV treated PC‐3 cells were TUNEL+. The difference in apoptosis between treatment groups was confirmed by measuring caspase‐3 activity. Thus, XRT/RSV increases apoptotic cell death in PC‐3 cells through the upregulation of Fas and TRAILR1.
In the analysis of cell cycle molecules, we unexpectedly found the addition of RSV to PC‐3 cells without XRT increased the expression of cyclin D. We believe the increase in cyclin D in this scenario is part of an adaptative response to cell injury to prevent cells from further damage. As well, the mRNA expression of p18 was increased in cells treated with XRT compared to that in controls, but its expression was decreased in cells treated with XRT/RSV compared to that of cells treated with XRT alone, although its expression was still higher than in control cells. When analyzing the mRNA expression of other pro‐and antiproliferative molecules, consistent with studies by others,17, 46, 47, 48 we also found that RSV alone increased the expression of pro‐apoptotic molecule FasL, TRAIL, and Bax, as well as anti‐apoptotic molecule Bcl‐2, but the addition of XRT partially or fully abolished this effect. Furthermore, FLIP contributed little to the synergistic effect of RSV with radiation. It is possible that there might be some other unexamined and/or unidentified pro‐ and antiproliferative and/or pro‐ and anti‐apoptotic molecules that also play roles in PC‐3 cell proliferation and/or apoptosis. Thus, it is reasonable to argue that it is not a specific pro‐ or antiproliferative and/or pro‐ and anti‐apoptotic molecule, but it is the balance between pro‐ and antiproliferative molecules and the balance between pro‐ and anti‐apoptotic molecules that dictates the fate of PC‐3 cells for their proliferation, quiescence, or apoptosis.
Cellular senescence occurs by irreversible growth arrest. Alongside apoptosis, senescence is a critical anticancer mechanism.49 We have established a role for senescence in the XRT/RSV inhibition of PC‐3 PCA cells as evidenced by increased expression of p‐H2A.X in cells treated with XRT/RSV compared to cells treated with XRT alone. p21, p27, and p53 are involved in the process of senescence.49, 50 We have shown increased expression of p21 in XRT/RSV treated cells (Figs 2, 3) and this also might contribute to senescence. Further studies are needed to examine the role of mp53 in this process.
Although RSV is thought to be safe for humans, optimal dosing has yet to be established and the side‐effects at therapeutic levels are unknown. An oral dosage above 2.5 g per day may be associated with gastrointestinal discomfort or diarrhea,51 whereas side‐effects are much less common at lower doses. Studies are still ongoing for the evaluation of RSV dosing, side‐effects, and therapeutic benefits in humans.51
In summary, RSV enhances radiation sensitivity in PCA by inhibiting cell proliferation and promoting cell senescence and apoptosis in vitro. Our data highlight the potential of RSV as a radiation sensitizer for PCA. Further in vivo studies and potentially clinical trials using XRT/RSV in PCA treatment are warranted to address the true therapeutic potential of this combination.
Disclosure Statement
The authors have no conflicts of interest.
Acknowledgments
We thank Drs. Susan L. Deutscher and Jessica R. Newton from the University of Missouri (Columbia, MO, USA) for providing the PC‐3 cells.
References
- 1. Siegel R, Ward E, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61: 212–36. [DOI] [PubMed] [Google Scholar]
- 2. Leith JT. In vitro radiation sensitivity of the LNCaP prostate tumor cell line. Prostate 1994; 24: 119–24. [DOI] [PubMed] [Google Scholar]
- 3. Jang M, Cai L, Udeani GO et al Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275: 218–20. [DOI] [PubMed] [Google Scholar]
- 4. Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med 2003; 69: 589–99. [DOI] [PubMed] [Google Scholar]
- 5. Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator‐activated receptor alpha in mice. Neurosci Lett 2003; 352: 203–6. [DOI] [PubMed] [Google Scholar]
- 6. Ingham JL. 3,5,4′‐Trihydroxystilbene as a phytoalexin from groundnuts (Arachis hypogea). Phytochem 1976; 15: 1791–3. [Google Scholar]
- 7. Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stylbene compounds of the roots of Ploygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem Pharm Bull 1982; 30: 1766–70. [DOI] [PubMed] [Google Scholar]
- 8. Uenobe F, Nakamur S, Miyazawa M. Antimutagenic effect of Resveratrol against Trp‐P‐1. Mutat Res 1997; 373: 197–200. [DOI] [PubMed] [Google Scholar]
- 9. Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clin Biochem 1997; 30: 91–113. [DOI] [PubMed] [Google Scholar]
- 10. Shishodia S, Aggarwal BB. Resveratrol: a polyphenol for all seasons. Boca Raton, FL: CRC Press Taylor and Francis; 2006; 1–15. [Google Scholar]
- 11. Bhardwaj A, Sethi G, Vadhan‐Raj S et al Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down‐regulation of STAT3 and nuclear factor‐ҝB‐regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007; 109: 2293–302. [DOI] [PubMed] [Google Scholar]
- 12. Scarlati F, Sala G, Ricci C et al Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett 2007; 253: 124–30. [DOI] [PubMed] [Google Scholar]
- 13. Fulda S, Debatin KM. Sensitization for anticancer drug‐induced apoptosis by the chemopreventive agent resveratrol. Oncogene 2004; 23: 6702–11. [DOI] [PubMed] [Google Scholar]
- 14. Fulda S, Debatin KM. Resveratrol‐mediated sensitisation to TRAIL‐induced apoptosis depends on death receptor and mitochondrial signalling. Eur J Cancer 2005; 41: 786–98. [DOI] [PubMed] [Google Scholar]
- 15. Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal 2005; 7: 1630–47. [DOI] [PubMed] [Google Scholar]
- 16. Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS ONE 2010; 5: e15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su JL, Lin MT, Hong CC et al Resveratrol induces FasL‐related apoptosis through Cdc42 activation of ASK/JNK‐dependent signaling pathway in human leukemia HL‐60 cells. Carcinogenesis 2005; 26: 1–10. [DOI] [PubMed] [Google Scholar]
- 18. Diaz R, Nguewa PA, Diaz‐Gonzalez JA et al The novel Akt inhibitor Palomid 529 (P529) enhances the effect of radiotherapy in prostate cancer. Br J Cancer 2009; 100: 932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao L, Wientjes MG, Au JLS. Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res 2004; 10: 7994–8004. [DOI] [PubMed] [Google Scholar]
- 20. Fang Y, Wei Y, DeMarco V, Chen K, Sharp GC, Braley‐Mullen H. Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol 2007; 171: 875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang Y, Sharp GC, Yagita H, Braley‐Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol 2008; 216: 505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berridge MV, Tan AS, McCoy KD, Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica 1996; 4: 12–20. [Google Scholar]
- 23. Fang Y, Yu S, Braley‐Mullen H. TGF‐β promotes proliferation of thyroid epithelial cells in IFN‐γ‐/‐ mice by downregulation of p21 and p27 via AKT pathway. Am J Pathol 2012; 180: 650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol 1999; 39: 295–312. [DOI] [PubMed] [Google Scholar]
- 25. Sherr CJ. G1 phaseprogression: cycling on cue. Cell 1994; 79: 551–5. [DOI] [PubMed] [Google Scholar]
- 26. Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1‐to‐S phase transition. Mol Cell Biol 1995; 15: 2612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sherr CJ. D‐type cyclins. Trends Biochem Sci 1995; 20: 187–90. [DOI] [PubMed] [Google Scholar]
- 28. Thompson, CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456–62. [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Xu Q, Krajewski S et al BAR: an apoptosis regulator at the intersection of caspases and Bcl‐2 family proteins. Proc. Natl Acad Sci USA 2000; 97: 2597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson FA. Fas ligand‐induced apoptosis as a mechanism of immune privilege. Science 1995; 270: 1189–92. [DOI] [PubMed] [Google Scholar]
- 31. Fang Y, Braley‐Mullen H. Cultured murine thyroid epithelial cells expressing transgenic Fas‐associated death domain‐like interleukin‐1β converting enzyme inhibitory protein are protected from Fas‐mediated apoptosis. Endocrinology 2008; 149: 1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF‐β signalling through a direct interaction with Smad3. Nat Cell Biol 2004; 6: 358–65. [DOI] [PubMed] [Google Scholar]
- 33. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 34. Assinder SJ, Dong Q, Kovacevic Z, Richardson DR. The TGF‐β, PI3K/Akt and PTEN pathways: established and proposed biochemical integration in prostate cancer. Biochem J 2009; 417: 411–21. [DOI] [PubMed] [Google Scholar]
- 35. Lawless C, Wang C, Jurk D, Merz A, Zglinicki T, Passos JF. Quantitative assessment of markers for cell senescence. Exp Gerontol 2010; 45: 772–8. [DOI] [PubMed] [Google Scholar]
- 36. Bonner WM, Redon CE, Dickey JS et al γH2AX and cancer. Nat Rev Cancer 2008; 8: 957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Camphausen K, Brady KJ, Burgan WE et al Flavopiridol enhances human tumor cell radiosensitivity and prolongs expression of γH2AX foci. Mol Cancer Ther 2004; 3: 409–16. [PubMed] [Google Scholar]
- 38. Li WX, Franklin WA. Radiation‐ and heat‐induced apoptosis in PC‐3 prostate cancer cells. Radiat Res 1998; 150: 190–4. [PubMed] [Google Scholar]
- 39. Zellweger T, Chi K, Miyake H et al Enhenced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res 2002; 8: 3276–84. [PubMed] [Google Scholar]
- 40. Nayak BK, Krishnegowda NK, Galindo CA, Meltz ML, Swanson GP. Synergistic effect between curcumin (difruloylmethane) and radiation on clonogenic cell death independent of p53 in prostate cancer cells. J Cancer Sci Ther 2010; 2: 171–81. [Google Scholar]
- 41. Bernard D, Martinez‐Leal JF, Rizzo S et al CBX7 controls the growth of normal and tumor‐derived prostate cells by repressing the Ink4a/Arf locus. Oncogene 2005; 24: 5543–51. [DOI] [PubMed] [Google Scholar]
- 42. Carroll AG, Voeller HJ, Sugars L, Gelmann EP. p53 oncogene mutations in three human prostate cancer cell lines Prostate 1993; 23: 123–34. [DOI] [PubMed] [Google Scholar]
- 43. Kelavkar UP, Cohen C, Kamitani H, Eling TE, Badr KF. Concordant induction of 15‐lipoxygenase‐1 and mutant p53 expression in human prostate adenocarcinoma: correlation with Gleason staging. Carcinogenesis 2000; 21: 1777–87. [DOI] [PubMed] [Google Scholar]
- 44. Zhou B, Liu X, Mo X et al The human ribonucleotide reductase subunit hRRM2 complements p53R2 in response to UV‐induced DNA repair in cells with mutant p53. Cancer Res 2003; 63: 6583–94. [PubMed] [Google Scholar]
- 45. Kelavkar UP, Badr KF. Effects of mutant p53 expression on human 15‐lipoxygenase‐promoter activity and murine 12/15‐lipoxygenase gene expression: evidence that 15‐lipoxygenase is a mutator gene. Proc Natl Acad Sci USA 1999; 96: 4378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci 2007; 12: 4839–54. [DOI] [PubMed] [Google Scholar]
- 47. Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5‐trihydroxy‐trans‐stilbene) and its interaction with TNF‐related apoptosis inducing ligand (TRAIL) in androgen‐insensitive prostate cancer cells. Mol Cell Biochem 2007; 304: 273–85. [DOI] [PubMed] [Google Scholar]
- 48. Zunino SJ, Storms DH. Resveratrol alters proliferative responses and apoptosis in human activated B lymphocytes in vitro. J Nutr 2009; 139: 1603–8. [DOI] [PubMed] [Google Scholar]
- 49. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192: 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gao FH, Hu XH, Li W et al Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c‐myc. BMC Cancer 2010; 10: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vang O, Ahmad N, Baile CA et al What is new for an old molecule? Systematic review and recommendations on the use of resveratrol PLoS ONE 2011; 6: e19881. [DOI] [PMC free article] [PubMed] [Google Scholar]


