Abstract
There is strong evidence that chronic, systemic inflammation hastens onset of the diseases of old age that ultimately lead to death. Importantly, several studies suggest that childhood adversity predicts chronic inflammation. Unfortunately, this research has been plagued by retrospective reports of childhood adversity, an absence of controls for adult stressors, and a failure to investigate various competing models of the link between childhood adversity and chronic inflammation. The present study was designed to address these limitations. Using 18 years of data collected from 413 African Americans (58% female) included in the Family and Community Health Study, hierarchical regression analyses provided support for a nuanced early life sensitivity explanation for the link between early adversity and adult chronic inflammation. Controlling for health risk behaviors and adult SES, late childhood (ages 10–12) adversity amplified the association between adult adversity (age 29) and chronic inflammation. This interaction operated in a domain-specific fashion. Harsh parenting amplified the relation between intimate partner hostility and inflammation, whereas early discrimination amplified the relation between adult discrimination and inflammation. These findings suggest that individuals may be primed to respond physiologically to adverse adult circumstances that resemble those experienced earlier in life.
Keywords: childhood adversity, discrimination, early life sensitivity model, harsh parenting, inflammation
Introduction
Modern perspectives on mental health are built on the developmental premise that early childhood trauma increases the chances of adult mental health problems (Carter, Dellucci, Turek, and Mir 2015; Fisher and Brown 2018; Kerig, Ward, Vanderzee, and Moeddel 2009; Zona and Milan 2011). In recent years, it has become evident that exposure to childhood adversity also elevates the risk of adult physical health problems (Bae & Wickrama 2017; Fagundes and Way 2014; Basu, McLaughlin, Misra, and Koenen 2017; Kliewer 2016). Further, there is reason to believe that chronic, system-wide inflammation is one of the chief biological mechanisms whereby childhood environments influence adult illness. First, a profusion of studies have shown that elevations in biomarkers of systemic inflammation are associated with the onset of chronic diseases such as cardiovascular disorders, type II diabetes, osteoporosis, rheumatoid arthritis, Alzheimer’s disease, and certain cancers (Maggio et al. 2006; Libby and Theroux 2005). Second, numerous studies have reported that childhood adversity increases the chances of inflammation across the life span (Miller et al. 2011; Fagundes and Way 2014). Indeed, the evidence is so strong that the American Heart Association (AHA) recently released a scientific statement indicating that there is now compelling evidence that child and adolescent adversity are linked to indicators of cardiometabolic health such as inflammation (Suglia et al., 2018). Together these findings suggest that childhood stress fosters chronically elevated inflammation which eventuates in adult illness.
The most popular framework for explaining the link between childhood adversity and adult inflammation is the early life sensitivity model. Examples of this approach are the predictive adaptive response (PAR) perspective (Miller et al. 2011) and the Developmental Origins of Health and Disease (DOHaD) hypothesis (Gluckman, Hanson, Cooper, and Thornburg 2008). These models view childhood and adolescence as sensitive periods during which cognitive and biological programming primes an individual to be vigilant for anticipated threats and to react intensely when adverse conditions are encountered (Miller et al. 2011; Fagundes and Way 2014).
Past research on the early life sensitivity model has employed rather narrow conceptualizations of stress and adversity. Assessments of childhood stress usually focus upon either family SES or harsh parenting (Fagundes and Way 2014; Hostinar et al. 2015) and SES is typically utilized as an indicator of adult stress (Loucks et al. 2010). Using 20 years of longitudinal data collected from a sample of 421 African Americans, the present study examines the effects of two rather distinct types of early adversity – childhood exposure to harsh/rejecting parenting and to racial discrimination – as well as to two adult stressors – exposure to romantic partner hostility and to racial discrimination. Controlling for adult stressors and health risk behaviors, the current study investigates the extent to which late childhood adversities have either a direct effect on adult inflammation, or a moderating effect where they amplify the impact of the adult stressors on inflammation. Further, in testing for amplification effects, this study explores the possibility that early life sensitivity operates in a domain specific manner. That is, it investigates whether individuals who have experienced a particular type of adversity as a child tend to show an elevated inflammatory response to adult stressors in general or only to adult circumstances that resemble adversities experienced earlier in life. To the authors’ knowledge, this possibility has never been tested. These various theoretical explanations for the link between early adversity and inflammation, including the potential for domain specificity in early life sensitization, are discussed in more detail in the following sections.
Causes and Consequences of Chronic Inflammation
Inflammation is part of the body’s immune defense system whereby it recognizes and removes harmful stimuli and begins the healing process. In general, there are two types of inflammation: acute and chronic. Acute inflammation is a short term response designed to address tissue damage in a specific area of the body due to trauma or microbial invasion. Chronic inflammation, on the other hand, is a slow, long-term increase in inflammation that occurs in various systems throughout the body. Chronic inflammation has been shown to be a robust predictor of the age-related chronic diseases that eventually cause the death of most humans such as heart disease, stroke, diabetes, Alzheimer’s, Parkinson’s, and cancer (Maggio et al. 2006; Libby and Theroux 2005). Modern medicine’s explanation for chronic inflammation emphasizes the role of aging, exercise, diet, and unhealthy habits such as smoking. While these are all important health risk factors, together they still leave much of the variance in inflammation unexplained. In recent years, several studies have reported a link between exposure to social adversity and inflammation. Findings suggest that social conditions such as loneliness, bereavement, PTSD, and caring for a terminally ill family member are associated with increased inflammation and that this relation holds even after controlling for health risk behaviors such as smoking, excess drinking, lack of exercise, BMI, etc. (Slavich and Cole 2013; Cole 2014). Perhaps the most compelling explanation for this association has been provided by Steven Cole and his colleagues (Cole 2013, 2014).
Cole and colleagues note that the inflammatory program of the immune system designed to is designed to combat tissue damage, bacteria, and other extracellular pathogens. They argue that adversity leads to increased expression of this inflammatory program as the organism prepares for possible attack and injury. Presumably, this pattern of gene expression evolved to help adapt molecular physiology to the types of sporadic and transient physical threats that characterized our ancestral environments (Cole 2014). The dangers faced in contemporary society, however, tend to be symbolic or social threats to well-being (Cole 2014). These persistent pressures and anticipated hazards undermine health by fostering chronic activation of the inflammatory program and risk for inflammation-related diseases (Cole 2013, 2014). If our immune system is wired to produce a heightened inflammatory response to threat, it makes sense that individuals who have recently endured stressful circumstances would manifest elevated inflammation. But, why would early adversity also be linked to adult inflammatory levels?
Early Life Sensitivity and Adult Chronic Inflammation
As noted above, the early life sensitivity model views early adversity as having “an independent and privileged role” (Hostinar et al. 2015, p. 1633) in shaping later risk for disease. Childhood and adolescence are considered stages of plasticity during which cognitive schemas are acquired and biological systems are calibrated in a manner designed to prepare the organism for the threatening future events that are expected based upon early developmental experiences (Gluckman et al. 2008; Fagundes and Way 2014). It is assumed that adverse conditions such as harsh or unpredictable family environments provide cues about the severe circumstances that are likely to be faced throughout the life course. At the cognitive level, harsh environmental conditions are viewed as fostering a distrustful, vigilant orientation that prepares the person for anticipated threats (Glaser, van Os, Portegijs, and MyinGermeys 2006; Miller et al. 2011; Fagundes and Way 2014). At the biological level, early adversity is thought to act as a programming agent that shapes operating tendencies of monocytes/macrophages in at least two ways (Miller et al. 2011): cells show more pronounced inflammatory responses when exposed to challenges, and they are less able to terminate these responses given their reduced sensitivity to inhibitory hormonal signals (e.g., cortisol).
Based upon this cognitive and biological programming, the early life sensitivity model makes two predictions regarding the impact of early adversity on adult inflammation. First, it posits that exposure to childhood adversities will elevate adult levels of inflammation irrespective of subsequent risk exposure. The distrustful schemas and monocyte programming acquired during childhood foster an elevated inflammatory response that continues unabated into adulthood (Hostnar et al. 2015; Miller et al. 2011). Second, harsh childhoods are seen as heightening sensitivity to subsequent stressors encountered across the life course. Distrustful schemas prime individuals to perceive adult events as threatening and a hypersensitive immune system increases the probability that perceived threats will result in a strong inflammatory response. The result is an interaction effect where early stressors, given their impact on cognitive and biological programming, enhance the effect of adult stressors on level of inflammation (Hostinar et al. 2015).
Much of the support for the early life sensitivity model comes from studies showing that early adversity, assessed using measures of either family-of-origin SES or harsh parenting, is related to adult inflammation (e.g., Lawlor, Smith, Rumley, Lowe, and Ebrahim 2005; Phillips et al. 2009). Virtually none of these studies, however, controlled for adult adversity beyond inclusion of a general measure of SES. Further, childhood adversity was almost always assessed using cross-sectional or retrospective reports (Fagundes and Way 2014). An exception is a longitudinal investigation by Danese, Pariante, Caspi, Taylor, and Poulton (2007) which found an association between parental neglect during childhood and inflammation at age 32. Finally, prior research has rarely been able to test the prediction that early adversity will amplify the effect of later adversity on inflammation. This has largely been because most studies lack a measure of adult adversity. Three studies have had the data necessary to investigate this interaction effect. Gouin et al. (2012) found that a history of child maltreatment amplified the association between adult daily stressors and inflammation, and Kiecolt-Glaser et al. (2011) reported that adults caring for family members with dementia were more likely to display elevated inflammation if they had experienced maltreatment as a child. In contrast, Hostinar et al. (2015) found that self-reported adverse childhood experiences did not interact with recent life experiences to predict adult inflammation. All three of these studies used retrospective reports to assess childhood adversity. Thus, although the early life sensitivity model is quite popular, the data supporting it is somewhat limited.
There is a need for studies that use prospective, longitudinal assessments of childhood and adult adversity to investigate the extent to which the early life sensitivity model is supported after controlling for adult stressors. Further, there is a need for research that attempts to disentangle the effects of different types of early adversity (Fagundes and Way 2014). Perhaps some types of early adversity amplify the effect of a particular class of adult stressors whereas another type amplifies the effect of a different class of adult stressors.
Domain Specific Sensitivity
Early life sensitivity research usually assumes that any type of serious childhood adversity will have a direct impact on adult inflammation and amplify the effect of adult stressors on inflammation. But, there is reason to believe that this may be an oversimplification. There is a wide range of stressful circumstances that an individual might experience during childhood and adolescence. This includes harsh parenting, sexual abuse, family financial challenges, academic failure, living in a dangerous neighborhood, exposure to racial discrimination, and the like. Although such adversities tend to be correlated, the correlations are usually rather modest. Many low SES children, for example, have wonderfully supportive parents even though the family struggles financially or lives in a dangerous neighborhood (Hardaway, Sterett-Hong, Larkby and Cornelius 2016). Or they may have uninvolved parents but live in a cohesive neighborhood filled with good friends (Fagan, Wright and Pinchevsky 2014). The point is that some aspects of an individual’s environment may be stressful while other aspects are not. This reality would seem to require a more nuanced interpretation of the early life sensitivity model.
Learning (Bandura 1986; Ferster and Perrott 1968) and social schematic (Bowlby 1982; Bourdieu 1990; Dodge 1980) perspectives might be interpreted as predicting domain specificity in social and biological sensitivities derived from these contrasting early circumstances. As a consequence of respondent conditioning and stimulus generalization, learning theory predicts that various indicators of a particular class of stimuli will come to evoke the same emotional and behavioral response. Similarly, social schematic theories argue that childhood experiences foster cognitive structures (tacit assumptions and dispositions) regarding particular types of events and situations and that these schemas color the way an individual interprets and reacts to similar conditions and settings later in life. Studies have shown, for example, that childhood experiences of parental rejection foster hypersensitivity to rejection in adult romantic relationships (Downey & Feldman, 1996; Nowland, Talbot, & Qualter, 2018). Thus learning and schematic theories would seem to suggest that individuals are primed to respond emotionally and physically to adverse adult circumstances that resemble those experienced earlier in life (rather than to adult adversity in general). A particular type of stressor, say exposure to harsh parenting, may predispose an individual to demonstrate a heightened vigilance, and to display an emotional and biological response, to interpersonal challenges such as intimate partner conflict while having little impact upon the person’s attentiveness and reaction to other types of stressors such as time pressures at work. It may be that the inconsistent results obtained in prior research are a consequence of the fact that individuals learn to be emotionally and biologically hypersensitive to certain classes or domains of adverse circumstances rather than to adversity in general. This domain specificity idea is investigated in the current study.
The Current Study
The present study is an attempt to overcome many of the limitations of past research regarding the link between childhood adversity and adult inflammation. First, the study uses longitudinal data collected from a sample of roughly 400 African Americans. The respondents averaged 10 years of age at Wave 1 and 29 years of age at Wave 7 which included blood draws and inflammatory assays. This dataset avoids the problem of retrospective reporting that has plagued most prior research. Further, use of a black sample is particularly relevant for our purposes given the high levels of adversity (Geronimus et al. 2016), inflammation (Nowakowski and Sumerau 2015; Paalani et al. 2011) and health inequities (Geronimus et al. 2016; Williams 2012) faced by this group. Second, the the study tests for various avenues whereby early adversity impacts adult inflammation. Controlling for various demographic characteristics and health risk behaviors, it examines the extent to which early adversity continues to predict inflammation after controlling for adult adversity. The study also investigates the early life sensitivity prediction that childhood adversity amplifies the effect of adult adversity on inflammation.
The analyses focus on two types of early adversity – exposure to harsh parenting and early experiences of racial discrimination. Decades of research has established that harsh parenting is an important childhood stressor that predicts a number of negative outcomes in adulthood including poor health (Bae and Wickrama, 2017; Felitti et al. 1998). Similarly, several recent studies have linked early exposure to racial discrimination to adult problems including chronic illness (Gerrard et al. 2012; Gibbons et al. 2007; Simons et al., 2018). In addition, the present study includes two types of adult stressors – intimate partner hostility and adult experiences of racial discrimination. Importantly, romantic partner hostility and adult racial discrimination might be seen as adult versions of our two childhood stressors, thereby providing an opportunity to test for possible domain specificity in the early life sensitivity model. The current study examines whether early racial discrimination amplifies the effect of both adult experiences of discrimination and intimate partner hostility (a general early life sensitivity effect) on inflammation, or whether its moderating influence is limited to the effects of adult discrimination (a specific early life sensitivity effect). Likewise, it explores whether early exposure to harsh parenting amplifies the effect of both adult discrimination and intimate partner hostility (a general early life sensitivity effect) or is limited to an intensification of the effect of intimate partner hostility (a specific early life sensitivity effect).
Another possibility is that harsh parenting trumps other dimensions of the childhood environment. Early life sensitivity proponents often argue that parents are the most central feature of the childhood milieu in terms of teaching lessons regarding environmental threats and the types of events and circumstances that are likely to be encountered across the life course (Del Giudice et al. 2011; Gluckman et al. 2008). To the extent that this is true, harsh/rejecting parenting would be expected to have a significant main effect on inflammation whereas childhood exposure to racial discrimination would not. Further, if parenting plays such a central role in terms of preparing the individual for the future, it may amplify the effect of both intimate partner hostility and adult discrimination, whereas early discrimination will have no moderating effect.
These various ideas are tested using a more comprehensive measure of inflammation than has commonly been utilized in past research. Prior studies have almost always assessed inflammation using just one or two markers such as IL6 or TNFα. The inflammatory system, however, is extensive and complex; one inflammatory protein (cytokine) often stimulates and amplifies the production of others, setting up a cascade of reactions (Abbas, Lichtman, and Pillai 2011). Further the system consists of both pro-inflammatory (e.g., IL-1β, IL-6, TNFα, IFN) and anti-inflammatory factors (e.g., IL-4, IL-10, IL-13), with the latter serving to regulate and limit the inflammatory process. Indeed, it has been suggested that most of the inconsistent and insignificant findings associated with research on inflammation is a consequence of using only one or two biomarkers to assess such a complex biological system (Morrisette-Thomas et al. 2014).
While past research has relied upon simplex ELISA assays to assess one or two cytokines (usually IL6 or TNFα), multiplex approaches such as the Bio-Plex Pro Human Cytokine 17-Plex Immunoassay are now available and provide assays for an array of pro- and anti-inflammatory cytokines. This approach requires decisions, however, regarding the most appropriate strategy for combining these two classes of cytokines into a single index. Simply summing would not seem to be appropriate as anti-inflammatory cytokines have been shown in both animal and human experiments to tamp down the expression of pro-inflammatory cytokines (e.g., Pajkart et al., 1997). In large measure, pro- and anti-inflammatory cytokines tend to be linked; pro-inflammatory cytokines such as TNFα are triggered in reaction to injury or bacterial infection and this response is then followed by the expression of potent anti-inflammatory cytokines such as IL10 which serve to control and reduce the pro-inflammatory response once it has served its purpose (Abbas et al. 2015). Thus pro- and anti-inflammatory cytokines tend to be yoked so that they rise and fall together. In response to unresolved pro-inflammatory stimulation, however, this yoking may breakdown and the organism may produce only a weak anti-inflammatory response (Dhabar et al. 2009; Libby, Nahrendorf, and Swirski, 2016). This suggests that chronic or pathological inflammation might be viewed as a condition characterized by elevated expression of pro-inflammatory cytokines combined with relatively low expression of anti-inflammatory cytokines. Although the pro-inflammatory response is usually a healthy and necessary response to the injuries and infections endemic to everyday life, it becomes problematic when the subsequent anti-inflammatory response is insufficient to terminate progression of the inflammatory process. In other words, the critical issue is balance. Consonant with this idea, studies have indicated that it is often the balance of pro- and anti-inflammatory cytokines that is crucial for health (Uchino et al., 2018; see Online Supplement). Based on this observation, the inflammatory index used in the present study involved summing across pro- and anti-inflammatory cytokines, respectively, and then calculating the ratio of these two summary scores (Simons et al. 2018).
Methods
Participants
The current study utilizes the seven waves of data that have been collected for the Family and Community Health Study (FACHS), a multi-site (Georgia and Iowa) investigation of neighborhood and family processes that contribute to African American children’s development in families living in a wide variety of community settings (see Gibbons et al. 2004; Simons et al. 2011). The first wave of FACHS data was collected in 1997–1998 from 889 African American children (467 from Iowa and 422 from Georgia), their primary caregiver, and a secondary caregiver when one was present in the home. The target child in each study family was in the 5th grade and roughly 10 years of age at the time of recruitment.
The second through sixth waves were collected between 1999 and 2012 to capture information when the target children were ages 12–13, 14–15, 18–19, 21–22, and 24–25, respectively. Of the 889 targets interviewed at wave 1, 699 were reinterviewed at wave 6 (78% of the original sample). In 2014–2015 a 7th wave of data collection was completed that included blood draws. Given the logistics of scheduling home visits by phlebotomists, only members of the sample residing in Georgia, Iowa, or a contiguous state were identified as eligible. After also excluding persons who were deceased, incarcerated, or otherwise unreachable, we were left with a pool of 479 individuals. Of these individuals, 413 (86%) were interviewed and provided blood. Average education for these individuals was 13.1 years (9% < high school, 38% High school or GED, 18% vocational school, 24% 2–3 years of college, 14% college graduate, and 2% graduate school). Median income was roughly $25,000 (30% < $13,000, 20% > $36,000, 9% > $52,000). Analyses indicated that those individuals who did not participate in wave 7 did not differ significantly from those who participated with regard to wave 1 scores on caregivers’ education, household income, family structure, or neighborhood characteristics. Compared to wave 1, however, a higher percentage of those interviewed at wave 7 were female.
Procedures
The protocol and all study procedures were approved by the Institutional Review Board at the University of Georgia (Title: FACHS IV; Protocol # Study00000172). The questions were administered using laptop computers in the respondent’s home and took on average about 2 hours to complete. In addition to the interview data, at Wave 7 participants were also asked to provide a blood sample. A certified phlebotomist drew five tubes of blood at each participant’s home. Two of the tubes were spun immediately to separate serum into 3 cryo-vials that were then frozen and stored in a −80° freezer until used for the analyses described in the Measures section.
Measures
Chronic Inflammation.
Levels of cytokines in plasma were determined using a Bio-Plex 200 system (Bio-Rad, USA ) and a standard 17-plex cytokine detection kit according to the manufacturer’s protocol. The Bio-Plex assay combines fluorescent flow cytometry and ELISA technology. For each of the 17 cytokines in each sample; 100 beads specific for that cytokine were assayed and the mean cytokine binding for the sample was determined. Thus the Bio-Plex runs 100 duplicates of each analyte. The manufacturer reports that the assay accuarately measures cytokine values in the range of 1–2,500 pg/ml. The assays are precise (intra-assay CV,10%, interassay CV,15%) and show less than 1% cross-reactivity among cytokines or with other molecules. The process provides simultaneous quantificiation of each of the 17 cytokines assayed in the sample. The assays for 12 individuals were flawed and therefore they were deleted from the analysis. This left a sample of 401 for the present study.
Using the 17-plex array, aliquots of blood for each of the 401 remaining participants were randomly assigned to eight “plates.” To correct for potential method variance reflecting “plate” rather than variables of interest, the cytokines used in the study were corrected for plate-to-plate variation by linear regression that included the eight plates as categorical covariates. For each of the cytokines, we used the residuals after removal of plate effects in subsequent analyses. Because IL-2, GM-CSFR, and MCP-1 were undetectable in most of the samples (≥ 95%), they were excluded, leaving 14 of the 17 cytokines to be included in our index of inflammatory cytokines. This consisted of 11 pro-inflammatory cytokines (IL-1, IL-5, IL-6, IL-7, IL-8, IL-12, IL-17, G-CSF, IFN-γ, MIP-1, and TNF) and three anti-inflammatory cytokines (IL4, IL10, and IL13).
To correct for skew, the scores for each cytokine were recoded using a three category approach: cytokines with no detectable values were coded as 1, those with detectable values below the upper quartile were coded as 2, and those above this value were coded as 3 (see van Kammen et al. 1999 and Conraads et al. 2006 for examples of using categorical coding to address non-detectable scores and skew). To capture the relative balance of pro-inflammatory to anti-inflammatory activity, scores for the pro-inflammatory cytokines were summed separately from the scores for the anti-inflammatory cytokines. The following equation was then used to calculate the relative balance of pro- to anti-inflammatory cytokines:
Using this ratio, higher scores indicated increased dominance of the pro-inflammatory response. Scores for the index were roughly normally distributed (see Online Supplement Figure 1).
Racial Discrimination.
At each wave of data collection, respondents completed 13 items from the Schedule of Racist Events (Landrine & Klonoff 1996). This instrument has strong psychometric properties and has been used extensively in studies of African Americans (Brody et al 2015; Burt, Simons, & Gibbons 2012; Simons et al. 2018). The items assess the frequency (1 = never, 4 = several times) with which various discriminatory events have been experienced in the past year. For example, respondents were asked how often someone yelled a racial slur/insult or issued a physical threat, or how often they experienced police harassment, disrespectful treatment by sales clerks, false accusations by authority figures, and exclusion from social activities because of being African American. At Wave 1, respondents were asked to report on lifetime experiences of discrimination, whereas at all subsequent waves they were asked to report how often they had experienced each of these events during the past year. Coefficient alpha for the scale was between .75 and .80 at every wave. A measure of early discrimination was created by standardizing and averaging the discrimination scores for Waves 1 and 2, and a measure of adult discrimination was created by standardizing and averaging the discrimination scores for Waves 5, 6, and 7.
Harsh/Rejecting Parenting.
This measure was designed to assess the extent to which parents were harsh and rejecting vs. being warm and supportive. A 21-item scale was used that asked respondents at Waves 1 and 2 to report how often during the preceding year (1=never; 4=always) that their primary caregiver had engaged in actions such as shouting or yelling at them, calling them bad names, criticizing them, insulting them, swearing at them, or pushed, hit, or shoved them. The scale also contained items that asked about parental warmth, support, inductive reasoning, and monitoring which were reverse coded. The monitoring items were included given strong evidence that monitoring items assess the willingness of children to self-disclose to their parents (rather than parental surveillance) and such sharing is an indication of the closeness of the parent-child relationship. Coefficient alpha for the 21-item scale was .75 at Wave 1 and .78 at Wave 2. This scale has been used in numerous publications (see L.G. Simons et al. 2014) and had an alpha coefficient of roughly .85 at both waves 1 and 2. Scores were standardized and averaged across waves to form a measure of persistent exposure to a parenting style that coupled high levels of hostility and rejection with an absence of warmth, nurturance, and support.
Romantic Partner Hostility.
At Waves 6 and 7, respondents used the Relationship Hostility Scale (Cui et al. 2005; L.G. Simons et al. 2014) to report how often during the previous 3 months that their partner had behaved in a hostile/rejecting fashion when interacting with them. This 5-item scale consisted of 4 items concerned with verbal hostility (“insult or swear at you?” “criticize you?” “shout or yell at you?”) and one that involved physical aggression “slap you”) The response format ranged from 1 (never) to 4 (always). Coefficient alpha for the scale was roughly .70 at both waves. At wave 6, 245 individuals reported having a romantic partner (i.e., they were in a committed dating relationship, cohabitating, or married), and 285 (70%) had a committed relationship at wave 7. Individuals reporting no romantic partner received a score of 1 (never) on all of the items. Scores were standardized and averaged to form a measure of persistent exposure to partner hostility.
Several statistical covariates that have been linked to health, race/ethnicity, and/or discrimination were included in order to minimize risk of confounding in the associations of interest.
Gender (male =1) is controlled in all analyses and is examined in exploratory analyses as a potential source of differential response. Demographic controls included level of
Education was assessed dichotomously as less than high school vs. high school graduate.
Age was assessed in years.
Work status was assessed dichotomously (1 = employed).
Financial hardship was assessed using scores from waves 4–7 on a 4-item economic strain scale developed by Simons et al. (2016). The items focus on the extent (1 = strongly disagree; 5 = strongly agree) to which respondents were unable to afford the basic necessities of life such as food, clothing, housing, and medical care and had difficulty paying their monthly bills. Coefficient alpha was roughly .85 at each wave and scores were averaged and summed across waves.
Respondents also reported on various health risk behaviors. At Waves 4–7,
Smoking was assessed by summing across waves 4–7 responses to the question: During the prior 12 months have you smoked cigarettes (1=yes).
Alcohol consumption was assessed by summing across waves 4–7 responses to the question: How frequently do you consume alcohol (0=never, 5 = every day).
Healthy diet was assessed using two items that asked about frequency of fruit and vegetable consumption during the previous 7 days (1=none to 5=twice a day or more). The relationship between the two diet items was significant (r > .25 at each wave). Scores were summed across waves 4 – 7.
Exercise was measured using the following item averaged across Waves 4–7: On how many of the past 7 days did you exercise or participate in physical activity for at least 30 min that made you breathe hard such as running or riding a bicycle fast? The response categories ranged from 1 (0 days) to 5 (all 7 days).
As noted above, the inflammation measure had missing data on 11 individuals who were therefore deleted from the analyses. There was no missing data on the independent or dependent variables among the remaining individuals. Last observation carried forward was used to impute missing values for the control variables. Missing data on these variables was, however, very rare (less than 1% on any of the items).
Analytic Strategy
The various predictions of the early life sensitivity perspective were tested using hierarchical regression models with robust standard errors in STATA 14.0 (StataCorp 2015). Controlling for a variety of demographic and health-risk behaviors, this approach allowed us to examine the unique effects of childhood exposure to harsh parenting and racial discrimination, as well as adult exposure to intimate partner hostility and discrimination, on inflammation. The regression models also investigated the extent to which the two types of early adversity interacted with the two types of adult adversity in predicting inflammation. When interaction effects were significant, the simple slopes were examined in order to interpret the results (Aiken and West 1991). Finally, sensitivity analyses were conducted by rerunning our hierarchical regressions using alternative measures of systemic inflammation.
Results
The correlations matrix for the study variables is presented in Table 1. Consonant with the early life sensitivity model, the table shows that exposure to harsh parenting and to early discrimination are related to inflammation. Adult discrimination is also related to inflammation, but intimate partner hostility only approaches significance. The two measures of early adversity show a modest correlation (r = .275), but there is no association between the two adult stressors. Both exposure to harsh parenting and early discrimination are correlated with adult discrimination (r = .159 and .366, respectively), but neither is related to intimate partner hostility. The various demographic and health-risk behaviors are inter-correlated but show little relation with inflammation. Indeed, the only health risk behavior that shows a significant association with inflammation is healthy diet, and contrary to expectation this correlation is positive. Consistent with past arguments and findings (Assari 2018; Geronimus et al. 2016; Pearson 2008; Simons et al. 2016) regarding the continued social and political marginalization of Blacks in the U.S., Table 1 shows that neither education nor income is related to inflammation, suggesting that increased SES brings little health advantage for our sample.
Table 1.
Correlation Matrix for the Study Variables (N=401)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Inflammation | — | |||||||||||
| 2. Harsh/Reject Parenting | .180** | — | ||||||||||
| 3. Child Discrimination | .162** | .275** | — | |||||||||
| 4. Rom Partner Hostility | .093† | .084† | .043 | — | ||||||||
| 5. Adult Discrimination | .135** | .159** | .366** | .065 | — | |||||||
| 6. Males | −.061 | −.014 | −.019 | .055 | .157** | — | ||||||
| 7. Economic Hardship | .029 | .036 | −.020 | −.022 | .046 | −.073 | — | |||||
| 8. Education | .073 | −.061 | −.056 | −.079 | .028 | −.054 | −.094† | — | ||||
| 9. Healthy Diet | .108* | −.015 | .123* | .059 | .046 | −.163** | −.048 | .124* | — | |||
| 10. Exercise | .011 | −.031 | .113* | −.007 | .140** | .480** | −.106* | .037 | .196** | — | ||
| 11. Smoking | .021 | .056 | .171** | .197** | .149** | .069 | .093† | −.181** | −.103* | .021 | — | |
| 12. Alcohol | .056 | .168** | .158** | .069 | .307** | .164** | .033 | .127* | −0.94† | .038 | .293** | — |
| Mean | 3.721 | .000 | .000 | .000 | .000 | .384 | .000 | .915 | .000 | .000 | .000 | .000 |
| SD | .764 | 1.000 | 1.000 | 1.000 | 1.000 | .497 | 1.000 | .279 | 1.000 | 1.000 | 1.000 | 1.000 |
p ≤ .10
p = .05
p = .01
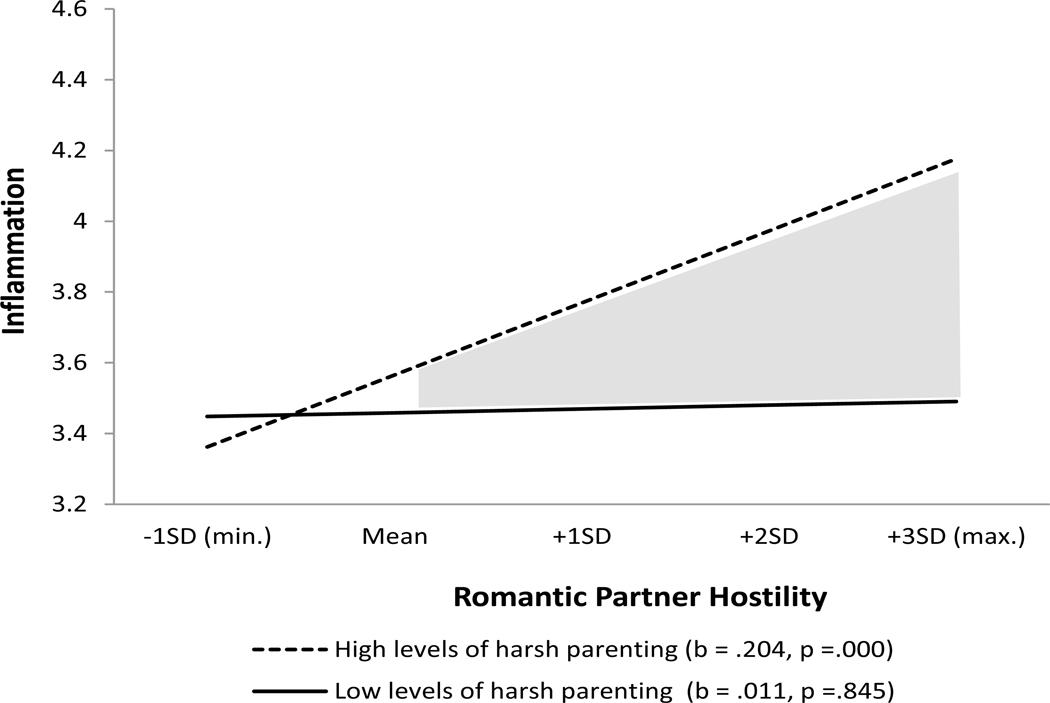
Table 2 shows the results of using hierarchical regression with robust standard errors to examine the effects of each of the early and adult stressors on inflammation while controlling for the others. Gender, childhood economic hardship and the various health risk behaviors are also included as controls. Model 1 shows that harsh parenting is the only social adversity variable to have a significant effect (β = .143, p = .006) after taking into account all of the various controls. Model 2 adds the interaction of harsh parenting and intimate partner hostility to the regression. The interaction term is significant (β = .120, p =.050) consonant with the argument that exposure to harsh parenting as a child increases the chances that adult intimate partner violence will be accompanied by an elevation in inflammation. Harsh parenting continues to demonstrate a main effect (β = .138, p =.007) on inflammation after entering the interaction term into the model, consistent with the contention by ELS theorists that parenting is a particularly important determinant of adult inflammation.
Table 2.
Regression models depicting the effects of hostile parenting and discrimination on inflammation (N=421)
| Inflammation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
| β / (t) | sig. | β / (t) | sig. | β / (t) | sig. | β / (t) | sig. | β / (t) | sig. | β / (t) | sig. | |
| Harsh/Rej Parenting | .143 (2.76) | .006 ** | .138 (2.69) | .007 ** | .147 (2.83) | .005 ** | .141 (2.74) | .007 ** | .145 (2.82) | .005 ** | .140 (2.74) | .006 ** |
| Early Discrim | .087 (1.56) | .119 | .086 (1.56) | .120 | .088 (.115) | .115 | .082 (1.48) | .141 | .064 (1.14) | .254 | .062 (1.10) | .270 |
| Romantic Part Host | .081 (1.60) | .110 | .071 (1.42) | .155 | .083 (1.52) | .102 | .081 (1.61) | .108 | .080 (1.59) | .112 | .070 (1.41) | .160 |
| Adult Discrim | .079 (1.43) | .153 | .069 (.1.24) | .214 | .085 (1.52) | .129 | .079 (1.44) | .152 | .048 (.843) | .400 | .035 (.610) | .542 |
| Control variables Male | −.060 (−1.01) | .314 | −.055 (−.918) | .359 | −.061 (1.02) | .307 | −.059 (−.979) | .328 | −.049 (−.817) | .414 | −.042 (−.709) | .479 |
| Economic Pressure | .032 (.652) | .515 | .037 (.742) | .459 | .033 (.662) | .508 | .035 (.704) | .482 | .030 (.608) | .543 | .034 (.700) | .484 |
| Education (≤ high school) | .082 (1.58) | .114 | .078 (1.51) | .130 | .085 (1.65) | .100 | .081 (1.58) | .115 | .085 (1.65) | .099 | .081 (1.59) | .114 |
| Healthy Diet | .069 (1.29) | .196 | .079 (1.47) | .140 | .067 (1.25) | .212 | .074 (1.39) | .166 | .084 (1.57) | .116 | .095 (1.79) | .075 |
| Exercise | .001 (.854) | .854 | .010 (.177) | .860 | .009 (.154) | .878 | .007 (.112) | .911 | .001 (.010) | .992 | −.001 (−.010) | .992 |
| Smoking | −.005 (−.094) | .925 | −.003 (−.074) | .963 | −.006 (−.116) | .908 | −.003 (−.059) | .953 | 001 (.004) | .997 | .003 (.062) | .951 |
| Alcohol | .−.005 (−.092) | .927 | −.004 (−.074) | .941 | −.006 (−.102) | .919 | −.004 (−.074) | .941 | −.012 (−.218) | .827 | −.011 (−.210) | .834 |
| Interactions Host Par X RP Host |
.120 (2.45) | .015 * | .120 (2.25) | .025 * | ||||||||
| Host Par X Adult Disc | −.033 (−.653) | .514 | ||||||||||
| Early Disc X RP Host | .055 (1.11) | .268 | ||||||||||
| Early Disc X Adult Disc | .112 (2.08) | .037 * | .126 (2.59) | .010 ** | ||||||||
| Constant | 3.607 (.141) | 3.634 (.141) | 3.604 (.141) | 3.612 (.142) | 3.570 (.142) | 3.597 (.142) | ||||||
| R-square | .076 | .090 | .077 | .079 | .086 | .102 | ||||||
Note: N = 413
p =.01
p =.05
Model 3 shows that the interaction of harsh parenting and adult discrimination is not significantly associated with inflammation, and Model 4 indicates that this is also the case for the interaction of early discrimination and intimate partner hostility. In contrast, Model 5 shows that the interaction of early discrimination and adult discrimination is related to inflammation (β = .112, p = .037). Importantly, Model 6 demonstrates that both the interaction of harsh parenting with intimate partner hostility and the interaction of early discrimination with adult discrimination remain significant (β = .120, p = .037 and .126, p = .010, respectively) when they are entered simultaneously into the regression model. These interaction terms were significant whether the control variables were or were not in the model. This pattern of findings is contrary to the usual interpretation of the early life sensitivity model where serious childhood adversity of any sort increases an individual’s biological responsiveness to virtually any type of adult stressor. Rather, our findings appear to support the domain specificity interpretation of the early life sensitivity model.
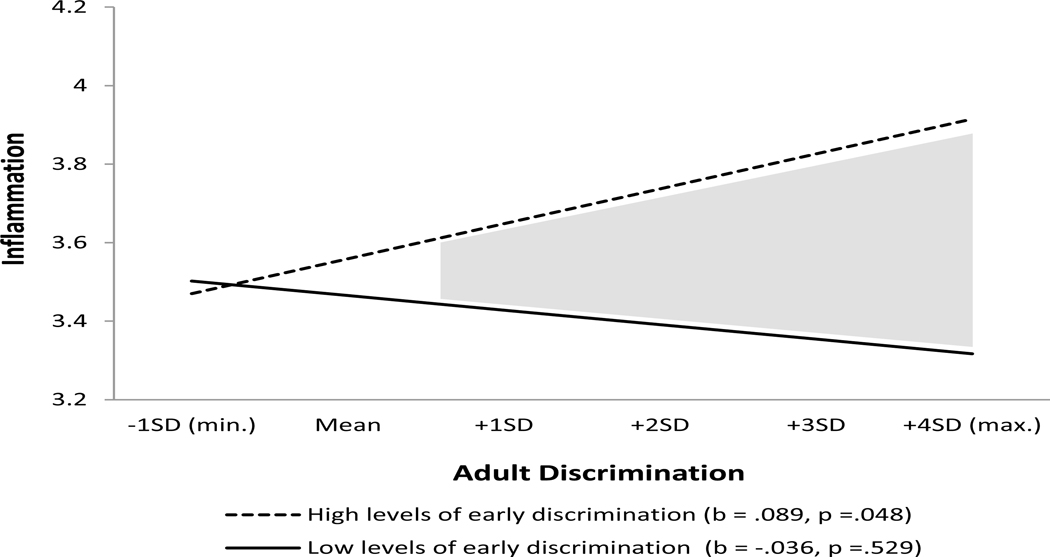
As an aid to interpretation, Figures 1 and 2 present graphs of the two interactions, respectively. Based on the simple slope test (Aiken and West 1991), Figure 1 shows that the association between intimate partner hostility and inflammation is significant (b = .204, p < .001) for respondents exposed to high levels of harsh parenting, whereas the relation between intimate partner hostility and inflammation does not approach significance (b = .011, p = .529) for those who experienced low levels of harsh parenting. Similarly, Figure 2 shows that the relation between adult discrimination and inflammation is significant (b = .089, p = .048) for respondents exposed to high levels of early discrimination, whereas the relation between adult discrimination and inflammation does not approach significance (β = −.036, p = −.529) for those who experienced low levels of early discrimination. This pattern of findings supports the domain specificity interpretation of the ELS model which posits that individuals are primed to respond emotionally and physically to adverse adult circumstances that resemble those experienced earlier in life.
Figure 1. The effect of intimate partner hostility on inflammation for individuals high and low on exposure to harsh parenting.
Note: Analysis uses Johnson-Neyman 95% confidence bands; gray areas are significant confidence regions (Roisman et al., 2012)
Figure 2. The effect of adult discrimination on inflammation for individuals high and low on early discrimination.
Note: Analysis uses Johnson-Neyman 95% confidence bands; gray areas are significant confidence regions (Roisman et al., 2012).
Given the well-known instability of interaction terms, the regression analyses were repeated using alternative approaches to constructing the inflammatory index. The first alternative involved constructing a log-transformed difference measure that entailed replacing non-detectable scores with 50% of the lower limit of detection, and then log-transforming, standardizing, and averaging across pro- and anti-inflammatory cytokines, respectively. Then, rather than forming difference scores were used to represent the relative balance of pro- to anti-inflammatory cytokines.
The second alternative approach involved dichotomizing (non-detectable vs. detectable) all 17 of the cytokines included in the Bio-Plex platform. A ratio measure was then formed using the sum of the pro- and anti-inflammatory cytokines, respectively. Finally, the third alternative involved reconstructing our trichotomous index (non-detectable, detectable, top quartile) using only those cytokines that had non-detectable levels of less than 60%. This consisted of five pro-inflammatory cytokines (IL-1β, IL-7, IL-8, MIP-1β, TNFα) and two anti-inflammatory (IL-10, IL-13) cytokines.
To begin, it should be noted that these indices were highly correlated with each other (see Online Supplement Table 2) suggesting that they were measuring the same construct. Although the new approaches reduced the skew associated with the cytokines, none was quite as effective as our original trichotomous index. Most importantly, however, all four indices (trichotomous, log-transformed difference, dichotomous, and trimmed trichotomous) generated a similar pattern of findings. First, regardless of approach, the only adversity variable to demonstrate a significant main effect was harsh parenting (see Online Supplement Table 3). Turning to the interaction effects, in every case the cross-domain interactions (harsh parenting x adult discrimination and early discrimination x hostile RP) did not approach significance (p > .5) regardless of measure whereas the within domain interactions (harsh parenting x hostile RP and early discrimination x adult discrimination) were either significant (p<.05) or approached significance (p <.08). Together, these sensitivity analyses indicate that our findings were not a function of our approach to coding the inflammatory index.
Finally, it should be noted that early in the analyses models were investigated that included mental health measures such depression and anxiety. Including these variables did not influence the study results as they were not related to chronic inflammation. Thus the pattern of results reported are not spurious to or mediated by level of mental health.
Discussion
Past research has reported that systemic inflammation is a robust predictor of the onset of chronic illness such as cardiovascular disease, diabetes, dementia, and cancer (Maggio et al. 2006; Libby and Theroux 2005 ) Importantly, studies have also found that exposure to childhood adversity increases the chances of elevated inflammation in adulthood (Miller et al. 2011; Fagundes and Way 2014). This latter finding is interpreted as support for the early life sensitivity model which posits that childhood and adolescent experiences of adversity increase an individual’s risk for poor health as an adult. Unfortunately, however, most research on the link between childhood adversity and adult inflammation has used retrospective reports of adversity, has failed to either control for adult stressors or examine the interaction between childhood and adult stressors, and has assumed a simple model where any of a wide variety of childhood adversities are seen as having a similar effect on inflammation. The current study was an attempt to overcome these limitations. Longitudinal, prospective data was used to assess early adversity, both childhood and adult stressors were assessed, both main and interaction effects were investigated, and systemic inflammation was assessed using s more comprehensive index than has been utilized in most past research.
The results provided rather strong support for elements of the early life sensitivity model. First, exposure to harsh parenting had a direct effect on adult inflammation. This is consistent with the assertion of the early life sensitization model that quality of parenting is particularly important in terms of preparing individuals for a threatening future (Del Giudice et al. 2011; Gluckman et al. 2008). It suggests that harsh parenting may foster cognitive biases and/or biological adaptations that lead to an elevated inflammatory response regardless of the adult environment. Second, the results showed significant interactions between early adversity and adult stress, supporting the idea that early adversity amplifies a person’s response to stressors encountered later in life. The early life sensitivity model, however, is generally interpreted as positing that exposure to any of a broad range of adverse childhood conditions will foster an increased inflammatory response to any and all adult stressors. Findings from the present study supported a more nuanced understanding of the early life sensitivity model. The results indicated that harsh parenting increased the probability of elevated inflammation in response to intimate partner hostility (but not to adult discrimination), whereas early discrimination increased the probability of a heightened inflammatory response to adult discrimination (but not to intimate partner hostility). This general pattern held across various approaches to constructing our inflammatory index, although in some cases the interactions only approached significance. These findings suggest that individuals may be primed to respond physiologically to adverse adult circumstances that resemble or fall into the same domain as those experienced earlier in life. If this is the case, it may be that some of the inconsistent findings reported in past research are a consequence of lumping childhood adversities together (e.g., ACE), rather than assessing separate domains.
Unfortunately, our findings do not provide information regarding the mechanisms that account for this link between early adverse experiences and adult inflammatory responses. It is likely, however, that either cognitive schemas, biological calibration, or some combination of the two, explain this pattern. With regard to cognitive schemas, a wide variety of developmental (Bowlby 1982) and social psychological (Dodge 1980) research has demonstrated that particular types of childhood experiences give rise to cognitive beliefs and assumptions that tacitly color the way an individual processes and responds to certain categories of situations. In sociology, Bourdieu’s (1990) popular theoretical perspective emphasizes the way that individuals internalize events and conditions from their personal history to form a habitus consisting of various schemata, dispositions, and assumptions that shape, in a largely unconscious fashion, the way particular situations are processed, defined, and experienced. Bourdieu, like other social schematic theorists, emphasizes the importance of early life conditions in forming fundamental and relatively durable cognitive structures that operate across the life course. This suggests that the main effect of harsh parenting on adult inflammation might be explained by distrustful dispositions that lead to a guarded approach to life. And, the interaction of harsh parenting with intimate partner hostility might be a consequence of a dispositional hypersensitivity to rejection in family or intimate relationships. Similarly, early discrimination may give rise to assumptions and expectations that promote heightened feelings of threat and guardedness in situations where racial stereotyping, identity threat, or similar processes appear to be operating.
An alternative explanation is that early adversity calibrates the inflammatory program of the immune system so that it becomes hyper-responsive to certain classes of stimuli. It is widely accepted among early life sensitivity researchers that adversity acts as a programming agent that shapes operating tendencies of the inflammatory program of the immune system in at least two ways (Ehrlich et al. 2016; Miller et al. 2011): cells show more pronounced inflammatory responses when exposed to challenge, and they are less able to terminate these responses given their reduced sensitivity to inhibitory hormonal signals (e.g., cortisol). As a response to this recalibration, an individual is expected to show an exaggerated inflammatory response to stressors that occur later in life. Evidence supporting the idea of recalibration comes from in-vitro laboratory studies showing that inflammatory white blood cells drawn from adolescents who have experienced high adversity or low SES show greater responsiveness to bacterial stimulation and less responsiveness to cortisol than the cells of adolescents who had experienced less stressful environments (Ehrlich et al. 2016; Miller et al. 2011). Other studies, however, have failed to find these effects (e.g., John-Henderson et al. 2016) and no studies have demonstrated that this pattern of cell reactivity is related to adult inflammation, that it increases the inflammatory response to adult stress, or that it enhances the chance of illness. Thus, at this point, it is not clear how much the link between early adversity and adult inflammation is a function of the development of schemas and how much is due to calibration of the immune system to be more reactive. There is a need for longitudinal research that assesses the relative contribution of these two processes.
Although the current study overcame several of the weaknesses of past research, it also suffered from certain limitations. First of all, the dataset used for the analyses contains no measures of early childhood exposure to adversity. Assessments of childhood adversity were collected at ages 10 and 12. Some versions of the early live sensitivity model emphasize the importance of early childhood programming and it may be that exposure to adversity during the preschool or early elementary school years has an even bigger impact on adult inflammation than were evident using assessments from late childhood.
Second, the study focused upon only two childhood and two adult stressors. While past research has shown these childhood stressors to be distressing and consequential for health, it might be that a different pattern of findings would be obtained with a wider variety of types of adversity (e.g., school problems, extreme poverty). Future research needs to examine the domain specificity hypotheses using additional stressors.
Third, failure to find a main effect for intimate partner hostility on inflammation may have been due to the low frequency of hostility, or to our decision to code the absence of a romantic relationships as absence of exposure to partner hostility. Also, all of the measures of family adversity and racial discrimination were self-report, and hence we cannot rule out the possibility that response bias or personality characteristics contributed to some of our findings. This concern is mitigated, however, by the fact that the childhood and adult assessments occurred nearly 20 years apart.
Fourth, chronic inflammation was the only marker of health risk used in the present study. Although inflammation has been shown to be involved in the onset of virtually all age-related chronic illnesses, it would be interesting to see if a similar pattern of results would be obtained using transcriptional or methylomic measures of aging, as these biomarkers also predict chronic illness. Further, our measure of inflammation was only available at Wave 7. Although observational studies rarely include longitudinal assessments of biomarkers such as inflammation, a better understanding of the mechanisms that link childhood adversity to adult inflammation will require data sets that contain both childhood and adult measures of inflammation.
Lastly, it should be noted that lifestyle factors such as diet, exercise, and substance use were largely unrelated to inflammation in our analyses. The marginal effect of these variables might be attributable, at least in part, to the well-known limitations of using self-reports to measure them, as well as to the limited variation associated with some of the variables (e.g., most of the respondents were eating an unhealthy diet). Alternatively, it may be that the inflammatory consequences of these lifestyle behaviors take a while to build up and will become more evident as the respondents become older.
Conclusion
Consistent with the early life sensitivity model, several studies have found that exposure to childhood adversity increases the chances of elevated inflammation in adulthood. Much of this research is limited, however, by the use of retrospective reports to assess adversity, a failure to either control for adult stressors or to examine the interaction between childhood and adult stressors, and the assumption of a simple model where any of a wide variety of childhood adversities are seen as having a similar effect on adult inflammation. In large measure the current study was able to overcome these limitations. The results indicated that respondents who had experienced little adversity as children showed biological resilience to the adult stressors included in our study. For these individuals, adult exposure to either intimate partner hostility or discrimination had no significant impact on their level of chronic inflammation. Conversely, exposure to harsh parenting produced lasting effects on an individual’s risk for elevated inflammation. And, childhood exposure to either harsh parenting or racial discrimination amplified the effects of analogous adult stressors. These findings provide further evidence that childhood adversity represents a significant health risk that continues to exert a deleterious effect in adulthood. Although there is little evidence regarding the extent to which these childhood risks, whether they be negative schemas or immune system programming, can be mitigated by interventions or a supportive environment, it is possible that an adult social environment characterized by high and consistent levels of nurturance and support might foster more positive schemas and recalibrate the inflammatory program (Johnson and Acabchuk 2018). This idea is bolstered by studies reporting that cognitive therapy designed to foster a more positive outlook (Zhang et al. 2016) and lifestyle interventions that include a focus on increased social support (Pischke et al. 2008; Silberman et al. 2010) are associated with increased cardiovascular and metabolic health. Such findings point to the importance of future studies that explore the extent to which such interventions or naturally occurring corrective experiences that occur in close relationships can be beneficial in countering the pro-inflammatory consequences of having faced particular adversities such as harsh parenting and early discrimination.
Supplementary Material
Acknowledgments
Funding. This work was supported by the National Heart, Lung, Blood Institute (R01 HL118045), the National Institute on Child Health and Human Development (R01 HD080749), the National Institute on Aging (R01 AG055393), the National Institute on Drug Abuse (R21 DA034457), and the National Institute of Mental Health (R01 MH62699, R01 MH62666). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography
Ronald L. Simons is Distinguished Research Professor in the Department of Sociology and Co-Director of the Center for Biological Embedding of Social Conditions and Relationships at the University of Georgia. His research uses a life course perspective to study crime, mental health, speed of biological aging, morbidity, and mortality.
David Woodring is a doctoral candidate in the Department of Sociology at the University of Georgia. His research interests include causes of crime, police shootings, and the biological embedding of stress.
Leslie Gordon Simons is Professor of Sociology at the University of Georgia. Her program of research focuses on the social contextual predictors and consequences of various family processes as well as the mediators and moderators of the relationship between experiences in the family of origin and outcomes for adolescents and emerging adults.
Tara E. Sutton is Assistant Professor of Sociology at Mississippi State University. Her research focuses on the social and familial contexts of intimate partner violence, sexual assault, child abuse and other criminal and deviant behaviors among adolescents and adults.
Man-Kit Lei is Assistant Professor of Sociology at the University of Georgia. His research interests include social determinants of health, aging, social epidemiology, criminology and advanced quantitative methods and measurements.
Steven S.R. Beach is Distinguished Professor in the Department of Psychology and Co-Director of the Center for Family Research as well as the Center for Biological Embedding of Social Conditions and Relationships at the University of Georgia. His research interests include epigenetics, marital and family processes, and depression and anxiety in close relationships.
Ashley Barr is Assistant Professor of Sociology at the State University of New York at Buffalo. Her research interests include the individual, interpersonal, and contextual factors that influence the formation and well-being of romantic and family relationships, and the way in which these relationships play out across domains such as health, education, and criminal behavior.
Frederick X. Gibbons is Professor Psychology at the University of Connecticut. He applies social psychology to the study of health (substance use, risky sex, cancer risk behavior), with a particular focus on the effects of racial prejudice and discrimination.
Footnotes
Data Sharing Declaration
The datasets generated or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Standards of ethical responsibility have been followed. None of the findings in this paper have been published elsewhere. All authors read and approved the final manuscript. The order of authorship reflects the relative level of contribution make by each of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical Approval. The Institutional Review Board of the University of Georgia approval the study and its informed consent procedures.
Informed Consent. Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
The authors report no conflicts of interest.
Contributor Information
Ronald L. Simons, Department of Sociology, University of Georgia, Athens, GA 30602
David Woodring, Department of Sociology, University of Georgia, Athens, GA.
Leslie Gordon Simons, Department of Sociology, University of Georgia, Athens, GA 30602.
Tara E. Sutton, Department of Sociology, University of Georgia, Athens, GA 30602
Man-Kit Lei, Center for Family Research, University of Georgia, Athens, GA 30605.
Steven R. H. Beach, Department of Psychology, University of Georgia, Athens GA 30602
Ashley B. Barr, Department of Sociology, SUNY Buffalo, Buffalo, NY 14260
Frederick X. Gibbons, Department of Psychological Sciences, University of Connecticut, Storrs, CT 06269
References
- Abbas A, Lichtman AH, & Pillai S. (2011). Cellular Molecular Immunology. 7th ed. Philadelphia: Saunders. [Google Scholar]
- Aiken LS & West SG. (1991). Multiple Regression: Testing and Interpreting Interactions. Newbury Park: Sage. [Google Scholar]
- Assari S. (2018). Health disparities due to diminished return among black Americans: Public policy solutions. Social Issues and Policy Review, 12, 1–34. [Google Scholar]
- Bae D, & Wickrama KA (2017). Stress Processes Linking Parent–Child Disconnection to Disease Risk in Young Adulthood: Amplification by Genotype. Journal of Youth and Adolescence, 46(5), 1137–1148. [DOI] [PubMed] [Google Scholar]
- Bandura A. (1986). Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Basu A, McLaughlin KA, Misra S, & Loenen K. (2017). Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clinical Psychology, 24, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista ML, Rosa JC, Lopes RD, Lira FS, Martins E, Yamashita AS, et al. (2010). Exercise training changes IL-10/TNF-alpha ratio in the skeletal muscle of post-MI rats. Cytokine, 49, 102–108. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Lei M, Simons RL, Barr A, Simons LG, Ehrlich K, et al. (Forthcoming). Parenting experiences in childhood affect African American young adult’s inflammation, depression, and their covariation: Longitudinal effects through stress and romantic partner relationships. Development and Psychopathology. [Google Scholar]
- Bourdieu P. (1990). The Logic of Practice. Stanford University Press. [Google Scholar]
- Bowlby J. (1982). Attachment and Loss: Vol. 1 (2nd ed.). New York: Basic Books. [Google Scholar]
- Brody GH, Yu T, Miller GE, Chen C. (2015). Discrimination, racial identity, and cytokine levels among African-American adolescents. Journal of Adolescent Health 56:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt CH, Simons RL, & Gibbons FX (2012). Racial discrimination, ethnic-racial socialization, and crime. American Sociological Review, 77, 648–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JS, Dellucci T, Turek C, & Mir S. (2015). Predicting depressive symptoms and weight from adolescence to adulthood: stressors and the role of protective factors. Journal of Youth and Adolescence, 44(11), 2122–2140. [DOI] [PubMed] [Google Scholar]
- Cassidy J, & Shaver PR, eds. (2008). Handbook of Attachment: Theory, Research, and Clinical Applications. New York: Guilford Press. [Google Scholar]
- Chen E, Cohen S, & Miller GE (2010). How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science, 21, 31–37. [DOI] [PubMed] [Google Scholar]
- Cole SW (2013). Social regulation of human gene expression: Mechanisms and implications for public health. American Journal of Public Health 103:S84–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW (2014). Human social genomics. PLoS Genetics 10(8):e1004601. 10.1371/journal.pgen.1004601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conraads VM, Denollet J, De Clerck LS, Stevens WJ, Bridts C, & Vronts, C.J. (2006). Type D personality is associated with increased levels of tumour necrosis factor (TNF)-alpha an TNF-alpha receptors in chronic heart failure. International Journal of Cardiology, 113, 34–38. [DOI] [PubMed] [Google Scholar]
- Cui M, Lorenz FO, Conger RD, Melby JN, & Bryant CM (2005). Observer, self- and partner reports of hostile behaviors in romantic relationships. Journal of Marriage and Family, 67, 1169–1181. [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R( 2007). Childhood maltreatment predicts adult inflammation in a life-course study.” Proceedings of the National Academy of Sciences 104:1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, & Belsky J. (2011). Parent-child relationships Pp. 65–82 in The Oxford Handbook of Evolutionary Family Psychology, edited by Salmon C and Shackleford TK. New York: Oxford University. [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, & Wolkowitz OM (2009). Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. Journal of Psychiatric Research, 43, 962–969. 10.1016j/j.jpsychires.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Dodge KA (1980). Social cognition and children’s aggressive behavior. Child Development, 51,162–170. [PubMed] [Google Scholar]
- Ehrlich KB, Ross KM, Chen E, & Miller GE (2016). Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Development and Psychopathology, 28, 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G & Feldman SI (1996). Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology.70, 1327–1343. [DOI] [PubMed] [Google Scholar]
- Fagan AA, Wright EM, & Pinchevsky GM (2014). The protective effects of neighborhood collective efficacy on adolescent substance use and violence following exposure to violence. Journal of Youth and Adolescence, 43(9), 1498–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, & Way B. (2014). Early-life stress and adult inflammation. Current Directions in Psychological Science, 23, 277–283. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DR, Spitz AM, Edwards V, et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study.” American Journal of Preventative Medicine, 14, 245–58. [DOI] [PubMed] [Google Scholar]
- Ferster CB, & Perrott MC (1968). Behavior Principles. New York: Appleton-Century-Crofts. [Google Scholar]
- Fisher JH, & Brown JL (2018). A prospective, longitudinal examination of the influence of childhood home and school contexts on psychopathic characteristics in adolescence. Journal of Youth and Adolescence, 1–19. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Algoe SB, Firestine AM, Jesusa MG Ma JA, & Cole SW (2015). Psychological well-being and the human conserved transcriptional response to adversity. PLoS ONE, 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, James SA, Destin J, Graham LG, Hatzenbuehler ML, Murphy MC, et al. (2016). Jedi public health: Co-creating an identity-safe culture to promote health equity. Social Science and Medicine – Population Health, 2, 105–116. 10.1016/j.ssmph.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard M, Stock ML Roberts ME, Gibbons FX, O’Hara RE, Weng C-Y, & Wills T. (2012). Coping with racial discrimination: The role of substance use. Psychological Addictive Behavior, 26, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons FX, Gerrard M, Cleveland MJ, Wills TA, & Brody GH 2004. Perceived discrimination and substance use in African American parents and their children: A panel study. Journal of Personality and Social Psychology, 86, 517–529. [DOI] [PubMed] [Google Scholar]
- Gibbons FX, Yeh H-C, Gerrard M, Cleveland MJ, Cutrona C, Simons RL, & Brody GH (2007). Early experience with racial discrimination and conduct disorder as predictors of subsequent drug use: A critical period hypothesis. Drug and Alcohol Dependence 88:S27–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J-P, van Os J, Portegijs PJM, & Myin-Germeys I. (2006). Childhood trauma and emotional reactivity to daily life stress in adult frequent attenders of general practitioners. Journal of Psychosomatic Research, 61, 229–236. [DOI] [PubMed] [Google Scholar]
- Gluckman P, & Hanson M. (2006). (Eds.) Developmental Origins of Health and Disease. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Gluckman P, Hanson M, Cooper C & Thornburg KL (2008). Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine, 359, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf DQ, & Kiecolt-Glaser JK (2012). Childhood abuse and inflammatory responses to daily stressors. Annuals of Behavioral Medicine, 44, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway CR, Sterrett-Hong E, Larkby CA, & Cornelius MD (2016). Family resources as protective factors for low-income youth exposed to community violence. Journal of Youth and Adolescence, 45(7), 1309–1322. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Lackman ME, Mroczek DK, Seeman TE, & Miller GE (2015). Additive contributions of childhood adversity and recent stressors to Inflammation at midlife: Findings form the MIDUS Study. Developmental Psychology, 51, 1630–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. [Google Scholar]
- John-Henderson NA, Marsland AL, Kamarck TW Muldoon MF & Manuck SB (2016). Childhood socioeconomic status and the occurrence of recent native life events as predictors of circulating and stimulated levels of IL6. Psychosomatic Medicine, 78, 91–101. 10.1097/PSY.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BT, & Acabchuk RL (2018). What are the keys to a longer, happier life? Answers from five decades of health psychology. Social Science and Medicine, 196, 218–226. 10.1016/j.socscimed.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerig PK, Ward RM, Vanderzee KL, & Moeddel MA (2009). Posttraumatic stress as a mediator of the relationship between trauma and mental health problems among juvenile delinquents. Journal of Youth and Adolescence, 38(9), 1214–1225. [DOI] [PubMed] [Google Scholar]
- Kerr M, Stattin H, & Burk WJ (2010). A reinterpretation of parental monitoring in longitudinal perspective. Journal of Research on Adolescence, 20, 39–64. [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, & Glaser R. (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine, 73, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer W. (2016). Victimization and biological stress responses in urban adolescents: Emotion regulation as a moderator. Journal of Youth and Adolescence, 45(9), 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Smith GD, Rumley A, Lowe G, & Ebrahim S. (2005). Associations of fibrinogen and c-reactive protein with prevalent and incidental coronary heart disease are attenuated by adjustment of confounding factors. Thrombosis and Haemostatis, 93, 955–963. [DOI] [PubMed] [Google Scholar]
- Libby P, & Theroux P. (2005). Pathophysiology of coronary artery disease. Circululation, 111, 3481–3488. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Pilote L Lynch JW, Richard H, Almeida ND, Benjamin EJ, & Murabito JM (2010). Life course socioeconomic position is associated with inflammatory markers: The Framingham Offspring Study. Social Science & Medicine, 71, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, & Ferrucci L. (2006). Interleukin-6 in aging and chronic disease: A magnificent pathway. Journals of Gerontology Series a-Biological Sciences and Medical Sciences, 61, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanism. Psychological Bulletin,137, 959–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RC III (2017). Breast cancer survivors, common markers of inflammation, and exercise: A narrative review. Breast Cancer: Basic and Clinical Research, 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisette-Thomas V, Cohen A, Fulop T, Riesco E, Legault V, Li Q, et al. (2014). Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mechanisms of Ageing Development, 139, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, & Muthen BO (2015). Mplus version 7.04: Base program and combination add-on. Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Nowakowski ACH, & Sumerau JE. (2015). Swell foundations: Funadmental causes and chronic inflammation. Sociological Spectrum 35:161–178. [Google Scholar]
- Nowland R, Talbot R, & Qualter P. (2018).Influence of loneliness and rejection sensitivity on threat sensitivity in romantic relationships in young and middle-aged adults. Personality and Individual Differences, 131, 185–190. [Google Scholar]
- Paalani M, Lee JW, Haddad E, & Tonstad S. (2011). Determinants of inflammatory markers in a bi-ethnic population. Ethnicity & Disease, 21, 142–149. [PMC free article] [PubMed] [Google Scholar]
- Pajkart D, Camoglio L, Tiel-van Buul MCM, et al. (1997). Attenuation of pro-inflammatory response by recombinant human IL-10 in human endotoxemia: the effect of timing of rhIL-10 administration. Journal of Immunology, 158, 3971–3977 [PubMed] [Google Scholar]
- Peres A, Perotto DL, Dorneles GP, Fuhro MIS, & Monteiro MB (2015). Effects of intradialytic exercise on sysstemic cytokine in patients with chronic kidney disease. Renal Failulre, 37, 1430–1434. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, & Manuck SB (2009). Parental education is related to c-reactive protein among female middle-aged community volunteers. Brain, Behavior, and Immunity, 23, 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischke CR, Scherwitz L, Weidner G,& Ornish D. (2008). Long-term effects of lifestyle changes on well-being and cardiac variables among coronary heart disease patients. Health Psychology, 27, 584–592. [DOI] [PubMed] [Google Scholar]
- Rickard IJ, &Lummaa V. (2007). The predictive adaptive response and metabolic syndrome: challenges for the hypothesis. Trends in Endocrinology & Metabolism 18:94–99. [DOI] [PubMed] [Google Scholar]
- Simons LG, Simons RL, Bryant AM, Bryant CM, & Beach SRH (2014). Factors linking childhood experiences to adult romantic relationships among African Americans. Journal of Family Psychology, 28:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Beach SRH, Brody GH, Philibert R, & Gibbons FX (2011). Social environment, genes, and aggression: Evidence supporting the differential susceptibility perspective. American Sociological Review 76:883–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Beach SRH, Barr AB, Simons LG, Gibbons FX, & Philibert RA Philibert. (2018). Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Developmental Psychology, 54, 1993–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Beach SRH, Philbert RA, Cutrona CE, Gibbons FX, & Barr AB 2016. Economic hardship and biological weathering: Epigenetic aging in a U.S. sample of black women.” Social Science & Medicine 150:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Murry V, McLoyd V, Cutrona C & Conger RD (2002). Discrimination, crime, ethnic identity, and parenting as correlates of depressive symptoms among African American children: A Multilevel Analysis. Development and Psychopathology. [DOI] [PubMed] [Google Scholar]
- Slavich GM, & Cole SW (2013). The emerging field of human social genomics. Clinical Psychological Science, 1, 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Danese A,….Zachariah JP (2018). Childhood and adolescent adversity and cardiometabolic outcomes. Circulation, 137:e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Trettevik R, Kent de Grey RG, Cronan S, Hogan J, & Baucom BRW (2018). Social support, social integration, and inflammatory cytokines: A meta-analysis. Health Psychology, Online First publication, March 22, 2018. 10.1037/hea0000594. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, McAllister-Sistilli CG, Kelley ME, Gurklis JA, & Yao JK (1999). Elevated interleukin-6 in schizophrenia. Psychiatry Research, 87(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Williams DR (2012). Miles to go before we sleep: Racial inequities in health. Journal of Health & Social Behavior, 53, 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Songli M, Yang R, Chen L, Gao H, Li L. (2016). Effects of lifestyle intervention using patient-centered cognitive behavioral therapy among patients with cardio-metabolic syndrome: A randomized, controlled trial. BMC Cardiovascular Disorders 16, 227 DOI 10.1186/s12872-016-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zona K, & Milan S. (2011). Gender differences in the longitudinal impact of exposure to violence on mental health in urban youth. Journal of Youth and Adolescence, 40(12), 1674–1690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.