Abstract
Intravenous propofol, fentanyl, and midazolam are utilized commonly in critical care for metabolic suppression and anesthesia. The impact of propofol, fentanyl, and midazolam on cerebrovasculature and cerebral blood flow (CBF) is unclear in traumatic brain injury (TBI) and may carry important implications, as care is shifting to focus on cerebrovascular reactivity monitoring/directed therapies. The aim of this study was to perform a scoping review of the literature on the cerebrovascular/CBF effects of propofol, fentanyl, and midazolam in human patients with moderate/severe TBI and animal models with TBI. A search of MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and the Cochrane Library from inception to May 2020 was performed. All articles were included pertaining to the administration of propofol, fentanyl, and midazolam, in which the impact on CBF/cerebral vasculature was recorded. We identified 14 studies: 8 that evaluated propofol, 5 that evaluated fentanyl, and 2 that evaluated midazolam. All studies suffered from significant limitations, including: small sample size, and heterogeneous design and measurement techniques. In general, there was no significant change seen in CBF/cerebrovascular response to administration of propofol, fentanyl, or midazolam during experiments where PCO2 and mean arterial pressure (MAP) were controlled. This review highlights the current knowledge gap surrounding the impact of commonly utilized sedative drugs in TBI care. This work supports the need for dedicated studies, both experimental and human-based, evaluating the impact of these drugs on CBF and cerebrovascular reactivity/response in TBI.
Keywords: brain injury, cerebral blood flow, cerebrovascular response, fentanyl, midazolam, propofol
Introduction
Intravenous anesthesia is used universally within care for patients with severe brain injury for its neuroprotective properties.1 Its use is not limited to its ability to moderate cerebral metabolism; it also provides a more stable cerebral physiology in the presence of the severe trauma.1,2 Despite large-scale use of intravenous anesthetic agents, the impact that these commonly employed drugs have on various aspects of cerebral physiology in critical care patients, especially those with a traumatic brain injury (TBI), is largely unknown. This is in spite of their widespread adoption and recommendation through consensus-based guidelines for the management of moderate/severe TBI.3–5
Of particular interest is the impact on cerebral blood flow (CBF) and cerebrovascular reactivity of such sedative agents in TBI care, as current clinical guidelines focus on improving cerebral perfusion, CBF, and end-organ nutrient delivery.3,6–9 The body of literature surrounding the link between impaired cerebrovascular reactivity and poor patient outcome after TBI is growing,10–14 with data suggesting that in modern TBI care much of the ongoing cerebral physiological insult seen is dominated by impaired cerebrovascular reactivity.9,12,13,15 Further, cerebrovascular reactivity-based individual cerebral physiological targets, such as optimal cerebral perfusion pressure (CPPopt)8,16–18 or individual intracranial pressure (iICP) thresholds,19,20 are emerging as novel methods to personalize treatment in TBI. Understanding the effects these commonly employed sedative agents have on CBF/cerebrovascular reactivity in the patient with severe TBI is a pivotal step in advancing personalized care.
The goal of this study was to perform a systematically conducted scoping review of all available literature on the impact of three commonly employed sedative agents used in moderate/severe TBI care (i.e., propofol, fentanyl, and midazolam) on cerebrovascular responsiveness/CBF response in human patients with moderate/severe TBI and animal TBI models.
Methods
A systematic review of the available literature was conducted using the methodology outlined in the Cochrane Handbook for Systematic Reviews of Interventions.21 The data were reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).22 Supplementary Table S1 provides the PRISMA checklist. The review questions and search strategy were decided upon by the supervisor (F.A.Z.) and primary author (L.F.).
Ethical considerations
All articles are from previously published journals and have been vetted by their respective journals.
Search question, population, and inclusion and exclusion criteria
The question posed for systematic review was: “What is the effect of exogenous systemically administered propofol, fentanyl, or midazolam on the cerebrovascular response/CBF in human patients with moderate/severe TBI and animal models with TBI?” All studies, prospective and retrospective, of any size, based on humans and animals were included.
The primary outcome measure was the impact on CBF or the cerebrovascular responsiveness as documented by any objective means of CBF/cerebrovascular reactivity assessment, including continuous measures and neuroimaging-based or blood sampling-based techniques.
All original studies, whether prospective or retrospective, of all sizes, of any human age category or animal TBI model design, with the use of propofol/fentanyl/midazolam, and with formal documentation of cerebrovascular response/CBF during administration were eligible for inclusion in this review. Exclusion criteria were as follows: mild TBI literature, non-TBI human literature, being a non-English language study, or conducting CBF mediation with a substance other than propofol/fentanyl/midazolam.
Search strategy
MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and the Cochrane Library from inception to May 2020 were searched using individualized search strategies for each database. The search strategy for MEDLINE can be found in Supplementary Table S2, and a similar search strategy was used for the other databases. Finally, the reference lists of reviewed articles on the cerebral blood vessels/CBF response to propofol, fentanyl, and midazolam were examined to ensure no references were left out.
Study selection
Using two reviewers (L.F. and J.D.), a two-step review of all articles returned by our search strategies was performed. First, the reviewers independently screened all titles and abstracts of the returned articles to decide whether they met the inclusion criteria. Second, full text of the chosen articles was assessed to confirm whether the articles met the inclusion criteria and that the primary outcome of CBF/cerebrovascular response to propofol, fentanyl, and midazolam was documented. Any discrepancies between the two reviewers were resolved by a third party (F.A.Z.).
Data collection
Data were extracted from the selected articles and stored in multiple electronic databases to ensure data integrity.
Human studies
Data fields included the following: number of patients/animals, type of study, patient/model characteristics, the goal of the study, dose of anesthetic administered, type of anesthetic administered, technique of CBF/vasculature assessment, CBF/cerebral vasculature response to drug, other outcomes, and general conclusions.
Bias assessment
Given the goal of this review was to provide a comprehensive scoping overview of the available literature, a formal bias assessment was not conducted.
Statistical analysis
A meta-analysis was not performed in this study because of the heterogeneity of model types, study designs, and data.
Results
Search results and study characteristics
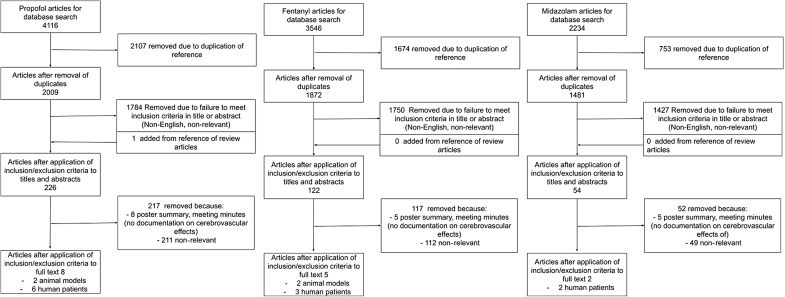
The results of the search strategy across all databases and reference sections of articles are summarized in Figure 1. Overall, a total of 9896 articles were identified, all from the databases searched. A total of 4534 were removed because of duplication of references, leaving 5362 to review. By applying the inclusion/exclusion criteria to the title and abstract of these articles, we identified 400 articles that fit these criteria. One article was added from reference sections of pertinent review articles, leaving a total of 401 articles to review. The portable document formats (PDFs) of these 401 were then gathered. Applying the inclusion/exclusion criteria to these PDFs, only 14 articles were found eligible for inclusion in the systematic review.
FIG. 1.
PRISMA flow diagram. PRISMA, preferred reporting in systematic reviews and meta-analysis.
Within the 14 TBI studies identified, there were 10 human TBI studies, and 4 animal TBI model studies. In the 10 human TBI studies, all the patients suffered a moderate/severe TBI, with human patients having a Glasgow Coma Scale (GCS) score of 12 or less on presentation. All studies measured CBF response to propofol, fentanyl, midazolam, and other agents: 5 used arterio-jugular differences of oxygen (AVDO2),10,23–26 2 studies used a Xenon133 diffusion technique,27,28 1 study used laser speckle imaging,29 1 study used radiolabeled microsphreres,30 4 studies used transcranial-Doppler flow velocity,10,26,28,31 and 4 studies used CPP/PO224,32–34 as a surrogate for CBF.35 There were 3 studies that evaluated cerebrovascular reactivity/responsiveness, as measured by response of CBF to CO2 reactivity25,26 or a variety of other methods that used CBF and CBF velocity (CBFv).28 Regarding specific sedative agent studies, there were 8 studies that used propofol (2 of which used rat models29,31), 5 studies that used fentanyl (1 used rats36 and 1 used cats30), and 2 studies that used midazolam. The characteristics of the studies can be found in Table 1, Table 2, and Supplementary Table S3.
Table 1.
Included Studies: General Characteristics and Study Goals
| References | No. patients/animals | Study type | Article location | Mean age | Patient/Animal characteristics | Primary and secondary goal of study |
|---|---|---|---|---|---|---|
| Human studies | ||||||
| Lee et al.28 | 28 patients | Prospective cohort study | Journal | 33 ± 13 years | TBI patients with GCS <7 | Primary: Assess influence of CO2 reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury |
| Secondary: Compare hemisphere response in CBF velocity | ||||||
| Steiner et al.10 | 10 patients | Prospective cohort study | Journal | 35 ± 12 years | TBI patients with GCS score ≤12, 7 men and 3 women, with evacuated mass lesion in 8, diffuse injury 2 in 2, large bilateral lesions in 5, and 7 had a craniectomy | Primary: Effect of propofol plasma concentration on pressure autoregulation |
| James et al.34 | 8 patients | Prospective randomized unblinded single crossover observational pilot study | Journal | Not mentioned | 4 patients with TBI, 3 with subarachnoid hemorrhage and one with intracerebral hemorrhage with median GCS score of 6.1 and acute physiology and chronic health evaluation 2 scores of 13.5 | Primary: Effects of dexmedetomidine and propofol on cerebral physiology in acute brain injury patients |
| Johnston et al.23 | 10 patients | Prospective cohort study | Journal | 21–53 years | TBI patients with GCS score 3–9, 8 patients with evacuated mass lesion and 2 with diffuse injury 2 | Primary: Assess the effect of propofol on cerebral oxygenation and metabolism in head-injured patients |
| Secondary: Use propofol to achieve EEG burst suppression and evaluate overall effects on ischaemic burden | ||||||
| Pinaud et al.27 | 10 patients | Prospective cohort study | Journal | 14–40 years | TBI patients with GCS score ≤6 | Primary: Effects of propofol on cerebral hemodynamics and metabolism in TBI patients |
| Tanguy et al.33 | 30 patients | Retrospective cohort study | Journal | 35 ± 18 years | TBI patients with acute physiological score 2–4 with mean GCS score 5 | Primary: Compare the cerebral microdialysis effects of propofol vs midazolam in TBI patients |
| Albanèse et al.24 | 6 patients | Randomized unblended crossover study | Journal | 20–44 years | TBI male patients with GCS score 4–8 | Primary: Assess the effects of sufentanyl, fentanyl, and alfentanil on cerebral hemodynamics |
| de Nadal et al.26 | 30 patients | Randomized crossover study | Journal | 30 ± 13 years | TBI patients with GCS score ≤8 | Primary: Evaluate the cerebral hemodynamic effects of morphine and fentanyl in TBI patients |
| Secondary: Correlation of morphine and fentanyl to cerebral autoregulation | ||||||
| de Nadal et al.25 | 30 patients | Prospective cohort study | Journal | 30.2 ± 13.2 years | TBI patients with GCS score ≤8 | Primary: Evaluate the effects of fentanyl in TBI patients |
| Papazian et al.32 | 12 patients | Prospective cohort study | Journal | 14–44 years | TBI patient with GCS score ≤6 | Primary: Effect of midazolam on ICP and CPP in TBI |
| Secondary: Evaluate cerebral damage by CPP increase | ||||||
| Animal studies | ||||||
| Feuerstein et al.29 | 28 rats | Four-arm study | Journal | Not applicable | Male Wistar rats initially anesthetized with isoflurane, TBI method not mentioned | Primary: Evaluation of different methods to detect CBF and tissue deterioration after TBI |
| Kahveci et al.31 | 16 rats | Two-arm study | Journal | Not applicable | Female Wistar rates with hypothermia and TBI caused from accelerated impact | Primary: Effects of propofol and isoflurane on cerebral hemodynamics during hypothermic conditions |
| Bedell et al.30 | 17 cats | Two arm-study | Journal | Not applicable | Cats initially anesthetized with isoflurane and nitrous oxide, then TBI was induced with a fluid percussion injury | Primary: Influence of fentanyl on CBF during hypotension after TBI |
| Statler et al.36 | 51 rats | Two-arm study | Journal | Not applicable | Sprague-Dawley rats initially anesthetized with nitrous oxide and isoflurane, TBI was induced with control cortical impact | Primary: Evaluate the effects of isoflurane and fentanyl in TBI rats |
| Secondary: Lesion volumes after TBI in rats | ||||||
CBF, cerebral blood flow; CPP, cerebral perfusion pressure; EEG, electroencephalogram; GCS, Glasgow Coma Scale; TBI, traumatic brain injury.
Table 2.
Sedation Treatment and Cerebrovascular Response: Summary of Study Details
| References | medication and dose | CBF/Cerebrovascular response | Limitations | Conclusions |
|---|---|---|---|---|
| Human studies | ||||
| Lee et al.28 | Propofol: 1 mg/kg | Metabolic reactivity was induced through propofol burst suppression -CPP increase by 5% (p < 0.01) -SjvO2 increase by 3% (p < 0.01) -MAP was constant -MCAv in most models decrease by 30%, CBF also demonstrated a decrease but was not significant -Trend to deteriorate vasoreactivity with 20% of patients having reduced response PCO2 and PO2 levels were controlled through ventilation |
MAP during burst suppression was maintained with phenylephrine, which may interfere with CBF | Propofol through metabolic suppression decreased CBFv |
| Steiner et al.10 | Propofol: 3-4 mg/kg/h | Propofol -Higher doses decreased MCAv by 8% -Little change to CPP or AVDO2 -MAP remained relatively constant -Static rate of autoregulation on average decreased from 56 ± 36 to 28 ± 35% but increased in some patients PCO2 and PO2 levels were controlled through ventilation |
MAP was maintained with norepinephrine, which may interfere with CBF | Propofol decreases MCAv, which is a surrogate measure of CBFv |
| James et al.34 | Propofol: 25.5 μg/kg/min Dexmedetomine: 0.54 μg/kg/h |
Propofol -Slight decrease in ICP and no change in PbtO2 although both lacked statistical significance -CPP increased during and fell after injection by about 6% -Lactate/Pyruvate ratio increased drastically after injection -CBF had minimal changes, based on limited response in ICP and PO2 Dexmedetomine -A slight increase in ICP with no change in PbtO2 though both lacked statistical significance -CPP fell slightly by 2% -Lactate/Pyruvate ratio increased drastically after injection -CBF had minimal changes, based on limited response in ICP and PO2 PCO2 and PO2 levels were controlled through ventilation From ICP and CPP, MAP can be assumed to be near constant |
Based on limited number of patients | Propofol demonstrated little to no effect on CBF derived from the ICP/PO2 comparison |
| Johnston et al.23 | Propofol: 3-4 mg/kg/h | Propofol -AVDO2, ICP, and PCO2 all slightly decreased as compared with baseline values -PO2 and PbtO2 slightly increased -CPP and lactate/pyruvate ratio had little variation -All changes were not significant |
ICP, CPP, and MAP can be assumed to be near constant | Propofol demonstrates a slight non-significant increase to CBF in the setting of TBI |
| Pinaud et al.27 | Propofol: 2 mg/kg then 150 μg/kg/min (3-5 μg/mL) |
Propofol -rCBF decrease by 25% (p < 0.01) -ICP decreased by 18% (p < 0.001), this decrease was then inverted after propofol infusion ceased -CPP dropped by28% (p < 0.001) -AVDO2 decreased by (6%) but was not significant -CVR increased then decrease as a result from propofol although this was not significant apart from 1 patient -PCO2 remained constant at 33 ± 2 mm Hg |
Large variation within individual patients was not accounted for | Propofol caused a varying decrease in rCBF in all patients; this was associated with a decrease in ICP and CPP This indicates that rCBF drop is caused by MAP decrease |
| Tanguy et al.33 | Propofol: 1 mg/kg/h and increased by same increment with 5 mg/kg/h being max Midazolam: 0.03 mg/kg/h and increased by 0.01 mg/kg/h |
Propofol -ICP of 19 ± 12 mm Hg -PO2 of 97 ± 2% -PCO2 of 38 ± 7 mm Hg -CPP of 73 ± 11 mm Hg -MAP of 91 ± 11 mm Hg -CBF had minimal changes, based on limited response in CPP and PO2 Midazolam -ICP of 20 ± 12 mm Hg -PO2 of 98 ± 1% -PCO2 of 35 ± 10 mm Hg -CPP of 73 ± 11 mm Hg -MAP of 100 ± 16 mm Hg -CBF had minimal changes, based on limited response in CPP and PO2 PCO2 and PO2 levels were controlled through ventilation No difference was seen in the lactate/pyruvate ratio was seen |
MAP was maintained with catecholamines, which may interfere with CBF Therapeutic goals and sedation levels were independent from microdialysis biomarkers |
Using the CPP/PO2 to find CBF, it is indicated that propofol and midazolam are near identical in CBF effect, both demonstrating no significant response |
| Albanèse et al.24 | Sufentanil: 1 μg/kg then 0.005 μg/kg/min Alfentanil: 100 μg/kg then 0.7 μg/kg/min Fentanyl: 10 μg/kg then 0.075 μg/kg/min |
Sufentanil, alfentanil, and fentanyl -Initial increase ICP (25%) then after 60 min ICP returned to baseline -CPP decreased by 41% (p < 0.05) -SvjO2 remained relatively unchanged -Based on CPP/ SvjO2, CBF was indicated to increase -Based on CMRO2/AVDO2, CBF slightly decreased PCO2 levels were maintained between 32 and 35 torr and CPP stayed between 27 to 37 mm Hg PCO2 and PO2 levels were controlled through ventilation No changes in lactate-oxygen index or MAP |
Based on limited number of patients | Sufentanil, alfentanil, and fentanyl had a slight increase and decrease to CBF found through the surrogate measure of CPP/SvjO2 and CMRO2/AVDO2 although it was not maintained or significant |
| de Nadal et al.26 | Morphine: 0.2 mg/kg Fentanyl: 2 μg/kg |
Morphine -Slight increase in CBF (10%) with no change in MCAv -When comparing autoregulation there was little difference in MCAv; however for CBF, impaired autoregulation demonstrated lower overall response (7%) then intact autoregulation (13%) Fentanyl -Slight increase in CBF (10%) and a slight decrease in CBFv (10%) -There was little difference in impaired vs. intact autoregulation in CBF and CBFv response All changing in AVDO2 were adjusted for PCO2 levels MAP remained relatively constant in all groups Slight decrease in CPP associated with ICP then increase to baseline |
CBF was approximated or found from the MCA MAP was maintained with phenylephrine, which may interfere with CBF |
Fentanyl showed little change in CBF in any group whether with intact or impaired autoregulation although the direct measurement by CBFv and 1/AVDO2 contradicted in response Morphine showed a slight increase in CBF with no significant change in CBFv. Intact autoregulation had a higher response then impaired autoregulation in any group whether intact or impaired autoregulation although the direct measurement by CBFv and 1/AVDO2 contradicted in response |
| de Nadal et al.25 | Fentanyl: 2 μg/kg | Fentanyl -ICP: Increased then slowly decreased in both the group with intact and impaired autoregulation -CPP: Moderately decreased by 6% -AVDO2 initially decreased (11%) then returned to the baseline at 60 min but was not significant MAP showed a similar decrease as CPP All changing in AVDO2 were adjusted for PCO2 levels |
Fentanyl showed a decrease in CPP with a small increase in 1/AVDO2 as a surrogate measure for CBF; this was a small and nonsignificant increase Fentanyl had little influence on autoregulation based on limited differences |
|
| Papazian et al.32 | Midazolam: 0.15 mg/kg | Midazolam -Reduced CPP by 26% (p < 0.0001) -Non-significant change to ICP >18 mm Hg before TBI, when ICP <18 mm Hg before TBI an increase in ICP was observed (20%) -CBF had little change apart from mentioned ICP <18 mm Hg in which case midazolam caused a slight increase in CBF (10%) PCO2 and PO2 were measured and maintained |
CBF assumed through CMRO2 coupling | CBF had little change apart from the mentioned ICP <18 mm Hg, in which case midazolam caused a slight increase in CBF Midazolam was assumed to have limited influence on autoregulation due to limited difference in ICP groups |
| Animal studies | ||||
| Feuerstein et al.29 | Isoflurane at 2% Propofol: 33 to 53 mg/kg/h |
Isoflurane -rCBF increased initially with injection then returned to baseline after 1 min (19.8 ± 27.2%) Propofol -rCBF increased initially with injection then returned to baseline after 1 min (27.5 ± 38.2%) -Atrial diameter decrease of 50% where isoflurane had no response Blood gasses were maintained through ventilation MAP had little change in each group |
Limited number of subjects | There was little response in rCBF in both groups, with propofol demonstrating a constriction of cerebral pial vessels |
| Kahveci et al.31 | Propofol: 12 mg/kg/h Isoflurane: 0.9 ± 0.04% |
Propofol -Decrease ICP from 50% (p < 0.01) -CPP increased by 10% -No significant change to PO2, CBFv, or MAP Isoflurane -No significant effect on CBFv, ICP, or PO2 -MAP and CPP decrease over time by 30% Blood gasses were maintained through ventilation |
Subjects were also in a hypothermic state, which influences CBF | Despite the limited result, it was indicated that propofol is the better choice in hypothermic conditions, with no response in CBFv The limited CBF effects of isoflurane are exaggerated by hypothermia indicating that isoflurane either caused no change or an increase in CBF |
| Bedell et al.30 | Isoflurane: 1-1.5% Fentanyl: 50 μg/kg/h |
Isoflurane -ICP increased -CPP decreased by 7% then returned to baseline -MAP, CBF, and CVR remain relatively constant Fentanyl -ICP decreased then slightly increased -CPP decrease by 30% -MAP decreased from 30% -CBF decreased by 22% at 75 min then increased to baseline -CVR decreased by 28% EEG, ICP, PCO2, PO2, pH, and temperature were similar between groups |
Surgery may influence CBF | In the presence of hypotension fentanyl demonstrated a prevention of CBF indicating the fentanyl may increase CBF; along with this there was a decrease in CVR indicating an vasoconstrictive effect Isoflurane had little influence on cerebral vasculature |
| Statler et al.36 | Isoflurane at 4% then reduced to 1% Fentanyl: 50 μg/mL then 50 μg/kg/h |
Fentanyl MAP was higher than isoflurane; however during infusion the MAP and CPP remained constant throughout the experiment CPP after 4 h was greater in fentanyl than isoflurane group by10%, but both constant CBF was 2 to 3 times higher in isoflurane then fentanyl group PO2 and PCO2were controlled by ventilation |
Subjects also sedated with nitrous oxide | The increase in CPP by isoflurane indicates that CBF is increased in contrast to the fentanyl demonstrating only minor change in CPP and therefor demonstrated little effect to CBF |
AVDO2, arterio-jugular venous oxygen differences; CBF, cerebral blood flow; CBFv, cerebral blood flow velocity; CMRO2, cerebral metabolic rate of oxygen; CPP, cerebral perfusion pressure; CVR, cerebrovascular resistance; EEG, electroencephalogram; h, hour; ICP, intracranial pressure; MAP, mean arterial pressure; MCA, middle cerebral artery; MCAv, middle cerebral artery velocity; min, minute; mm Hg, millimeters of mercury; PbtO2, brain tissue oxygen tension; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; rCBF, regional cerebral blood flow; sec, second; SvjO2, jugular venous oxygen saturation; TBI, traumatic brain injury.
Propofol, fentanyl, and midazolam impact on objectively measured CBF
The following subsections provide a narrative summary of the impact of propofol, fentanyl, and midazolam administration on objectively measured cerebrovascular response/CBF in human patients followed by a brief summary of the four animal model studies. A summary of main study results can be found in Table 2, with more details for the interested reader in Supplementary Table S3. Of note, the following sections describe the trends presented in the parent articles. In all the human studies but one,34 partial pressure of carbon dioxide (PCO2) levels were either maintained or accounted for in cerebral response. PO2 was controlled in all studies through constant ventilation parameters. MAP was maintained at a constant level for most of these human studies, except for three studies where MAP was changed due to the sedative agent.25,27,32
Propofol
Within the six studies10,23,27,28,33,34 that evaluated propofol and CBF in human patients with TBI, most had a non-significant change in CBF. However, one study had a trend toward decrease to regional CBF when measured through a Xenon133 diffusion technique. Although it should be noted that there was also a significant drop in CPP and MAP, which could account for the decrease in CBF seen.27 Also, in this study individual patient responses were measured, demonstrating that most patients had a drop in CBF by at least 10 mL/100 g/min27; further, in one patient cerebrovascular resistance (CVR; measured by CPP/CBF) was found to increase by 90% from baseline values (other patients had a limited response).
Two other studies displayed a non-significant response in CBF to propofol. Using transcranial-Doppler (TCD) to measure middle cerebral artery velocity (MCAv; which is a surrogate measure of CBF), these studies found the MCAv trended toward a decrease during propofol administration.10,28 In contrast to this CBFv change, CBF measured through AVDO2 methods demonstrated little response to propofol infusions.10 MAP, PCO2, and PO2 were relatively constant throughout, in both studies.
Finally, the three remaining studies demonstrated a non-significant CBF response to intravenous propofol administration. However, they did demonstrate a trend toward a decrease in CPP with no change in PO2. Such CPP and PO2 responses may indicate a decrease in CBF, based on CPP/PO2 as a surrogate measure for CBF.23,33,34 CPP and MAP remained unchanged in these studies.
Fentanyl
Within the three studies24–26 that evaluated the CBF effects of fentanyl in patients with TBI, all three had a non-significant response to fentanyl. However, there was a trend toward a decrease in CBFv found through TCD,26 with this drop found to be similar in patients with intact and impaired autoregulation (autoregulation was measured by comparing response of CBF with CO2 reactivity37). In contrast to the CBFv decrease seen in these studies, a trend toward a CBF increase was demonstrated with an increase in 1/AVDO2 (surrogate measure for CBF). This difference in CBF and CBFv response remained similar in patients with intact and impaired cerebral autoregulation.25,26 The PCO2 in these studies was between 29 and 35 torr, and MAP remained relatively unchanged during CBFv measurements.
Midazolam
In the two studies that evaluated CBF and midazolam in patients with TBI, there was a non-significant response to midazolam in CPP, PO2, and CBF. Although in one study the CPP and PO2 values were slightly higher in the midazolam group, compared with the propofol group.33 The second study demonstrated that midazolam decreases MAP by over 15 mm Hg, with patients who had an ICP <18 mm Hg before infusion demonstrating a slight increase in CPP.32 No definitive conclusions regarding the CBF/cerebrovascular reactivity response of midazolam can be made at this time.
Animal studies
In the four animal studies two compared propofol with isoflurane29,31 and the other two compared fentanyl with isoflurane.30,36 In all studies PO2 and PCO2 remained constant in all models. In the two studies in which propofol was evaluated in rat models, MAP was relatively constant. Both studies demonstrated a decrease in CBFv (measured through TCD of the MCA)31 or a decrease in CBF measured through laser speckle imaging with propofol administration.29 One of these studies had ICP drastically decreasing from 18 ± 2 to 7 ± 1 mm Hg (CPP decrease of 10%),31 and the other demonstrated a constriction of pial cerebral vessels by 50% (through direct visualization of vessels).29
In the two remaining animal studies, the effects of fentanyl on CBF was evaluated. Both studies found the fentanyl groups displayed lower CBF and CPP values compared with the isoflurane groups, although CPP did trend toward increasing with fentanyl administration.30,36 The one study with feline models found fentanyl decreased MAP from 120 to 80 mm Hg with a significant drop in CBF (measured through radiolabel microspheres) and a slight decrease in CVR (calculated from MAP/CBF).30 Whereas the other study with rodents found that the fentanyl group had a CBF value that was 2 to 3 times lower than that in the isoflurane group, although the technique used and true value of CBF were not indicated.36
Discussion
Through this systematically conducted scoping review of the literature surrounding the impact of propofol, fentanyl, and midazolam on CBF/cerebrovascular response in human and animal TBI, we have identified a significant knowledge gap. Although 14 studies were identified, they all suffered from some significant limitations, which restricted our ability to derive concrete conclusions regarding the CBF/cerebrovascular effects of these sedative agents. However, some general trends were seen in these studies.
First, in the studies identified propofol had a tendency to decrease CBF23,27,34 and CBFv.10,28 This has been previously described in healthy patients.2,38 However, it must be acknowledged that with the reduction in CPP seen in some of these studies with propofol, this alone may account for the CBF reductions.27 Further, some of the propofol studies estimated CBF using the 1/AVDO2 method, which is predicated on a relatively constant cerebral metabolic rate of oxygen (CMRO2).10,23 This may be the case in healthy patient populations, but likely does not hold true in the setting of TBI, where both regional and global changes in CMRO2 may fluctuate. Further, literature exists suggesting propofol may alter flow-metabolism coupling,39 further muddying the interpretation of CBF using the 1/AVDO2 technique. As such, no conclusive comments regarding the impact of propofol in CBF can be made at this time in patients with TBI, highlighting the need for future work.
Second, a decrease in CPP after fentanyl was seen in these TBI studies24,25; this has been commented on in past review articles.2,38 Along with this, there was a limited response in CBF in the three TBI studies with PCO2 being constant through the studies, indicating that fentanyl has little influence on CBF in the setting of ventilatory and cardiovascular support/control seen during treatment in an intensive care unit (ICU). In the animal models there was a decrease in CBF seen with fentanyl administration, compared with isoflurane, although the true influence of response is hard to identify. In one of the animal studies the decrease in CBF occurred with a concurrent decrease in MAP.30 Thus, as with propofol, we are limited in the conclusions that can be made, although there appears to be a no significant impact on CBF.
Third, midazolam was only evaluated in two studies with patients with TBI where CBF was objectively assessed and did not appear to have any significant impact on CBF. In one study there was a significant decrease in MAP from 89 to 71 mm Hg with a non-significant response to CBF.32 In healthy patients, midazolam has been documented to decrease CBF and increase in CPP.2 Based on the studies identified, it appears that in the setting of cardiorespiratory control in the ICU, midazolam does not appear to significantly impact CBF, although it must be acknowledged that further work is required in this area.
Finally, there was a limited response in CVR to administration of sedative agents. For example, propofol was found to have limited effects on CVR (CPP/CBF), with one patient having a significant response in CVR.27 Similarly, there was in one animal study that analyzed cerebral pial vessel response to propofol through direct visualization; vessels constricted by 50% as compared with baseline diameter.29 Whereas, fentanyl found a trend toward a decrease in CVR in one animal study, from 1.68 ± 0.46 to 1.21 ± 0.58 (as estimated through CPP/CBF).30
Limitations
As mentioned above, the identified literature carries significant limitations, which hinder our ability to make conclusive statements regarding the CBF/cerebrovascular response of propofol, fentanyl, and midazolam in moderate/severe TBI. First, the literature body is low in number, consisting mainly of small case series with limited sample sizes. As well, many studies only demonstrated a weak non-significant response, which could be influenced by publication bias, therefore only trends may be commented on. Second, the studies were heterogeneous in nature, with different dosing and co-administration of medications. Further, some patients were on vasopressor drugs to support MAP and CPP during the recorded CBF response. These drugs have known cerebral vasoconstrictive properties and may therefore have confounded the results. Third, most studies employed the 1/AVDO2 method for CBF estimation. This method estimated CBF under the assumption of relatively fixed CMRO2. This may be the case in non-TBI patient populations but does not hold true in the setting of moderate/severe TBI. Similarly, propofol is known to impact flow-metabolism coupling in the brain40,41 and systemic blood pressure changes could have caused the CBF response in many of these studies.
These outlined limitations of the CBF measurement technique further limit our ability to interpret if these agents have a true impact on CBF. As well, CBFv methods to evaluate MCAv make the assumption that medium/large vessel changes in CBFv reflect downstream CBF/cerebrovascular responses. Finally, there is a lack of recorded high temporal physiology responses of each drug with respect to CBF, relying mainly on serological information for CBF estimation. Thus, the true temporal CBF/cerebrovascular response to these sedative agents in moderate/severe TBI remains unknown.
Future directions
It is clear from this review that knowledge of the impact of commonly administered sedative agents on CBF/cerebrovascular response in TBI is limited. As such, we believe this review both highlights the knowledge gap and provides evidence to support further work in this area. Future investigations would benefit from both experimental animal TBI models and in vivo human studies in TBI. Both types of research require the use of continuous high temporal frequency CBF/cerebrovascular reactivity measurement techniques. These data would need to be time-linked to medication dosing information, to provide the optimal platform for exploring the temporal impact of such sedation agents on CBF/cerebrovascular reactivity. A multi-modal cerebral physiological monitoring approach would be preferred, employing ICP, brain tissue oxygen tension (PbtO2), thermal diffusion CBF, near-infrared oximetry, and cerebral microdialysis. Similarly, objective assessments of sedation depth, such as via processed electroencephalogram (EEG) data, may remove the uncertainty around individual dose-response to sedative agents. Capturing, curating, and analyzing such data require a multi-disciplinary team, consisting of clinicians, biomedical engineers, physiologists, and data scientists, like those formed by research networks such as CENTER-TBI in Europe42,43 and CAHR-TBI in Canada.44 Leveraging advances in machine learning may facilitate analysis of complex data that would be captured in these large collaborative networks.
Conclusion
There were a limited number of articles objectively documenting the CBF/cerebrovascular response of propofol, fentanyl, and midazolam in human patients with moderate/severe TBI and in animal TBI models. All studies suffered from significant limitations and small sample sizes, limiting the conclusions that can be drawn. In general, none of the agents had a significant impact on estimated CBF in the TBI populations described. This review highlights a significant knowledge gap present regarding the CBF/cerebrovascular response of these sedative agents in moderate/severe TBI, emphasizing the need for future dedicated experimental and human studies.
Supplementary Material
Abbreviations Used
- AVDO2
arterio-jugular differences of oxygen
- CBF
cerebral blood flow
- CBFv
CBF velocity
- CMRO2
cerebral metabolic rate of oxygen
- CPPopt
optimal cerebral perfusion pressure
- CVR
cerebrovascular resistance
- EEG
electroencephalogram
- GCS
Glasgow Coma Scale
- ICU
intensive care unit
- iICP
individual intracranial pressure
- MAP
mean arterial pressure
- MCA
middle cerebral artery
- MCAv
middle cerebral artery velocity
- PbtO2
brain tissue oxygen tension
- PCO2
partial pressure of carbon dioxide
portable document format
- rCBF
regional cerebral blood flow
- SvjO2
jugular venous oxygen saturation
- TBI
traumatic brain injury
- TCD
transcranial-Doppler
Funding Information
This work was supported by funding from a University of Manitoba UMG GFT grant, and the University of Manitoba URGP grant programs.
F.A.Z. receives research support from the Manitoba Public Insurance (MPI) Neuroscience/TBI Research Endowment, the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS) (NIH grant #R03NS114335-01), the Canadian Institutes of Health Research (CIHR) (CIHR grant #432061), the Canada Foundation for Innovation (CFI) (CFI grant #38583), Research Manitoba, the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Centre on Aging, the University of Manitoba Rudy Falk Clinician-Scientist Professorship, and the Health Sciences Centre Foundation Winnipeg. L.F. is supported through a University of Manitoba – Department of Surgery GFT Research Grant, and the University of Manitoba Office of Research Services (ORS) – University Research Grant Program (URGP). C.B. is supported through a University of Manitoba Centre on Aging Fellowship Grant. A.G. is supported through the University of Manitoba Clinician Investigator Program.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
Cite this article as: Froese L, Dian J, Batson C, Gomez A, Bertram Unger B, Zeiler FA (2020) Cerebrovascular response to propofol, fentanyl, and midazolam in moderate/severe traumatic brain injury: A scoping systematic review of the human and animal literature, Neurotrauma Reports 1:1, 100–112, DOI:10.1089/neur.2020.0040.
References
- 1. Khandelwal, A., Bithal, P.K., and Rath, G.P. (2019). Anesthetic considerations for extracranial injuries in patients with associated brain trauma. J. Anaesthesiol. Clin. Pharmacol. 35, 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oddo, M., Crippa, I.A., Mehta, S., Menon, D., Payen, J.-F., Taccone, F.S., and Citerio, G. (2016). Optimizing sedation in patients with acute brain injury. Crit. Care 20, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carney, N., Totten, A.M., O'Reilly, C., Ullman, J.S., Hawryluk, G.W.J., Bell, M.J., Bratton, S.L., Chesnut, R., Harris, O.A., Kissoon, N., Rubiano, A.M., Shutter, L., Tasker, R.C., Vavilala, M.S., Wilberger, J., Wright, D.W., and Ghajar, J. (2017). Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 [DOI] [PubMed] [Google Scholar]
- 4. Hawryluk, G.W.J., Aguilera, S., Buki, A., Bulger, E., Citerio, G., Cooper, D.J., Arrastia, R.D., Diringer, M., Figaji, A., Gao, G., Geocadin, R., Ghajar, J., Harris, O., Hoffer, A., Hutchinson, P., Joseph, M., Kitagawa, R., Manley, G., Mayer, S., Menon, D.K., Meyfroidt, G., Michael, D.B., Oddo, M., Okonkwo, D., Patel, M., Robertson, C., Rosenfeld, J.V., Rubiano, A.M., Sahuquillo, J., Servadei, F., Shutter, L., Stein, D., Stocchetti, N., Taccone, F.S., Timmons, S., Tsai, E., Ullman, J.S., Vespa, P., Videtta, W., Wright, D.W., Zammit, C., and Chesnut, R.M. (2019). A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 45, 1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chesnut, R., Aguilera, S., Buki, A., Bulger, E., Citerio, G., Cooper, D.J., Arrastia, R.D., Diringer, M., Figaji, A., Gao, G., Geocadin, R., Ghajar, J., Harris, O., Hoffer, A., Hutchinson, P., Joseph, M., Kitagawa, R., Manley, G., Mayer, S., Menon, D.K., Meyfroidt, G., Michael, D.B., Oddo, M., Okonkwo, D., Patel, M., Robertson, C., Rosenfeld, J.V., Rubiano, A.M., Sahuquillo, J., Servadei, F., Shutter, L., Stein, D., Stocchetti, N., Taccone, F.S., Timmons, S., Tsai, E., Ullman, J.S., Vespa, P., Videtta, W., Wright, D.W., Zammit, C., and Hawryluk, G.W.J. (2020). A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 46, 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kellum, J.A., and Pinsky, M.R. (2002). Use of vasopressor agents in critically ill patients. Curr. Opin. Crit. Care 8, 236. [DOI] [PubMed] [Google Scholar]
- 7. Auchet, T., Regnier, M.-A., Girerd, N., and Levy, B. (2017). Outcome of patients with septic shock and high-dose vasopressor therapy. Ann. Intensive Care 7, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeiler, F.A., Ercole, A., Cabeleira, M., Carbonara, M., Stocchetti, N., Menon, D.K., Smielewski, P., Czosnyka, M., and CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators. (2019). Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult traumatic brain injury: a Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J. Neurotrauma 36, 1505–1517 [DOI] [PubMed] [Google Scholar]
- 9. Zeiler, F.A., Lee, J.K., Smielewski, P., Czosnyka, M., and Brady, K. (2018). Validation of intracranial pressure-derived cerebrovascular reactivity indices against the lower limit of autoregulation, Part II: experimental model of arterial hypotension. J. Neurotrauma 35, 2812–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steiner, L.A., Johnston, A.J., Chatfield, D.A., Czosnyka, M., Coleman, M.R., Coles, J.P., Gupta, A.K., Pickard, J.D., and Menon, D.K. (2003). The effects of large-dose propofol on cerebrovascular pressure autoregulation in head-injured patients. Anesth. Analg. 97, 572–576, table of contents. [DOI] [PubMed] [Google Scholar]
- 11. Budohoski, K.P., Czosnyka, M., Kirkpatrick, P.J., Smielewski, P., Steiner, L.A., and Pickard, J.D. (2013). Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat. Rev. Neurol. 9, 152–163 [DOI] [PubMed] [Google Scholar]
- 12. Czosnyka, M., Smielewski, P., Kirkpatrick, P., Laing, R.J., Menon, D., and Pickard, J.D. (1997). Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41, 11–19 [DOI] [PubMed] [Google Scholar]
- 13. Zeiler, F.A., Ercole, A., Beqiri, E., Cabeleira, M., Thelin, E.P., Stocchetti, N., Steyerberg, E.W., Maas, A.I.R., Menon, D.K., Czosnyka, M., Smielewski, P., Anke, A., Beer, R., Helbok, R., Bellander, B.-M., Nelson, D., Buki, A., Chevallard, G., Citerio, G., Chieregato, A., Czeiter, E., Depreitere, B., Eapen, G., Frisvold, S., Jankowski, S., Kondziella, D., Koskinen, L.-O., Meyfroidt, G., Moeller, K., Piippo-Karjalainen, A., Raj, R., Radoi, A., Sahuquillo, J., Ragauskas, A., Rocka, S., Rhodes, J., Rossaint, R., Stevanovic, A., Sakowitz, O., Sundström, N., Takala, R., Tamosuitis, T., Tenovuo, O., Vajkoczy, P., Vargiolu, A., Vilcinis, R., Wolf, S., and Younsi, A. (2019). Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult traumatic brain injury: a CENTER-TBI Study. J. Neurotrauma 37, 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeiler, F.A., Ercole, A., Beqiri, E., Cabeleira, M., Aries, M., Zoerle, T., Carbonara, M., Stocchetti, N., Smielewski, P., Czosnyka, M., Menon, D.K., Anke, A., Beer, R., Bellander, B.-M., Buki, A., Chevallard, G., Chieregato, A., Citerio, G., Czeiter, E., Depreitere, B., Eapen, G., Frisvold, S., Helbok, R., Jankowski, S., Kondziella, D., Koskinen, L.-O., Meyfroidt, G., Moeller, K., Nelson, D., Piippo-Karjalainen, A., Radoi, A., Ragauskas, A., Raj, R., Rhodes, J., Rocka, S., Rossaint, R., Sahuquillo, J., Sakowitz, O., Stevanovic, A., Sundström, N., Takala, R., Tamosuitis, T., Tenovuo, O., Vajkoczy, P., Vargiolu, A., Vilcinis, R., Wolf, S., Younsi, A., and the CENTER-TBI High Resolution ICU (HR ICU) Sub-Study Participants and Investigators. (2019). Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir. (Wien) 161, 1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budohoski, K.P., Czosnyka, M., de Riva, N., Smielewski, P., Pickard, J.D., Menon, D.K., Kirkpatrick, P.J., and Lavinio, A. (2012). the relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery 71, 652–661 [DOI] [PubMed] [Google Scholar]
- 16. Aries, M.J., Czosnyka, M., Budohoski, K., Steiner, L., Lavinio, A., Kolias, A., Hutchinson, P., Brady, K., Menon, D., Pickard, J., and Smielewski, P. (2012). Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med. 40, 2456–2463 [DOI] [PubMed] [Google Scholar]
- 17. Malhotra, A.K., Schweitzer, J.B., Fox, J.L., Fabian, T.C., and Proctor, K.G. (2003). Cerebral perfusion pressure directed therapy following traumatic brain injury and hypotension in swine. J. Neurotrauma 20, 827–839 [DOI] [PubMed] [Google Scholar]
- 18. Howells, T., Johnson, U., McKelvey, T., and Enblad, P. (2015). An optimal frequency range for assessing the pressure reactivity index in patients with traumatic brain injury. J. Clin. Monit. Comput. 29, 97–105 [DOI] [PubMed] [Google Scholar]
- 19. Donnelly, J., Czosnyka, M., Adams, H., Robba, C., Steiner, L.A., Cardim, D., Cabella, B., Liu, X., Ercole, A., Hutchinson, P.J., Menon, D.K., Aries, M.J.H., and Smielewski, P. (2017). Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit. Care Med. 45, 1464–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beqiri, E., Smielewski, P., Robba, C., Czosnyka, M., Cabeleira, M.T., Tas, J., Donnelly, J., Outtrim, J.G., Hutchinson, P., Menon, D., Meyfroidt, G., Depreitere, B., Aries, M.J., and Ercole, A. (2019). Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 9, e030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., and Welch, V.A. (eds). (2020). Cochrane Handbook for Systematic Reviews of Interventions, version 6.1 (updated September 2020). Cochrane. www.training.cochrane.org/handbook (Last accessed January5, 2020)
- 22. Moher, D., Liberati, A., and Tetzlaff, J. (2009). Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Ann. Intern. Med. 151, 264–269 [DOI] [PubMed] [Google Scholar]
- 23. Johnston, A.J., Steiner, L.A., Chatfield, D.A., Coleman, M.R., Coles, J.P., Al-Rawi, P.G., Menon, D.K., and Gupta, A.K. (2003). Effects of propofol on cerebral oxygenation and metabolism after head injury. Br. J. Anaesth. 91, 781–786 [DOI] [PubMed] [Google Scholar]
- 24. Albanèse, J., Viviand, X., Potie, F., Rey, M., Alliez, B., and Martin, C. (1999). Sufentanil, fentanyl, and alfentanil in head trauma patients: a study on cerebral hemodynamics. Crit. Care Med. 27, 407–411 [DOI] [PubMed] [Google Scholar]
- 25. de Nadal, M., Ausina, A., Sahuquillo, J., Pedraza, S., Garnacho, A., and Gancedo, V.A. (1998). Effects on intracranial pressure of fentanyl in severe head injured patients. Acta Neurochir. Suppl. 71, 10–12 [DOI] [PubMed] [Google Scholar]
- 26. Nadal, M. de, Munar, F., Poca, M.A., Sahuquillo, J., Garnacho, A., and Rosselló, J. (2000). Cerebral hemodynamic effects of morphine and fentanyl in patients with severe head injury: absence of correlation to cerebral autoregulation. Anesthesiol. J. Am. Soc. Anesthesiol. 92, 11–11 [DOI] [PubMed] [Google Scholar]
- 27. Pinaud, M., Lelausque, J.N., Chetanneau, A., Fauchoux, N., Ménégalli, D., and Souron, R. (1990). Effects of propofol on cerebral hemodynamics and metabolism in patients with brain trauma. Anesthesiology 73, 404–409 [DOI] [PubMed] [Google Scholar]
- 28. Lee, J.H., Kelly, D.F., Oertel, M., McArthur, D.L., Glenn, T.C., Vespa, P., Boscardin, W.J., and Martin, N.A. (2001). Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J. Neurosurg. 95, 222–232 [DOI] [PubMed] [Google Scholar]
- 29. Feuerstein, D., Takagaki, M., Gramer, M., Manning, A., Endepols, H., Vollmar, S., Yoshimine, T., Strong, A.J., Graf, R., and Backes, H. (2014). Detecting tissue deterioration after brain injury: regional blood flow level versus capacity to raise blood flow. J. Cereb. Blood Flow Metab. 34, 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bedell, E.A., DeWitt, D.S., and Prough, D.S. (1998). Fentanyl infusion preserves cerebral blood flow during decreased arterial blood pressure after traumatic brain injury in cats. J. Neurotrauma 15, 985–992 [DOI] [PubMed] [Google Scholar]
- 31. Kahveci, F.S., Kahveci, N., Alkan, T., Goren, B., Korfali, E., and Ozluk, K. (2001). Propofol versus isoflurane anesthesia under hypothermic conditions: effects on intracranial pressure and local cerebral blood flow after diffuse traumatic brain injury in the rat. Surg. Neurol. 56, 206–214 [DOI] [PubMed] [Google Scholar]
- 32. Papazian, L., Albanese, J., Thirion, X., Perrin, G., Durbec, O., and Martin, C. (1993). Effect of bolus doses of midazolam on intracranial pressure and cerebral perfusion pressure in patients with severe head injury. Br. J. Anaesth. 71, 267–271 [DOI] [PubMed] [Google Scholar]
- 33. Tanguy, M., Seguin, P., Laviolle, B., Bleichner, J.-P., Morandi, X., and Malledant, Y. (2012). Cerebral microdialysis effects of propofol versus midazolam in severe traumatic brain injury. J. Neurotrauma 29, 1105–1110 [DOI] [PubMed] [Google Scholar]
- 34. James, M.L., Olson, D.M., and Graffagnino, C. (2012). A pilot study of cerebral and haemodynamic physiological changes during sedation with dexmedetomidine or propofol in patients with acute brain injury. Anaesth. Intensive Care 40, 949–957 [DOI] [PubMed] [Google Scholar]
- 35. Heilbrun MP, Jorgensen PB, and Boysen G. (1972). Relationships between cerebral perfusion pressure and regional cerebral blood flow in patients with severe neurological disorders. Stroke 3 181–195 [DOI] [PubMed] [Google Scholar]
- 36. Statler, K.D., Kochanek, P.M., Dixon, C.E., Alexander, H.L., Warner, D.S., Clark, R.S., Wisniewski, S.R., Graham, S.H., Jenkins, L.W., Marion, D.W., and Safar, P.J. (2000). Isoflurane improves long-term neurologic outcome versus fentanyl after traumatic brain injury in rats. J. Neurotrauma 17, 1179–1189 [DOI] [PubMed] [Google Scholar]
- 37. Sahuquillo, J., Poca, M.A., Ausina, A., Báguena, M., Garcia, R.M., and Rubio, E. (1996). Arterio-jugular differences of oxygen (AVDO2) for bedside assessment of CO2-reactivity and autoregulation in the acute phase of severe head injury. Acta Neurochir. (Wien) 138, 435–444 [DOI] [PubMed] [Google Scholar]
- 38. Chong, K.Y., and Gelb, A.W. (1994). Cerebrovascular and cerebral metabolic effects of commonly used anaesthetics. Ann. Acad. Med. Singapore 23, 145–149 [PubMed] [Google Scholar]
- 39. Klein, K.U., Fukui, K., Schramm, P., Stadie, A., Fischer, G., Werner, C., Oertel, J., and Engelhard, K. (2011). Human cerebral microcirculation and oxygen saturation during propofol-induced reduction of bispectral index. Br. J. Anaesth. 107, 735–741 [DOI] [PubMed] [Google Scholar]
- 40. Oshima, T., Karasawa, F., and Satoh, T. (2002). Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol. Scand. 46, 831–835 [DOI] [PubMed] [Google Scholar]
- 41. Slupe, A.M., and Kirsch, J.R. (2018). Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J. Cereb. Blood Flow Metab. 38, 2192–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maas, A.I.R., Menon, D.K., Steyerberg, E.W., Citerio, G., Lecky, F., Manley, G.T., Hill, S., Legrand, V., Sorgner, A., and CENTER-TBI Participants and Investigators. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 43. Klein, S.P., De Sloovere, V., Meyfroidt, G., and Depreitere, B. (2019). Autoregulation assessment by direct visualisation of pial arterial blood flow in the piglet brain. Sci. Rep. 9, 13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernard, F., Gallagher, C., Griesdale, D., Kramer, A., Sekhon, M., and Zeiler, F.A. (2020). The CAnadian High-Resolution Traumatic Brain Injury (CAHR-TBI) Research Collaborative. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 47, 551–556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.