Abstract
Dengue is the most important arboviral disease world-wide with an estimated 400 million annual infections. Dengvaxia™ is a live attenuated tetravalent vaccine recently licensed for dengue seropositive individuals aged 9 – 45 years. There is great need for a dengue vaccine that be given to dengue-naïve individuals and very young children. To that end, the U.S. NIH developed a live attenuated tetravalent dengue vaccine using an iterative approach evaluating the safety, infectivity, and immunogenicity of different candidates. This approach identified poor candidates which were then discarded from further evaluation. Each of the components of the tetravalent vaccine formulation is able to replicate to very low titer, inducing a homotypic immune response to each. The immune response elicited by the tetravalent vaccine is balanced, without immunodominance of one component. The vaccine was licensed by several manufacturers for development, including the Instituto Butantan which initiated a Phase 3 efficacy trial.
Keywords: dengue, Vaccine, human challenge, efficacy
Introduction
Dengue is the world’s greatest arboviral threat with nearly 4 billion people at risk for infection in 141 countries and has become an increasing problem for travelers [1,2]. The incidence of dengue has increased substantially with estimates suggesting the number of symptomatic cases has doubled every decade between 1990 and 2013 [3]. Bhatt et al estimated 96 million apparent dengue infections occurred worldwide in 2010 in addition to an additional nearly 300 million inapparent infections [4]. A 2013 analysis estimated nearly 60 million symptomatic dengue infections at a cost of almost 9 billion USD [2]. More than 10 million of these infections were treated in the hospital setting, often overwhelming the health care facilities. The global distribution of dengue and magnitude of international travel has made dengue an important threat to travelers as well [5,6].
Dengue is caused by four antigenically distinct viruses; DENV-1, DENV-2, DENV-3, and DENV-4. Each of these viruses can cause the full spectrum of dengue infection: asymptomatic infection or undifferentiated febrile illness (most common), uncomplicated dengue fever, or severe dengue comprised of vascular leak syndrome leading to shock. Although a prognostic indicator for progression to severe dengue has not been identified [7,8], epidemiologic studies have demonstrated that pre-existing immunity to one DENV serotype is the greatest risk factor for severe dengue upon secondary infection with a heterotypic DENV [9]. Cross-reactive antibody induced by the primary DENV infection can bind to heterotypic virus of the secondary infection but, instead of neutralizing the virus, the antibody-virus complex enters monocytes and macrophages via the Fcγ receptor; a mechanism termed antibody-dependent enhancement (ADE) of infection [10]. This functional increase in infection of target cells leads to higher viral titers which have been associated with more severe dengue disease [11]. For this reason, partial immunity to dengue is a risk factor for severe disease. Interestingly, symptomatic and severe dengue is rarely observed with third or fourth dengue infection [12,13]. Second, heterotypic dengue infection is thought to broaden both the humoral and cellular immune response resulting in a more protective immune response against third and fourth infections. Indeed, Dejnirattisai et al isolated monoclonal antibodies from plasmablasts collected following secondary DENV infection which were broadly cross-reactive and highly neutralizing [14]. Additionally, Weiskopf et al demonstrated that secondary DENV infection honed CD8 T cell responses to conserved epitopes and that CD8+ T cell responses that were polyfunctional and of higher magnitude were associated with protection from dengue disease [15].
Although there is not an approved antiviral agent for the treatment of dengue, there is one licensed vaccine for the prevention of dengue and two other candidate dengue vaccines in Phase 3 clinical trial. Dengvaxia™, a live attenuated tetravalent vaccine, is the first dengue vaccine to be licensed. This vaccine is comprised of 4 chimeric viruses in which the prM and E proteins of YF17D are replaced with those of DENV-1, −2, −3, or −4. Because the non-structural proteins are those of YF17D and not of DENV, and because > 80% of the CD8 epitopes of DENV are found in the NS proteins [15], the CD8 T cell responses are directed primarily against YF and not dengue. Although the vaccine induced an overall efficacy of 60.3% against symptomatic dengue in the first 25 months, efficacy varied by serotype, age, and serostatus at vaccination [16–18]. Additionally, children who were 2 – 5 at the time of vaccination with Dengvaxia™, had a > 7-fold higher relative risk of hospitalization for dengue in year 3 of the study compared with those children who had received the control. Subsequent analysis linked the risk of more severe disease in the vaccinated group to being sero-naive to dengue at the time of vaccination [19,20]. For this reason, a pre-vaccination screening strategy was recommended for Dengvaxia™ [20,21]. Unfortunately, development of a screening assay with sufficient sensitivity and specificity to adequately determine serostatus prior to vaccination with Dengvaxia™ has been difficult [22,23].
The difference in efficacy by serotype and the risk of more severe disease in subjects who were dengue sero-naive at the time of vaccination is thought to be due an imbalance in the immune response to the vaccine [24]. The DENV-4 component of the vaccine is immunodominant; the neutralizing antibody titers to DENV-4 are significantly higher than the other serotypes following the first dose of vaccine in sero-naive subjects and additional doses do not boost DENV-4 titers but do boost titers to DENV-1, DENV-3, and DENV-3 [25,26]. Following vaccination of dengue sero-naive subjects, type-specific (homotypic) antibodies dominated the neutralizing antibody response to DENV-4 but more than 50% of the neutralizing antibody response to DENV-1, DENV-2, and DENV-3 was cross-reactive [27]. Because Dengvaxia™ is a live attenuated vaccine, the components of the vaccine must infect and replicate within the host to induce a protective immune response. However, the four vaccine components of Dengvaxia™ are not equally infective, contributing to its imbalanced humoral response. The DENV-4 component is more infectious than the other 3 components of the vaccine and induces a greater homotypic antibody response. The DENV-4 component was detected by RT-PCR in 44.2% of dengue-naïve subjects following first vaccination compared with only 12.6% for the DENV-3 component, and 7.4% for the DENV-1 component. The DENV-2 component of the vaccine was not detected in any subject following any vaccination [28].
The live attenuated dengue vaccine TV003/TV005 was developed by the U.S. National Institutes of Health. To ensure that each of the components of the vaccine was sufficiently attenuated and immunogenic, numerous clinical trials were conducted of both the individual monovalent vaccine components and different tetravalent admixtures (Table 1). Clinical studies were designed to assess the infectivity, immunogenicity, and reactogenicity of monovalent candidate and tetravalent candidate vaccines. In addition, these studies were used to determine the number of doses needed to induce a suitable neutralizing antibody response, to evaluate the effect of pre-existing flavivirus antibody on the response to the vaccine, the durability of the protective immune response against pseudo-challenge with a second dose of live tetravalent vaccine, and the protection against challenge with DENV-2. The DENV-2 challenge model was developed in direct response to the failure of neutralizing antibody to predict protection against DENV-2 in the Phase 2b study of Dengvaxia™[29]. Several additional challenge studies are in progress but will not be discussed in detail in this review.
Table 1:
Clinical trials of the NIH live attenuated tetravalent dengue vaccine and its components
| Vaccine | # vaccinees | # placebo recipients | Clinicaltrials.gov | Reference |
|---|---|---|---|---|
| DEN4Δ30 | 20 | 0 | Not available | Durbin 2001[32], Troyer 2001[37] |
| DEN4Δ30 | 80 | 16 | Not available | Durbin, 2005 [45] |
| DEN2/4Δ30 | 20 | 8 | Not available | Durbin, 2006 [46] |
| DEN1Δ30 | 20 | 8 | NCT89908 | Durbin, 2006 [47] |
| DEN4Δ30–200,201 | 40 | 16 | NCT270699 | McArthur, 2008 [48] |
| DEN4Δ30–4995 | 20 | 8 | NCT322946 | Wright, 2009 [49] |
| Heterologous vaccination | 30 | 6 | NCT458120 | Durbin, 2011 [50] |
| DEN3/4Δ30 | 40 | 16 | NCT375726 | Durbin, 2011 [39] |
| DEN1Δ30 2 doses | 50 | 10 | NCT473135 | Durbin, 2011 [51] |
| DEN2/4Δ30–2 doses | 20 | 5 | NCT920517 | Durbin, 2011 [39] |
| DEN3–3’D4Δ30 | 20 | 8 | NCT712803 | Durbin, 2011 [39] |
| DEN1Δ30-low dose | 15 | 3 | NCT1084291 | Lindow, 2012 [52] |
| DEN2/4Δ30-low dose | 15 | 3 | NCT2317900 | Durbin, 2011 [39] |
| DEN4Δ30 | 50 | 20 | NCT919178 | Durbin, 2011 [39] |
| DEN3Δ30/31 | 60 | 20 | NCT831012 | Durbin, 2011 [39] |
| TV001, TV002, TV003, TV004 | 100 | 40 | NCT01072786 | Durbin, 2013 [38] |
| TV003/TV005 | 80 | 32 | NCT01436422 | Kirkpatrick, 2013 [40] |
| TV003 | 40 | 16 | NCT01506570 | Whitehead, 2017 [53] |
| TV003 | 40 | 8 | NCT01782300 | Durbin, 2016 [54] |
| DEN2Δ30–7169 | 10 | 4 | NCT01931176 | Larsen, 2015 [41] |
| TV003 + DEN2Δ30 challenge | 24 | 24 | NCT02021968 | Kirkpatrick, 2016 [42] |
All of the monovalent DENV vaccine candidates were developed using recombinant DNA technology. Homologous 30-nucleotide deletions were introduced into 3′ untranslated regions (UTR) of rDEN1 Western Pacific (rDEN1Δ30) [30], rDEN2 Tonga/74 (rDEN2Δ30) [31], and rDEN4 Dominica (rDEN4Δ30) [32] to attenuate these viruses. Two chimeric candidate vaccines were created on the rDEN4Δ30 background by replacing the prM and E coding sequences of rDEN4Δ30 with those of DENV-2 NGC (rDEN2/4Δ30) [33] or DENV-3 Sleman/78 (rDEN3/4Δ30) [34]. Two additional DENV-3 candidate vaccines were created by adding 30- and 31-nucleotide deletions into the 3′ UTR of rDEN3 Sleman/78 (rDEN3Δ30,31) or by swapping the entire 3′UTR of rDEN4Δ30 with that of rDEN3 Sleman/78 (rDEN3–3′D4Δ30) [34]. The rDEN4Δ30 candidate vaccine was further modified by the introduction of mutations into nonstructural protein 5 (NS5), designated rDEN4Δ30–200,201) [35] or into NS3, designated rDEN4Δ30–4995 [36]. The infectivity of each component was evaluated by determining the titer of vaccine virus recovered from the blood of volunteers at multiple time-points post-vaccination, as described in the cited references. Nine different monovalent DENV vaccine candidates were evaluated to assess their infectivity and safety profile. Because they were administered as individually, infectivity could be assessed by recovery of infectious virus and immunogenicity as measured seroconversion to the wildtype parent virus determined by the PRNT60 assay (Table 2). These studies eliminated several monovalent vaccine candidates from consideration because they were insufficiently infectious (rDEN3/4Δ30) or thought to be under-attenuated when administered as monovalent vaccines (rDEN2Δ30 and rDEN4Δ30–4995). To further assess the safety of the recombinant DENV vaccine candidates, the transmissibility of the rDEN4Δ30 was assessed early in the vaccine development program. Volunteers were administered a dose of rDEN4Δ30 100-fold higher than the dose eventually selected for the tetravalent vaccine. Mosquitoes were fed on these volunteers 7, 8, and 9 days after vaccination; days on which volunteers had previously been shown to be viremic. rDEN4Δ30 was not recovered from more than 300 mosquitoes that had fed on volunteers during this period of expected viremia [37].
Table 2:
Infectivity of monovalent dengue vaccine candidates
| Virus | Dose (log10 PFU) | No. subjects | % viremic | Mean peak virus titer log10 PFU/mL | % seroconverting |
|---|---|---|---|---|---|
| rDEN1Δ30 | 3 | 71 | 61 | 1.0 | 93 |
| rDEN2/4Δ30 | 3 | 40 | 60 | 0.5 | 100 |
| rDEN2Δ30 | 3 | 10 | 100 | 2.5 | 100 |
| rDEN3/4Δ30 | 3 | 20 | 15 | 1.0 | 30 |
| rDEN3/4Δ30 | 4 | 20 | 0 | n/a | 25 |
| rDEN3Δ30/31 | 3 | 50 | 34 | 0.5 | 81 |
| rDEN3–3’D4Δ30 | 3 | 20 | 20 | 0.6 | 80 |
| rDEN4Δ30 | 3 | 50 | 26 | 0.7 | 93 |
| rDEN4Δ30–200,201 | 5 | 20 | 0 | n/a | 100 |
| rDEN4Δ30–4995 | 5 | 20 | 0 | n/a | 95 |
Six monovalent vaccines (rDEN1Δ30, rDEN2/4Δ30, rDEN3–3′D4Δ30, rDEN3Δ30/31, rDEN4Δ30, and rDEN4Δ30,31 were then evaluated in 5 different tetravalent admixtures (Table 3). Each of admixtures was well tolerated; a mild maculopapular rash was the only adverse event that occurred significantly more frequently in vaccine recipients than placebo-recipients [38]. Samples were collected approximately every other day post-vaccination through Study Day 16 to measure infectious viremia of each component of each admixture. The rDEN3Δ30,31 and the DEN4Δ30 components were more infectious in the tetravalent admixtures than the rDEN3–3′D4Δ30 and DEN4Δ30–200,201 components, respectively [38]. rDEN3–3′D4Δ30 was recovered from20% of subjects who received TV001 but was not recovered from any subject who received TV002. In contrast, rDEN3Δ30,31 was recovered from 40%, 75%, and 40% of subjects vaccinated with TV003, TV004, or TV005, respectively. rDEN4Δ30–200,201 was recovered from 5% of subjects vaccinated with TV002 but was not recovered from any subject vaccinated with TV004. In contrast, rDEN4Δ30 was recovered from 40%, 25%, and 45% of subjects vaccinated with TV001, TV003, and TV005, respectively. Differences in the infectivity of the four vaccine components affected the balance of the neutralizing response to the different serotypes, as shown in Table 4. The immune response to DENV-4 is immunodominant in TV001, likely due to the poor infectivity of rDEN3–3D4Δ30. However, the DENV-1 response is immunodominant in TV002 because of the poor infectivity of rDEN4Δ30–200,201. TV003 and TV005 induced the most balanced immune response of the five admixtures. The only difference between TV003 and TV005 is the dose of the rDEN2/4Δ30 component (103 PFU in TV003 and 104 PFU in TV005). The dose of the DENV-2 component was increased 10-fold in TV005 because the human infectious dose 50% (HID50) of DEN2/4Δ30 was higher (10 PFU) than rDEN1Δ30, rDEN3Δ30/31, and rDEN4Δ30 (<10 PFU) as determined in dose ranging studies [39]. TV005 induced seroconversion to DENV-2 in 80% of vaccinated subjects compared with 50% for TV003 (Table 4).
Table 3:
Tetravalent admixtures evaluated in clinical trial
| Administered dose of each component (log10 PFU) | Monovalent dengue vaccine component | ||||
|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | ||
| TV001 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3–3’D4Δ30 | rDEN4Δ30 |
| TV002 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3–3’D4Δ30 | rDEN4Δ30–200,201 |
| TV003 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30,31 | rDEN4Δ30 |
| TV004 | 3,3,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30,31 | rDEN4Δ30–200,201 |
| TV005 | 3,4,3,3 | rDEN1Δ30 | rDEN2/4Δ30 | rDEN3Δ30,31 | rDEN4Δ30 |
Bolded formulations are those chosen to move forward for further evaluation
Table 4:
Immunogenicity of tetravalent admixtures
| % Seropositive (PRNT60 ≥10) | Geometric mean peak titer, reciprocal, PRNT60 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Admixture | No. subjects | DENV-1 | DENV-2 | DENV-3 | DENV-4 | DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
| TV001 | 20 | 80 | 65 | 60 | 85 | 54 | 39 | 36 | 154 | |
| TV002 | 20 | 80 | 60 | 75 | 90 | 118 | 41 | 31 | 32 | |
| TV003 | 20 | 100 | 50 | 85 | 100 | 62 | 44 | 36 | 65 | |
| TV004 | 20 | 75 | 50 | 85 | 100 | 36 | 17 | 124 | 32 | |
| TV005 | 20 | 90 | 80 | 90 | 100 | 44 | 41 | 42 | 80 | |
The safety, infectivity, and immunogenicity of TV003 and TV005 were further evaluated head-to-head in a placebo controlled double-blind study, CIR279 [40]. The clinical, virologic, and serologic responses to TV003 and TV005 in flavivirus-naïve healthy adults are presented in Tables 5, 6, and 7 [38,40]. The data for each admixture from CIR268 and CIR279 were combined for this analysis to provide a larger pool for comparison. In addition, the neutralizing antibody data for these later studies was calculated as the PRNT50. Neutralizing antibody data from CIR268 was recalculated as a PRNT50 for these comparisons. Both admixtures were well tolerated by volunteers with a mild, maculopapular rash being the only adverse event that occurred significantly more frequently in vaccine recipients compared with placebo recipients (61.7% of both TV003 and TV005 recipients. All four components of both TV003 and TV005 are highly infectious; ≥75% of vaccinated volunteers had one more infectious vaccine components recovered post-vaccination. In addition, the infectivity of the four components is balanced, particularly the DENV-1, DENV-3, and DENV-4 components. The infectivity of the DENV-2 component improved with the 10-fold higher dose contained in TV005; 22% of TV005 vaccinated individuals had infectious rDEN2/4Δ30 recovered post-vaccination compared with 7% of TV003 recipients. This was also reflected in the proportion of TV005 recipients who seroconverted to DENV-2 following a single dose (86% compared with 64% of subjects who received TV003. The geometric mean peak neutralizing antibody titers induced by the two different admixtures were not significantly different (Table 7). A higher proportion of TV005 recipients developed a tetravalent neutralizing antibody response following a single dose (79.7% vs 65.5%) however, the trivalent or better response was nearly indistinguishable between the two admixtures; 94.8% of TV003 recipients developed a trivalent or better response compared to 95.0% of TV003 recipients.
Table 5:
Clinical response to Dose 1 of TV003 or TV005 (CIR268 & 279 and combined)
| Adverse event | Treatment group | |||||
|---|---|---|---|---|---|---|
| TV003 (n=60) | Placebo (n=48) | p value (1-sided)2 | TV005 (n=60) | Placebo (n=48) | p value (1-sided)3 | |
| Injection site: | ||||||
| Erythema | 4.8% | 4.2% | 0.6784 | 5.0% | 4.2% | 0.6784 |
| Pain | 0.0% | 8.3% | 1.0000 | 3.3% | 0.0% | 0.5077 |
| Tenderness | 5.0% | 4.2% | 0.6784 | 1.7% | 4.2% | 0.9208 |
| Induration | 3.3% | 0.0% | 0.5077 | 1.2% | 0.0% | 0.7143 |
| Systemic: | ||||||
| Fever | 0.0% | 0.0% | n/a | 3.3% | 0.0% | 0.5077 |
| Headache | 36.7% | 29.2% | 0.3488 | 51.7% | 41.7% | 0.2792 |
| Rash | 61.7% | 0.0% | <0.0001 | 61.7% | 0.0% | <0.0001 |
| Neutropenia1 | 8.3% | 8.3% | 0.6833 | 6.7% | 0.0% | 0.2527 |
| Elevated ALT | 5.0% | 0.0% | 0.3591 | 3.3% | 4.2% | 0.8050 |
| Myalgia | 8.3% | 8.3% | 0.6833 | 11.7% | 4.2% | 0.2713 |
| Arthralgia | 3.3% | 4.2% | 0.8050 | 0.0% | 0.0% | n/a |
| Retro-orbital Pain | 6.7% | 8.3% | 0.7767 | 6.7% | 8.3% | 0.7767 |
| Fatigue | 16.7% | 4.2% | 0.1159 | 28.3% | 20.8% | 0.3393 |
| Photophobia | 0.0% | 4.2% | 1.0000 | 5.0% | 4.2% | 0.6784 |
| Elevated PT | 6.7% | 12.5% | 0.9012 | 5.0% | 4.2% | 0.6784 |
| Elevated PTT | 6.7% | 8.3% | 0.7767 | 0.0% | 4.2% | 1.0000 |
| Thrombocytopenia | 0.0% | 0.0% | n/a | 0.0% | 0.0% | n/a |
Neutropenia was defined as an ANC ≤ 1,000/mm3 in CIR279. Neutropenia was defined as an ANC≤1,500/mm3 for CIR268.
Probability is greater for TV003 vs placebo
Probability is greater for TV005 vs placebo
Table 6:
Viremia induced by TV003 and TV005 (replication in tissue culture)
| Admixture | Vaccine components | No. Subjects with viremia (%) | Mean peak titer ± SE (log10 PFU/mL) | Maximum titer (log10 PFU/mL) | Mean day of onset (range) | Mean duration in days (range) |
|---|---|---|---|---|---|---|
| TV003 (N=60) | DEN1Δ30 | 15 (25) | 0.6 ± 0.1 | 1.4 | 10.9 (8 – 15) | 2.1 (1 – 5)) |
| DEN2/4Δ30 | 4 (7) | 0.5 ± 0.0 | 0.5 | 7.5 (6 – 10) | 1.0 (all 1 day) | |
| DEN3Δ30,31 | 23 (38) | 0.6 ± 0.0 | 1.2 | 9.3 (5 – 14) | 2.5 (1 – 7) | |
| DEN4Δ30 | 18 (30) | 0.5 ± 0.0 | 0.7 | 9.3 (6 – 16) | 1.9 (1 – 6) | |
| Total Viremic | 45 (75) | |||||
| TV005 (N=60) | DEN1Δ30 | 17 (28) | 0.6 ± 0.1 | 1.7 | 12.3 (8 – 16) | 2.2 (1 – 7) |
| DEN2/4Δ30 | 13 (22) | 0.5 ± 0.0 | 0.5 | 9.1 (2 – 14) | 1.8 (1 – 8) | |
| DEN3Δ30,31 | 25 (42) | 0.6 ± 0.0 | 1.0 | 9.2 (6 – 14) | 2.8 (1 – 9) | |
| DEN4Δ30 | 18 (30) | 0.6 ± 0.1 | 1.5 | 10.1 (6 – 16) | 2.2 (1 – 10) | |
| Total Viremic | 46 (77) |
Table 7:
Cumulative serologic response following a single dose of TV003 or TV005 in flavivirus-naïve subjects
| % seroconverted | ||||
|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
| TV003 (N=581) | 97.0 | 64.0 | 98.0 | 100.0 |
| TV005 (n=591) | 91.5 | 86.4 | 94.9 | 96.6 |
| Geometric Mean Peak Titer (Median)2,3 | ||||
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
| TV003 (N=821) | 70.6 (75) | 45.2 (50) | 68.1 (57) | 123.3 (133) |
| TV005 (n=591) | 54.4 (43) | 89.3 (101) | 80.6 (78) | 164.7 (172) |
| Percent of TV005 recipients with indicated multivalent response (cumulative) | ||||
| TETRA | TRI | BI | MONO | |
| TV003 (N=821) | 65.5 | 29.3 (94.8) | 5.2 (100) | 0 (100) |
| TV005 (n=591) | 79.7 | 15.3 (95.0) | 1.7 (96.7) | 3.3 (100) |
From CIR268 and CIR279
Based on PRNT50. Seropositive response is defined as a PRNT50 ≥ 1:10 by Study Day 90.
PRNT50 titer calculated only for those subjects who seroconverted
Results from the Phase 2b trial of Dengvaxia™ cast doubt on the ability of neutralizing antibody titers to predict the efficacy of a dengue vaccine. For this reason, we developed a DENV-2 challenge model to evaluate the protective efficacy of TV003. We chose to develop a DENV-2 challenge model because rDEN2/4Δ30 has the highest HID50 of the four vaccine components and a lower proportion of TV003 seroconverted to DENV-2 compared with the other 3 serotypes. We repurposed the under-attenuated DENV-2 rDEN2Δ30 to use as the challenge virus. rDEN2Δ30 is an American strain of DENV-2 (DEN2/Tonga78) whereas the DENV-2 parent virus of rDEN2/4Δ30 is an Asian strain. rDEN2Δ30 infected 100% of flavivirus-naïve healthy adults and induced a mean peak titer ~ 100-fold higher than the vaccine candidate rDEN2/4Δ30 (2.5 log10 PFU/mL compared to 0.5 log10 PFU/mL [41]. Forty-eight flavivirus subjects were enrolled in the challenge study (CIR287); 24 received TV003 at day 0 and 24 received placebo.
Six months later, 41 subjects were challenged with 103 PFU rDEN2Δ30 (21 had previously received TV003 and 20 had previously received placebo. TV003 induced DENV-2 neutralizing antibody in all vaccinated subjects post-vaccination, although the PRNT50 had declined to <1:5 in some subjects by Study Day 180. Infectious DENV-2 challenge virus was recovered from all challenged placebo recipients, however rDEN2Δ30 could not be recovered by culture or by nucleic acid testing (standard PCR and digital drop PCR) from any vaccinated subject [42]. This study provided conclusive evidence that TV003 is highly protective against DENV-2. Although challenge virus was not recovered from any vaccinated subject, 9 subjects did demonstrate a boost in the DENV-2 PRNT50, as defined as a ≥4-fold rise in titer, post-challenge; 12 subjects did not mount a ≥ 4-fold rise in antibody titer indicative of a sterilizing immune response.
The induction of homotypic neutralizing antibody is thought to be critical for vaccine-induced protection. Because the PRNT50 assay utilizes cell lines that do not express Fcγ receptors, the assay measures neutralizing antibody only and does not account for the effects of non-neutralizing antibody. To better assess the overall effect of neutralizing and non-neutralizing antibodies in a single assay, we engineered Vero cells to express CD32a, the Fcγ2a receptor and compared the PRNT50 in those cells with the PRNT50 performed with standard Vero cells [43]. Although the PRNT50 titers were lower in Vero-CD32a cells (Figure 1), enhancement of infection was not observed in samples post-vaccination or post-challenge in the net neutralization assay (data not shown). In addition, challenge with DENV-2 did not induce a boost in DENV-2 antibody titer, also suggesting strong protection against infection with the DENV-2 challenge virus. These data suggest that the majority of DENV-2 antibody induced by TV003 is homotypic and neutralizing; cross-reactive, non-neutralizing antibody did not abrogate the neutralizing effect of the DENV-2 antibody induced by TV003.
Figure 1:
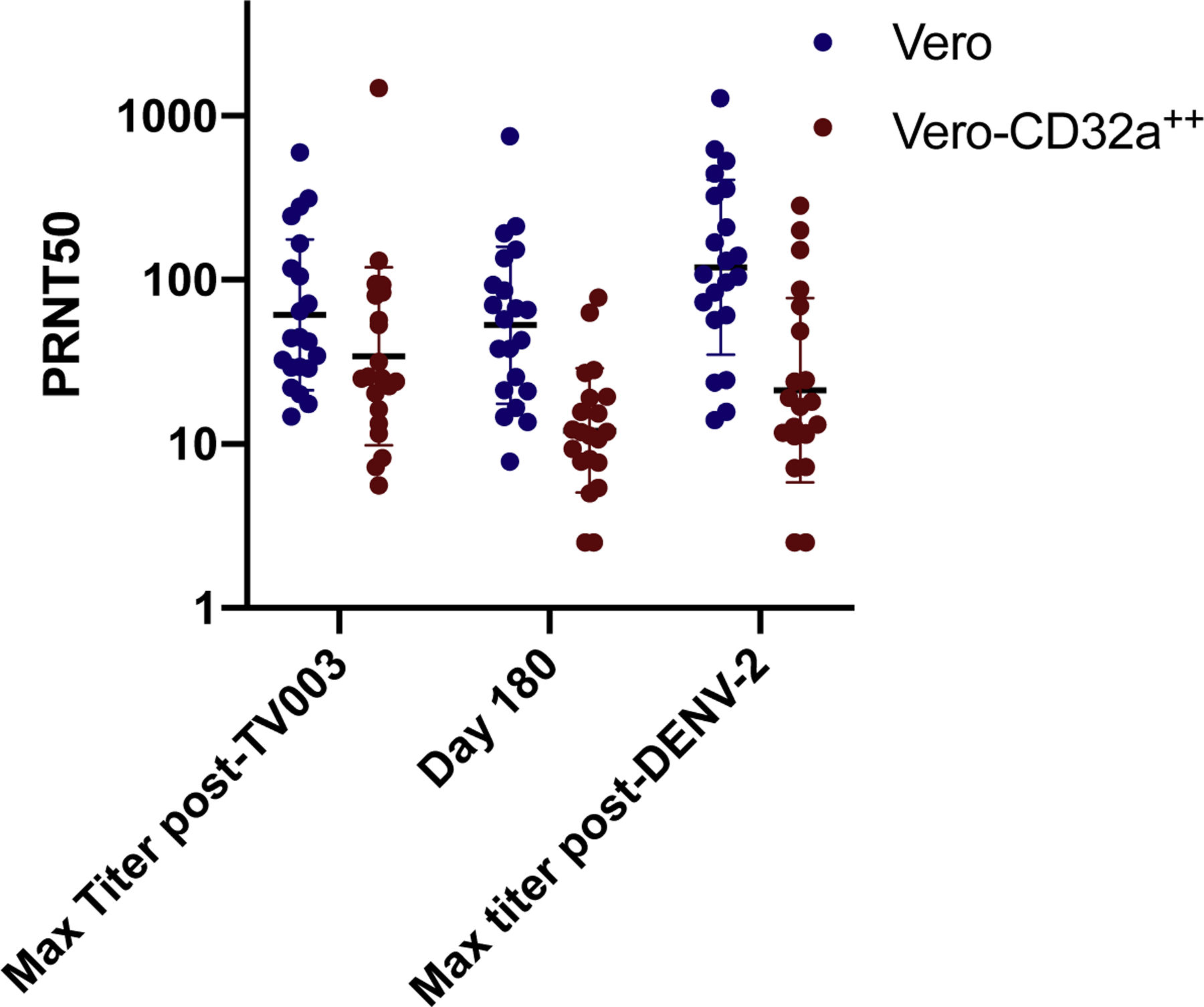
The neutralizing antibody titers induced by TV003 in CIR287 as measured in Vero cells (Blue) and Vero cells expressing CD32a (Vero-CD32a, Red). Neutralizing antibody titers were measured at study days 0, 28, 56, 90 post-vaccination and challenge. The study day 180 titer is the day of challenge. Seroconversion was defined as 1:10 post-vaccination or ≥ 4-fold rise in titer post-challenge. All subjects were flavivirus-naïve prior to challenge (PRNT50 < 5).
The ability of TV003 to protect flavivirus-naïve volunteers from DENV-2 challenge was critical to the decision of the Instituto Butantan to choose the TV003 formulation dengue vaccine development program. Butantan licensed TV003 and has completed its Phase 2 trial of the vaccine, Butantan-DV. Butantan-DV was well-tolerated and highly immunogenic in flavivirus-naïve and flavivirus-experienced volunteers in Brazil [44]. The vaccine is currently in Phase 3 clinical trial in Brazil with efficacy results expected to be released in 2021. In addition, 3 manufacturers in India have licensed the vaccine for in-country manufacture and Merck & Co. has licensed the vaccine for its dengue-development program. Given the billions of people currently at risk for dengue, it is hoped that multiple manufactures will be able to produce the vaccine to meet the needs of those in dengue-endemic areas.
In summary, an iterative approach was taken was to ensure that each component of the NIH live attenuated vaccine had an acceptable safety profile and was able to infect and replicate in the host post-vaccination. The ability to infect and replicate is critical for these live attenuated vaccines to ensure that each will induce a homotypic antibody response and reduce the risk for enhanced disease upon subsequent exposure to wild-type dengue. TV003 has demonstrated balanced infectivity and a balanced humoral and cellular immune response. Although these features suggest the vaccine will induce protection against dengue, only the Phase 3 efficacy study will definitively evaluate this.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
I was the Principal Investigator for the live attenuated dengue vaccine trials sponsored by the National Institutes of Health referenced in this article. I do not hold any patent rights to the vaccines. These trials were conducted under a contract between the National Institute of Allergy and Infectious Diseases and Johns Hopkins University.
References
- 1.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI: Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 2012, 6:e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD: The global economic burden of dengue: a systematic analysis. Lancet Infect Dis 2016, 16:935–941. [DOI] [PubMed] [Google Scholar]
- 3.Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castaneda-Orjuela CA, et al. : The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016, 16:712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. : The global distribution and burden of dengue. Nature 2013, 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead S, Wilder-Smith A: Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med 2019. [DOI] [PubMed] [Google Scholar]
- 6.Riddell A, Babiker ZO: Imported dengue fever in East London: a 6-year retrospective observational study. J Travel Med 2017, 24. [DOI] [PubMed] [Google Scholar]
- 7.Sherif NA, Zayan AH, Elkady AH, Ghozy S, Ahmed AR, Omran ES, Taha EA, Eldesoky EA, Ebied A, Tieu T, et al. : Mast cell mediators in relation to dengue severity: A systematic review and meta-analysis. Rev Med Virol 2020, 30:e2084. [DOI] [PubMed] [Google Scholar]
- 8.Rathore AP, Farouk FS, St John AL: Risk factors and biomarkers of severe dengue. Curr Opin Virol 2020, 43:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Innis BL, Eckels KH, Kraiselburd E, Dubois DR, Meadors GF, Gubler DJ, Burke DS, Bancroft WH: Virulence of a live dengue virus vaccine candidate: a possible new marker of dengue virus attenuation. J Infect Dis 1988, 158:876–880. [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB, Marchette NJ, Sung Chow JS, Lolekha S: Dengue virus replication enhancement in peripheral blood leukocytes from immune human beings. Proc Soc Exp Biol Med 1976, 151:136–139. [DOI] [PubMed] [Google Scholar]
- 11.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. : Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J Infect Dis 2000, 181:2–9. [DOI] [PubMed] [Google Scholar]
- 12.Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, Kochel TJ, Scott TW, Stoddard ST: Reduced risk of disease during postsecondary dengue virus infections. J Infect Dis 2013, 208:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP Jr., Srikiatkhachorn A: Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 2007, 77:910–913. [PubMed] [Google Scholar]
- 14.Tsai WY, Lai CY, Wu YC, Lin HE, Edwards C, Jumnainsong A, Kliks S, Halstead S, Mongkolsapaya J, Screaton GR, et al. : High-avidity and potently neutralizing cross-reactive human monoclonal antibodies derived from secondary dengue virus infection. J Virol 2013, 87:12562–12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, et al. : Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. : Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med 2015, 373:1195–1206. [DOI] [PubMed] [Google Scholar]; This paper identified key differences in vaccine efficacy based on serostatus and serotype and identified a higher relative risk of hospitalization in year 3 of the study in children who were aged 2 – 5 at the time of vaccination.
- 17.Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHJM, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. : Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. The Lancet 2014, 384:1358–1365. [DOI] [PubMed] [Google Scholar]
- 18.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. : Efficacy of a tetravalent dengue vaccine in children in Latin America. The New England journal of medicine 2015, 372:113–123. [DOI] [PubMed] [Google Scholar]
- 19.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. : Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
- 20.Wilder-Smith A: Serostatus-dependent performance of the first licensed dengue vaccine: implications for travellers. J Travel Med 2018, 25. [DOI] [PubMed] [Google Scholar]
- *21.Wilder-Smith A, Hombach J, Ferguson N, Selgelid M, O’Brien K, Vannice K, Barrett A, Ferdinand E, Flasche S, Guzman M, et al. : Deliberations of the Strategic Advisory Group of Experts on Immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis 2019, 19:e31–e38. [DOI] [PubMed] [Google Scholar]; This paper outlines the recommendations for use of the first licensed dengue vaccine and the rationale behind the recommendations.
- 22.Bonaparte M, Zheng L, Garg S, Guy B, Lustig Y, Schwartz E, DiazGranados CA, Savarino S, Ataman-Onal Y: Evaluation of rapid diagnostic tests and conventional enzyme-linked immunosorbent assays to determine prior dengue infection. J Travel Med 2019, 26. [DOI] [PubMed] [Google Scholar]
- 23.Hunsperger E, Peeling R, Gubler DJ, Ooi EE: Dengue pre-vaccination serology screening for the use of Dengvaxia(R). J Travel Med 2019, 26. [DOI] [PubMed] [Google Scholar]
- *24.Guy B, Jackson N: Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 2016, 14:45–54. [DOI] [PubMed] [Google Scholar]; This paper examines different theories related to the difference in vaccine efficacy of Dengvaxia based on serotype and serostatus at vaccination and concludes that there is an imbalance in the in immunogenicity of the four vaccine components.
- 25.Dayan GH, Thakur M, Boaz M, Johnson C: Safety and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine 2013, 31:5047–5054. [DOI] [PubMed] [Google Scholar]
- 26.Villar LA, Rivera-Medina DM, Arredondo-Garcia JL, Boaz M, Starr-Spires L, Thakur M, Zambrano B, Miranda MC, Rivas E, Dayan GH: Safety and Immunogenicity of a Recombinant Tetravalent Dengue Vaccine in 9–16 Year Olds: A Randomized, Controlled, Phase II Trial in Latin America. Pediatr Infect Dis J 2013, 32:1102–1109. [DOI] [PubMed] [Google Scholar]
- 27.Henein S, Swanstrom J, Byers AM, Moser JM, Shaik SF, Bonaparte M, Jackson N, Guy B, Baric R, de Silva AM: Dissecting Antibodies Induced by a Chimeric Yellow Fever-Dengue, Live-Attenuated, Tetravalent Dengue Vaccine (CYD-TDV) in Naive and Dengue-Exposed Individuals. J Infect Dis 2017, 215:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torresi J, Richmond PC, Heron LG, Qiao M, Marjason J, Starr-Spires L, van der Vliet D, Jin J, Wartel TA, Bouckenooghe A: Replication and Excretion of the Live Attenuated Tetravalent Dengue Vaccine CYD-TDV in a Flavivirus-Naive Adult Population: Assessment of Vaccine Viremia and Virus Shedding. J Infect Dis 2017, 216:834–841. [DOI] [PubMed] [Google Scholar]
- 29.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. : Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012, 380:1559–1567. [DOI] [PubMed] [Google Scholar]
- 30.Whitehead SS, Falgout B, Hanley KA, Blaney JE Jr., Markoff L, Murphy BR: A Live, Attenuated Dengue Virus Type 1 Vaccine Candidate with a 30-Nucleotide Deletion in the 3’ Untranslated Region Is Highly Attenuated and Immunogenic in Monkeys. J Virol 2003, 77:1653–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaney JE Jr., Hanson CT, Hanley KA, Murphy BR, Whitehead SS: Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect Dis 2004, 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, et al. : Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3’-untranslated region. Am J Trop Med Hyg 2001, 65:405–413. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead SS, Hanley KA, Blaney JE Jr., Gilmore LE, Elkins WR, Murphy BR: Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine 2003, 21:4307–4316. [DOI] [PubMed] [Google Scholar]
- 34.Blaney JE Jr., Hanson CT, Firestone CY, Hanley KA, Murphy BR, Whitehead SS: Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am J Trop Med Hyg 2004, 71:811–821. [PubMed] [Google Scholar]
- 35.Hanley KA, Lee JJ, Blaney JE Jr., Murphy BR, Whitehead SS: Paired charge-to-alanine mutagenesis of dengue virus type 4 NS5 generates mutants with temperature-sensitive, host range, and mouse attenuation phenotypes. J Virol 2002, 76:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley KA, Manlucu LR, Manipon GG, Hanson CT, Whitehead SS, Murphy BR, Blaney JE Jr.: Introduction of mutations into the non-structural genes or 3’ untranslated region of an attenuated dengue virus type 4 vaccine candidate further decreases replication in rhesus monkeys while retaining protective immunity. Vaccine 2004, 22:3440–3448. [DOI] [PubMed] [Google Scholar]
- *37.Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, Durbin AP, Murphy BR: A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg 2001, 65:414–419. [DOI] [PubMed] [Google Scholar]; This paper describes the transmissibility of the live attenuated DENV-4 candidate vaccine from vaccine recipients to mosquitoes.
- 38.Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, Tibery C, Sabundayo BP, Shaffer D, Talaat KR, et al. : A Single Dose of Any of Four Different Live Attenuated Tetravalent Dengue Vaccines Is Safe and Immunogenic in Flavivirus-naive Adults: A Randomized, Double-blind Clinical Trial. J Infect Dis 2013, 207:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS: Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 2011, 29:7242–7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Kirkpatrick BD, Durbin AP, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Diehl SA, Elwood D, Jarvis AP, et al. : Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J Infect Dis 2015, 212:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the balanced infectivity and immunogenicity of each component of the live attenuate tetravalent dengue vaccine candidate vaccine TV003/TV005.
- 41.Larsen CP, Whitehead SS, Durbin AP: Dengue human infection models to advance dengue vaccine development. Vaccine 2015. [DOI] [PubMed] [Google Scholar]
- **42.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, et al. : The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Science Translational Medicine 2016, 8:330ra336–330ra336. [DOI] [PubMed] [Google Scholar]; Recipeints of the live attenuated tetravalent dengue vaccine candidate TV003 were challenged 6 months after vaccination with a DENV-2 challenge virus and were completely protected against viremia and rash. These results contributed to the choice of the TV003 formulation for evaluation in a Phase 3 clinical efficacy trial.
- 43.Durbin A, Fang X, Luo W, Whitehead S: Use of a neutralization capacity assayto better understand vaccine induced protection in a CHIM In Annual Meeting of the American Society of Tropical Medicine and Hygiene. Edited by Balitmore, MD; 2017. [Google Scholar]
- *44.Kallas EG, Precioso AR, Palacios R, Thome B, Braga PE, Vanni T, Campos LMA, Ferrari L, Mondini G, da Graca Salomao M, et al. : Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine Butantan-DV in adults in Brazil: a two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect Dis 2020. [DOI] [PubMed] [Google Scholar]; This paper describes the results of the Phase1/II clinical trial of the Instituto Butantan produced vaccine TV003. Instituto Butantan produced TV003 is currently in Phase 3 clinical trial in Brazil
- 45.Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney JE, Thumar B, Murphy BR, Karron RA: rDEN4 Delta 30, a Live Attenuated Dengue Virus Type 4 Vaccine Candidate, Is Safe, Immunogenic, and Highly Infectious in Healthy Adult Volunteers. J Infect Dis 2005, 191:710–718. [DOI] [PubMed] [Google Scholar]
- 46.Durbin AP, McArthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K, Murphy BR, Whitehead SS: rDEN2/4Delta30(ME), A Live Attenuated Chimeric Dengue Serotype 2 Vaccine Is Safe and Highly Immunogenic in Healthy Dengue-Naive Adults. Hum Vaccin 2006, 2:255–260. [DOI] [PubMed] [Google Scholar]
- 47.Durbin AP, McArthur J, Marron JA, Blaney JE Jr., Thumar B, Wanionek K, Murphy BR, Whitehead SS: The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin 2006, 2:167–173. [DOI] [PubMed] [Google Scholar]
- 48.McArthur JH, Durbin AP, Marron JA, Wanionek KA, Thumar B, Pierro DJ, Schmidt AC, Blaney JE Jr., Murphy BR, Whitehead SS: Phase I clinical evaluation of rDEN4Delta30–200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity. Am J Trop Med Hyg 2008, 79:678–684. [PMC free article] [PubMed] [Google Scholar]
- 49.Wright PF, Ankrah S, Henderson SE, Durbin AP, Speicher J, Whitehead SS, Murphy BR, Pletnev AG: Evaluation of the Langat/dengue 4 chimeric virus as a live attenuated tick-borne encephalitis vaccine for safety and immunogenicity in healthy adult volunteers. Vaccine 2008, 26:882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durbin AP, Schmidt A, Elwood D, Wanionek KA, Lovchik J, Thumar B, Murphy BR, Whitehead SS: Heterotypic dengue infection with live attenuated monotypic dengue virus vaccines: implications for vaccination of populations in areas where dengue is endemic. J Infect Dis 2011, 203:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durbin AP, Whitehead SS, Shaffer D, Elwood D, Wanionek K, Blaney JE Jr., Murphy BR, Schmidt AC: A single dose of the DENV-1 candidate vaccine rDEN1Δ30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS neglected tropical diseases 2011, 5:e1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindow JC, Borochoff-Porte N, Durbin AP, Whitehead SS, Fimlaid KA, Bunn JY, Kirkpatrick BD: Primary vaccination with low dose live dengue 1 virus generates a proinflammatory, multifunctional T cell response in humans. PLoS Negl Trop Dis 2012, 6:e1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitehead SS, Durbin AP, Pierce KK, Elwood D, McElvany BD, Fraser EA, Carmolli MP, Tibery CM, Hynes NA, Jo M, et al. : In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl Trop Dis 2017, 11:e0005584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durbin AP, Kirkpatrick BD, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Opert K, Jarvis AP, Sabundayo BP, et al. : A 12-Month-Interval Dosing Study in Adults Indicates That a Single Dose of the National Institute of Allergy and Infectious Diseases Tetravalent Dengue Vaccine Induces a Robust Neutralizing Antibody Response. J Infect Dis 2016, 214:832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]


