Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has spread through more than 180 countries, leading to diverse health systems overload around the world. Because of the high number of patients and the supply chain disruption, it generated a shortage of medical devices and personal protective equipment. In this context, initiatives from the additive manufacturing community emerged to fight the lack of devices. Diverse designs were produced and are currently being used in hospitals by patients and health workers. However, as some devices must follow strict standards, these products may not fulfill these standards. Therefore, to ensure the user’s health, there is a need for understanding each device, their usage, and standards. This study reviews the use of additive manufacturing during COVID-19 pandemic. It gathers the source of several 3D printed devices such as face shields, face masks, valves, nasopharyngeal swabs, and others, discussing their use and regulatory issues. In this regard, the major drawbacks of the technology, addressed for the next pandemic scenario, are highlighted. Finally, some insights of the future of additive manufacturing during emergency are given and discussed.
Keywords: Additive manufacturing, 3D printing, COVID-19, Pandemic, PPE, Medical devices
Introduction
The coronavirus disease 2019 (COVID-19) had its first documented patient in December 2019, Wuhan, China [1]. Since then, the virus has spread through the world, and the World Health Organization (WHO) defined the disease as pandemic in 11 March 2020 [2]. Just 9 months after identified “patient zero”, the disease has spread, on October 9th, in 188 countries and regions with more than 36.6 million confirmed cases and more than 1,063,000 global deaths according to the official data of the countries in the COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [3].
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can be transmitted though droplets [4], aerosol, and direct and indirect physical contact through contaminated surfaces, in which the virus may remain up to 72 h [4, 5]. Regarding the transmission modes involving droplets and aerosol, they are still under research and a consensus has not been reached [6, 7]. Some recent works point out that aerosol transmission may be more significant than previously considered [8, 9].
Infected people might be asymptomatic [10, 11], but may also present severe acute respiratory syndrome (SARS), resulting in intensive care unit (ICU) admission [11, 12].
These are all factors that contribute to the virus spread ratio, leading to health systems overload around the world. The high number of patients created a supply chain disruption, generating a shortage of personal protection equipment (PPE) and medical devices in hospitals for fighting off the virus.
To fight the lack of devices and equipment, companies, universities, independent groups, and individuals started a movement of production of diverse items to supply hospitals and people that are facing the shortage [13]. Various devices have been produced, such as face masks, face shields, test swabs, door handle attachments, valves [13–17] etc. and are being massively shared by social media [18, 19]. In this context, additive manufacturing (AM), commonly named as 3D printing, has emerged as one remarkable fabrication process because of its accessibility and flexibility to quickly produce complex and monolithic parts or even mechanical systems [20–22]. Another reason is that people involved with AM are open-mind, creative, with interdisciplinary relationships and applications. Therefore, AM technology allows the user to easily produce parts directly from a STL file format (designed in a CAD system), which can be easily found and shared in makers hubs or even at social media [19]. Finally, during the last decade, because of patent period expiration, desktop 3D printers have become accessible as low-end-machines for common people by open access projects that dramatically reduced their costs [22, 23] and the entrance barriers for low range AM equipment.
However, 3D printed parts may present different properties and finishes because of differences of materials used, technology, and individual machine software and calibration, even for a same STL project [24]. As PPE and medical devices must follow strict standards, these do it yourself (DIY) products may not fulfill the requirements of these standards, representing a risk to the users’ health. Therefore, there is a need for understanding each device, usage, and standards to ensure that these products can in fact be helpful for the purpose intended with the lowest risk for patients and healthcare personal.
This study aims at reviewing the use of additive manufacturing during COVID-19 pandemic. It focuses on various PPE, medical devices, and other applications, providing their source and discussing their use and regulatory topics. Finally, a perspective for the future of AM during emergency situations is given.
Additive manufacturing
As defined by ISO/ASTM 52900 standard [25], additive manufacturing is a “process of joining materials to make parts from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing and formative manufacturing methodologies”. The 3D printing terminology is commonly associated with machines that are low end in price and/or overall capability [25].
The first commercial system of AM dates from the year 1987 and consists of the stereolithography (SLA) technique, which uses photo-curable polymers [20, 21]. Nowadays, a wide range of techniques and materials are available in AM technologies. The International Standard ISO/ASTM 52900 [25] divides AM processes in seven different categories: vat photopolymerization, material extrusion, material jetting, binder jetting, powder bed fusion, sheets addition, and direct energy deposition. These processes use polymers, ceramics, metals, and composites as feedstocks to build a part [20, 21, 25, 26]. Some techniques available on market are SLA, fused deposition modeling (FDM), fused filament fabrication (FFF), PolyJet, direct light projection (DLP), multi jet fusion (MJF), selective laser sintering (SLS), selective laser melting (SLM), and electron beam melting (EBM).
Because of its wide range of materials and techniques, AM can be used in a myriad of areas and applications, from rapid prototyping to medicine and aerospace end-use parts [20, 27]. The direct-from-CAD production, freedom of design, ease of customization, low number of steps, and rapid production of unique or small batches of parts are some of the main advantages of AM [20, 22, 27, 28]. However, there are a few common drawbacks for AM techniques that are still present, like parts anisotropy [20], high cost of industrial equipment, high cost of feedstocks (when compared to traditional techniques) [21], and high cost for big batches [22, 28]. Another serious concern still present in AM is the repeatability among machines of the same brand and models and even between consecutives processes [20].
Pandemic and additive manufacturing
At the same time that the COVID-19 pandemic increased the demand for medical devices and created a new market of products for general public, it provoked various supply chain disruptions, rapidly generating a shortage of many critical items, such as PPE [14, 16, 29–31]. Following this issue, companies, groups and individuals started to cooperate to supply people and hospitals that are facing items shortage [14, 24, 28, 32, 33]. In this regard, AM has emerged as a potential flexible solution for locally supplying various types of these items, demonstrating the potential of AM for a local and flexible production of strategic items, reducing supply chain dependency [13, 27, 34].
One of the main reasons that made AM an important tool during the pandemic is the great number of users around the world [19], many of them very specialized, innovative and creative. During the last decade, the decrease in the cost of processors and the expiration of patents made desktop 3D printers as low-end-machines [22, 23]. Furthermore, the feedstock price, mainly filament polymers, for these printers are in constant lowering [22]. Thus, the ability to produce parts for supplying the pandemic shortage is not exclusive for industry: makers, people who own or have access to rapid manufacturing machines such as 3D printers, have been proven to significantly contribute during the disease [23].
A second reason is that people who have a 3D printer do not require CAD-design knowledge to produce parts. 3D printable files can be easily found in internet via 3D printing open-design repositories, makers hubs or even at social media [19], only requiring a desktop printer to start production.
A third reason is that complex parts that would require a long period of time of manufacturing planning via traditional processes can be quickly put into production by AM. It has a minimal lead time to deliver small batches of parts, producing an almost immediate response to the crisis [22, 28, 35]. Consequently, when media started to share information on shortage of items, people could start helping as soon as they had STL files. The technique ability for rapid prototyping also allowed the fast production of the first designs and machine prototypes. Furthermore, an AM facility for low-end machines does not require complex planning or infrastructure. As example, François et al. [36] presented a technical note on the production of devices related to the COVID-19 pandemic for Greater Paris University Hospitals and various state institutions. They reported that 60 FFF machines were ordered, delivered, and settled in a period of 4 days. Therefore, if there is an urgent demand of emergency items, a 3D printer facility can be quickly installed and put into work. Using a single desktop 3D printer, Thomas et al. [37] took 21 days from conception to delivery of 50 reusable personalized face masks based on the Stopgap surgical face mask design [38].
Data sharing
One of the main advantages of AM is the direct-from-CAD production. The user can easily produce parts from CAD files, which can be designed or obtained from internet specialized repositories. Currently, there are various file repositories like Thingiverse [39], NIH 3D Print Exchange [40], GrabCAD [41], Pinshape [42], Cults [43], Yeggi [44], MyMiniFactory [45] and others. These websites store 3D printable files, usually in STL file format, which are shared by users. These repositories allow the upload, download, exchange, and even edition and new upload of 3D printing files and information by anyone.
During the pandemic, data sharing has rapidly increased to spread initiatives and solutions as an attempt to fight off the virus. From this perspective, social media [19, 33], company websites [46, 47] and file repositories have played a main role in sharing data [23, 33, 35]. As social distancing was the main strategy for most governments to reduce transmission rates, people at homework increased social media use [19]. After the virus was announced by China, a wave of information sharing occurred though social media as never seen before. Unfortunately, part of these were unreliable information like conspiracy theories or misleading rumors, generating panic and depletion of stockpiles [48]. However, social media also played a role in sharing initiatives aiming at providing PPE and medical devices for people in need [18, 19, 49]. In line with social media, file repositories websites were used for sharing downloadable CAD files for 3D printing parts to be used during the pandemic [19, 49]. Furthermore, there are several websites hosting platforms like github and gitlab, dedicated to open projects related to COVID-19. These platforms facilitate quick design interaction among different professionals by allowing access to anyone interested to contribute and share their developments [50]. Global network put together volunteers and experts via dedicated hubs to provide diverse types of products to supply the community [28]. These interactions, associated with the use of desktop 3D printers, act as an iteration chain process for optimizing the project and reaching a final design in a short period of time.
In addition, various companies worked on more engineered and reliable data and designs solutions using 3D printing workforce around the world. Some examples of notable projects, just to cite a few, are ISINNOVA’s valves [51, 52], Prusa’s face shields [53] and Materialise’s [46] solutions for non-contact tools and masks. Furthermore, there are projects like the Open Source Medical Supplies that gathered together multidisciplinary professionals, such as engineers, medical professionals, makers, researchers etc. to lead and inform people who are trying to fight the equipment shortage [54]. Finally, scientific papers [12, 50, 55, 56] were also used as a way of data and information sharing. In this case, more detailed data in fabrication methods and results are given, as well as more reliable data because of the peer-reviewing process. However, they are less accessible to common public since they can be found only in specific scientific media and some publications are not open access.
3D printed devices
The following sections present and discuss diverse types of solutions produced by AM during COVID-19 pandemic.
Personal protective equipment—PPE
PPE are the most printed devices during the pandemic because of their relative simplicity, low geometrical tolerance requirements and lower-risk classification within Food and Drug Administration (FDA) or other regulatory bodies, when compared more complex devices like ventilators and valves [23].
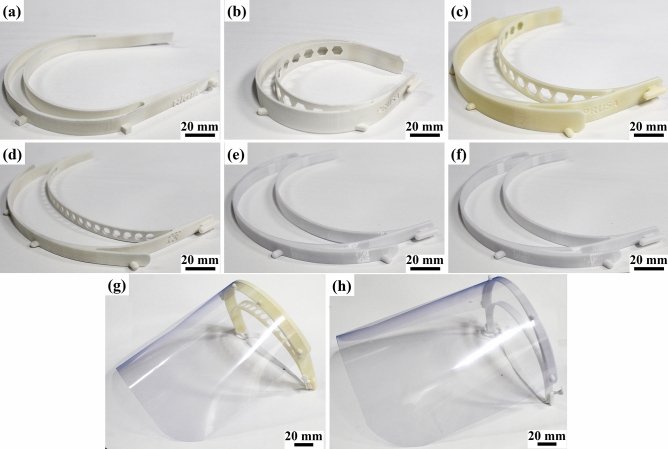
Face shields constitute majority of the projects among PPE [23, 57]. FDA considers face shields as Class I medical devices [4, 58], and WHO considers them as an alternative to medical masks during shortages [59]. They are a head-worn frame with a clear plastic sheet to protect the user’s face from direct contamination via sprays and splashes [4, 13, 23, 60]. Another requirements are that they must be easy to disinfect, comfortable during long periods, durable and allow additional PPE wearing [61]. It is important to remark that in highly infectious situations the face shield must be used with additional PPE equipment [4, 61]. The frame consists of a simple geometry that can easily be 3D printed [53, 56, 61, 62]. A further approach uses SLA to produce a hollow frame to be used with an external airflow, defogging the visor and preventing ambient air from entering [63]. The plastic sheet is cut manually, using scissors and an office puncher [56], or automatically, using laser cutters [60]. Some material of choice are polycarbonate, Propionate, acetate, polyvinyl chloride, and polyethylene terephthalate glycol (PETG) [60]. An elastic band may be suitable in some models [61, 64]. The face shield design must cover the sides of the face and below the chin [59]. Printing time and price of face shields are design, machine, material, and parameters dependents [4, 56, 57]. Figure 1 shows diverse 3D printed face shields models built using different AM techniques and materials. Designs of face shields can be found in various file repositories [39, 40, 42, 43, 45].
Fig. 1.
Various face shields designs made by different AM techniques. a Hígia model [62]—SLS/PA12; b Prusa RC1 model [53]—SLS/PA12; c Prusa RC2 model [53]—FDM/ABS; d adapted Prusa model 1—SLS/PA12; adapted Prusa model 2—FDM/PC; adapted Prusa model 3—FDM/PC. Face shields assembly with a Prusa RC1 model and b adapted Prusa model 3
Safety goggles act in a similar way of face shields: they work as a physical barrier for the eyes. Eye protection is indicated as a protection from infected patients’ exhaled droplets [10]. Farsoon, Huaxiang and LEHVOSS companies designed, validated, and made available for download two sizes of safety googles to be made by SLS, SLA or FFF technologies. The lens are to be cut separately in acrylic material [65]. There are other available models, such as PUC-Rio’s [66], FRC Team’s [67] and Creality’s [68] designs. However, there is no information if these models were validated.
N95 respirators (class II device by FDA [58]) and surgical masks (class I device by FDA [58]) are critical PPE. Both are recommended by the WHO for health workers during this pandemic [59], and are intended to filter particles of bacteria and virus to avoid contamination [31]. N95 respirators have a minimum efficiency reporting value (MERV) of 95% for particles larger than 0.3 µm [32, 69]. Even though COVID-19 virus particle size are under 0.16 µm [1, 70], these respirators are capable of filtering droplets that work as vehicles of virus transportation [32]. Surgical masks cannot assure the same protection as a N95 respirators [64, 69], but they can provide significant reduction, leading various countries and states to incentive or obligate people to use even homemade fabric masks to reduce contamination ratio [59].
Because of the critical function and high demand, diverse 3D printable projects and designs were proposed. 3D printed adaptor valves to be connected with full-face snorkeling masks and a high-efficiency particulate air (HEPA) filter (Fig. 2) were suggested for health workers usage [49, 71, 72]. These full-face snorkel masks have three diaphragm valves and airways that ensure the air inlet only through the HEPA filter and prevents the visor from fogging. In a study made by Kroo et al. [71], this PPE exceeded the standards of N95 respirators. However, in a study made by Gierthmuehlen et al. [73], the full-face snorkel mask obtained only a 85% filtering efficiency for 580–660 nm particle size, which is below the N95 filters efficacy. These conflicting results raise the need for more tests to reach a definitive conclusion.
Fig. 2.
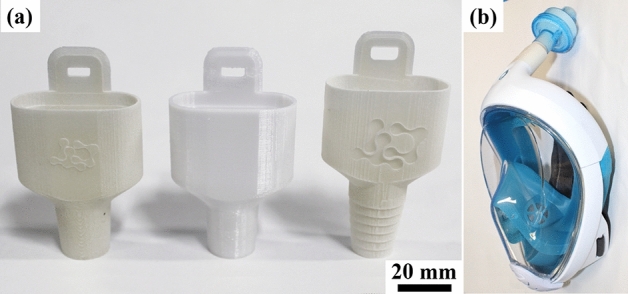
a Different versions of valve adaptors made using SLS/PA12 and FDM/PC. b PPE mask assembly with snorkeling mask, adaptor and HEPA filter
Another approach for PPE is the production of open-source powered air-purifying respirators (PAPR), classified as class II devices by FDA [58]. In this case, AM is being used for producing parts of the mask and the pump for the equipment assembly [74] or reposition parts [75]. Furthermore, Erickson et al. [76] proposed 3D printing manifolds to adapt surgical helmet systems as PPE during the pandemic since these systems are going unused because of elective surgeries delay.
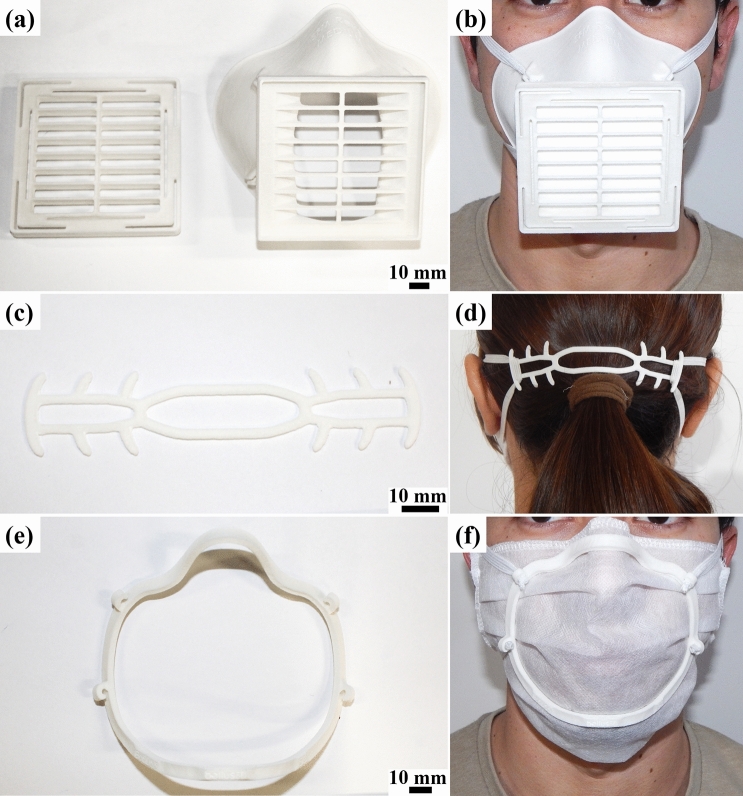
A simpler approach, which acts as a substitute to surgical masks and does not require prefabricated masks is 3D prints, a half-face mask, and uses diverse materials such as filters in an air inlet/outlet. The mask efficiency will depend on the AM technique used, the print quality, the user’s anatomy, and the filter material used [77]. Tino et al. [15] reviewed five different types of half-face masks made by FFF and found out some models are inviable by long printing time or inefficient sealing. Swennen, Pottel and Haers [77] presented a proof of concept of a 3D printed mask composed by two reusable components made by SLS and two disposable components (head band and filter membrane). They propose using 3D facial scanning software from smartphones to obtain a 3D model of the user’s anatomy to improve the mask fit.
It is important to remark that these masks require regulatory approval [14, 15]. Nevertheless, a collaboration between the NIH 3D Print Exchange website, FDA, the Veterans Healthcare Administration, and America Makes in 3D printing solutions inform which models undergone review in a clinical setting and were found appropriate when fabricated with the specified parameters [40]. One reviewed and validated model is the Stopgap surgical face mask [38], to be fabricated via SLS or MJF, shown in Fig. 3a and b. It consists of two sterilizable 3D printed components, elastic straps, and a filter material, which must be disposed after each use. However, a preprinted work by Bezek et al. [78] tested the Stopgap [38], La Factoria 3D COVID-19 [79] and Montana face masks [80] made by powder bed fusion industrial and FFF industrial and desktop equipment. In almost all cases, masks without post-processing steps presented an overall filtering efficiency under 60% for particles between 100 and 300 nm, which is comparable to fabric masks. The only exception was the Montana design made in ULTEM material using an industrial FFF printer, which presented a 90–95% efficiency. Further, they showed that post-processing steps can improve the masks filtering efficiency. Their work indicates the need for further quantitative testing and validation of 3D printed PPE and for development and standardization of post-processing steps to improve the masks efficiency. In agreement with the previous work, Gierthmuehlen et al. [73] obtained a filtering efficacy of only 39% for La Factoria’s mask model, which is a home-made vacuum cleaner bag mask model (70%). They highlight that a tight fit is essential for the masks to perform optimally.
Fig. 3.
Face mask and support parts made by SLS/PA12. a Stopgap surgical face mask [38] b Ear saver designed by tech4primary user from NIH 3D Print Exchange [81]. c Face fitter designed by Bellus3D [82]
Finally, there are parts that were designed to be used as support to PPE. N95 or surgical masks are critical and required PPE for healthcare professionals [59]. Because of the long shifts, the delicate skin behind the ears starts to breakdown by the contact with the mask bands. 3D printed ear savers, or mask extenders [35], were designed to improve the wellness of health workers and people who work for long periods with face masks. In the NIH 3D Print Exchange repository [40], there are various reviewed models such as the one submitted by tech4primary user [81], shown in Fig. 3c and d. Additionally, to improve the fit of surgical masks, Bellus3D designed a face mask fitter (Fig. 3e and f), based on a 3D printed plastic frame [82]. The design uses the company’s face scan software (for smartphones) for creating an adapted geometry to the user’s face.
Valves
COVID-19 patients may present SARS, with high respiratory rates and low oxygen levels [83]. Patients with these critical hypoxemic symptoms are normally submitted to invasive mechanical ventilation (IMV) procedure [84]. During this procedure, droplets and aerosols from the patient are released, creating a propitious environment for contamination of health workers [83, 85–87]. In these cases, patients remain in induced coma for long periods of time, increasing the risk of death using sedatives and the occupation of intensive care unit (ICU) beds. These are critical facts during the COVID-19 pandemic because of the shortage of ICU beds, mechanical ventilators, and high risk of contamination for health workers. A possible way for reducing intubation is using non-invasive ventilation (NIV) procedures, such as continuous positive airways pressure (CPAP). The WHO recommends the use of NIV as a first approach before intubation of patients [10]. Wang et al. [88] made a study in which 27 COVID-19 patients presenting SARS were submitted to NIV. From these patients, only 4 had to be intubated. However, because COVID-19 can be transmitted through aerosols and droplets [4, 14], NIV with inappropriate seals should not be used [87, 89]. Hence, exhaled air from the patients submitted to NIV cannot be returned to the environment or must be filtered with a HEPA filter.
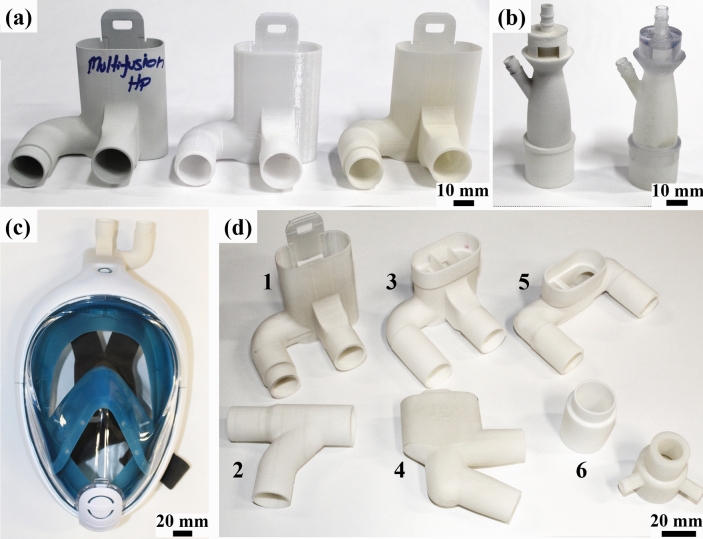
Italian ISINNOVA company created a solution using 3D printing connector valves to be used with Decathlon’s Surface Snorkeling Mask Easybreath® as full-face masks for CPAP procedure (Fig. 4c). The valves, named as Charlotte (Fig. 4a) and Dave (Fig. 4d), can be downloaded at ISINNOVA’s website. FFF/polylactic acid (PLA) are the recommended fabrication method/material [51]. This assembly can be used with an oxygen flow or mechanical ventilator, may minimize environment contamination [12], and can be sterilized and reused [32].
Fig. 4.
a ISINNOVA’s Charlotte valve [51] made by different processes and materials—from left to right: MJF/PA12, FDM/PC and SLS/PA12. b Venturi valve models by SLS/PA12 and PolyJet/VeroClear®. c Snorkeling mask connected to an ISINNOVA’s valve for being used during CPAP procedure. d Diverse types of valves: 1—Charlotte [51]; 2—Dave [51]; 3,4,5—Modified Charlottes; 6–adaptors made by SLS/PA12
Another approach related to the shortage of available mechanical ventilators are 3D printed splitters (class II devices by FDA [58]), which allow the use of a single mechanical ventilator by two different patients [15, 90]. Furthermore, there are also open-source NIV helmets projects like the Bubble Helmet from COSMIC Medical [91] that may use 3D printed valves as ports for the assembly.
Venturi valves (Fig. 4b) operate by Bernoulli’s principle of jet gas mixing [11] and can deliver a fixed fraction of inspired oxygen (FiO2) [69, 87], depending on the valve geometry [87]. They are used with a mechanical ventilator and a Venturi mask during NIV procedures [11, 87]. They are also described as part of the CPAP system when connected to a mechanical ventilator [12]. Therefore, these valves are essential during COVID-19 pandemic because of the high demand for respiratory devices. In Brescia, Italy, a supply chain disruption made these valves scarce. Again, ISINNOVA 3D printed by FFF Venturi valves in a short period of time, based on reverse engineering, and have saved lives [52]. These valves were also further produced in SLS and SLA [52]. Filip Kober further created 3D models to be printed in SLA, downloadable at GrabCAD [92]. SLA is the technique of choice because part porosity may alter the functionality of the valves, changing delivered FiO2 [15]. The models were simulated using computational fluid dynamics (CFD) to ensure the correct values of FiO2 [52].
Isolation chambers and wards
A measure to prevent the SARS-CoV-2 dissemination is the isolation of suspected or confirmed patients. As explored before, patients in hospitals may spread the virus through aerosols and droplets [4, 14]. Therefore, some devices can reduce environment contamination. Cubillos et al. [93] developed a negative air flow isolation chamber for COVID-19 patients. Their device consists in a rigid cubic frame chamber draped with a clear plastic bag in which a continuous negative air flow prevents air from inside the chamber from contaminating the external environment. It uses 3D printed ports that allows suction, oxygen delivery and nebulization. The instructions and plans are available under a Creative Commons License, Attribution-NonCommercial-ShareAlike 4.0 International. A collaboration of Sector 67 with University of Wisconsin-Madison Department of Surgery, Dr. Hau Le, and UW-Madison College of Engineering [94] developed a negative pressure isolation head box, named as “badger box”, which uses AM to produce glove grommets. Another example of isolation chambers, although more simplistic, were produced in Texas A&M University [95]. The chambers work as a physical barrier for contaminated patients and consist in CNC cut vinyl and 3D printed parts.
In large-scale projects, the creation of 3D printed isolation wards by the Chinese company WinSun can be highlighted [96, 97]. The wards are used as a professional and comfortable environment for patient isolation and treatment. They can also serve as a properly sanitized and protected resting place for healthcare professionals working directly to combat the pandemic. The standard model is 10 m2 and 2.8 m tall. The 3D printed wards construction process is automated, resulting in low labor cost and rapid construction. The buildings can be moved to different locations and can be easily connected to a power supply network [13, 34]. Finally, build process uses as feedstock material industrial or solid construction waste and, after demolition, can be reused in new 3D printed buildings [96].
Field respirators
An alliance between the Consorci de la Zona Franca de Barcelona, HP, CatSalut and Leitat Technological Center developed a field respirator, named as LEITAT 1, using AM for prototyping and parts production. The device, a mechanical bag valve mask, is to be used for short term emergency ventilation. The emergency ventilator, tested in ICU patients and approved by the Spanish Medicines Agency, has parts produced by MJF and is industrially scalable [98, 99]. Moreover, Petsiuk et al. [100] designed an automated fully open source bag valve mask-based ventilator, which also serve as a temporary emergency ventilator. In their work, they give a detailed protocol and links to the downloadable files, which include 3D printable parts of the ventilator assembly. The reported results exceed human capabilities in bag valve mask-based manual ventilation.
Nasopharyngeal swabs
Massive testing is an important measure for fighting the COVID-19 pandemic. For this, specimens from the upper respiratory tract must be collected and tested by reverse transcription polymerase chain reaction (RT-PCR) [10]. In this sense, nasopharyngeal swabs provide the highest sensitivity for the virus detection [50]. These are FDA Class I medical devices [58] with a rod-shape and a head coated with short synthetic filaments or spun fiber, used to collect secretions from the upper respiratory tract from the patient. The swab usually has a breakpoint on the shaft for enabling the head storage in a vial with viral transport media. Then, the sealed vial is sent for testing. To ensure a safe and reliable test, the swab must be flexible and sterilizable [15, 101]. Cotton head swabs are not indicated for COVID-19 [10, 50].
Cox and Koepsell [29] created a FFF printable and sterilizable PETG nasopharyngeal swab. They made clinical tests using commercial swabs as control and found the 3D printed model has the same efficiency of the control group. Callahan et al. [50] made a multi-step preclinical evaluation on 160 designs and 48 materials of test swabs and validated 4 prototypes. From this study, a consortium including Carbon, FormLabs, Envisiontec, Origin and Abiogenix companies was created and can produce up to 4 million FDA registered test swabs per-week via vat photopolymerization with medical grade resin [102]. In the same work, swabs designed for MJF medical grade nylon were also validated [103]. Arnold et al. [101] developed a swab with an open lattice design to be produced by DLP process. The model was tested and obtained a 90% match of results taking commercial swabs as golden standard, showing the design is adequate for test. Ford et al. [104] designed and developed over 20 nasopharyngeal tips. Their final design showed a 94% concurrence with the current synthetic version in an initial study and multisite clinical trials are currently underway. The STL models from the previous works are only available by request to the companies.
Finally, some nasopharyngeal swabs designs can be found at file repositories, such as the model from Fig. 5, designed by MarianneE (Johns Hopkins University Applied Physics Laboratory) user in the NIH 3D Print Exchange repository [105]. This design is for production using SLS/PA12. However, it is important to remark that there is no evidence these items have undergone any clinical test or validation, and the manufacturers should be certified by ISO 13485 (Medical Devices Quality Management System) for ensuring safe and reliable tests.
Fig. 5.
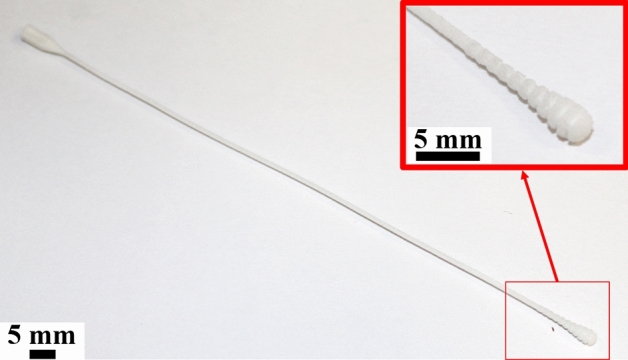
Nasopharyngeal swab from MarianneE user from NIH 3D Print Exchange repository [105] made by SLS/PA12. This design has not undergone any validation process and is exhibited only as example
Hands-free tools
The COVID-19 virus may remain on different surfaces for periods up to 72 h [5]. Thus, avoiding direct contact with surfaces is an important way to reduce contamination during the pandemic, especially in public and medical centers [15]. Some objects such as buttons, door handlers, switches etc. are potentially virus spreaders because of the high number of interactions with different people [36].
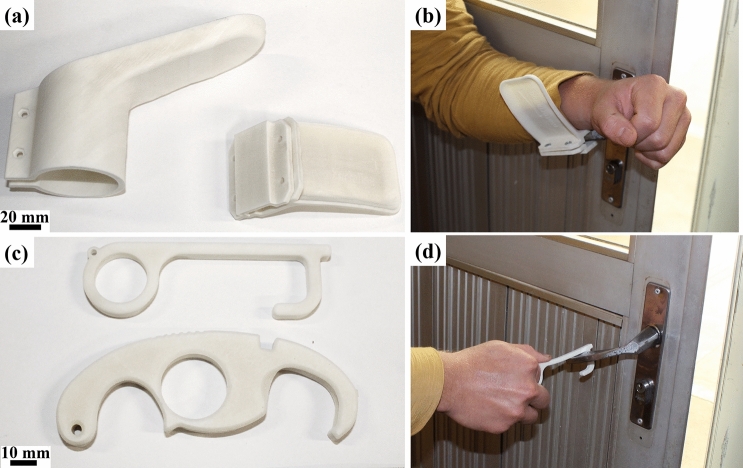
As a solution for reducing direct hand contact, various hands-free tools were designed. François et al. [36] designed and produced by FFF a high volume of various hands-free tools to be used in Greater Paris University Hospitals and other sites.
Hands-free door openers are devices that are fixed to door handles and allow door opening and closing with elbows or forearms [36]. Materialise company designed different types of hands-free door openers for cylindrical, rectangular, circular, and spherical handlers that can be 3D printed by MJF, SLS or FFF technologies [46]. Figure 6a and b show Materialise’s rectangular and circular hands-free door openers, and the rectangular model usage. Furthermore, hands-free tools for opening doors, pushing buttons, hooking handles, opening water taps, and other daily tasks were created by various designers for personal use. These hands-free designs can be found in various repositories websites such as Thingiverse [39], NIH 3D Print Exchange [40] and others. Figure 6c and d shows hands-free tools by SimonSolar2C [106] and bgiovanny [107] users from Thingiverse repository and an example of use.
Fig. 6.
Hands-free models made using SLS/PA12. a Materialise’s hands-free circular and rectangular door openers [46]. b Rectangular model usage. c Hands-free tools from SimonSolar2C [106] and bgiovanny [107] users from Thingiverse repository. d Tool being used for opening door
Antimicrobial devices
One major drawback of using AM polymers in some applications, such as masks, valves, and no-contact tools, is that SARS-CoV-2 presents a high stability (up to 72 h) on these surfaces. On the other hand, the virus stability on copper surfaces are reduced to 4 h, as found by van Doremalen et al. [5]. The previous work is in accordance with literature related to the use of some metallic coatings or nanoparticles into polymers. Some metals such as copper and silver have antimicrobial characteristics and can be used in the development of bioactive polymers [108]. Borkow et al. [109] showed that a face mask doped with copper and copper oxide particles reduce the risk of influenza (H1N1 and H9N2) contamination.
In this context, Copper 3D company uses the antiviral properties of copper to produce a face mask (named as NanoHack) using the company’s bioactive materials PLACTIVE® and MDflex®. The filtering system still require non-printed material (non-woven polypropylene embedded in copper nanoparticles) [110].
Another approach for antimicrobial devices is using antimicrobial coatings on surfaces to reduce biofilm formation. Muro-Fraguas et al. [111] used plasma-polymerization to create an acrylic acid (AcAc) coating on 3D printed PLA Petri dishes and obtained antibacterial properties using different Pseudomonas aeruginosa and Staphylococcus aureus strains.
Training and education
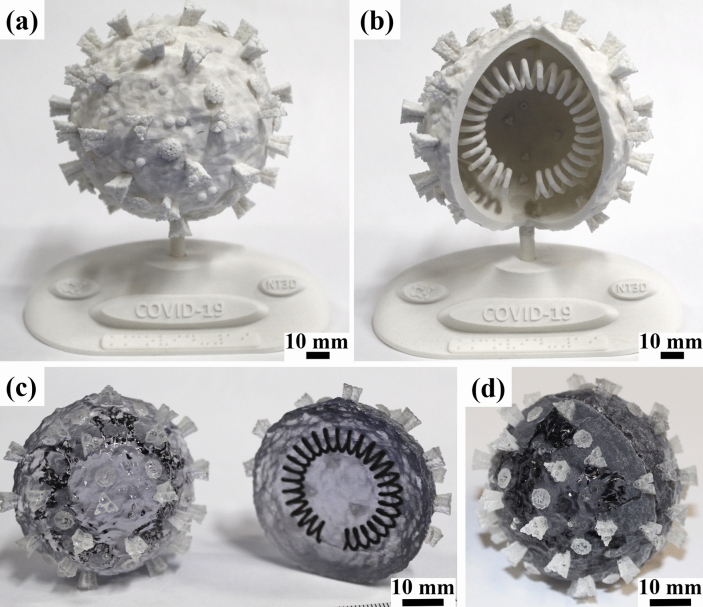
One of the various applications for AM is the education area. It can be used from printing simple forms to help children to develop abilities and skills [112, 113] to adults and professionals, where complex replicas to improve diagnosis, surgical planning and patient education can be printed [113, 114]. In this regard, AuMed [115] designed a manikin to help training of inexperienced healthcare workers in COVID-19 diagnosis by nasopharyngeal swab testing. In the education context, according to Hervás-Gómez et al. [113], the use of AM in education is growing, following the development of the Industry 4.0. Moreno Martínez and Morales Cevallos [116] point out the opportunity to use the AM notoriety during the COVID-19 pandemic to use this technology to improve students interest, participation and proactivity in experimentation and manipulation. The use of 3D printed tactile models can also be a tool for communication with individuals who are visually impaired [112]. Figure 7 presents two visual-tactile models of the SARS-CoV-2 morphology. Both models explore the proteins, lipid membrane and RNA from the virus. The models shown in Fig. 7a and b also contain support bases with Braille transcription for people who are visually impaired. Finally, because of the rapid shift to online education that occurred in various countries, simple solutions, such as 3D printed mounts to point webcams downwards, may help instructors to present a board-based pedagogical method [117].
Fig. 7.
Visual and tactile educative models of SARS-CoV-2, showing RNA, proteins, and lipid membrane. a SLS/PA12 model with base containing braille writing and b PolyJet/VeroClear® + TangoBlackPlus® two-halves model. Models from own authorship and available by request to the main author
Mask manufacturing tools
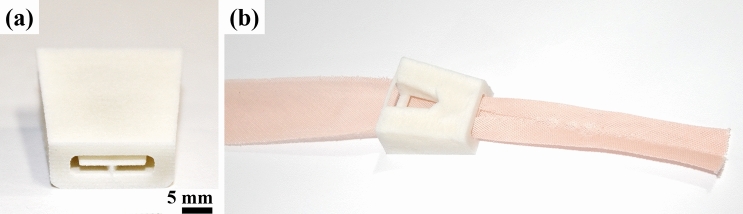
In a document with advice on the uses of masks during the pandemic [59], the WHO guided governments to encourage the general public to wear face masks. Because of the shortage of surgical and N95 masks, homemade fabric masks emerged as an interim solution. Consequently, some people started to sew their own fabric masks and, in some cases, to sell or donate these items. As a way for helping people to increase the production of these wearables, 3D printable mask pleaters and bias tape makers were designed and made available for download at diverse repositories, such as Thingiverse [39]. Bias tape makers are folders that assist the fabrication of cloth bias tape, as shown in Fig. 8, used in finishing edges of fabric masks. Mask pleaters help the production of pleated face masks. Both are time-consuming tasks, and these simple designs act as tools for increasing production.
Fig. 8.
a Bias tape maker made by SLS/PA12 used for b folding cloth. Model designed by ongaroo user from Thingiverse repository [118]
Endoscopic procedure mask
The risks to health workers associated with aerosols and droplets during IMV procedures were discussed in Sect. 5.2. However, there are other medical procedures, like endoscopic skull base surgery, that also create high risks of pathogens transmission. In this context, Helman et al. [55] developed a 3D printed mask for endoscopic skull base surgery. They redesigned the mask edge circumference from open-source mask models and added an inlet and outlet to the sides of the masks for tubing. Then, they used a surgical glove to provide a flexible physical barrier that allows usage of medical instrumentation. They performed various tests in two cadaveric models and found the device offers the surgeon appropriate surgical degrees of freedom, maneuverability and reduces risks of contamination. The mask model is available by request to the main author of the work.
Metallic devices
As explored in Sect. 5.7, the COVID-19 virus stability on copper surface is up to only 4 h [5]. Following these findings, ExOne company and the University of Pittsburgh worked in collaboration to develop sterilizable copper filters made by binder jetting technology. They use the process intrinsic porosity after sintering to produce filters with specific porosity levels to meet N95 filtration standard. One major advantage of the filter is that it is reusable, reducing waste disposal [119].
To summarize the data from all the 3D printed devices presented in the previous sections to fight against COVID-19 pandemic, Table 1 gathers information about used AM technologies and their respective references.
Table 1.
3D printed parts produced during COVID-19 pandemic
| Item | AM technologies | References/Sources |
|---|---|---|
| Face shields | FFF, SLA, SLS | [53, 62, 63] |
| Safety goggles | FFF, SLA, SLS | [65–68] |
| N95 respirators similar | FFF | [49, 71, 72] |
| Half-face masks | FFF, MJF, SLS | [38, 77, 79, 80] |
| Antimicrobial mask | FFF | [110] |
| PAPR parts | FFF | [74–76] |
| Ear Savers | FFF | [81] |
| Mask fitters | FFF | [82] |
| Isolation chambers | FFF | [93–95] |
| Isolation wards | Not specified | [97] |
| Valves for CPAP | FFF | [51] |
| Splitter valves | Not specified | [90, 120] |
| Valves for NIV helmets | Not specified | [91] |
| Venturi valves | FFF, SLA, SLS | [92] |
| Field Respirators parts | Not specified | [98, 100] |
| Nasopharyngeal swabs | DLP, FFF, MJF, SLS, SLA | [29, 101–105] |
| Hands-free door openers | MJF, FFF, SLS | [46] |
| Hands-free tools | FFF | [106, 107] |
| Manikin for swab testing training | Not specified | [115] |
| Educational models | SLS, PolyJet | Own authorship |
| Mounts to point webcams downward | FFF | [117] |
| Bias tape makers | FFF | [118] |
| Mask pleaters | FFF | [121] |
| Copper filters | Binder jetting | [119] |
| Surgery mask | FFF | [55] |
Drawbacks
As AM has assumed an important role during COVID-19 pandemic, there are some major drawbacks that must be discussed.
Different parts produced from a same STL file may present significant variations in geometry and mechanical properties because there are differences in material type and quality, software, calibration and equipment [24, 28]. In a work by Mueller et al. [28], the authors raise several questions about the material selection and quality control of 3D printed parts for COVID-19 pandemic. Most of medical devices must comply with strict standards to ensure a safe and reliable use, avoiding any preventable harm for patients and professionals. As an example, FFF filament may retain moisture, which could act as a virus transmitter during use or reuse [24]. In addition, 3D printed parts have different characteristics from parts produced by conventional methods: the layer upon layer process may generate poor interfaces, delamination, and unintentional porosity [122]. Thus, as pointed by Larrañeta, Dominguez-Robles and Lamprou [14], these parts may not provide the same security levels. In a preprinted work from Bezek et al. [78], face masks produced by FFF presented defects that decreased their efficiency in filtering particles. Novak and Loy [57] indicate the need for design for additive manufacturing (DfAM), which considers the AM process characteristics and particularities during project. Therefore, makers must be aware of optimized printing parameters for the application and the required quality of each printed part [123].
In addition, since there is no review process for any STL file uploaded in file repositories, there is no assurance that the final models will be functional. Another issue is the high number of different types of projects that can be found for a same device, such as face-shields and face masks. As an example, Novak and Loy [57] studied the manufacturability of 37 and 31 different face-shield and face mask models, respectively, from companies, groups, and individuals. They found large differences in terms of cost and manufacturing time between the models. This may be a concern when choosing which model to produce. Another issue is choosing valid models that were previously tested and reviewed by medical workers, since there are various designs that lack this information. In this regard, companies and scientific papers are a better source of projects that were generally developed by multidisciplinary teams and went through a validation process.
With regards to issues, the regulatory agencies and intellectual property are two relevant topics. Beyond medical review and validation, medical devices must be approved by the country’s regulatory agency, such as FDA for USA, the Medicines and Healthcare products Regulatory Agency (MHRA) for the United Kingdom and the Brazilian Health Regulatory Agency (Anvisa) for Brazil. Hence, it is worth to say that every single person involved in the production of medical devices must be aware of his/her legal responsibility even as a philanthropic action. Usually, the process for approval by a regulatory agency is expensive and requires considerable time [23]. Regarding this, during the pandemic, some agencies had published official declarations on the use of unapproved medical products during the emergency [124, 125]. Previous to the pandemic, in December 2017, FDA had published a guidance with technical considerations for additive manufactured medical devices [126]. However, Pecchia et al. [18] point that emergency situations demand universal regulations, mainly for low- and mid-income countries. Further, the authors also argue about the importance of regulations and standards for guiding the manufacturers for ensuring high safety levels, since even small defects may harm the user. For the intellectual property theme, most of the medical equipment from the existing medical system is based on patented equipment from medical suppliers [31]. Regarding this, Tino et al. [15] alert for the reverse-engineering process and its possible legal issues. They point that goodwill from regulators, legal experts, and governments are essential for saving lives during an emergency such as the COVID-19 pandemic. As an example, ISINNOVA company rapidly patented their Charlotte valve to prevent any price speculation and left it free to use [51]. Furthermore, Mahr and Dickel [127] discuss how the COVID-19 pandemic rapidly changed our thinking on intellectual property and highlight the need for regulators, ethicists, lawyers, and members of the industry community to discuss and create guidelines for the maker community during global crisis scenarios.
Finally, as shown by the current study and summarized in Table 1, majority of parts and projects are developed in polymer AM. Larrañeta, Dominguez-Robles and Lamprou [14] pointed FFF, SLS and SLA as the main AM techniques used for producing COVID-19-related parts. Besides, Novak and Loy [23] studied the AM projects availability and also found that FFF, SLA, MJF, SLS and other polymer-based techniques are the flagship AM processes during the pandemic. Beyond polymers, AM can work with metals, ceramics, and composites. In 2017, the unit sales for metal AM systems grew by 79.9%, well above the 12.6% growth of unit sales of overall AM industrial system [21]. However, even with an exponential growth, metallic AM solutions during COVID-19 pandemic are almost zero. This statement could be related to the high costs of metal AM machines and feedstocks, leading to a low number of disponible workforce. Thus, metal AM fully potential was not explored during the pandemic.
Potential uses of AM in future pandemics
In the history of mankind there are records of several pandemics such as smallpox, cholera, Black Death (plague), AIDS, influenza, SARS, West Nile disease and tuberculosis [128]. The “Spanish flu” influenza pandemic, the most lethal pandemic in recent history, killed more than 20 million people worldwide [128, 129]. Influenza A (H1N1), a more recent pandemic, occurred in 2009 and contaminated over 1.6 million people and had 18,449 confirmed deaths [130]. In a more localized spread, Ebola outbreak in West Africa in 2014–2015 resulted in 28,600 cases, resulting in 11,350 deaths, though it is estimated that up to 70% of cases may not have been accounted [131]. Even though infectious diseases have always plagued humanity, during the last decade, there has been a significant increase in the number of emerging and reemerging infections, reaching more than 20 infectious agents [132]. In fact, since 1980 the number of infectious diseases has increased by nearly fourfold because of the population growth, increased number of domesticated species, and globalized economy—with an increased people mobility [133]. Therefore, there is no reason in expecting COVID-19 pandemic will be the last, or it will take a long time for a new outbreak.
In this regard, beyond the already developed applications for AM during emergency situations, there might be new uses that will come from the technology advancements. As explored previously, AM is a relatively recent technology, having its first commercial system dated from 1987. The technology has currently attracted attention from research and industry, with new technologies and materials coming up daily. The next sections show and discuss some specific AM fields that may affect possible future applications for AM during future pandemics.
New materials
New material development will make AM more competitive toward conventional production technologies as it will create opportunities to new design freedoms and applications [26]. As a previously discussed example, bioactive materials, such as bioactive plastics, which present antimicrobial properties [108–110], are still being developed and will reach common public, opening a window for mass fabrication of bioactive devices, such as valves, hands-free tool and masks. Regarding this, Zuniga e Cortes [134] highlight the importance of developing these materials as they may be an important tool for fighting off the COVID-19 and future pandemics.
Metal and ceramic additive manufacturing
As reported, metal and ceramic AM did not show significant engagement during the COVID-19 pandemic. Even though metal AM has grown exponentially in the last years [21], it is still an expensive technology with less facilities around the world. However, this growth will create new opportunities for the technology expansion, development, and accessibility, making it an important tool for possible next pandemics.
Design for additive manufacturing
The advantages of AM, such as freedom of design, allow using lattice structures, organic shapes, stacking, cooling channels, topology-optimized geometries and more. These features are related to AM-engineering design, named as design for additive manufacturing. The DfAM also relates to process particularities, such as layer-by-layer manufacturing, thermal history, and residual stress [135]. This area is quickly evolving, aided by software development, and will strongly impact AM industry and development. In a review work, Thompson et al. [135] study the importance of DfAM and highlight that the future will bring educational materials for institutions, industry and hobby community. Consequently, DfAM will evolve and leverage AM productivity, suitability of design, lower production costs, and open new opportunities of applications.
Rapid tooling
AM is a powerful tool for prototyping and small batch production. However, when dealing with big batches, conventional techniques, such as injection molding, are economic favorable [136–138]. As an example, a preprinted work by Kunkel et al. [139] presented a qualitative comparison between AM and injection molding capabilities for large-scale production of face shields. For a single AM machine, the production capability was of about 10 per day, while for an injection molding machine it reached a capability of 2000. Nevertheless, AM can be used for rapid tooling, an accelerated production of tools for injection molding. Furthermore, conformal cooling channels can be added in the design. Conformal cooling channels, which cannot be produced by conventional production technologies, are cooling channels in tooling that follow the shape of the designed parts. These channels provide enhanced thermal control with improved heat exchange, implying in faster production cycles, better products, and increased injection tool life [69, 135, 136, 140]. This area is related to the DfAM field and is developing as research is done [135]. Therefore, AM will be able to accelerate the production of tooling devices for optimized mass production. As an example made reality during the pandemic, to achieve mass production, Catalysis Additive Tooling company took 2 weeks to design and produce a 3D printed metallic injection mold for manufacturing headbands for face shields [141].
Bioprinting
A technology with great potential to help fight pandemic diseases is bioprinting. This is a technology, emerged from 3D printing in 2003 [142], that uses bioink as deposition material [143]. Groll et al. [144] define bioink as “a formulation of cells suitable for processing by an automated biofabrication technology that may also contain biologically active components and biomaterials”. Still in its infancy, the technology can be used in regenerative medicine to support the manufacture of tissues and organs for transplantation [145, 146]. It could also be used for producing lung models—or any other organ model -, which could be used to study a disease dynamic and analyze an infected organ response to drug targets, detecting the disease progression in vitro [147, 148]. In this regard, Berg et al. [148] have shown the model ability to mimic the distribution of an influenza A virus strain in a real lung, something impossible for traditional cell culture.
3D printed medicines
As the pandemic diseases can easily overpass frontiers, it is important to develop strategies for producing and distributing drugs to all parts of the world during supply chain disruptions. Regarding this, there is the possibility to 3D print oral solid dosage forms (OSDF) with different drugs in a customized way [140, 149]. These medicines can be produced with controlled dosages, according to patients’ characteristics, with controlled release and, most importantly in a pandemic scenario, in a decentralized way [140]. Therefore, AM can be used for producing drugs at hot spots where conventional drugs cannot reach [140]. However, quality control, costs lowering, and the selection of the AM technology according to the formulation requirements are some key challenges that must be addressed for this application to be viable possible future pandemic scenarios [140].
Industry 4.0
The AM technology is described as part of Industry 4.0 [28, 113]. Regarding this, Choong et al. [34] highlights that AM machines will continue to be key parts in the cyber-physical post-pandemic scenario. In a review by Khan and Javaid [150], the authors point AM machines as part of an automated emergency system composed by innovative technologies, such as drones, machine learning, and artificial intelligence, to quickly deliver PPE at required places without human contact. Therefore, AM will benefit from Industry 4.0 development.
Final considerations
Additive manufacturing has emerged as an essential technology during COVID-19 pandemic. Because of the supply chain disruption, various devices, such as face shields, face masks, valves, nasopharyngeal swabs, and other devices were in shortage. Quick and innovative design associated with AM were successfully used for production of these items, saving lives from health workers, patients, and general population. These devices could be almost instantaneously produced in diverse parts of the world, as soon a project file was projected or shared through global network, pointing out for a future reduction in logistics with local production. Other major features that leveraged AM role during pandemic were the great number of 3D printers spread around the world, even in developing countries, the ease of sharing and downloading printable parts, and its ability for rapid prototyping first designs and equipment parts. Accompanied from diverse initiatives of companies, groups, and individuals in helping people in need, a mass production of devices was reached.
As this massive production gained the notoriety of people and media, it also brought up some issues to be overcome before the next emergency. The lack of centralized standards and guidelines from regulatory agencies caused different people, groups, and companies to take the initiative for producing non-validated parts from their own experience. This issue called the need for producing pre-approved guidelines for these situations that will allow people to help in a short period of time, without being threatened by legal issues and harming final users. Furthermore, intellectual property emergency laws must be developed to ensure people lives, as during the pandemic the supply chain disruption created a shortage of diverse essential devices from medical suppliers. In this line, public domain open-source equipment and devices can be developed and act as an emergency option during these situations. Within that, scientists must work in testing and publishing results. These works will remain in literature as a reliable peer-reviewed data, beyond media and social media volatile information, and will help the development of guidelines and projects that may be centralized and stay as resource during future pandemics.
Finally, AM is still a relatively recent technology. Materials, techniques, software, and applications are being developed daily. This will ensure that additive manufacturing will continue to be fundamental to help humanity fight the next coming pandemics.
Author contributions
GAL: main idea; literature search; data analysis; draft; critical review. GBN: literature search; draft; critical review. GC: critical review. JVLS: literature search; draft; critical review.
Funding
This study was funded by the National Council for Scientific and Technological Development (CNPq), Grants nos. 139963/2019-7, 301626/2020-0, and 301624/2020-8 and the Scientific Research Foundation for the State of São Paulo (FAPESP), Grant no. 2020/05612-8.
Availability of data and materials
Not applicable.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020 (2020) https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 19 May 2020
- 3.COVID-19 Map - Johns Hopkins Coronavirus Resource Center (2020) https://coronavirus.jhu.edu/map.html. Accessed 2 Sep 2020
- 4.Wesemann C, Pieralli S, Fretwurst T et al (2020) 3-D printed protective equipment during COVID-19 pandemic. Mater (Basel) 13:1–9. 10.3390/MA13081997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N, Bushmaker T, Morris DH et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Ning Z, Chen Y et al (2020) Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582:557–560. 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 7.Jayaweera M, Perera H, Gunawardana B, Manatunge J (2020) Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res 188:109819. 10.1016/j.envres.2020.109819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EL, Turnham P, Griffin JR, Clarke CC (2020) Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal 40:902–907. 10.1111/risa.13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Ji Z, Yue Y et al (2020) Infection risk assessment of COVID-19 through aerosol transmission: a case study of South China Seafood Market. Environ Sci Technol. 10.1021/acs.est.0c02895 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization (2020) Clinical management of COVID-19: Interim guidance (27 May 2020)
- 11.Tabashi S, Mirkheshti A, Dahi M et al (2020) Supplemental oxygen therapy and non-invasive ventilation in coronavirus disease 2019 (COVID-19). J Cell Mol Anesth 5:27–31 [Google Scholar]
- 12.Cavallo L, Marcianò A, Cicciù M, Oteri G (2020) 3D Printing beyond dentistry during COVID 19 epidemic: a technical note for producing connectors to breathing devices. Prosthesis 2:46–52. 10.3390/prosthesis2020005 [Google Scholar]
- 13.Advincula RC, Dizon JRC, Chen Q et al (2020) Additive manufacturing for COVID-19: devices, materials, prospects and challenges. MRS Commun. 10.1557/mrc.2020.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larrañeta E, Dominguez-Robles J, Lamprou DA (2020) Additive Manufacturing can assist in the fight against COVID-19 and other pandemics and impact on the global supply chain. 3D Print Addit Manuf 2020:1–3. 10.1089/3dp.2020.0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tino R, Moore R, Antoline S et al (2020) COVID-19 and the role of 3D printing in medicine. 3D Print Med 6:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsikala Vafea M, Atalla E, Georgakas J et al (2020) Emerging technologies for use in the study, diagnosis, and treatment of patients with COVID-19. Cell Mol Bioeng. 10.1007/s12195-020-00629-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das H, Patowary A (2020) Uses of 3D Printing for Production of Ppe for Covid 19 like situations: scope and future. Am J Prev Med Public Heal 6:76. 10.5455/ajpmph.20200413022349 [Google Scholar]
- 18.Pecchia L, Piaggio D, Maccaro A et al (2020) The inadequacy of regulatory frameworks in time of crisis and in low-resource settings: personal protective equipment and COVID-19. Health Technol (Berl). 10.1007/s12553-020-00429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vordos N, Gkika DA, Maliaris G et al (2020) How 3D printing and social media tackles the PPE shortage during COVID-19 pandemic. Saf Sci 130:104870. 10.1016/j.ssci.2020.104870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo N, Leu MC (2013) Additive manufacturing: technology, applications and research needs. Front Mech Eng 8:215–243. 10.1007/s11465-013-0248-8 [Google Scholar]
- 21.Wohlers T, Campbell I, Diegel O, Kowen J (2018) Wohlers Report 2018—3D printing and additive manufacturing state of the industry—annual worldwide progress report. Wohlers Associates Inc., Fort Collins, Colorado
- 22.Franchetti M, Kress C (2017) An economic analysis comparing the cost feasibility of replacing injection molding processes with emerging additive manufacturing techniques. Int J Adv Manuf Technol 88:2573–2579. 10.1007/s00170-016-8968-7 [Google Scholar]
- 23.Novak JI, Loy J (2020a) A critical review of initial 3D printed products responding to COVID-19 health and supply chain challenges. Emerald Open Res 2:24. 10.35241/emeraldopenres.13697.1 [Google Scholar]
- 24.Clifton W, Damon A, Martin AK (2020) Considerations and cautions for three-dimensional-printed personal protective equipment in the COVID-19 crisis. 3D Print Addit Manuf 2020:3–5. 10.1089/3dp.2020.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ISO/ASTM (2015) International standard ISO/ASTM 52900 additive manufacturing—general principles—terminology. Int Organ Stand 5:1–26. 10.1520/ISOASTM52900-15 [Google Scholar]
- 26.Bourell D, Kruth JP, Leu M et al (2017) Materials for additive manufacturing. CIRP Ann Manuf Technol 66:659–681. 10.1016/j.cirp.2017.05.009 [Google Scholar]
- 27.Silva JVL, Rezende RA (2013) Additive manufacturing and its future impact in logistics. IFAC Proc Vol. 2013;46(24):277–282. 10.3182/20130911-3-BR-3021.00126
- 28.Mueller T, Elkaseer A, Charles A et al (2020) Eight weeks later—The unprecedented rise of 3D Printing during the COVID-19 pandemic—a case study, lessons learned, and implications on the future of global decentralized manufacturing. Appl Sci 10:4135. 10.3390/app10124135 [Google Scholar]
- 29.Cox JL, Koepsell SA (2020) 3D-printing to address COVID-19 Testing supply shortages. Lab Med. 10.1093/labmed/lmaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shokrani A, Loukaides EG, Elias E, Lunt AJG (2020) Exploration of alternative supply chains and distributed manufacturing in response to COVID-19; a case study of medical face shields. Mater Des 192:108749. 10.1016/j.matdes.2020.108749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belhouideg S (2020) Impact of 3D printed medical equipment on the management of the Covid19 pandemic. Int J Health Plann Manage. 10.1002/hpm.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livingston E, Desai A, Berkwits M (2020) Sourcing Personal protective equipment during the COVID-19 pandemic. JAMA J Am Med Assoc. 10.1001/jama.2020.5317 [DOI] [PubMed] [Google Scholar]
- 33.Fiorillo L, Leanza T (2020) Worldwide 3D printers against the new coronavirus. Prosthesis 2:87–90. 10.3390/prosthesis2020009 [Google Scholar]
- 34.Choong YYC, Tan HW, Patel DC et al (2020) The global rise of 3D printing during the COVID-19 pandemic. Nat Rev Mater. 10.1038/s41578-020-00234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manero A, Smith P, Koontz A et al (2020) Leveraging 3D printing capacity in times of crisis: recommendations for COVID-19 distributed manufacturing for medical equipment rapid response. Int J Environ Res Public Health 17:1–17. 10.3390/ijerph17134634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.François P-M, Bonnet X, Kosior J et al (2020) 3D-printed contact-free devices designed and dispatched against the COVID19 pandemic: the 3D COVID initiative. J Stomatol Oral Maxillofac Surg. 10.1016/j.jormas.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas CN, Schroder LK, Cole PA (2020) Ten days to implementation of 3D-printed masks for a level-I orthopaedic trauma practice during the COVID-19 pandemic. J Bone Joint Surg Am 102:1–6. 10.2106/JBJS.20.00881 [DOI] [PubMed] [Google Scholar]
- 38.Stopgap Surgical Face Mask (SFM) Revision B|NIH 3D Print exchange (2020) https://3dprint.nih.gov/discover/3dpx-014168. Accessed 4 Jul 2020
- 39.Thingiverse—Digital Designs for Physical Objects (2020) https://www.thingiverse.com/. Accessed 19 May 2020
- 40.COVID-19 Response|NIH 3D Print Exchange (2020) https://3dprint.nih.gov/collections/covid-19-response. Accessed 24 Jun 2020
- 41.Popular models|3D CAD Model Collection|GrabCAD Community Library (2020) https://grabcad.com/library. Accessed 20 Jul 2020
- 42.Free 3D Printable Files and Designs|Pinshape (2020) https://pinshape.com/. Accessed 20 Jul 2020
- 43.Cults—Download for free 3D models for 3D printers (2020) https://cults3d.com/en. Accessed 20 Jul 2020
- 44.yeggi—3D Printer Models Search Engine (2020) https://www.yeggi.com/. Accessed 20 Jul 2020
- 45.MyMiniFactory|100,000+ 3D Print Files & Models (2020) Guaranteed. https://www.myminifactory.com/. Accessed 20 Jul 2020
- 46.Materialise Acts: Our 3D Printing Response to COVID-19 (2020) https://www.materialise.com/en/3d-printing-response-to-covid-19. Accessed 23 Jun 2020
- 47.COVID-19 Digital Manufacturing and 3D Printing response|3D Systems (2020) https://www.3dsystems.com/covid-19-response. Accessed 24 Jun 2020
- 48.Depoux A, Martin S, Karafillakis E et al (2020) The pandemic of social media panic travels faster than the COVID-19 outbreak. J Travel Med 27:1–2. 10.1093/jtm/taaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greig PR, Carvalho C, El-Boghdadly K, Ramessur S (2020) Safety testing improvised COVID-19 personal protective equipment based on a modified full-face snorkel mask. Anaesthesia. 10.1111/anae.15085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Callahan CJ, Lee R, Zulauf KE et al (2020) Open Development and clinical validation of multiple 3D-printed nasopharyngeal collection swabs: rapid resolution of a critical COVID-19 testing Bottleneck. J Clin Microbiol. 10.1128/jcm.00876-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Easy–Covid19 ENG|Isinnova (2020) https://www.isinnova.it/easy-covid19-eng/. Accessed 19 May 2020
- 52.Coronavirus and 3D printing—3D Printing Media Network (2020) https://www.3dprintingmedia.network/covid-19-3d-printed-valve-for-reanimation-device/. Accessed 25 Jun 2020
- 53.3D printed face shields for medics and professionals—Prusa3D—3D Printers from Josef Průša (2020) https://www.prusa3d.com/covid19/. Accessed 23 Jun 2020
- 54.Open Source Medical Supplies—Open Source Medical Supplies (2020) https://opensourcemedicalsupplies.org/. Accessed 30 Jun 2020
- 55.Helman SN, Soriano RM, Tomov ML et al (2020) Ventilated upper airway endoscopic endonasal procedure mask: surgical safety in the COVID-19 era. Oper Neurosurg 2020:1–10. 10.1093/ons/opaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amin D, Nguyen N, Roser SM, Abramowicz S (2020) 3D printing of face shields during COVID-19 pandemic: a technical note. J Oral Maxillofac Surg 78:1275–1278. 10.1016/j.joms.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novak JI, Loy J (2020b) A quantitative analysis of 3D printed face shields and masks during COVID-19. Emerald Open Res 2:42. 10.35241/emeraldopenres.13815.1 [Google Scholar]
- 58.Sinha MS, Bourgeois FT, Sorger PK (2020) Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health 110:1162–1164. 10.2105/AJPH.2020.305753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization (2020) Advice on the use of masks in the context of COVID-19: interim guidance—June 2020
- 60.Kalyaev V, Salimon AI, Korsunsky AM (2020) Fast mass-production of medical safety shields under COVID-19 quarantine: optimizing the use of university fabrication facilities and volunteer labor. Int J Environ Res Public Health 17:3418. 10.3390/ijerph17103418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neijhoft J, Viertmann T, Meier S et al (2020) Manufacturing and supply of face shields in hospital operation in case of unclear and confirmed COVID-19 infection status of patients. Eur J Trauma Emerg Surg. 10.1007/s00068-020-01392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.English|Projeto Higia (2020) https://www.projetohigia.com.br/english. Accessed 23 Jun 2020
- 63.Maracaja L, Blitz D, Maracaja DLV, Walker CA (2020) How 3D printing can prevent spread of COVID-19 among healthcare professionals during times of critical shortage of protective personal equipment. J Cardiothorac Vasc Anesth 2020:1–3. 10.1053/j.jvca.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearce JM (2020) Distributed manufacturing of open source medical hardware for pandemics. J Manuf Mater Process 4:49. 10.3390/jmmp4020049 [Google Scholar]
- 65.Safety goggles to protect against COVID-19—3D Printing Media Network (2020) https://www.3dprintingmedia.network/safety-goggles-to-fight-covid-19/. Accessed 26 Jun 2020
- 66.Oculos de Protecao—Google Drive (2020) https://drive.google.com/drive/folders/1O5yAkDuu6TZSS_KA0XeO9hOJu2UZJwbH?sort=13&direction=a. Accessed 26 Jun 2020
- 67.Help Infinite Resource—FRC TEAM 2471 (2020) https://team2471.org/infinite-resource/help/. Accessed 26 Jun 2020
- 68.3D Printed Face Mask—No worries on mask shortage and coronavirus infection (2020) https://creality.com/info/makers-guide-3d-printed-face-mask-no-worries-on-mask-shortage-and-virus-infection-i00248i1.html. Accessed 26 Jun 2020
- 69.Ishack S, Lipner SR (2020) Applications of 3D printing technology to address COVID-19–related supply shortages. Am J Med 2019:2019–2021. 10.1016/j.amjmed.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu C, Yang Y, Gao Y et al (2020) Viral architecture of SARS-CoV-2 with post-fusion spike revealed by Cryo-EM. bioRxiv 2020:1–17 [Google Scholar]
- 71.Kroo L, Kothari A, Hannebelle M et al (2020) Pneumask: modified full-face snorkel masks as reusable personal protective equipment for hospital personnel. medRxiv. 10.1101/2020.04.24.20078907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Addi RA, Abdelhafid Benksim MC (2020) Easybreath decathlon mask: an efficient personal protective equipment (PPE) against COVID-19 in Africa. J Thorac Oncol 11:1–3. 10.5799/jcei/7894 [Google Scholar]
- 73.Gierthmuehlen M, Kuhlenkoetter B, Parpaley Y et al (2020) Evaluation and discussion of handmade face-masks and commercial diving-equipment as personal protection in pandemic scenarios. PLoS ONE 15:e0237899. 10.1371/journal.pone.0237899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.COVID—19—RKID (2020) https://www.rkid.co.za/covid-19-industrial-design-innovation/?fbclid=IwAR2JYE70YHg-lB3fIALZFGJnk5XKVcOmlVnKa6YAs44H0GeF352klQAH4Lw. Accessed 27 Jun 2020
- 75.Something Labs-CAPR Mounting Tabs (2020) https://www.somethinglabs.org/capr-mounting-tabs. Accessed 27 Jun 2020
- 76.Erickson MM, Richardson ES, Hernandez NM et al (2020) Helmet modification to PPE with 3D printing during the COVID-19 pandemic at Duke University Medical Center: a novel technique. J Arthroplasty 35:S23–S27. 10.1016/j.arth.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swennen GRJ, Pottel L, Haers PE (2020) Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg 49:673–677. 10.1016/j.ijom.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bezek LB, Pan J, Harb C, Zawaski CE, Molla B, Joseph R (2020) Particle Transmission through respirators fabricated with fused filament fabrication and powder bed fusion additive manufacturing. preprints
- 79.lafactoria3D COVID-19 Mask (2020) https://www.thingiverse.com/thing:4225667. Accessed 3 Sep 2020
- 80.Make The Masks (2020) https://www.makethemasks.com/. Accessed 3 Sep 2020
- 81.Ear Savers for health workers|NIH 3D Print Exchange (2020) https://3dprint.nih.gov/discover/3dpx-013860. Accessed 4 Jul 2020
- 82.How to make Bellus3D’s Face mask fitter|Bellus3D: High-quality 3D face scanning (2020) https://bellus3d.com/solutions/facemask.html. Accessed 26 Jun 2020
- 83.Zuo M, Huang Y, Ma W et al (2020) Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chinese Med Sci J. 10.24920/003724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murthy S, Gomersall CD, Fowler RA (2020) Care for critically ill patients with COVID-19. JAMA 323:1499–1500. 10.1001/jama.2020.3633 [DOI] [PubMed] [Google Scholar]
- 85.Asenjo JF (2020) Safer intubation and extubation of patients with COVID-19. Can J Anesth Can d’anesthésie. 10.1007/s12630-020-01666-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coccolini F, Perrone G, Chiarugi M et al (2020) Surgery in COVID-19 patients : operational directives. World J Emerg Surg 2:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pavlov I, Sacchetti AD, Whittle JS et al (2020) Respiratory support for adult patients with COVID-19. J Am Coll Emerg Physicians Open. 10.1002/emp2.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang K, Zhao W, Li J et al (2020) The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care 10:1–4. 10.1186/s13613-020-00653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winck JC, Ambrosino N (2020) COVID-19 pandemic and non invasive respiratory management: every Goliath needs a David. Ann Pulmonol. 10.1016/j.pulmoe.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johns Hopkins engineers develop 3D-printed ventilator splitters|Hub (2020) https://hub.jhu.edu/2020/04/02/3d-printed-ventilator-splitters-for-covid-19/. Accessed 12 Sep 2020
- 91.Bubble Helmet—COSMIC Medical (2020) https://cosmicmedical.ca/bubble-helmet. Accessed 26 Jun 2020
- 92.Printable ventilator-free respiratory: Venturi Valve|3D CAD Model Library|GrabCAD (2020) https://grabcad.com/library/respirator-free-reanimation-venturi-s-valve-1. Accessed 25 Jun 2020
- 93.Cubillos J, Querney J, Rankin A et al (2020) A multipurpose portable negative air flow isolation chamber for aerosol-generating procedures during the COVID-19 pandemic. Br J Anaesth 125:e179–e181. 10.1016/j.bja.2020.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Badger Boxes–UW Makerspace–UW–Madison (2020) https://making.engr.wisc.edu/box/. Accessed 11 Sep 2020
- 95.How Texas A&M is using 3D Printing To Respond To Coronavirus—Texas A&M Today (2020) https://today.tamu.edu/2020/05/26/how-texas-am-is-using-3d-printing-to-respond-to-coronavirus/. Accessed 11 Sep 2020
- 96.News-Yingchuang Building Technique (Shanghai)Co.Ltd. (WinSun) (2020) http://www.winsun3d.com/En/News/news_inner/id/543. Accessed 9 Sep 2020
- 97.WinSun deploys 3D printed isolation wards for coronavirus medical staff - 3D Printing Media Network. https://www.3dprintingmedia.network/winsun-3d-printed-isolation-wards-coronavirus-medical-workers/. Accessed 9 Sep 2020
- 98.LEITAT 1 reaches ICU patients as an accredited field ventilator—Covid Leitat (2020) https://covid-leitat.org/en/leitat-1-reaches-icu-patients-as-an-accredited-field-ventilator/. Accessed 2 Jul 2020
- 99.3D printed ventilator from Leitat—3D Printing Media Network (2020) https://www.3dprintingmedia.network/leitat-presents-first-medically-validated-industrialized-3d-printed-ventilator/. Accessed 2 Jul 2020
- 100.Petsiuk A, Tanikella NG, Dertinger S et al (2020) Partially RepRapable automated open source bag valve mask-based ventilator. HardwareX 8:e00131. 10.1016/j.ohx.2020.e00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arnold FW, Grant G, Bressoud PF et al (2020) A comparison efficacy study of commercial nasopharyngeal swabs versus a novel 3D printed swab for the detection of SARS-CoV-2. Univ Louisv J Respir Infect. 10.18297/jri/vol4/iss1/41 [Google Scholar]
- 102.Home|COVID Swabs (2020) https://printedswabs.org/. Accessed 26 Jun 2020
- 103.Nasopharyngeal swabs|NIH 3D Print Exchange (2020) https://3dprint.nih.gov/discover/3dpx-014487. Accessed 26 Jun 2020
- 104.Ford J, Goldstein T, Trahan S et al (2020) A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med 6:1–7. 10.1186/s41205-020-00076-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.3D-Printed Test Swabs|NIH 3D Print Exchange (2020) https://3dprint.nih.gov/discover/3dpx-013730. Accessed 4 Jul 2020
- 106.Ergonomic “Deri” Door Opener/grabber—Covid-19/Corona by SimonSolar2C—Thingiverse (2020) https://www.thingiverse.com/thing:4260659. Accessed 4 Jul 2020
- 107.GB 3D—Hands Free—Door Pull Opener—Button Press—Door Open by bgiovanny—Thingiverse (2020) https://www.thingiverse.com/thing:4310110. Accessed 4 Jul 2020
- 108.Palza H (2015) Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 16:2099–2116. 10.3390/ijms16012099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Borkow G, Zhou SS, Page T, Gabbay J (2010) A novel anti-influenza copper oxide containing respiratory face mask. PLoS ONE. 10.1371/journal.pone.0011295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hack the Pandemic—Copper 3D|Antibacterial 3D Printing (2020) https://copper3d.com/hackthepandemic/. Accessed 27 Jun 2020
- 111.Muro-Fraguas I, Sainz-García A, López M et al (2020) Antibiofilm coatings through atmospheric pressure plasma for 3D printed surgical instruments. Surf Coatings Technol 399:126163. 10.1016/j.surfcoat.2020.126163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buehler E, Kane SK, Hurst A (2014) ABC and 3D: opportunities and obstacles to 3D printing in special education environments. In: ASSETS14—Proc 16th Int ACM SIGACCESS Conf Comput Access, vol 107–114. 10.1145/2661334.2661365
- 113.Hervás-Gómez C, Román Graván P, Domínguez-González MÁ, Reina Parrado M (2020) Diseño e impresión en 3d de protectores de pantallas faciales por docentes universitarios para proteger al personal sanitario ante el Covid-19. IJERI Int J Educ Res Innov. 10.46661/ijeri.4970 [Google Scholar]
- 114.D’Urso PS, Atkinson RL, Lanigan MW et al (1998) Stereolithographic (SL) biomodelling in craniofacial surgery. Br J Plast Surg 51:522–530. 10.1054/bjps.1998.0026 [DOI] [PubMed] [Google Scholar]
- 115.Why 3D Printed Medical Mankins make swab testing easier—Creatz3D (2020) https://creatz3d.com.sg/3d-printed-medical-manikins-become-effective-training-aids-for-respiratory-swab-collection/. Accessed 9 Sep 2020
- 116.Moreno Martínez NM, Morales Cevallos MB (2020) COVID-19 desde una óptica tecno-educativa a través de markerspaces. IJERI Int J Educ Res Innov. 10.46661/ijeri.4898 [Google Scholar]
- 117.Davis EJ, Wheeler K (2020) Use of 3D printing to manufacture document camera mounts in support of online education shifts during the COVID-19 pandemic. J Chem Educ. 10.1021/acs.jchemed.0c00629 [Google Scholar]
- 118.4cm/5cm Bias Tape Maker, zakladac prouzku by ongaroo—Thingiverse (2020) https://www.thingiverse.com/thing:4232886. Accessed 4 Jul 2020
- 119.ExOne|ExOne and Pitt collaborate to produce promising reusable respirators with 3d printed metal Filters (2020) https://www.exone.com/en-US/News/ExOne-Reusable-Metal-3D-Printed-Filter-Respirator. Accessed 30 Jun 2020
- 120.no2covid.com (2020) https://no2covid.com/. Accessed 16 Jul 2020
- 121.Face Mask Pleater by j_wigger—Thingiverse (2020) https://www.thingiverse.com/thing:4259706. Accessed 16 Jul 2020
- 122.Ngo TD, Kashani A, Imbalzano G et al (2018) Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos Part B Eng 143:172–196. 10.1016/j.compositesb.2018.02.012 [Google Scholar]
- 123.Salmi M, Akmal JS, Pei E et al (2020) 3D printing in COVID-19: productivity estimation of the most promising open source solutions in emergency situations. Appl Sci 10:1–15. 10.3390/app10114004 [Google Scholar]
- 124.RESOLUÇÃO—RDC No 356, DE 23 DE MARÇO DE 2020 (*)—RESOLUÇÃO—RDC No 356, DE 23 DE MARÇO DE 2020 (*)–DOU—Imprensa Nacional (2020) http://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-356-de-23-de-marco-de-2020-*-250404719. Accessed 4 Jul 2020
- 125.Emergency Use Authorization|FDA (2020) https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. Accessed 2 Jul 2020
- 126.Center for Devices and Radiological Health (2017) Technical considerations for additive manufactured medical devices: guidance for Industry and Food and Drug Administration Staff
- 127.Mahr D, Dickel S (2020) Rethinking intellectual property rights and commons-based peer production in times of crisis: the case of COVID-19 and 3D printed medical devices. J Intellect Prop Law Pract 2020:1–7. 10.1093/jiplp/jpaa124 [Google Scholar]
- 128.Qiu W, Rutherford S, Mao A, Chu C (2017) The pandemic and its impacts. Heal Cult Soc 9:1–11. 10.5195/hcs.2017.221 [Google Scholar]
- 129.Shanks GD (2015) Insights from unusual aspects of the 1918 influenza pandemic. Travel Med Infect Dis 13:217–222. 10.1016/j.tmaid.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 130.WHO|Pandemic (H1N1) 2009—update 112 (2015) WHO
- 131.Scarpino SV, Iamarino A, Wells C et al (2015) Epidemiological and viral genomic sequence analysis of the 2014 Ebola outbreak reveals clustered transmission. Clin Infect Dis 60:1079–1082. 10.1093/cid/ciu1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balkhair AA (2020) Covid-19 pandemic: a new chapter in the history of infectious diseases. Oman Med J 35:2–3. 10.5001/OMJ.2020.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Covid-19: The history of pandemics—BBC Future (2020) https://www.bbc.com/future/article/20200325-covid-19-the-history-of-pandemics. Accessed 16 Jul 2020
- 134.Zuniga JM, Cortes A (2020) The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev Med Devices 17:477–481. 10.1080/17434440.2020.1756771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thompson MK, Moroni G, Vaneker T et al (2016) Design for Additive manufacturing: trends, opportunities, considerations, and constraints. CIRP Ann Manuf Technol 65:737–760. 10.1016/j.cirp.2016.05.004 [Google Scholar]
- 136.Shinde MS, Ashtankar KM (2017) Additive manufacturing-assisted conformal cooling channels in mold manufacturing processes. Adv Mech Eng 9:1–14. 10.1177/1687814017699764 [Google Scholar]
- 137.Mazur M, Leary M, McMillan M et al (2016) SLM additive manufacture of H13 tool steel with conformal cooling and structural lattices. Rapid Prototyp J 22:504–518. 10.1108/RPJ-06-2014-0075 [Google Scholar]
- 138.Huang R, Riddle ME, Graziano D et al (2017) Environmental and economic implications of distributed additive manufacturing: the case of injection mold tooling. J Ind Ecol 21:S130–S143. 10.1111/jiec.12641 [Google Scholar]
- 139.Kunkel ME, Perfeito JAJ, Zambrana NRM et al (2020) Mass-production and distribution of medical face shields using additive manufacturing and injection molding process for healthcare system support during COVID-19 pandemic in Brazil. Res Sq. 10.21203/rs.3.rs-63872/v1 [Google Scholar]
- 140.Hsiao W-K, Lorber B, Paudel A (2020) Can 3D printing of oral drugs help fight the current COVID-19 pandemic (and similar crisis in the future)? Expert Opin Drug Deliv 2020:1–4. 10.1080/17425247.2020.1772229 [DOI] [PubMed] [Google Scholar]
- 141.3D Printing and Coronavirus: US additive manufacturers share their experiences (2020) https://www.additivemanufacturing.media/blog/post/3d-printing-and-coronavirus-us-additive-manufacturers-share-their-experiences. Accessed 17 Jul 2020
- 142.Mironov V, Boland T, Trusk T et al (2003) Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol 21:157–161. 10.1016/S0167-7799(03)00033-7 [DOI] [PubMed] [Google Scholar]
- 143.Włodarczyk-Biegun MK, del Campo A (2017) 3D bioprinting of structural proteins. Biomaterials 134:180–201. 10.1016/j.biomaterials.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 144.Groll J, Burdick JA, Cho DW et al (2019) A definition of bioinks and their distinction from biomaterial inks. Biofabrication. 10.1088/1758-5090/aaec52 [DOI] [PubMed] [Google Scholar]
- 145.Mandrycky C, Wang Z, Kim K, Kim DH (2016) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34:422–434. 10.1016/j.biotechadv.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785. 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 147.Singh AK, Mishra G, Maurya A et al (2020) Biofabrication: an interesting tool to create in vitro model for COVID-19 drug targets. Med Hypotheses 144:110059. 10.1016/j.mehy.2020.110059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berg J, Hiller T, Kissner MS et al (2018) Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci Rep 8:1–13. 10.1038/s41598-018-31880-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hsiao WK, Lorber B, Reitsamer H, Khinast J (2018) 3D printing of oral drugs: a new reality or hype? Expert Opin Drug Deliv 15:1–4. 10.1080/17425247.2017.1371698 [DOI] [PubMed] [Google Scholar]
- 150.Khan I, Javaid M (2020) Automated COVID-19 emergency response using modern technologies. Apollo Med 14:198–201. 10.4103/am.am_68_20 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.