Abstract
Scope
Diet rich in bilberries is considered cardioprotective, but the mechanisms of action are poorly understood. Cardiovascular disease is characterized by increased proatherogenic status and high levels of circulating microvesicles (MVs). In an open‐label study patients with myocardial infarction receive an 8 week dietary supplementation with bilberry extract (BE). The effect of BE on patient MV levels and its influence on endothelial vesiculation in vitro is investigated.
Methods and results
MVs are captured with acoustic trapping and platelet‐derived MVs (PMVs), as well as endothelial‐derived MVs (EMVs) are quantified with flow cytometry. The in vitro effect of BE on endothelial extracellular vesicle (EV) release is examined using endothelial cells and calcein staining. The mechanisms of BE influence on vesiculation pathways are studied by Western blot and qRT‐PCR. Supplementation with BE decreased both PMVs and EMVs. Furthermore, BE reduced endothelial EV release, Akt phosphorylation, and vesiculation‐related gene transcription. It also protects the cells from P2X7‐induced EV release and increase in vesiculation‐related gene expression.
Conclusion
BE supplementation improves the MV profile in patient blood and reduces endothelial vesiculation through several molecular mechanisms related to the P2X7 receptor. The findings provide new insight into the cardioprotective effects of bilberries.
Keywords: bilberries, cardiovascular diseases, microvesicles, P2X7 (P2X purinoreceptor 7)
Bilberries are considered beneficial in cardiovascular disease (CVD). In this study, patients with myocardial infarction receive an 8 week dietary supplementation with bilberry extract (BE). While CVD patients are characterized by high levels of circulating microvesicles (MVs), BE supplementation improves MV profile in patient blood by reducing the endothelial vesiculation through molecular mechanisms related to P2X7 receptor.
1. Introduction
A diet rich in bilberry (Vaccinium myrtillus) or Nordic wild blueberry has been suggested to be cardioprotective,[ 1 ] possibly because of their content of polyphenolic pigments, anthocyanins, that can be absorbed by the human intestine and further detected in plasma.[ 1 a] However, the oral bioavailability is low in humans—after ingestion of 1.2 g anthocyanins from freeze‐dried blueberries, the maximal plasma concentration reached 36 nmol L−1.[ 2 ] In vitro and in vivo studies on bilberry‐derived compounds have identified several different possible target tissues: cardiomyocytes,[ 3 ] endothelial cells (ECs),[ 4 ] and leukocytes.[ 1 b] Protective effects of bilberries are thought to be mediated by several molecular mechanisms involving inhibition of oxidative stress[ 1 b, 4 a, 5 ] and inflammation,[ 6 ] increased nitric oxide (NO) production,[ 4 b] and improved lipid profile.[ 6 b, 7 ] Importantly, studies in humans report improvements of numerous cardiovascular disease (CVD) subsets,[ 8 ] for example, metabolic syndrome,[ 9 ] hypertension,[ 10 ] and hypercholesterolemia.[ 6 b] Consequently, a diet rich in anthocyanins has been advocated as beneficial in the prevention of CVD and myocardial infarction (MI).[ 11 ]
One of the hallmarks of vascular dysfunction and CVD are high levels of circulating microvesicles (MVs) in blood. MVs belong to a group of cell‐derived particles, called extracellular vesicles (EVs), that also include exosomes and apoptotic bodies.[ 12 ] Although no direct studies have focused on a basic MV output, the fact that healthy individuals have a steady MV population indicates that a basal output exists. There are strong indications that MVs are directly related to pathological processes.[ 13 ] While MVs circulate in the blood of both healthy individuals and patients with diseases, increased concentrations of MVs in plasma have been described in several vascular‐related pathologies, such as coronary artery disease (CAD),[ 14 ] atherosclerosis,[ 15 ] hypertension,[ 16 ] and MI.[ 12 , 17 ] It has previously been shown that platelet aggregation is enhanced ex vivo by MVs originating from activated ECs, via crosslinking with von Willebrand factor.[ 18 ] Endothelial‐derived MVs (EMVs) facilitate proatherogenic processes through many molecular mechanisms, involving enhanced proliferation of vascular smooth muscle cells,[ 19 ] ECs, activation of clotting cascade, as well as monocyte interactions with endothelium.[ 20 ]
MVs circulating in plasma stem not only from blood cells, like platelets,[ 12 , 17 a] but also from the vasculature, mainly ECs.[ 21 ] Both platelet‐derived MV (PMV) and EMV blebbing from the cells is triggered by procoagulant and proinflammatory conditions.[ 21 , 22 ] The blebbing process is complex and involves numerous intracellular and membrane‐bound proteins. As MVs originate directly from plasma membranes, membrane‐bound receptors and phospholipids from the cell are retained on the surface.[ 23 ] The molecular cargo of the vesicular lumen includes proteins,[ 24 ] miRNAs,[ 25 ] and long non‐coding RNAs, all originating from the mother cell.[ 26 ] All these properties render MVs promising in cardiovascular biomarker discovery.[ 27 ]
In vivo experiments showed rapid removal of MVs by liver and spleen. Some of the proposed mechanisms of MV clearance involve lipid‐ or protein‐directed phagocytosis, depending both on the origin of MVs, as well as the recipient cell type.[ 28 ]
The data presented in this publication comes from a sample analysis of the already published BilbErry as A dietaRy SuppleMent After myocaRdial infarcTion (BEARSMART) clinical trial, an open‐label clinical study in patients with MI, randomized to 8 weeks of dietary supplementation with bilberry extract (BE) and standard medical therapy or to standard medical therapy alone.[ 29 ] The original study aimed to investigate the effect of BE supplementation on traditional risk factors, such as cholesterol levels and exercise capacity in MI patients. The present study is an exploratory sub‐study, evaluating the effects of bilberry consumption on plasma MV concentration. We found that supplementation with BE significantly reduced PMVs and EMVs, respectively, in patient plasma. We also investigated the influence of BE on endothelial vesicle release. Our results showed that BE decreased endothelial vesiculation through several molecular mechanisms connected with the P2X7 signaling pathway, such as protein phosphorylation and vesiculation‐related gene transcription.
2. Results
2.1. Study Design and Patient Information
The BEARSMART study, by Arevström et al.[ 29 ] is a clinical investigation, where MI patients were divided into groups receiving BE dietary intervention or no supplementation. Fifty patients (42 male) of median age 68 years completed the study. Blood samples were obtained at baseline and after 8 weeks. Patient information, BE administration protocol, as well as its characteristics are included in Tables S1 and S2 and Figure S1, Supporting Information. The aim of the original study was to determine the effect of BE supplementation in MI patients on cardiovascular risk markers. The results showed that in the BE‐supplemented group there was a reduction in oxidized low‐density lipoprotein (oxLDL) in plasma.[ 29 ] As oxLDL contributes to development of CVD and has been reported to stimulate vesiculation,[ 30 ] MVs appeared as another possible target affected by BE supplementation. As MV release is stimulated by proatherosclerotic conditions[ 21 , 22 ] and no study has investigated the effects of bilberry on plasma MV concentration, we analyzed patient plasma samples to measure MV levels. We also combined the clinical approach with an experimental one by analyzing BE influence on endothelial vesiculation in vitro.
2.2. Effect of Bilberry Extract on Microvesicles in Plasma
As the BE supplementation in the BEARSMART trial was associated with a decrease in oxLDL in plasma[ 29 ] and oxLDL stimulates vesiculation,[ 30 ] we investigated the effect of BE dietary intervention on the concentration of EMVs and PMVs. The BE intervention group had significantly lower concentration of PMVs in plasma after 8 weeks of the supplementation, in contrast to the control group (204CD42a+MVs per μL vs 524 CD42a + MVs per μL, Figure 1A). Similarly, we observed significantly reduced levels of plasma EMVs in the BE intervention group compared to the non‐intervention group (193CD62E+ MVs per μL vs 237 CD62E+ MVs per μL, Figure 1B).
Figure 1.
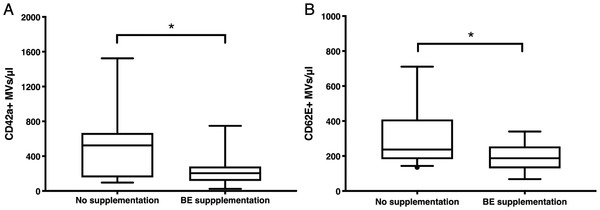
Effect of bilberry extract consumption on plasma MVs. CD42a+ PMV (A) and CD62E+ EMV (B) levels decreased after 8 weeks of BE intake by MI patients. Box plot. Y‐axis values represent MV concentration in 1:4 diluted samples. Mann–Whitney U test. *Statistically significant, p < 0.05. No supplementation: n = 18, BE supplementation: n = 16.
As the majority of subjects were male, we additionally analyzed the impact of BE on plasma MV levels after exclusion of the female patients. Similarly to the analysis of all patients the BE intervention group exhibited a significantly lower concentration of PMVs in plasma in contrast to the control group after 8 weeks of the supplementation (208 CD42a+ MVs per μL vs 492 CD42a+ MVs per μL, Figure S4, Supporting Information). We also observed significantly reduced levels of plasma EMVs in the BE intervention group compared to the non‐intervention group (187 CD62E+ MVs per μL vs 237 CD62E+ MVs per μL, Figure S4, Supporting Information).
2.3. Nanoparticle Tracking Analysis of Plasma Extracellular Vesicles
NanoSight‐based nanoparticle tracking analysis (NTA) was performed to verify the presence of CD62E+ and CD42+ EVs in our sample set. The analysis of non‐fluorescent vesicles revealed two major particle peaks between 200 and 400 nm that correspond to MVs (Figure 2A). We were also able to confirm the presence of CD62E+ EMVs (Figure 2B) and CD42a+ PMVs (Figure 2C). For both CD62E+ and CD42a+ EVs, the peak was at 100–150 nm that represents very small MVs. Moreover, CD62E+ EVs appear as 200–500 nm peaks and CD42a+ EVs as 300–500 nm that also matches the MV size.
Figure 2.
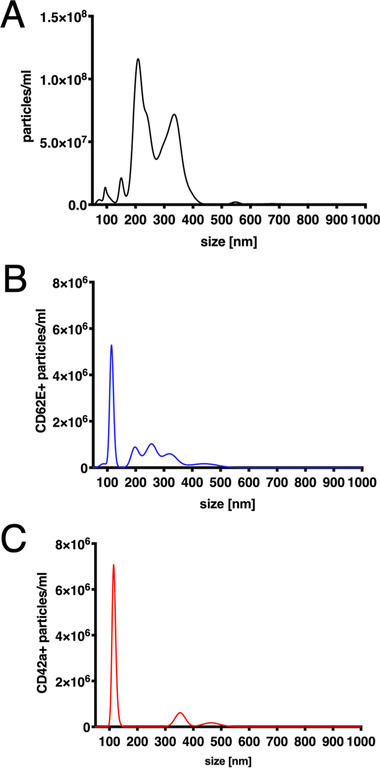
Nanoparticle tracking analysis of patient plasma. A) Concentration of all EVs (unstained). B) Concentration of CD62E+ EVs. C) Concentration of CD42a+ EVs.
2.4. Bilberry Extract Effect on Endothelial Vesiculation
Based on the BE influence on plasma MVs, we designed in vitro experiments to determine if BE acts directly on ECs and leads to a decreased vesiculation. We performed a dose response of BE on human umbilical vein endothelial cell (HUVEC) viability and proliferation and the concentration of 1000 µg mL−1 did not exhibit any toxic effects (Figures S2 and S3, Supporting Information). This led to the use of this concentration, and lower BE doses were not further investigated.
To induce vesicle release from ECs, the endothelial P2X7 receptor was activated with 2′(3′)‐O‐(4‐benzoylbenzoyl)adenosine‐5′‐triphosphate tri(triethylammonium) salt (bzATP), as this receptor is known to take part in EV formation and budding.[ 31 ] To visualize EC vesiculation, we performed calcein staining of HUVECs.[ 32 ] The cells were pre‐incubated with BE and stimulated with P2X7 agonist bzATP or inhibited with P2X7 antagonist AZ11645373.
BE reduced HUVEC vesiculation (Figure 3 ), measured as calcein fluorescence in the culture medium. The P2X7 agonist bzATP stimulated vesiculation, which was blocked by the P2X7 antagonist AZ11645373. It is noteworthy that similar results were seen with BE pre‐incubation and subsequent agonist stimulation.
Figure 3.
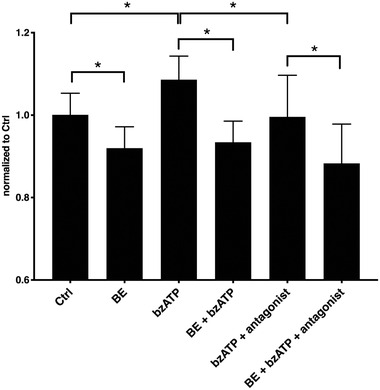
HUVEC vesiculation in the presence of BE, bzATP, and P2X7 antagonist. P2X7 activator bzATP increased HUVEC vesiculation via a P2X7‐dependent mechanism. BE decreased basic vesiculation of HUVECs. Pre‐incubation with BE was able to inhibit the effect of bzATP on HUVECs. Data is represented as normalized calcein fluorescence. Error bars represent SD. One‐way ANOVA with FDR correction. *Statistically significant, p < 0.05, n = 4.
2.5. Bilberry Extract Effect on Protein Phosphorylation
Based on the above results and current knowledge about P2X7 and anthocyanins, we selected Akt and p38 as candidate proteins for further investigation, as both are involved in P2X7 signaling pathways and vesiculation.[ 33 ] Also, both Akt and p38 phosphorylation status has previously been reported to be affected by BE.[ 34 ] As protein phosphorylation is a moderately rapid reaction, we chose 3 and 6 h as time points. None of the tested BE concentrations exhibited toxic effect on HUVECs, and we thus chose the highest dose, 1 mg mL−1, for further experiments. BE decreased Akt phosphorylation after 3 h, but not after 6 h (Figure 4A,B , and Figure S5, Supporting Information). However, p38 phosphorylation was unchanged at both time points (Figure 4C,D), suggesting that this protein is not involved in BE‐stimulated vesiculation decrease.
Figure 4.
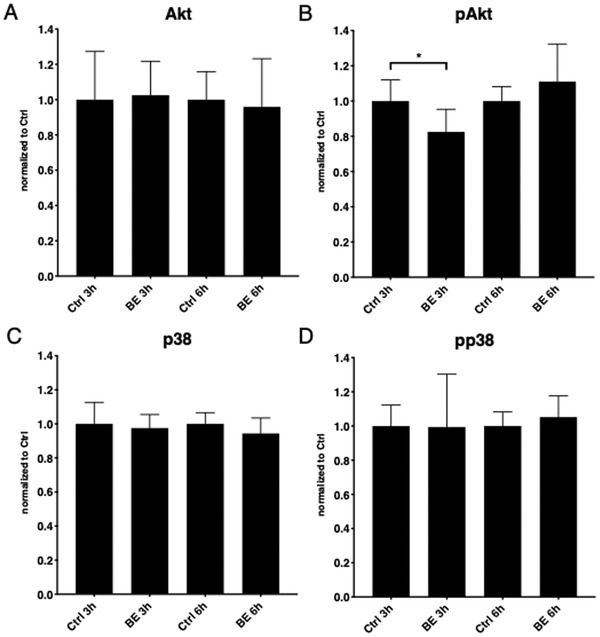
Effect of bilberry extract on Akt and p38 signaling in endothelial cells. 3 h incubation with BE decreased Akt phosphorylation in HUVECs (A), while 6 h incubation had no effect (B). Lack of BE influence on p38 phosphorylation was observed (C and D). Error bars represent SD. One‐way ANOVA with FDR correction, n = 3. *Statistically significant, p < 0.05; pAkt, phosphorylated Akt; pp38, phosphorylated p38.
2.6. Bilberry Extract Effect on P2X7 Transcription
Next, we investigated if the decrease in vesiculation also could be associated with a BE‐induced effect on P2X7 expression. A decline in P2X7 mRNA in HUVECs was observed following BE treatment (Figure 5 ). Interestingly, a similar picture was found with bzATP alone, which could be explained by an inhibitory effect of P2X7 stimulation on its transcription.[ 35 ] BE or BE plus antagonist with subsequent bzATP treatment also profoundly decreased P2X7 expression.
Figure 5.
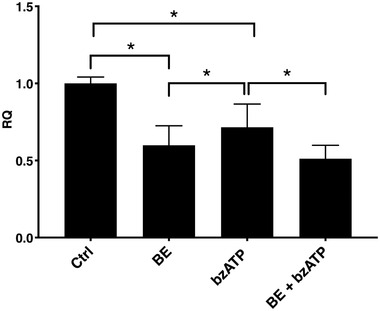
P2X7 expression in HUVECs in the presence of BE and bzATP. Both bzATP and BE decreased P2X7 expression. Pre‐incubation with BE was able to additionally decrease P2X7 expression in HUVECs, following agonist stimulation. Error bars represent SD. One‐way ANOVA with FDR correction, n = 4. *Statistically significant, p < 0.05.
2.7. Bilberry Extract Effect on Transcription of Vesiculation‐Related Genes
Last, we examined the mechanism of BE inhibition of vesiculation. Rab27b, Rab27a, SMPD1, and ARF6 genes were chosen as candidate genes, as the proteins are involved in EV release.[ 36 ] BE reduced expression of Rab27b and Rab27a and prevented bzATP‐induced increase of Rab27b, Rab27a, and SMPD1 gene expression (Figure 6 ). We did not obtain any conclusive results for ARF6 transcription, as it was decreased after all combinations of treatments and did not present any obvious pattern.
Figure 6.
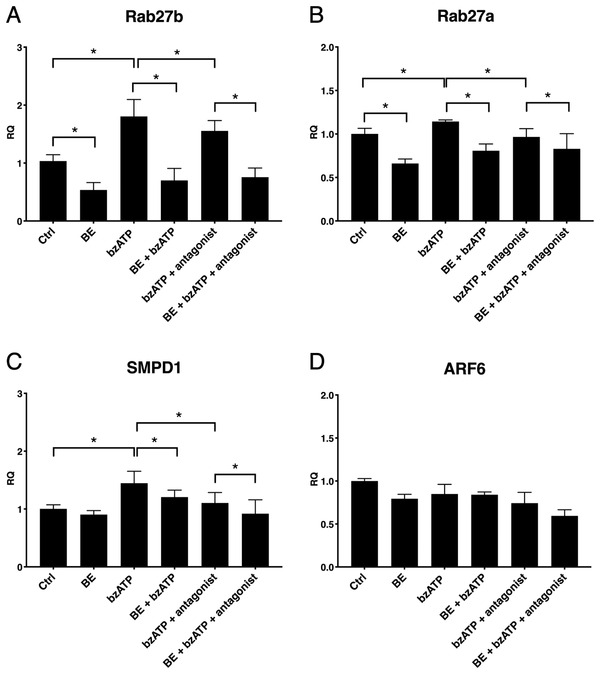
BE influence on vesiculation‐related gene expression in the presence of bzATP and P2X7 antagonist. BzATP increased A) Rab27b, B) Rab27a, and C) SMPD1 expression in HUVECs via P2X7‐dependent mechanism. BE decreased basic expression of Rab27b (A) and Rab27a (B). Pre‐incubation with BE was able to inhibit the effect of bzATP on Rab27b, Rab27a, and SMPD1 genes. D) ARF6 expression following the treatments did not present any pattern. Error bars represent SD. One‐way ANOVA with FDR correction, n = 4. *Statistically significant, p < 0.05.
3. Discussion
Bilberry is a widely consumed berry with suggested cardiovascular health benefits.[ 1 a, 37 ] In this study, we investigated if BE dietary intervention improves EMV and PMV concentrations in plasma of MI patients. The mechanism whereby it affects endothelial vesiculation in vitro was also analyzed. We found that BE supplementation reduced MV levels in patient plasma. Furthermore, it decreased P2X7‐dependent endothelial EV release, Akt phosphorylation, and vesiculation‐related gene expression.
In the BEARSMART study, Arevström et al. observed a positive influence of BE on oxLDL levels in patient plasma.[ 29 ] While it is well known that oxLDL plays a role in atherosclerosis progression and promotes EV release,[ 30 ] a possible link between vesiculation and BE has not been addressed before.
Our results clearly showed that BE supplements significantly decreased PMV levels in plasma. Yang et al. reported inhibition of platelet activation by the anthocyanin delphinidin‐3‐glucoside,[ 38 ] a pigment also present in bilberries,[ 39 ] which could explain the reduced vesiculation, and thus the lower PMV counts we observed in patient plasma. This is further supported by studies by Erlund et al. and Thompson et al., reporting reduced platelet aggregation after 8 and 4 weeks of diet with berry extracts (mixtures of bilberries, lingonberries, strawberries, black currants, chokeberries, and raspberries).[ 10 , 40 ] Furthermore, Song et al. showed a reduction of platelet granule secretion after in vitro incubation with an anthocyanin mixture.[ 41 ]
Similar to the PMVs, plasma EMVs were also significantly reduced. Malvidin, an anthocyanidin highly abundant in bilberries, is transported into ECs,[ 42 ] where it inhibits oxidative stress, promotes NO production, and decreases inflammation.[ 43 ] This in turn leads to diminished activation, thus the procoagulant and proinflammatory state of ECs,[ 44 ] and decreased E‐selectin (CD62E) presentation.[ 45 ] Furthermore, reduced endothelial activation decreases membrane blebbing and, as a consequence, vesiculation.[ 21 ]
Our results demonstrate a potent clinical effect of BE supplementation on plasma MV levels in MI patients. As MVs are directly connected to pathological processes[ 13 ] and increased levels of MVs in plasma are associated with atherosclerosis‐related pathologies,[ 12 , 14 , 15 , 16 , 17 ] the data presented here points at the preventive role of bilberries in CVD. Since there is very limited information regarding the influence of food‐derived compounds on EV release in vitro and in vivo, this further supports the importance of our findings for dietary guidance of cardiovascular patients, as well as healthy individuals.
As BE dietary intervention resulted in a decrease of EMV concentration in patient plasma, we decided to investigate how it affects endothelial vesiculation in vitro. P2X7 receptor was selected as a target for our experimental setup due to its presence in HUVECs and its verified contribution to EV release.[ 31 a–c, 46 ] Since the potential effects of BE on platelets and PMV release have been previously described, we decided to focus our research on ECs. Thompson et al. reported that a 4 week bilberry and black currant extract supplementation inhibited ADP‐induced platelet aggregation ex vivo[ 40 ] that is directly linked to P2Y12 receptor‐induced platelet activation.[ 47 ]
The concentration of BE used in the in vitro study (1 mg mL−1), as well as the time pattern of the experiments (24 h) differed from the clinical design (40 g daily for 8 weeks), as mentioned in the Dosage Information section. Due to the nature of in vitro cultured ECs, it was not feasible to perform experiments using the same time frame, as the clinical study. Thus, to mimic long‐term incubation time, we used the highest tested concentration for further experiments. However, oscillations of low concentration of BE and its metabolites in blood in the clinical study may act differently on vesiculation, compared to our in vitro experimental design.
Our results showed that the P2X7 agonist, bzATP, increased endothelial vesicle release and that AZ11645373 inhibited this response. Importantly, basic HUVEC vesiculation was decreased by incubation with BE alone, but BE pre‐incubation was able to prevent the effect of subsequent agonist stimulation. These results show that BE affects non‐stimulated, as well as P2X7‐stimulated vesicle release in ECs.
After establishing the inhibitory effect of BE on EC vesiculation, we next investigated the potential mechanisms. Akt is a downstream target of P2X7 in astrocytes[ 33 c] and Hao et al. demonstrated inhibition of Akt in cardiac fibroblasts after pretreatment with berry‐derived malvidin.[ 34 c] Akt was also reported to take part in vesiculation of red blood cells.[ 33 d] Our results showed that Akt phosphorylation was diminished after 3 h of incubation with BE, but returned to the original phosphorylation state after 6 h. In line with our findings, Matsunaga et al. reported decreased Akt phosphorylation after BE treatment of HUVECs.[ 48 ]
Activation of p38 has previously been reported to increase EV production.[ 49 ] Furthermore, p38 activation in TNFα‐stimulated ECs resulted in EMV production.[ 49 ] Anthocyanins are known to enter ECs through bilitranslocases[ 42 , 50 ] and Ogawa et al. reported that BE decreases p38 phosphorylation in retinal cells.[ 34 d] We have previously shown that p38 is involved in P2X7‐related EC activation after high glucose and palmitate treatment.[ 33 b] Moreover, p38 takes part in P2X7 signaling pathway leading to MV release from microglia.[ 33 a, 36 b] Surprisingly, we did not observe any significant effect of BE on pp38 after 3 and 6 h of stimulation. A possible explanation is that in our experimental setup the phosphorylation of p38 is not involved in BE‐evoked decrease in vesiculation or that the time frame of the experiments did not allow us to detect such changes.
Since it has been demonstrated that activation of the PI3K/Akt pathway enhances transcription of P2X7 receptor in neuroblastoma cells,[ 51 ] we next investigated if BE is capable of modulating the P2X7 expression level. We found that incubation with BE significantly lowered the basic levels of P2X7 mRNA which, in turn, can in part explain part of the inhibitory effect of BE on Akt phosphorylation. A similar inhibitory effect was obtained with bzATP alone, which is well in line with the report of Salvestrini et al., who described a negative feedback effect of P2X7 stimulation on its transcription.[ 35 ]
The effect of BE on transcription of genes related to EV release was then further investigated. Four genes were selected: Rab27b, Rab27a, SMPD1, and ARF6, that all have been reported to take part in EV release.[ 36 ] Rab27b and Rab27a were chosen as potential candidates to examine the effect of BE, as they control different stages of EV release.[ 36 d] The results showed a gene expression pattern matching the EV release results. BzATP significantly increased Rab27b and Rab27a expression in HUVECs, while pre‐incubation with BE alone decreased it.
We finally investigated two other genes, SMPD1 and ARF6. SMPD1 was chosen as it was described to trigger MV release from glial cells via P2X7‐dependent phosphorylation of p38.[ 36 b] Although neither P2X7 agonist nor BE had any effect on p38 phosphorylation, the SMPD1 transcription was affected by both, thus suggesting a p38‐independent mechanism. No conclusive results were obtained regarding ARF6 transcription, as its levels were decreased after all treatments, without any evident pattern.
Based on our results, we suggest a model where anthocyanins from BE decrease Akt phosphorylation and P2X7 transcription, which in turn reduces EV release (Figure 7 ). We also speculate that the observed BE in vitro influence on EC vesiculation reflects the decreased EMV levels in plasma from BE‐treated patients.
Figure 7.
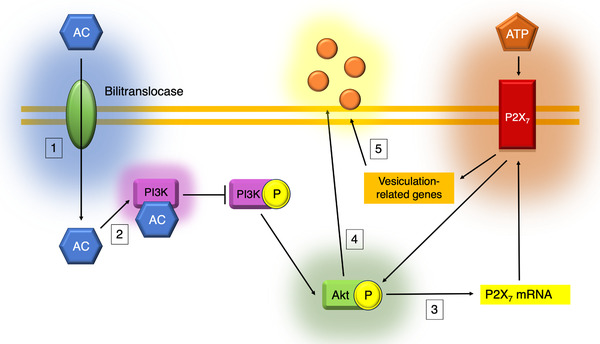
Proposed molecular mechanisms of BE‐induced reduction of vesicle release. Previous results demonstrated that anthocyanins (AC) enter ECs via bilitranslocases (1) and inhibit PI3K activation (2). This prevents Akt phosphorylation and reduces P2X7 transcription (3) that stands in line with our results. According to the literature, P2X7 signaling pathway leads to Akt phosphorylation and further EV release (4). Moreover, our data shows that P2X7 cascade also augments transcription of vesiculation‐related genes Rab27b and Rab27a that are inhibited by BE treatment (5), possibly due to a decrease in P2X7 expression. We propose that P2X7‐evoked increase in Rab27b and Rab27a transcription reflects increased cellular demands after P2X7‐stimulated vesiculation.
However, as the concentration of BE used in the in vitro study was higher than the concentration in the patient blood, other mechanisms could also be responsible for the decrease in plasma MVs. BE‐derived anthocyanins exhibit a number of antioxidant properties.[ 1 a] Thus, while circulating in blood, anthocyanins could act locally as free radical scavengers. Free radicals can activate ECs via decreasing NO production;[ 44 ] and antioxidants, such as anthocyanins, could reduce radical activation‐related MV release by the endothelium.[ 21 ] If taken up via bilitranslocases or by other mechanisms, anthocyanins could also potentially influence other vesiculation‐related pathways within ECs. There is also the possibility that an active uptake, in combination with a longer time frame, results in an intracellular accumulation of BE that in turn could explain the different dose/response findings in the in vivo study.
Furthermore, BE is a complex mixture that undergoes molecular transformations in the body, and anthocyanin‐derived metabolites could also potentially play an important role in reducing vesiculation. Edwards et al. reported an inhibitory effect of vanillic acid, a metabolite of cyanidine‐3‐glucoside, on NADPH oxidase (enzyme producing superoxide anions) expression in HUVECs.[ 52 ] Thus anthocyanin metabolites could also act against vascular activation and MV release into the circulation.
Most of the currently available clinical trials point to favorable effects of berries in CVD patients, compared to healthy subjects.[ 53 ] As an increase in the vesicle formation reflects the endothelial activation status in a negative way, the reduction in vesiculation can be interpreted as CVD protective. Thus, long‐term intake of anthocyanin‐rich diet in an otherwise healthy population is probably beneficial for maintaining proper vascular status and preventing development of CVD in the future.
The major strength of this study is that it combines both clinical, as well as experimental findings. Another strength was the rapid initiation of BE administration within 24 h after PCI. The original design of the study involved administration of placebo to the control group subjects. However, the placebo powder caused gastrointestinal stress, and the study was halted and restarted with new study subjects to avoid the side effects.[ 29 ] Furthermore, a larger population would have allowed for a better examination of the subgroups.
The effect of bilberry intake on MV concentration in plasma has not been explored before. Here we show the BEARSMART clinical trial that investigated if an 8 week BE consumption affects MV status in patients after MI. According to our results, such dietary intervention decreased PMV and EMV levels in patient plasma. Since the majority of the studied subjects were male and because men and women differ in morbidity in CVD, larger studies across both genders should be performed to establish effectiveness of BE on MI patients. Moreover, in vitro studies revealed that BE reduced EV release by ECs. Further experiments revealed that BE decreased Akt phosphorylation, as well as transcription of P2X7 and vesiculation‐related genes. BzATP stimulation of HUVECs increased vesiculation and the expression of Rab27b, Rab27a, and SMPD1 genes, while BE pre‐incubation prevented it. All these findings show that P2X7 receptor is important for EC vesiculation and BE reduces EV release through different molecular mechanisms, related to the P2X7 signaling pathway.
In conclusion, BE reduced both EMVs and PMVs in the plasma of MI patients. A decrease in EMV levels is caused by intracellular mechanisms, known to be important for vesiculation. Our findings also provide new insight into the protective effects of bilberries, which can have impact on vascular health of CVD patients.
4. Experimental Section
Study Design
The BEARSMART trial was an open‐label, randomized clinical study (ClinicalTrials.gov identifier: NCT01958034), where patients with MI, after providing informed consent, were randomized within 24 h of percutaneous coronary revascularization in a 1:1 ratio to BE supplementation or no dietary intervention. All subjects received standard medical treatment. The trial was approved by the regional ethics review board in Uppsala, Sweden (Drn: 2013/311). Patient information, BE characteristics, as well as administration protocols are included in Tables S1 and S2 and Figure S1, Supporting Information. Subject exclusion criteria had previously been described.[ 29 ] Patients randomized to BE‐supplemented group ingested three doses of 13.3 g of BE each day, totaling 40 g daily that corresponded to 480 g fresh bilberries. The extract (Immune, Sweden, www.immun.se) contained 2250 mg of anthocyanins per 100 g; thus, the patients consumed 300 mg anthocyanins in a single dose.
Sample Collection
Citrated blood samples were collected at baseline and after 8 weeks. The blood was drawn according to hospital procedures and regulations, and centrifuged within 30 min. Before freezing at −80 °C, plasma samples were centrifuged to avoid contamination with platelet fragments. The obtained cell‐free plasma was aliquoted and stored at −80 °C.
Extracellular Vesicle Preparation with Acoustic Trapping Platform
The AcouTrap acoustic trapping platform (AcouSort AB, Sweden) for EV enrichment from plasma samples had previously been described.[ 27 b, 54 ] In the acoustic trapping method of MV enrichment, 12 µm polystyrene beads, so‐called seed particles, were retained within a glass capillary by an acoustic standing wave. Diluted plasma sample was then aspirated and MVs were retained within the cluster of the seed particles. For the flow cytometry experiments, the samples were thawed at 37 °C and diluted 1:2 in PBS. 50 µL, of the diluted plasma was aspirated into the seed particle cluster, at a speed of 25 µL min−1. The trapped MVs were then washed with 50 µL of PBS and released in 100 µL of PBS.
Sample Exclusion Criteria
Some samples were excluded from further analysis, because of the physical properties of these samples (e.g., high viscosity and lipid content) that interfered with platelet elimination during centrifugation. Due to these issues, values from 16 and 18 subjects in the bilberry intervention and the non‐intervention groups were reported, respectively.
Flow Cytometry
100 µL of eluted MV suspension was stained with 3 µL of PE‐conjugated antibodies (Abs) against CD62E or glycoprotein IX (CD42a) (BD Biosciences, US). Samples were analyzed with Accuri C6 (BD Biosciences, US) cytometer as previously described.[ 27 b]
Nanoparticle Tracking Analysis
Trapped plasma samples were diluted with DPBS, stained with CD62E and CD42a Abs, further diluted with DPBS, and analyzed by NanoSight LM10 (Malvern, UK) equipped with 488 nm blue laser. Samples were measured for 60 s in quadruplicates using manual gain, camera level set to 5 and detection threshold to 33 (unstained), or camera level set to 13 and detection threshold to 4 (stained). Ab‐only and PBS controls were used to ensure rigorous measurement of the vesicles. The obtained data was analyzed with NTA 3.2.16 software (Malvern, UK).
Dosage Information
BE was prepared from V. myrtillus as described by Arevström et al.[ 29 ] For measuring the effect on in vitro toxicity and cell proliferation, HUVECs were seeded in 6‐well dishes covered with attachment factor and cultured in Medium 200 with LSGS (final serum concentration 2%) and gentamycin/amphotericin (Thermo Fisher Scientific, US). 100 mg of bilberry powder was diluted in 1 mL water, incubated in 60 °C for 1 h, and centrifuged at 20 000 × g for 10 min. The resulting supernatant (the BE) was aspirated and added to fresh culture medium to prepare BE‐containing medium. The mixture was further incubated with confluent cells to obtain 1000, 100, and 10 µg mL−1 final concentrations. After 24 h incubation, the cells were harvested, stained with trypan blue for viability and cell proliferation assessment. 1000 µg mL−1 BE was chosen as the working concentration for further experiments due to the lack of toxic effects on the cells (Figures S2 and S3, Supporting Information). The concentration of BE chosen for the in vitro study (1 mg mL−1 BE, thus 0.0225 mg mL−1 anthocyanins) clearly exceeded the concentration of anthocyanins in the clinical study (300 mg three times per day). The reasons for this were that both cellular context and time frame were different from the clinical study (hours instead of weeks). According to the literature, 1200 mg of blueberry anthocyanins resulted in a maximal plasma concentration of 36 nmol L−1.[ 2 ] The concentration of total anthocyanins in blood was not determined in the original study but, based on the report by Manach et al.,[ 2 ] it could be assumed that the maximal plasma concentration of anthocyanins could end up to be 9 nmol L−1, whereas for the in vitro study it would be about 12 µmol L−1 (22.5 µg mL−1). However, in the original study the concentrations of anthocyanin‐derived metabolites in blood were measured and high concentrations were found. For example, the concentrations reached 875 nmol L−1 for vanillic acid‐4‐O‐sulfate, 21 nmol L−1 for gallic acid, 26.9 nmol L−1 for caffeic acid 4‐β‐d‐glucuronide and, 6.4 nmol L−1 for p‐coumaric acid.[ 29 ] All the above‐mentioned compounds were significantly different from the control group.
Compound Effect on Endothelial Vesiculation
12 × 103 HUVECs were seeded in 12‐well dishes and cultured for 24 h. 1000 µg mL−1 BE or fresh culture medium (control) were added and cultured for 24 h. 300 µm of P2X7 agonist bzATP (Sigma‐Aldrich, Germany) and 100 nm of P2X7 antagonist AZ11645373 (Sigma‐Aldrich, Germany) were prepared from stock solutions in the culture medium. The diluted compounds or fresh culture medium were added to the calcein‐stained cells (see below) and incubated for the next 24 h. Three different HUVEC lots below passage 4 were used in the in vitro experiments.
Calcein Staining
2 mm calcein AM (Thermo Fisher Scientific, US) stock was prepared in DMSO and serially diluted in HBSS with 0.1% BSA to obtain 2 µm working solution with trace amounts of DMSO. After the compound incubation, the cells were washed twice with HBSS. 1 mL of calcein solution was added per well and the cells were incubated at 37 °C for 15 min. The cells were then washed with HBSS and fresh culture medium was added. After 24 h incubation, the medium was collected, centrifuged at 2000 × g for 10 min at 4 °C to remove cellular debris and analyzed using CLARIOstar plate reader (BMG Labtech, Germany). All values were normalized to that of the control cells.
Western Blot
HUVECs were plated in 6‐well plates. After 24 h, 1000 µg mL−1 BE was added and the cells were harvested after 3 or 6 h with 200 µL RIPA buffer (Cell Signaling, US) containing phosphatase inhibitors. The samples were vortexed, incubated at 4 °C for 5 min, sonicated and centrifuged at 15 000 × g for 10 min at 4 °C. The lysates were then transferred to new tubes and kept frozen at −80 °C. Protein concentration was measured using BCA Protein Assay Kit (Thermo Fisher Scientific, US) according to the manufacturer's instructions. 10 µg of protein were loaded on pre‐cast polyacrylamide midi gels (Thermo Fisher Scientific, US). Electrophoresis was performed at 150 V and dry transfer was performed on nitrocellulose membrane via iBlot 2 transferring system (Thermo Fisher Scientific, US). The nitrocellulose membrane was blocked with blocking buffer (Thermo Fisher Scientific, US) for 1 h. Primary Abs against phospho‐Akt (pAkt) clone D9E, Akt clone 11E7, phospho‐p38 (pp38) clone D3F9, p38 clone D13E1, and β‐actin clone 13E5 (Cell Signaling, US) were diluted as per manufacturer's instructions in 5% BSA in TBST and incubated overnight at 4 °C. Secondary goat anti‐rabbit Ab (Thermo Fisher Scientific, US) was diluted 1:2500 in 5% BSA in TBST and incubated for 1 h at room temperature. The bands were visualized with Pico Chemiluminescent Assay (Thermo Fisher Scientific, US) and analyzed with Odyssey Fc (LI‐COR, US) and Image Studio v. 3.1.4.
Quantitative Real‐Time PCR
HUVECs were plated and treated with 1000 µg mL−1 BE or combinations of bzATP and AZ11645373 as previously described. The cells were harvested with 700 µL QIAzol (Qiagen, Germany), incubated for 5 min in RT, and frozen at −80 °C. RNA isolation was performed with miRNeasy kit with DNase digestion (Qiagen, Germany) as per manufacturer's instructions. High Capacity RNA to cDNA kit (Thermo Fisher Scientific, US) was used for reverse transcription. The mRNA levels for P2X7, Rab27b, Rab27a, SMPD1, and ARF6 with GAPDH as a reference gene were assessed using TaqMan Fast Universal PCR Master Mixes (Thermo Fisher Scientific, US) and 20 ng template as per manufacturer's instructions.
Statistics
Shapiro test of normality showed that the MV concentration in patient plasma was not normally distributed. Thus, Mann–Whitney U test was applied to compare differences between BE supplementation and no supplementation after 8 weeks. In vitro compound effects on ECs were analyzed with one‐way ANOVA with false discovery rate (FDR) correction of Benjamini and Hochberg. p‐values < 0.05 were considered statistically significant. All analyses were performed with GraphPad Prism software version 7.0a (GraphPad Software Inc., USA).
Conflict of Interest
Thomas Laurell is a founder, board member, and shareholder of AcouSort AB, a University spin‐off company that commercializes acoustofluidic technology. The rest of authors declare no conflict of interest.
Author Contributions
Data collection—P.B.‐G., R.S.; Data analysis and interpretation—P.B.‐G., R.S.; Clinical study design and analysis—L.A., O.F., R.L., C.B.; Drafting the article—P.B.‐G., B.O.; Critical revision of the article—D.E., B.O., R.S., T.L., L.A., O.F., R.L., C.B., M.E.; Final approval of the version to be published—all authors.
Supporting information
Supporting Information
Acknowledgements
This work was supported by the Swedish Foundation for Strategic Research, the Knut and Alice Wallenberg Foundation, the Swedish Heart‐Lung Foundation, the Swedish Research Council, Region of Scania and ALF‐funds. The authors would like to thank Siv Svensson for laboratory support.
Bryl‐Górecka P., Sathanoori R., Arevström L., Landberg R., Bergh C., Evander M., Olde B., Laurell T., Fröbert O., Erlinge D., Bilberry Supplementation after Myocardial Infarction Decreases Microvesicles in Blood and Affects Endothelial Vesiculation. Mol. Nutr. Food Res. 2020, 64, 2000108 10.1002/mnfr.202000108
References
- 1.a) Rodriguez‐Mateos A., Heiss C., Borges G., Crozier A., J. Agric. Food Chem. 2014, 62, 3842; [DOI] [PubMed] [Google Scholar]; b) Rodriguez‐Mateos A., Rendeiro C., Bergillos‐Meca T., Tabatabaee S., George T. W., Heiss C., Spencer J. P., Am. J. Clin. Nutr. 2013, 98, 1179. [DOI] [PubMed] [Google Scholar]
- 2. Manach C., Williamson G., Morand C., Scalbert A., Rémésy C., Am. J. Clin. Nutr. 2005, 81, 230S. [DOI] [PubMed] [Google Scholar]
- 3. Louis X. L., Thandapilly S. J., Kalt W., Vinqvist‐Tymchuk M., Aloud B. M., Raj P., Yu L., Le H., Netticadan T., Food Funct. 2014, 5, 1785. [DOI] [PubMed] [Google Scholar]
- 4.a) Bornsek S. M., Ziberna L., Polak T., Vanzo A., Ulrih N. P., Abram V., Tramer F., Passamonti S., Food Chem. 2012, 134, 1878; [DOI] [PubMed] [Google Scholar]; b) Park S. H., Jeong S. O., Chung H. T., Pae H. O., Plant Foods Hum. Nutr. 2015, 70, 263. [DOI] [PubMed] [Google Scholar]
- 5. Del Bo C., Riso P., Campolo J., Moller P., Loft S., Klimis‐Zacas D., Brambilla A., Rizzolo A., Porrini M., Nutr. Res. 2013, 33, 220. [DOI] [PubMed] [Google Scholar]
- 6.a) Vendrame S., Daugherty A., Kristo A. S., Riso P., Klimis‐Zacas D., J. Nutr. Biochem. 2013, 24, 1508; [DOI] [PubMed] [Google Scholar]; b) Zhu Y., Xia M., Yang Y., Liu F., Li Z., Hao Y., Mi M., Jin T., Ling W., Clin. Chem. 2011, 57, 1524. [DOI] [PubMed] [Google Scholar]
- 7. Roopchand D. E., Kuhn P., Rojo L. E., Lila M. A., Raskin I., Pharmacol. Res. 2013, 68, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cutler B. R., Petersen C., Anandh Babu P. V., Mol. Nutr. Food Res. 2017, 61, 1600271. [DOI] [PubMed] [Google Scholar]
- 9.a) Stull A. J., Cash K. C., Champagne C. M., Gupta A. K., Boston R., Beyl R. A., Johnson W. D., Cefalu W. T., Nutrients 2015, 7, 4107; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kolehmainen M., Mykkanen O., Kirjavainen P. V., Leppanen T., Moilanen E., Adriaens M., Laaksonen D. E., Hallikainen M., Puupponen‐Pimia R., Pulkkinen L., Mykkanen H., Gylling H., Poutanen K., Torronen R., Mol. Nutr. Food Res. 2012, 56, 1501. [DOI] [PubMed] [Google Scholar]
- 10. Erlund I., Koli R., Alfthan G., Marniemi J., Puukka P., Mustonen P., Mattila P., Jula A., Am. J. Clin. Nutr. 2008, 87, 323. [DOI] [PubMed] [Google Scholar]
- 11. Cassidy A., Mukamal K. J., Liu L., Franz M., Eliassen A. H., Rimm E. B., Circulation 2013, 127, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hafiane A., Daskalopoulou S. S., Metab., Clin. Exp. 2018, 85, 213. [DOI] [PubMed] [Google Scholar]
- 13. Panagiotou N., Neytchev O., Selman C., Shiels P. G., Cells 2018, 7, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu S. S., Zhang H. G., Zhang Q. J., Xiu R. J., PLoS One 2014, 9, e104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mallat Z., Hugel B., Ohan J., Leseche G., Freyssinet J. M., Tedgui A., Circulation 1999, 99, 348. [DOI] [PubMed] [Google Scholar]
- 16. Helbing T., Olivier C., Bode C., Moser M., Diehl P., World J. Cardiol. 2014, 6, 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Jansen F., Nickenig G., Werner N., Circ. Res. 2017, 120, 1649; [DOI] [PubMed] [Google Scholar]; b) Eichner N. Z. M., Erdbrugger U., Malin S. K., J. Diabetes Res. 2018, 2018, 7807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jy W., Jimenez J. J., Mauro L. M., Horstman L. L., Cheng P., Ahn C. R., Bidot C. J., Ahn Y. S., J. Thromb. Haemostasis 2004, 3, 1301. [DOI] [PubMed] [Google Scholar]
- 19. Buendia P., Montes de Oca A., Madueno J. A., Merino A., Martin‐Malo A., Aljama P., Ramirez R., Rodriguez M., Carracedo J., FASEB J. 2015, 29, 173. [DOI] [PubMed] [Google Scholar]
- 20. Curtis A. M., Edelberg J., Jonas R., Rogers W. T., Moore J. S., Syed W., E. R. Mohler, 3rd , Vasc. Med. 2013, 18, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng F., Wang S., Xu R., Yu W., Wang X., Zhang L., J. Cell. Mol. Med. 2018, 22, 3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Badimon L., Suades R., Fuentes E., Palomo I., Padro T., Front. Pharmacol. 2016, 07, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Mause S. F., Weber C., Circ. Res. 2010, 107, 1047; [DOI] [PubMed] [Google Scholar]; b) El Andaloussi S., Mager I., Breakefield X. O., Wood M. J., Nat. Rev. Drug Discovery 2013, 12, 347. [DOI] [PubMed] [Google Scholar]
- 24.a) Milioli M., Ibanez‐Vea M., Sidoli S., Palmisano G., Careri M., Larsen M. R., J. Proteomics 2015, 121, 56; [DOI] [PubMed] [Google Scholar]; b) Haas B., Serchi T., Wagner D. R., Gilson G., Planchon S., Renaut J., Hoffmann L., Bohn T., Devaux Y., J. Proteomics 2011, 75, 229; [DOI] [PubMed] [Google Scholar]; c) Bosman G. J., Lasonder E., Groenen‐Dopp Y. A., Willekens F. L., Werre J. M., J. Proteomics 2012, 76, 203; [DOI] [PubMed] [Google Scholar]; d) Chaichompoo P., Kumya P., Khowawisetsut L., Chiangjong W., Chaiyarit S., Pongsakul N., Sirithanaratanakul N., Fucharoen S., Thongboonkerd V., Pattanapanyasat K., J. Proteomics 2012, 76, 239. [DOI] [PubMed] [Google Scholar]
- 25. Gidlöf O., van der Brug M., Öhman J., Gilje P., Olde B., Wahlestedt C., Erlinge D., Blood 2013, 121, 3908. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y., Zhang L., Wang Y., Ding H., Xue S., Qi H., Li P., Aging Dis. 2019, 10, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Gidlof O., Evander M., Rezeli M., Marko‐Varga G., Laurell T., Erlinge D., Sci. Rep. 2019, 9, 8991; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bryl‐Gorecka P., Sathanoori R., Al‐Mashat M., Olde B., Jogi J., Evander M., Laurell T., Erlinge D., Lab Chip 2018, 18, 3101. [DOI] [PubMed] [Google Scholar]
- 28. Boulanger C. M., Loyer X., Rautou P.‐E., Amabile N., Nat. Rev. Cardiol. 2017, 14, 259. [DOI] [PubMed] [Google Scholar]
- 29. Arevstrom L., Bergh C., Landberg R., Wu H., Rodriguez‐Mateos A., Waldenborg M., Magnuson A., Blanc S., Frobert O., Nutr. Res. 2019, 62, 13. [DOI] [PubMed] [Google Scholar]
- 30.a) Wang H., Wang Z. H., Kong J., Yang M. Y., Jiang G. H., Wang X. P., Zhong M., Zhang Y., Deng J. T., Zhang W., Mol Med 2012, 18, 159; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fu Z., Zhou E., Wang X., Tian M., Kong J., Li J., Ji L., Niu C., Shen H., Dong S., Liu C., Vermorken A., Willard B., Zu L., Zheng L., Am J Physiol Cell Physiol 2017, 313, C567. [DOI] [PubMed] [Google Scholar]
- 31.a) Morelli A., Chiozzi P., Chiesa A., Ferrari D., Sanz J. M., Falzoni S., Pinton P., Rizutto R., Olson M. F., Di Virgilio F., Molecular Biology of the Cell 2003, 14, 2655; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Constantinescu P., Wang B., Kovacevic K., Jalilian I., Bosman G. J., Wiley J. S., Sluyter R., Biochim Biophys Acta 2010, 1798, 1797; [DOI] [PubMed] [Google Scholar]; c) Verhoef P. A., Estacion M., Schilling W., Dubyak G. R., J Immunol 2003, 170, 5728; [DOI] [PubMed] [Google Scholar]; d) Park M., Kim J., Phuong N. T. T., Park J. G., Park J. H., Kim Y. C., Baek M. C., Lim S. C., Kang K. W., Sci Rep 2019, 9, 11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gray W. D., Mitchell A. J., Searles C. D., MethodsX 2015, 2, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.a) Li J., Li X., Jiang X., Yang M., Yang R., Burnstock G., Xiang Z., Yuan H., Purinergic Signal 2017, 13, 13; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sathanoori R., Sward K., Olde B., Erlinge D., PLoS One 2015, 10, e0125111; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jacques‐Silva M. C., Rodnight R., Lenz G., Liao Z., Kong Q., Tran M., Kang Y., Gonzalez F. A., Weisman G. A., Neary J. T., Br J Pharmacol 2004, 141, 1106; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kostova E. B., Beuger B. M., Klei T. R., Halonen P., Lieftink C., Beijersbergen R., van den Berg T. K., van Bruggen R., Biosci Rep 2015, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.a) Ahmet I., Spangler E., Shukitt‐Hale B., Joseph J. A., Ingram D. K., Talan M., PLoS One 2009, 4, e7975; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lim W., Jeong W., Song G., Mol Cell Endocrinol 2016, 422, 172; [DOI] [PubMed] [Google Scholar]; c) Hao J., Du H., Li W., Liu F., Lu J., Yang X., Cui W., Am J Transl Res 2016, 8, 1100; [PMC free article] [PubMed] [Google Scholar]; d) Ogawa K., Tsuruma K., Tanaka J., Kakino M., Kobayashi S., Shimazawa M., Hara H., J Agric Food Chem 2013, 61, 10345. [DOI] [PubMed] [Google Scholar]
- 35. Salvestrini V., Zini R., Rossi L., Gulinelli S., Manfredini R., Bianchi E., Piacibello W., Caione L., Migliardi G., Ricciardi M. R., Tafuri A., Romano M., Salati S., Di Virgilio F., Ferrari S., Baccarani M., Ferrari D., Lemoli R. M., Blood 2012, 119, 217. [DOI] [PubMed] [Google Scholar]
- 36.a) Muralidharan‐Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G., D'Souza‐Schorey C., Curr Biol 2009, 19, 1875; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., Saglietti L., Schuchman E. H., Furlan R., Clementi E., Matteoli M., Verderio C., EMBO J 2009, 28, 1043; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li W., Hu Y., Jiang T., Han Y., Han G., Chen J., Li X., APMIS 2014, 122, 1080; [DOI] [PubMed] [Google Scholar]; d) Ostrowski M., Carmo N. B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C. F., Schauer K., Hume A. N., Freitas R. P., Goud B., Benaroch P., Hacohen N., Fukuda M., Desnos C., Seabra M. C., Darchen F., Amigorena S., Moita L. F., Thery C., Nat Cell Biol 2010, 12, 19. [DOI] [PubMed] [Google Scholar]
- 37. Del Rio D., Rodriguez‐Mateos A., Spencer J. P., Tognolini M., Borges G., Crozier A., Antioxid Redox Signal 2013, 18, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Y., Shi Z., Reheman A., Jin J. W., Li C., Wang Y., Andrews M. C., Chen P., Zhu G., Ling W., Ni H., PLoS One 2012, 7, e37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lätti A. K., Riihinen K. R., Kainulainen P. S., J. Agric. Food Chem. 2008, 56, 190. [DOI] [PubMed] [Google Scholar]
- 40. Thompson K., Hosking H., Pederick W., Singh I., Santhakumar A. B., Br J Nutr 2017, 118, 368. [DOI] [PubMed] [Google Scholar]
- 41. Song F., Zhu Y., Shi Z., Tian J., Deng X., Ren J., Andrews M. C., Ni H., Ling W., Yang Y., Thromb Haemost 2014, 112, 981. [DOI] [PubMed] [Google Scholar]
- 42. Maestro A., Terdoslavich M., Vanzo A., Kuku A., Tramer F., Nicolin V., Micali F., Decorti G., Passamonti S., Cardiovasc Res 2010, 85, 175. [DOI] [PubMed] [Google Scholar]
- 43. Speciale A., Cimino F., Saija A., Canali R., Virgili F., Genes Nutr 2014, 9, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao J. K., J Clin Invest 2013, 123, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leeuwenberg J. F. M., Smeets E. F., Neefjes J. J., Shaffer M. A., Cinek T., Jeunhomme T. M. A. A., Ahern T. J., Buurman W. A., Immunology 1992, 77, 543. [PMC free article] [PubMed] [Google Scholar]
- 46. Furlan‐Freguia C., Marchese P., Gruber A., Ruggeri Z. M., Ruf W., J Clin Invest 2011, 121, 2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chyrchel B., Drozdz A., Dlugosz D., Stepien E. L., Surdacki A., Int J Med Sci 2019, 16, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsunaga N., Chikaraishi Y., Shimazawa M., Yokota S., Hara H., Evid Based Complement Alternat Med 2010, 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Curtis A. M., Wilkinson P. F., Gui M., Gales T. L., Hu E., Edelberg J. M., J Thromb Haemost 2009, 7, 701. [DOI] [PubMed] [Google Scholar]
- 50. Passamonti S., Vrhovsek U., Mattivib F., Biochem Biophys Res Commun 2002, 296, 631. [DOI] [PubMed] [Google Scholar]
- 51. Gomez‐Villafuertes R., Garcia‐Huerta P., Diaz‐Hernandez J. I., Miras‐Portugal M. T., Sci Rep 2015, 5, 18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Edwards M., Czank C., Woodward G. M., Cassidy A., Kay C. D., J Agric Food Chem 2015, 63, 2423. [DOI] [PubMed] [Google Scholar]
- 53. Huang H., Chen G., Liao D., Zhu Y., Xue X., Sci Rep 2016, 6, 23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rezeli M., Gidlof O., Evander M., Bryl‐Gorecka P., Sathanoori R., Gilje P., Pawlowski K., Horvatovich P., Erlinge D., Marko‐Varga G., Laurell T., Anal Chem 2016, 88, 8577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


