Abstract
Background
Canagliflozin reduces hospitalizations for heart failure (HF) in type 2 diabetes mellitus (T2DM). Its effect on cardiorespiratory fitness and cardiac function in patients with established HF with reduced ejection fraction (HFrEF) is unknown.
Methods
We conducted a double‐blind randomized controlled trial of canagliflozin 100 mg or sitagliptin 100 mg daily for 12 weeks in 88 patients, and measured peak oxygen consumption (VO2) and minute ventilation/carbon dioxide production (VE/VCO2) slope (co‐primary endpoints for repeated measure ANOVA time_x_group interaction), lean peak VO2, ventilatory anaerobic threshold (VAT), cardiac function and quality of life (ie, Minnesota Living with Heart Failure Questionnaire [MLHFQ]), at baseline and 12‐week follow‐up.
Results
The study was terminated early due to the new guidelines recommending canagliflozin over sitagliptin in HF: 17 patients were assigned to canagliflozin and 19 to sitagliptin, total of 36 patients. There were no significant changes in peak VO2 and VE/VCO2 slope between the two groups (P = .083 and P = .98, respectively). Canagliflozin improved lean peak VO2 (+2.4 mL kgLM −1 min−1, P = .036), VAT (+1.5 mL kg−1 min−1, P = .012) and VO2 matched for respiratory exchange ratio (+2.4 mL Kg−1 min−1, P = .002) compared to sitagliptin. Canagliflozin also reduced MLHFQ score (−12.1, P = .018).
Conclusions
In this small and short‐term study of patients with T2DM and HFrEF, interrupted early after only 36 patients, canagliflozin did not improve the primary endpoints of peak VO2 or VE/VCO2 slope compared to sitagliptin, while showing favourable trends observed on several additional surrogate endpoints such as lean peak VO2, VAT and quality of life.
Keywords: cardiorespiratory fitness, diabetes mellitus, DPP4 inhibitors, heart failure, SGLT2 inhibitors
1. INTRODUCTION
Heart failure (HF) remains one of the most common comorbidities in type 2 diabetes mellitus (T2DM). 1 The sodium‐glucose co‐transporter (SGLT)‐2 inhibitors, canagliflozin, 2 dapagliflozin 3 and empagliflozin, 4 are the only glucose‐lowering agents that reduce the incidence of HF and HF‐related hospitalizations in patients with T2DM, and dapagliflozin also in patients without T2DM and established HF with reduced ejection fraction (HFrEF). 5 The mechanisms explaining these benefits appear, at least in part, independent of glycemic control.
The cardiovascular outcomes trials with the SGLT2 inhibitors canagliflozin and empagliflozin do not provide data on the forms of HF and HF‐related events being prevented, with a small percentage of patients having HF at baseline. Dapagliflozin reduced the risk of worsening HF or death from cardiovascular causes in established HFrEF patients and improved quality of life (QoL) in patients with and without T2DM. 5
Exercise intolerance is a hallmark consequence of HF, typically defined as a reduction in cardiorespiratory fitness (CRF) measured as a reduced peak oxygen consumption (VO2) during cardiopulmonary exercise testing (CPX) and largely responsible for the reduced QoL in HF. 6 Peak VO2 is a strong prognostic factor in HFrEF, and its improvements have been associated with long‐term reduction of clinical events, making it a strong surrogate outcome measure. 7 Despite several therapeutics able to improve CRF, 6 , 8 patients with HFrEF still present with a markedly reduced CRF. The effects of SGLT2 inhibitors on CRF in patients with established HF have been only minimally investigated, 9 , 10 and, to date, no randomized controlled trials comparing the effects of SGLT2 inhibitors on CRF with other glucose‐lowering agents have been performed.
We investigated whether canagliflozin improved peak VO2 and ventilatory efficiency in patients with T2DM and HFrEF compared to sitagliptin, a dipeptidyl peptidase (DPP)4 inhibitor with proven cardiovascular safety with glucose‐lowering efficacy similar to SGLT2 inhibitors. 11 We hypothesized that improvements in CRF would be independent of glycemic control.
2. METHODS
2.1. Study design
The CANA‐HF study was a double blind, randomized, controlled study investigating the effects of canagliflozin 100 mg daily compared to sitagliptin 100 mg daily for 12 weeks in 88 patients with T2DM and stable chronic HFrEF on CRF started on September 2016. On 22 October 2018, due to the new guidelines released by the American Diabetes Association 12 and the European Association for the Study of Diabetes 13 recommending the use of SGLT2 inhibitors over sitagliptin in patients with HF, the study was interrupted prematurely after consultation with the sponsor, as we believed that an equipoise no longer existed between the two treatments. Thus, we enrolled a total of 36 patients (Figure 1).
FIGURE 1.
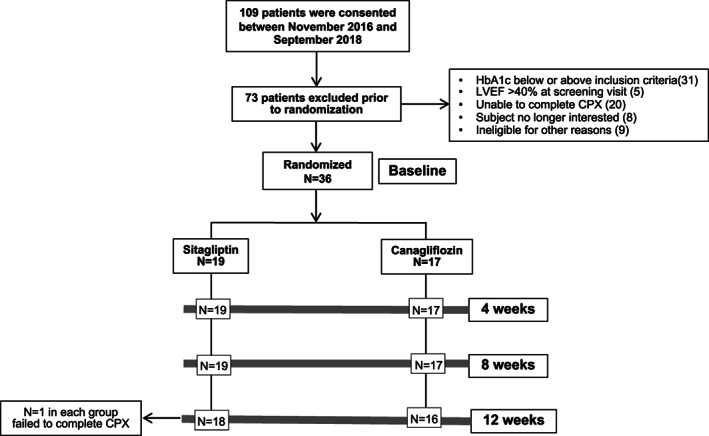
Screening and enrollment. CPX, cardiopulmonary exercise testing; HbA1c, glycated haemoglobin; LVEF, left ventricular ejection fraction
2.2. Inclusion and exclusion criteria
Briefly, stable patients with symptomatic HF (New York Heart Association [NYHA] class II‐III) with a left ventricular ejection fraction (LVEF) ≤40%, T2DM and reduced CRF were enrolled (Supporting Information for full list of inclusion and exclusion criteria). The Western Institutional Review Board approved the study and all patients provided written informed consent.
2.3. Cardiopulmonary exercise testing
At baseline and 12 weeks subjects underwent a symptom‐limited CPX, using a metabolic cart interfaced with a treadmill utilizing a conservative ramping protocol wherein the speed and grade increased approximately 0.6 estimated metabolic equivalents every minute, as previously described. 14 Peak VO2 was defined as the highest 10‐second interval average during the last 30 seconds of exercise. 6 The VE/VCO2 slope was calculated using 10‐second averaged VE and VCO2 data from the initiation of exercise to peak that was inserted into spreadsheet software (Microsoft Excel, Microsoft Corp., Bellevue, Washington) to calculate the slope via least squares linear regression. 6 The VE/VCO2 slope is a marker of HF severity defined as minute ventilation/carbon dioxide production (VE/VCO2) slope.
2.4. Doppler echocardiography
At baseline and at 12 weeks we performed resting transthoracic Doppler echocardiography. 15 We measured LV end‐diastolic and LV end‐systolic volumes, LVEF, and early transmitral velocity (E) on pulsed‐wave Doppler spectra and early mitral annular velocities by tissue Doppler averaged between the lateral and septal (E′) annulus; these measures were used to calculate the E/E′ ratio. We also measured deceleration time (DT) and calculated the E′ velocity indexed by DT (E′DT). 16
2.5. Quality of life
We measured QoL at baseline and 12 weeks using the Minnesota Living with Heart Failure Questionnaire (MLHFQ). The MLHFQ is a 21‐item graded questionnaire used to assess the impairments in QoL in HF, higher scores reflect a greater HF symptom burden. 17
2.6. Anthropometrics, fluid status and blood pressure
We measured body weight and height to calculate body mass index (BMI) and waist circumference at baseline and at 12 weeks. 18 We assessed lean mass (LM) to calculate lean peak VO2 using dual‐energy X‐Ray Absorptiometry (DEXA) (QDR 4500a, Hologic, Marlborough, Massachusetts) 19 and changes in fluid status: total body water (TBW), intracellular water (ICW) and extracellular water (ECW) using bioelectrical impedance analysis (BIA; Quantum IV, RJL System, Clinton Township, Michigan) at baseline and 12 weeks. Resting blood pressure was measured at baseline and after 12 weeks using a Tango automated blood pressure system (Suntech Medical, Morrisville, North Carolina).
2.7. Biomarkers
We measured non‐fasting biomarkers: HbA1c, N‐terminal pro‐brain natriuretic peptide (NTproBNP) and glomerular filtration rate (GFR), at baseline and 12 weeks.
2.8. Dietary intake
Because canagliflozin increases glucose and sodium urinary excretion resulting in a modest daily caloric deficit, we also assessed dietary caloric and sodium intake: the research dietitian performed a standardized 5‐pass 24‐hour dietary recall. 20 Food consumption was converted in calories and sodium using the Nutrition Data System for Research 2016. 20
2.9. Study endpoints
The co‐primary endpoints for the study were changes from baseline in peak VO2 and the VE/VCO2 slope after 12 weeks of treatment with canagliflozin 100 mg daily or sitagliptin 100 mg daily. We measured QoL using the MLHFQ and additional CRF variable: ventilatory anaerobic threshold (VAT), and DEXA‐measured lean peak VO2. 6 , 21 Lean peak VO2 has been proposed to be superior to peak VO2 in determining prognosis in patients with HFrEF, especially in patients with obesity, in which peak VO2 may underestimate the overall level of CRF. 6 , 21 We performed a post‐hoc exploratory analysis to investigate the effects of canagliflozin vs sitagliptin on respiratory exchange ratio (RER)‐matched VO2 after 12 weeks, using the VO2 values corresponding to the lowest RER achieved between baseline and follow‐up. Such analysis allowed for correction of differences in RER between baseline and follow‐up. Additional exploratory analyses included measures of resting cardiac function, anthropometrics, body water and blood pressure.
2.10. Statistical analysis and sample size calculation
Statistical analyses were performed with SPSS 24.0 package (Chicago, Illinois). Continuous data are reported as means and standard deviations for normally distributed variables or median and interquartile range for potential deviation from the Gaussian distribution, and discrete variables were reported as N and %. We used the nonparametric Wilcoxon signed‐rank test for repeated measures to assess the within‐group related changes compared to baseline. The differences in interval changes between the two groups were compared using a random‐effect analysis of variance model for repeated measures to analyse the effects of time and group allocation. Unadjusted P‐values were reported throughout, with statistical significance set at the two‐tailed .025 level for the co‐primary endpoints and .05 for the additional analyses. Missing data were omitted and not imputed and the cases excluded from the related analysis. A sample size of 40 patients per group provided sufficient power to detect a MD in the interval change in peak VO2 of 1.50 ± 1.76 mL kg−1 min−1 expected with canagliflozin compared to sitagliptin, which we predicted to have no significant effects on peak VO2 (0 ± 1.76 mL kg−1 min−1; power of >90%, α value .025 two‐sided). 9 , 22 , 23 We initially anticipated that a sample size of 88 patients would allow for a 10% loss to follow‐up. Study enrolment was, however, interrupted early, ultimately enrolling 36 individuals, providing a sufficient statistical power to detect a MD in the interval change in peak VO2 of 1.50 ± 1.76 mL kg−1 min−1 with canagliflozin compared to sitagliptin (0 ± 1.76 mL kg−1 min−1; power of 70%, α value .025 two‐sided).
3. RESULTS
3.1. Baseline characteristics
No statistically significant differences were found between the two groups at baseline, except for peak RER, which was significantly lower in the sitagliptin group compared to canagliflozin (Table 1). Patients randomized to canagliflozin tended to be more likely to receive insulin, had a lower BMI, and lower NTproBNP, although the differences did not reach statistical significance. Patients were followed for a mean follow‐up of 93 days.
TABLE 1.
Baseline demographics and clinical characteristics
| Sitagliptin n = 19 | Canaglifozin n = 17 | P value | |
|---|---|---|---|
| Male | 15 (78.9) | 13 (76.5) | .86 |
| Age, y | 54.3 ± 8.8 | 58 ± 6.1 | .15 |
| Caucasian | 7 (36.8) | 10 (58.8) | .19 |
| Non‐Hispanic | 18 (94.7) | 14 (82.4) | .33 |
| Body mass index, kg/m2 | 38.8 ± 7 | 34.5 ± 6.6 | .06 |
| Systolic blood pressure, mmHg | 130.4 ± 20.4 | 130.8 ± 17.1 | .96 |
| Diastolic blood pressure, mmHg | 72 ± 13.4 | 72.5 ± 13.2 | .93 |
| Hypertension | 17 (89.5) | 16 (94.1) | .62 |
| Hyperlipidemia | 16 (84.2) | 15 (88.2) | .73 |
| Atrial fibrillation | 5 (26.3) | 4 (23.5) | 1 |
| Current tobacco | 3 (15.8) | 5 (29.4) | .43 |
| Coronary artery disease | 7 (36.8) | 9 (52.9) | .33 |
| Myocardial infarction | 5 (26.3) | 4 (23.5) | 1 |
| CABG | 0 | 3 (17.6) | .10 |
| COPD | 2 (10.5) | 3 (17.6) | .65 |
| Peripheral artery disease | 3 (15.8) | 3 (17.6) | 1 |
| Stroke/TIA | 3 (15.8) | 1 (5.9) | .61 |
| Heart failure therapy | |||
| Aldosterone blockers | 13 (68.4) | 9 (52.9) | .34 |
| Angiotensin blockers | 11 (57.9) | 14 (82.4) | .11 |
| ARNI | 4 (21.1) | 2 (11.8) | .66 |
| Aspirin | 12 (63.2) | 13 (76.5) | .39 |
| Digoxin | 0 | 1 (5.9) | .47 |
| DOACs | 5 (26.3) | 3 (17.6) | .70 |
| Hydralazine | 3 (15.8) | 3 (17.6) | 1 |
| ICD | 7 (36.8) | 5 (29.4) | .64 |
| Loop diuretics | 18 (94.7) | 13 (76.5) | .11 |
| Nitrates | 6 (31.6) | 3 (17.6) | .45 |
| Statins | 17 (89.5) | 17 (100) | .17 |
| Warfarin | 3 (15.8) | 1 (5.9) | .61 |
| β‐adrenergic receptor blockers | 18 (94.7) | 16 (94.1) | .94 |
| Glucose‐lowering agents | |||
| Biguanides | 10 (52.6) | 10 (58.8) | .71 |
| DPP4 inhibitors | 1 (5.3) | 0 | .53 |
| GLP1 receptor agonists | 0 | 1 (5.9) | .47 |
| Insulin | 6 (31.6) | 11 (64.7) | .051 |
| Sulfonylureas | 5 (26.3) | 1 (5.9) | .13 |
| NYHA class and quality of life | |||
| II | 12 (63.2) | 10 (58.8) | .93 |
| III | 7 (36.8) | 7 (41.2) | .79 |
| MLHFQ score | 38.4 ± 26.6 | 49.2 ± 26.8 | .43 |
| Doppler echocardiography parameters | |||
| LVEF, % | 27.0 ± 6.8 | 31.6 ± 7.5 | .28 |
| E/e′ ratio | 13.8 (11.4‐18.2) | 12.4 ± (8.6‐30.0) | .42 |
| Deceleration time velocity, ms | 179 (153‐211) | 205 (159‐263) | .57 |
| LVESV index | 61 (43‐76) | 36 (27‐57) | .42 |
| LVEDV index | 78 (65‐108) | 58 (42‐83) | .42 |
| Cardiopulmonary exercise testing parameters | |||
| Exercise time, s | 551 ± 108 | 561 ± 143 | .82 |
| Peak VO2, mL Kg−1 min−1 | 15.3 ± 3.5 | 16.2 ± 3.4 | .43 |
| Peak VO2, mL KgLM −1 min−1 | 22.9 ± 5.0 | 25.2 ± 5.4 | .27 |
| Respiratory exchange ratio | 1.04 ± 0.4 | 1.12 ± 0.1 | .01 |
| VAT, mL Kg−1 min−1 | 11.6 ± 3 | 11.7 ± 2.2 | .95 |
| VE/VCO2 slope | 32.6 ± 7.2 | 34 ± 6.1 | .52 |
| Laboratory | |||
| Creatinine (mg/dL) | 1.09 ± 0.32 | 1.12 ± 0.24 | .74 |
| Glomerular filtration rate (mL min−1/1.73 m2) | 83.3 ± 22 | 74.7 ± 19 | .22 |
| HbA1c (%) | 8.3 ± 1.3 | 8.3 ± 1.4 | .91 |
| Haemoglobin (g/dL) | 13.1 ± 1.6 | 13.6 ± 1.6 | .37 |
| NTproBNP (pg/mL) | 492 (330‐961) | 243 (74‐750) | .10 |
| Red blood cells (×106/mL) | 4.5 ± 0.6 | 4.6 ± 0.6 | .66 |
| White blood cells (×103/mL) | 7.2 ± 2.2 | 7.6 ± 2.3 | .62 |
Abbreviations: CABG, coronary artery bypass surgery; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DOACs, direct oral anti‐coagulants; DPP, dipeptidyl peptidase; GLP, glucagon‐like peptide; HbA1c, glycosylated haemoglobin; ICD, implantable cardiac defibrillator; LM, lean mass; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NTproBNP, N‐terminal pro b‐type natriuretic peptide; NYHA, New York Heart association; TIA, transient ischemic attack; VAT, ventilatory anaerobic threshold; VE/VCO2, minute ventilation/carbon dioxide production; VO2, oxygen consumption.
3.2. Cardiorespiratory fitness
We did not observe any statistically significant improvements in the co‐primary endpoints of the study in any of the groups (Table 2). We did not detect any significant within‐group differences in the canagliflozin (peak VO2: from 16.2 ± 3.4 to 16.9 ± 4.0 mL kg−1 min−1, P = .23; VE/VCO2 slope: from 34.1 ± 6.1 to 33.8 ± 4.2, P = .66) and sitagliptin groups (peak VO2 from 15.3 ± 3.5 to 14.7 ± 3.9 mL kg−1 min−1, P = .16; VE/VCO2 slope: from 32.6 ± 7.2 to 32.5 ± 5.8, P = .65), nor between‐group differences (peak VO2 mean difference [MD] between groups: +1.3 mL kg−1 min−1, P = .083; VE/VCO2: MD between groups: −0.2, P = .98; Figure 2).
TABLE 2.
Cardiorespiratory fitness and cardiac function
| Cardiorespiratory fitness variables | Sitagliptin n = 19 | Canaglifozin n = 17 | P value (time_X_group interaction) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 wk | P value | Baseline | 12 wk | P value | ||
| Peak VO2, mL min−1 | 1769 ± 489 | 1710 ± 392 | 0.21 | 1583 ± 409 | 1600 ± 454 | 0.86 | 0.15 |
| VE/VCO2 slope | 32.6 ± 7.2 | 32.5 ± 5.8 | 0.65 | 34 ± 6.1 | 33.8 ± 4.2 | 0.66 | 0.98 |
| Peak VO2, mL Kg−1 min−1 | 15.3 ± 3.5 | 14.8 ± 3.9 | 0.16 | 16.2 ± 3.4 | 16.9 ± 4.0 | 0.23 | 0.08 |
| Peak VO2, mL KgLM −1 min−1 | 22.9 ± 5.0 | 22.0 ± 5.4 | 0.16 | 25.2 ± 5.0 | 26.7 ± 5.3 | 0.16 | 0.036 |
| Peak VO2 pulse, mL min−1 | 13.9 ± 3.5 | 13.6 ± 3.6 | 0.78 | 12.7 ± 3.1 | 12.9 ± 3.5 | 0.53 | 0.46 |
| VAT, mL Kg−1 min−1 | 11.6 ± 3.0 | 11.8 ± 2.9 | 0.93 | 11.7 ± 2.2 | 13.1 ± 2.3 | 0.004 | 0.01 |
| OUES | 2.11 ± 0.58 | 2.06 ± 0.55 | 0.64 | 1.72 ± 0.36 | 1.80 ± 0.43 | 0.09 | 0.15 |
| RER‐matched VO2, mL Kg−1 min−1 | 14.4 ± 3.9 | 14.7 ± 3.9 | 0.26 | 14.2 ± 4.2 | 16.9 ± 3.9 | 0.001 | 0.002 |
| Respiratory exchange ratio | 1.04 ± 0.04 | 1.03 ± 0.07 | 0.38 | 1.12 ± 0.11 | 1.0 ± 0.07 | <0.001 | 0.001 |
| Exercise time, s | 551 ± 108 | 534 ± 120 | 0.12 | 561 ± 143 | 573 ± 151 | 0.69 | 0.13 |
| Resting heart rate, bpm | 71 ± 10 | 73 ± 10 | 0.89 | 70 ± 10 | 69 ± 11 | 0.23 | 0.43 |
| Peak heart rate, bpm | 129 ± 23 | 126 ± 24 | 0.18 | 125 ± 17 | 125 ± 15 | 0.82 | 0.46 |
| Resting systolic blood pressure, mmHg | 130 ± 20 | 124 ± 15 | 0.27 | 131. ±17 | 130 ± 17 | 0.91 | 0.37 |
| Peak systolic blood pressure, mmHg | 159 ± 29 | 162 ± 26 | 0.42 | 174 ± 31 | 174 ± 30 | 0.26 | 0.19 |
| Resting diastolic blood pressure, mmHg | 71 ± 16 | 69 ± 13 | 0.39 | 72 ± 13 | 74 ± 14 | 0.78 | 0.99 |
| Peak diastolic blood pressure, mmHg | 72 ± 13 | 74 ± 13 | 0.41 | 77 ± 14 | 76 ± 16 | 0.85 | 0.25 |
| Echocardiographic Variables | Sitagliptin n = 19 | Canaglifozin n = 17 | P value (time_X_group interaction) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 wk | P value | Baseline | 12 wk | P value | ||
| Deceleration time velocity, ms | 179 (153–211) | 187 (159–216) | 0.13 | 205 (159–263) | 193 (152–233) | 0.08 | 0.02 |
| E/E′ | 13.9 ± 4.6 | 13.4 ± 4.6 | 0.45 | 18.5 ± 13.2 | 16.7 ± 9.4 | 0.43 | 0.63 |
| E/DT | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.82 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.06 | 0.46 |
| LVESV index | 61 (43–76) | 51 (44‐61) | 0.01 | 36 (27‐57) | 37 (23‐47) | 0.02 | 0.92 |
| LVEDV index | 78 (65–108) | 81 (58‐89) | 0.27 | 58 (42–83) | 54 (42‐73) | 0.86 | 0.68 |
| LVEF, % | 27.0 ± 6.8 | 30.3 ± 10.4 | 0.18 | 31.6 ± 7.5 | 38.8 ± 10.9 | 0.006 | 0.21 |
| TAPSE, cm | 2.18 ± 0.47 | 2.04 ± 0.46 | 0.34 | 2.15 ± 0.42 | 2.19 ± 0.54 | 0.29 | 0.16 |
Abbreviations: DT, deceleration time; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; OUES, oxygen uptake efficiency slope; RER, respiratory exchange ratio; TAPSE, tricuspid annular plane systolic excursion; VAT, ventilatory anaerobic threshold; VE/VCO2, minute ventilation/carbon dioxide production; VO2, oxygen consumption.
FIGURE 2.
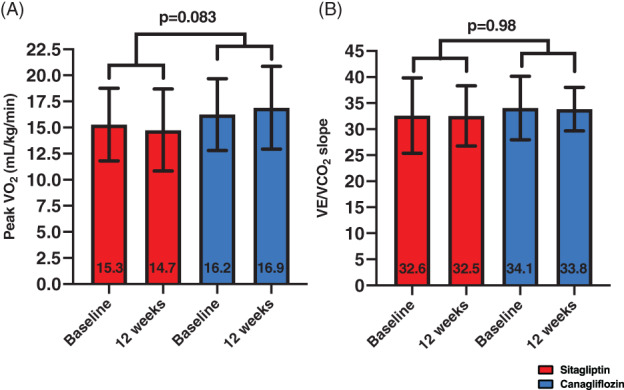
Effects of treatments on peak oxygen consumption (VO2) and minute ventilation/carbon dioxide production slope (VE/VCO2 slope). Treatment with canagliflozin nor sitagliptin did not result in significant changes in peak VO2, A and VE/VCO2 slope, B. Data are presented as mean ± SD
Canagliflozin improved lean peak VO2 compared to sitagliptin (mean difference [MD] between changes of +2.4 mL kgLM −1 min−1, P = .036; canagliflozin within group: from 25.2 to 26.7 mL kgLM −1 min−1, P = .15; sitagliptin within group: from 22.9 to 22.0 mL kgLM −1 min−1, P = .15; Figure 3). Canagliflozin also induced a significant improvement in VAT compared to sitagliptin (MD between groups: +1.5 mL kg−1 min−1, P = .012; canagliflozin within group: from 11.7 ± 2.2 to 13.1 ± 2.3 mL kg−1 min−1, P = .004; sitagliptin within group: from 11.7 ± 3.0 to 11.8 ± 2.9 mL kg−1 min−1, P = .93; Figure 3).
FIGURE 3.
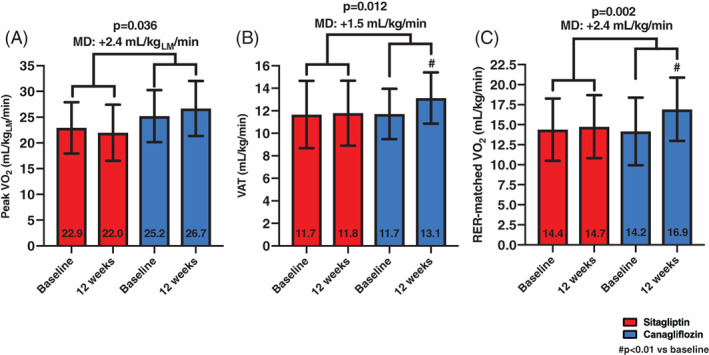
Effects of treatments on exploratory cardiopulmonary exercise testing (CPX) variables. Canagliflozin resulted in significant improvements in lean peak oxygen consumption (VO2), A, ventilatory anaerobic threshold (VAT), B, and RER‐matched VO2, C, compared to sitagliptin in a time_x_group interaction. Data are presented as mean ± SD. LM, lean mass measured with dual‐energy X‐ray absorptiometry; MD, mean difference between groups
RER was significantly reduced at 12 weeks in the canagliflozin group (MD between groups: −0.10, P < .001; from 1.12 ± 0.11 to 1.00 ± 0.08, in the canagliflozin group, P < .001, and from 1.04 ± 0.04 to 1.02 ± 0.07, in the sitagliptin group, P = .38). Therefore, we calculated the RER‐matched VO2, allowing for a VO2 comparison at similar levels of exercise effort. Canagliflozin showed a significant improvement in RER‐matched VO2 (MD between groups: +2.4 mL kg−1 min−1, P = .002; from 14.2 ± 4.2 to 16.9 ± 3.9 mL kg−1 min−1, in canagliflozin, P = .001; and from 15.3 ± 3.5 to 14.7 ± 3.9 mL kg−1 min−1, with sitagliptin, P = .26; Figure 3).
3.3. Quality of life
Canagliflozin reduced the MLHFQ score compared to sitagliptin, where a reduction of the score reflects improvements in QoL (MD between groups: −12.1, P = .018; canagliflozin within group: from 49.2 ± 26.8 to 41.3 ± 28.6, P = .073; sitagliptin from 38.4 ± 26.6 to 42.6 ± 29.5, P = .14; Figure 4).
FIGURE 4.
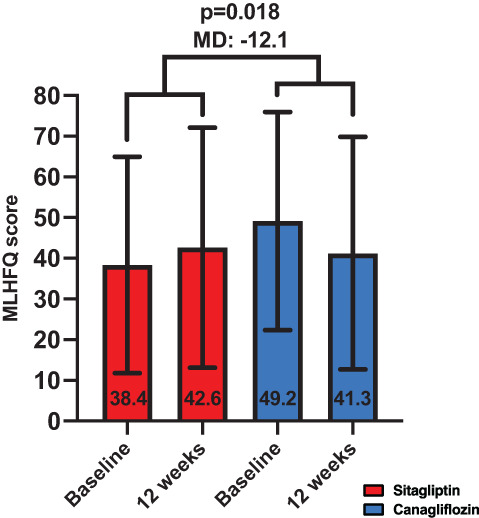
Effects of treatments on quality of life. Canagliflozin improved quality of life (QoL) as shown in the Minnesota Living with Heart Failure Questionnaire (MLHFQ) score, where a reduction in the score suggests improvements in QoL. Data are presented as mean ± SD. MD, mean difference between groups
3.4. Doppler echocardiography
Canagliflozin was associated with a small reduction in DT compared with sitagliptin (MD between groups: −20 ms, P = .023; canagliflozin: from 205 [159‐263] to 193 [152‐233] ms, P = .078; sitagliptin: from 179 [153‐211] to 187 [159‐216] ms, P = .13; Table 2 and Figure 5). No changes in E/e′ were observed in the within group as well as time_x_group interaction (Table 2). Treatment with canagliflozin, but not sitagliptin was associated with a significant within‐group improvement in LVEF (canagliflozin: from 31.6 ± 7.5 to 38.8 ± 10.9%, P = .006; sitagliptin: from 27.0 ± 6.8 to 30.3 ± 10.4%, P = .18), however, there was no significant time_x_group interaction (MD between groups: +3.9%, P = .21; Table 2 and Figure 5).
FIGURE 5.
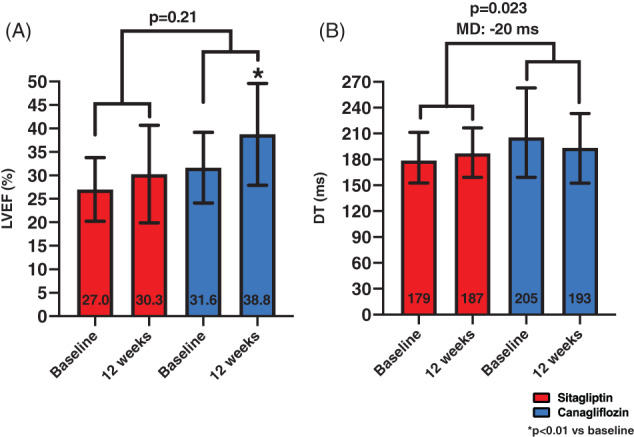
Effects of treatments on cardiac function. Canagliflozin was associated with improved systolic and diastolic function compared to baseline. Specifically, we observed improvements in left ventricular ejection fraction (LVEF), A, and deceleration time (DT), B. When compared to sitagliptin at time_x_group interaction analysis, however, the difference in LVEF was no longer statistically significant, while the difference with DT remained statistically significant. Data are presented as mean ± SD for LVEF, and median and interquartile ranges for DT. MD, mean difference between groups
3.5. Biomarkers
Glycemic control (ie, HbA1c) did not differ between canagliflozin and sitagliptin (P = .63 for time_x_group interaction; canagliflozin: from 8.3 ± 1.4 to 8.0 ± 1.0%, P = .29; sitagliptin: from 8.3 ± 1.3 to 7.6% ± 1.4, P = .10;). No significant changes for NTproBNP were found at time_x_group interaction (P = .47; canagliflozin within group: from 243 [74‐750] to 257 [106‐481] pg/mL, P = .83; sitagliptin: from 492 [330‐961] to 512 [132‐895] pg/mL, P = .91). No significant changes in estimated GFR were observed at time_x_group interaction analysis (MD between groups: +8.9 mL/min/1.73 m2, P = .09; canagliflozin: from 74.7 ± 18.9 to 79.2 ± 19.8 mL/min/1.73 m2, P = .20; sitagliptin: from 83.3 ± 22.1 to 78.9 ± 24.2 mL/min/1.73 m2, P = .60).
3.6. Dietary Intake
We did not observe any significant changes in reported daily caloric intake (time_x_group interaction analysis, P = .15) (canagliflozin: from 1635 ± 645 to 1852 ± 784 kcal, P = .21; sitagliptin: from 1846 ± 692 to 1702 ± 495 kcal, P = .53). Sodium intake was not significantly affected by either intervention at time_x_group interaction (P = .73; canagliflozin: from 3241 ± 1250 to 3470 ± 1697 mg, P = .86; sitagliptin: from 3145 ± 987 to 3183 ± 1253 mg, P = .84).
3.7. Anthropometrics, fluid status and blood pressure
Neither canagliflozin nor sitagliptin significantly affected blood pressure or fluid status (all P > .05; Figure S1). We observed a non‐significant trend for BMI reduction favouring canagliflozin (canagliflozin: from 34.5 ± 6.6 to 34.1 ± 6.7 kg/m2, P = .084; sitagliptin: from 38.9 ± 7.0 to 38.8 ± 7.1 kg/m2, P = .58; P = .053 for time_x_group interaction analysis). We did not observe a significant difference in waist circumference between groups (canagliflozin: from 116.7 ± 15.2 to 114.3 ± 15.3 cm, P = .073; sitagliptin: from 126.3 ± 17.0 to 124.4 ± 15.0 cm, P = .57; P = .14 for time_x_group interaction analysis).
3.8. Adverse events
As expected, the overall number of adverse events was low (Table 3). One event of worsening HF with sitagliptin was reported. No events of amputations, diabetic ketoacidosis nor fractures were reported.
TABLE 3.
Adverse events
| Sitagliptin n = 19 | Canaglifozin n = 17 | |
|---|---|---|
| Acute kidney injury | 3 (15.8) | 4 (23.5) |
| Arrhythmic events | 1 (5.3) | 1 (5.9) |
| Genital infections | 1 (5.3) | 1 (5.9) |
| Hypoglycemia | 0 | 2 (11.8) |
| Hypotensive events | 1 (5.3) | 2 (11.8) |
| Rehospitalizations for any cause | 1 (5.3) | 1 (5.9) |
| Unplanned revascularizations | 0 | 1 (5.9) |
| Worsening of heart failure | 1 (5.3) | 0 |
4. DISCUSSION
In the current study, we have shown that 12‐week treatment with an SGLT2 inhibitor, canagliflozin, was well‐tolerated and did not improve the primary endpoints of peak VO2 and VE/VCO2 slope. Canagliflozin, however, improved several surrogate functional markers of CRF, cardiac function and QoL compared to a DPP4 inhibitor, sitagliptin, in patients with stable HFrEF and T2DM. The trial was prematurely halted after only 40% of subjects were enrolled because of concerns given recent guideline recommendations to preferentially use SGLT2 inhibitors, instead of DPP4 inhibitors, in patients with T2DM and HF, thus, significantly reducing the overall power of the study. Possibly due to an early termination after 36 subjects, the study failed to meet the co‐primary endpoints, showing, however, a MD of 1.3 mL kg−1 min−1 for peak VO2 that failed to reach the pre‐specified statistical significance (P = .083).
Canagliflozin was associated with a significant improvement in QoL and additional CPX variables that have been found to consistently predict prognosis in patients with HF, and have proven to be more sensitive than peak VO2, such as lean peak VO2 and VAT. 6 , 21 Treatment with canagliflozin was associated with a significant reduction in the MLHFQ, indicating improved QoL related to improvements in HF‐related symptoms.
In patients with HF, lean peak VO2 may be a stronger prognosticator than peak VO2, particularly in individuals with obesity, in which peak VO2 adjusted by total body mass may result in an underestimate of CRF. 21 , 24 Of note, 86% of the patients enrolled in the study had obesity at baseline, 22% had class III obesity (BMI ≥ 40 kg/m2) and 100% had either overweight or obesity. VAT allows the quantification of the ability to sustain submaximal physical activity for prolonged periods, which approximates levels associated with activities of daily living in individuals with HF, who rarely perform higher intensity activities. 6 At the 12‐week visit, many subjects had a reduction in peak RER, which further reduced the power of our study to detect changes in peak VO2. Of note, an assessment of peak VO2 adjusted per RER showed a significant improvement with canagliflozin. It is unclear as to why subjects in the canagliflozin group exercised to a lower peak RER post‐intervention. This may be related to the imbalance in RER at baseline, with higher values in the canagliflozin group, but it could be related to other factors such as improved cardiac function allowing the individuals to reach the same or higher effort level while maintaining a greater cardiac reserve. It is indeed noteworthy that although canagliflozin patients tended to have lower RER at follow‐up this did not negatively affect the peak VO2, as it is was not reduced from baseline. It is possible that in this cohort of HFrEF patients with obesity and deconditioning, a treatment that improves cardiac function may fall short in improving peak VO2 due to supervening secondary limitation to maximal aerobic capacity. 25
On the other hand, the VE/VCO2 slope, an effort‐independent CPX variable, was not affected by either treatment. Of note, while peak VO2 was clearly reduced in the investigated population (64% ± 12% of predicted), offering room for significant improvements, the mean VE/VCO2 slope was only mildly elevated (33.3 ± 7), with 11 subjects (31%) presenting with a normal VE/VCO2 slope (<30), potentially minimizing the ability to detect significant changes with the intervention.
Canagliflozin was associated with greater LVEF at 12 weeks compared to baseline, while sitagliptin had neutral effects, however, the time_x_group analysis was not statistically significant. Importantly, prior studies have shown favourable changes in systolic function (ie, LVEF and echocardiography‐derived strains) in preclinical models of HF. 26 In addition to LVEF, we observed a small reduction in DT with canagliflozin compared to sitagliptin, which may reflect an improvement in myocardial relaxation. Of note, other more robust measures of cardiac diastolic function and estimate of filling pressures, such as E/E′, were not improved by canagliflozin.
These effects appear to be independent of glycemic control, which did not differ between the two groups. We did not observe significant changes in biomarkers of myocardial wall stress and renal function, such as NTproBNP and GFR, respectively. The lack of changes may be related to the study being underpowered to detect significant changes in such variables and/or short duration of treatment.
No changes in blood pressure and fluid status were found. While this may appear surprising, the baseline blood pressure and fluid status were within normal ranges, likely due to the fact that patients were required to be on maximally tolerated optimal medical therapy and stable without evidence of fluid overload prior to enrolment. We cannot exclude the possibility, however, that in this population, the blood pressure lowering effects of SGLT2 inhibitors 27 may not be as pronounced, due to concomitant therapies and perhaps require a larger sample size to detect a significant change. 5 We did not report any significant dietary changes in terms of daily caloric and sodium intake. We finally reported the safety profile of canagliflozin compared to sitagliptin in patients with established and well characterized HFrEF. This observation is, however, limited by the small sample size, the limited number of adverse events in the two groups, and the short duration of follow‐up.
Canagliflozin and other SGLT2 inhibitors have been shown to reduce hospitalizations for HF and renal events 2 , 3 , 4 , 5 in patients with T2DM and recently also in patients without T2DM. 5 , 28 Canagliflozin was shown to reduce cardiovascular and renal events, including hospitalizations for HF, even in patients with chronic kidney disease and albuminuria, 29 further supporting its role in patients with T2DM to reduce cardiovascular events. In small studies, canagliflozin has been associated with improved diastolic function in patients with T2DM, 30 however, its role on CRF and cardiac function in patients with established HF has not been previously investigated. We have previously shown that empagliflozin was associated with reduced E/E′ in patients with HFrEF 9 suggesting a reduction in filling pressures and improvements in peak VO2 when patients where concomitantly treated with loop diuretics. Empagliflozin has also been associated with improved peak VO2 in other non‐randomized studies in patients with established HF. 10 The recent EMPERIAL‐Preserved and EMPERIAL Reduced trials, 31 however, suggested no improvements in functional capacity measured with 6‐minute walk test distance with empagliflozin. Of note, the EMPERIAL trials did not investigate the effects of SGLT2 inhibitors on CRF, particularly on peak VO2, like we attempted to do in our trial. Peak VO2 is a more sensitive parameter of exercise capacity than 6‐minute walk test distance, which instead estimates functional capacity and does not provide insights on the determinants of reduced CRF. 6 Due to early interruption of our study, however, we cannot make definite conclusion on the effects of SGLT2 inhibitors on peak VO2, while we hope that ongoing clinical trials, such as the EMPA‐TROPISM will provide a final answer, also in patients without T2DM. 32
The mechanisms responsible for such effects are largely unknown. The hearts of patients with HF present with metabolic dysregulation resulting in reduced ability to utilize carbohydrates as an energetic substrate, with a resulting compensatory increased utilization of fatty acids and ketone bodies. By increasing fatty acid oxidation, ultimately increasing the production of ketone bodies, SGLT2 inhibitors, may increase the availability and utilization of these newly preferred energetic substrates in the setting of HF.
Sitagliptin was neutral on CRF, QoL, cardiac function and biomarkers, confirming its safety profile explored in a large cardiovascular outcome trial. 11
The study is not without limitations. In addition to the early termination, there were some differences in the baseline characteristics of the patients, which could have potentially affected the overall results of the study. Moreover, the small sample size of the study does not allow to definitely confirm the lack of differences of the safety profile of the two agents in the investigated population.
5. CONCLUSION
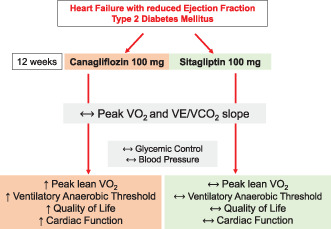
Despite the early interruption in enrolment and the failure to show differences in the primary endpoints of peak VO2 and VE/VCO2, we report for the first time that in patients with T2DM and HFrEF canagliflozin was associated with improvements in several measures of CRF such as lean peak VO2, VAT and RER‐matched VO2, and an improvement in the MLHFQ score reflecting a reduced HF symptom burden compared to sitagliptin, despite no significant differences in glycemic and hemodynamic control.
Central Illustration. Key Findings of the Study.
CONFLICT OF INTEREST
Dr Abbate has served as consultant to Janssen. Dr Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association. The other authors have nothing to disclose.
Supporting information
Supplemental Figure S1 Effects of treatments on blood pressure and body fluid. Canagliflozin nor sitagliptin were associated with significant changes in resting systolic blood pressure (BP) and diastolic BP (Panels A, B). Neither intervention affected body fluids, assessed with bioelectrical impedance analysis, including total body water (TBW) in litres (L) and percent (%) of body weight (Panels C, D), intracellular water (ICW) in L and % of TBW (Panels E, F), and extracellular water (ECW) in L and % of TBW (Panels G, H). All p values>0.05. Data are presented as mean ± SD.
Carbone S, Billingsley HE, Canada JM, et al. The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: The CANA‐HF study. Diabetes Metab Res Rev. 2020;36:e3335 10.1002/dmrr.3335
Funding information Janssen Research and Development, Grant/Award Number: Investigator initiated study to Antonio Abbate
REFERENCES
- 1. Rawshani A, Franzen S, Sattar N, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633‐644. [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 5. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. [DOI] [PubMed] [Google Scholar]
- 6. Del Buono MG, Arena R, Borlaug BA, et al. Exercise intolerance in patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73(17):2209‐2225. [DOI] [PubMed] [Google Scholar]
- 7. Greene SJ, Mentz RJ, Fiuzat M, et al. Reassessing the role of surrogate end points in drug development for heart failure. Circulation. 2018;138(10):1039‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbate A, Van Tassell BW, Canada JM, Dixon DL, Arena RA, Biondi‐Zoccai G. Pharmacologic and surgical interventions to improve functional capacity in heart failure. Heart Fail Clin. 2015;11(1):117‐124. [DOI] [PubMed] [Google Scholar]
- 9. Carbone S, Canada JM, Billingsley HE, et al. Effects of empagliflozin on cardiorespiratory fitness and significant interaction of loop diuretics. Diabetes Obes Metab. 2018;20(8):2014‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nunez J, Palau P, Dominguez E, et al. Early effects of empagliflozin on exercise tolerance in patients with heart failure: a pilot study. Clin Cardiol. 2018;41(4):476‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232‐242. [DOI] [PubMed] [Google Scholar]
- 12. Association AD . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42(Suppl 1):S90‐s102. [DOI] [PubMed] [Google Scholar]
- 13. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canada JM, Fronk DT, Cei LF, et al. Usefulness of C‐reactive protein plasma levels to predict exercise intolerance in patients with chronic systolic heart failure. Am J Cardiol. 2016;117(1):116‐120. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1‐64. [DOI] [PubMed] [Google Scholar]
- 16. Trankle C, Canada JM, Buckley L, et al. Impaired myocardial relaxation with exercise determines peak aerobic exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail. 2017;4(3):351‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garin O, Ferrer M, Pont A, et al. Disease‐specific health‐related quality of life questionnaires for heart failure: a systematic review with meta‐analyses. Qual Life Res. 2009;18(1):71‐85. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization . Waist circumference and waist‐hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008: World Health Organization; 2011:39.
- 19. Carbone S, Canada JM, Buckley LF, et al. Obesity contributes to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68(22):2487‐2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carbone S, Canada JM, Buckley LF, et al. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2017;2(5):513‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol. 2000;36(7):2126‐2131. [DOI] [PubMed] [Google Scholar]
- 22. Van Tassell BW, Canada J, Carbone S, et al. Interleukin‐1 blockade in recently decompensated systolic heart failure: Results from REDHART (recently decompensated heart failure Anakinra response trial). Circ Heart Fail. 2017;10(11):e004373. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301(14):1439‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carbone S, Billingsley HE, Rodriguez‐Miguelez P, et al. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol. 2019;100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Tassell BW, Trankle CR, Canada JM, et al. IL‐1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11(8):e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santos‐Gallego CG, Requena‐Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931‐1944. [DOI] [PubMed] [Google Scholar]
- 27. Baker WL, Buckley LF, Kelly MS, et al. Effects of sodium‐glucose cotransporter 2 inhibitors on 24‐hour ambulatory blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6(5):e005686‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Y, Geng Z, Wang X, Huang Y, Shen L, Wang Y. Meta‐analysis on the efficacy and safety of SGLT2 inhibitors and incretin based agents combination therapy vs. SGLT2i alone or add‐on to metformin in type 2 diabetes. Diabetes Metab Res Rev. 2020;36(2):e3223. [DOI] [PubMed] [Google Scholar]
- 29. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 30. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abraham WT, Ponikowski P, Brueckmann M, et al. Rationale and design of the EMPERIAL‐preserved and EMPERIAL‐reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail. 2019;21(7):932‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos‐Gallego CG, Garcia‐Ropero A, Mancini D, et al. Rationale and design of the EMPA‐TROPISM trial (ATRU‐4): are the "cardiac benefits" of empagliflozin independent of its hypoglycemic activity? Cardiovasc Drugs Ther. 2019;33(1):87‐95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 Effects of treatments on blood pressure and body fluid. Canagliflozin nor sitagliptin were associated with significant changes in resting systolic blood pressure (BP) and diastolic BP (Panels A, B). Neither intervention affected body fluids, assessed with bioelectrical impedance analysis, including total body water (TBW) in litres (L) and percent (%) of body weight (Panels C, D), intracellular water (ICW) in L and % of TBW (Panels E, F), and extracellular water (ECW) in L and % of TBW (Panels G, H). All p values>0.05. Data are presented as mean ± SD.


