Abstract
HIV is associated with disruptions in cognition and brain function. Marijuana use is highly prevalent in HIV but its effects on resting brain function in HIV are unknown. Brain function can be characterized by brain activity that is correlated between regions over time, called functional connectivity. Neuropsychiatric disorders are increasingly being characterized by disruptions in such connectivity. We examined the synergistic effects of HIV and marijuana use on functional whole-brain network organization during resting state. Our sample included 78 adults who differed on HIV and marijuana status (19 with co-occurring HIV and marijuana use, 20 HIV-only, 17 marijuana-only, and 22 controls). We examined differences in local and long-range brain network organization using eight graph theoretical metrics: transitivity, local efficiency, within-module degree, modularity, global efficiency, strength, betweenness, and participation coefficient. Local and long-range connectivity were similar between the co-occurring HIV and marijuana use and control groups. In contrast, the HIV-only and marijuana-only groups were both associated with disruptions in brain network organization. These results suggest that marijuana use in HIV may normalize disruptions in brain network organization observed in persons with HIV. However, future work is needed to determine whether this normalization is suggestive of a beneficial or detrimental effect of marijuana on cognitive functioning in HIV.
Keywords: HIV, Marijuana, Cannabis, Functional Magnetic Resonance Imaging (fMRI), Resting state, Graph theory
1. INTRODUCTION
Nearly 40 million people are living with human immunodeficiency virus (HIV) across the world, with over 1.5 million becoming newly infected each year (UNAIDS, 2019). An estimated 15–50% of individuals with HIV have HIV-associated neurocognitive disorder (HAND; McArthur, Steiner, Sacktor, & Nath, 2010). HAND can occur when the virus infiltrates the central nervous system and causes a neuroinflammatory cascade (Valcour, Sithinamsuwan, Letendre, & Ances, 2011). Even when the disease is well-managed, the latent viral reservoir can cause persistent inflammation, which can promote neuronal damage (Saylor et al., 2016). Alterations in brain structure and function accompany HAND, with diffuse degradation in gray and white matter volume (Becker et al., 2011; Cohen et al., 2010; Kuper et al., 2011; Pfefferbaum et al., 2012; Sarma et al., 2014), as well as changes in brain function (Ann et al., 2016; Chaganti, Heinecke, Gates, Moffat, & Brew, 2017; Chockanathan, DSouza, Abidin, Schifitto, & Wismuller, 2019).
Factors like drug use may contribute to the progression of HAND. Marijuana use is especially common among HIV-positive individuals, with rates of use ranging from 20% to 60% (Crane et al., 2017; D’Souza et al., 2012; Gamarel et al., 2016; Hartzler et al., 2017; Mimiaga et al., 2013; Okafor, Cook, et al., 2017; Okafor, Zhou, et al., 2017). Though marijuana use in individuals with HIV has been associated with worse learning and memory performance (Cristiani, Pukay-Martin, & Bornstein, 2004; Thames, Mahmood, Burggren, Karimian, & Kuhn, 2016), it has also been suggested that moderate marijuana use may protect against such cognitive declines in HIV and that marijuana-associated neurocognitive decline depend on the amount of marijuana used (Thames et al., 2017; Thames et al., 2016; Watson et al., 2020). This lack of consistency suggests that the effects of marijuana on neurocognitive functioning in HIV are complex and under-characterized.
Abnormalities in patterns of functional connectivity between brain regions are increasingly being used to characterize neurocognitive impairment and neuropsychiatric conditions (for reviews, see: Dennis & Thompson, 2014; Fornito, Zalesky, Pantelis, & Bullmore, 2012; Stam, 2014; Xia et al., 2018). Functional connectivity is defined as correlated brain activity over time. Functional connectivity between two brain regions suggests that these regions are communicating (Biswal, Yetkin, Haughton, & Hyde, 1995; Damoiseaux et al., 2006; Greicius, Krasnow, Reiss, & Menon, 2003; Salvador et al., 2005; van den Heuvel & Pol, 2010). Topological properties of brain function can be quantified by measuring functional connectivity within and between large-scale brain networks. Healthy brain networks operate efficiently by segregating into discrete networks with high local connectivity, and integrating those networks with few strategically placed long-range connections such that the distance between any two networks is minimized. During task-free resting state, HIV has generally been associated with decreases in local and long-range connectivity (Plessis et al., 2014; Samboju et al., 2018; Thomas, Brier, Snyder, Vaida, & Ances, 2013), though the literature is mixed (Toich et al., 2018; H. J. Wang et al., 2018), suggesting that the response to neuronal insult caused by HIV varies across regions. Independent of HIV, individuals who use marijuana generally show increased connectivity between regions across the brain during resting state (Cheng et al., 2014; Filbey et al., 2014; Lopez-Larson, Rogowska, & Yurgelun-Todd, 2015; C. Orr et al., 2013), but this literature is also mixed (C. Orr et al., 2013; Pujol et al., 2014).
Despite the work done investigating connectivity in HIV and marijuana alone, the synergistic effects of marijuana and HIV on brain network organization is unclear. Identifying differences in local versus long-range connectivity could help to explain the inconsistencies in these literatures. To our knowledge, only two studies have investigated the interaction between HIV and marijuana on brain structure and the blood oxygenation level dependent response during functional magnetic resonance imaging (fMRI). In the first, Thames and colleagues (2017) investigated brain structure and showed that HIV has different effects on brain structure than marijuana but did not find an HIV x marijuana interaction. In the second, Meade and colleagues (2019) investigated brain activity during a cognitive interference task. They found an interaction effect, with increased activity in the co-occurring HIV and marijuana group compared to individuals with either condition alone or controls. These results indicate that marijuana may not simply exacerbate the existing effects of HIV, but that there may be a synergistic effect of HIV and marijuana use that differs from either condition alone.
In the current study, we examined differences in functional brain network organization in a cohort of 79 individuals with and without HIV and individuals who used and did not use marijuana. We focused on local and long-range connectivity during resting state. Local connectivity is connectivity between neighboring regions or regions within the same network. Long-range connectivity is connectivity between non-neighboring regions or regions across networks. Individual brain networks are thought to underlie unique cognitive functions (Laird et al., 2011; Yeo et al., 2015), so strong local connectivity is thought to reflect strength in those separate domains. Long-range connectivity, by contrast, is thought to reflect functioning in complex cognitive processes that require multiple domains of cognition (Yeo et al., 2015). Understanding the synergistic effects of HIV and marijuana on local and long-range connectivity can contribute to understanding whether treatment for neurocognitive dysfunction in co-occurring HIV and marijuana use should focus on improving the single cognitive processes that serve as the building blocks of cognition or whether it should focus on improving the ability to integrate said cognitive processes. Because the only other study examining the synergistic effects of HIV and marijuana on brain function found that co-occurring HIV and marijuana had increased brain activity compared to either condition alone and compared to controls, we hypothesized that co-occurring HIV and marijuana would be associated with increased local and long-range connectivity compared to HIV-only, marijuana-only, and controls.
2. METHODS
2.1. Participant Recruitment and Eligibility
Adults aged 18–55 years were recruited from the Raleigh/Durham area in North Carolina, USA using advertisements in local newspapers, community-based organizations, websites, and infectious disease clinics. Four groups of participants were recruited: marijuana users with HIV (co-occurring), marijuana users without HIV (MJ-only), non-marijuana users with HIV (HIV-only), or non-marijuana users without HIV (control). The co-occurring and MJ-only groups met the following criteria: ≥10 days of marijuana use in the past 30 days and ≥1 year of regular marijuana use [i.e., consistent use at a frequency of ≥3 days/week (McLellan et al., 1992)]. The non-marijuana groups met the following criteria: no lifetime cannabis dependence, no history of regular marijuana use, 0 days of marijuana use in the past 90 days, and a marijuana negative drug screen. Alcohol and nicotine use were permitted in all groups, but individuals with a primary alcohol use disorder were excluded. Participants also could have no history of other drug abuse, including cocaine, amphetamine, benzodiazepines, and opioids, as defined by the following: history of regular use, lifetime dependence, use in the past 30 days, or positive drug screen (could screen positive for amphetamines, opioids or benzodiazepines if they had a prescription). HIV-related inclusion criteria included being diagnosed for > 3 months. All HIV-negative participants had their HIV status verified via OraQuick® rapid HIV test, and a self-reported HIV+ status was verified by medical record review. All individuals with HIV were currently engaged in HIV care.
Additional exclusion criteria were nonfluency in English, illiteracy, less than eighth-grade education, severe learning disability, severe mental illness, current use of antipsychotic or mood stabilizing medications, serious neurological disorders not caused by HIV, acute opportunistic brain infections or history of such infections without return to normal cognition, severe head trauma with loss of consciousness > 30 minutes and persistent functional decline, and other MRI contraindications.
2.2. Procedures
After a telephone pre-screen, eligible participants were invited for a thorough in-person screening and an MRI scan session. The Duke University Institutional Review Board approved all procedures and participants provided written informed consent. During the in-person screening, participants completed clinical interviews, questionnaires, a pregnancy test, and urine drug screening. We identified current and past substance use disorders using Module E of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision; First, Spitzer, Gibbon, & Williams, 1996). History of substance use and associated impairments was assessed using the Addiction Severity Index-Lite (McLellan et al., 1992). We assessed substance use in the past 90 days at the screening visit and the MRI visit using timeline follow-back methodology (Robinson, Sobell, Sobell, & Leo, 2014). Premorbid cognitive function was estimated using the Wechsler Test of Adult Reading (Wechsler, 2001). Demographics, smoking behavior, and medical history were assessed using an audio computer-assisted self-interview. Participants also provided releases for research staff to view medical records to verify self-report history. We reviewed health care records in all participants to confirm that no exclusionary neurologic, psychiatric, or medical conditions were present. For HIV+ participants, we also abstracted HIV disease indicators, including date of diagnosis, HIV viral load, and CD4 cell counts.
On the scan day, participants were asked to abstain from marijuana use for >6 hours prior to the scan. Though we did not use Draeger testing to verify recency of marijuana use, staff were trained to recognize signs of intoxication. No participant exhibited such signs. Participants also completed another pregnancy test prior to scanning. Participants then completed an hour-long MRI scan.
2.3. Imaging Protocol
All scans were performed at Duke University Hospital using a 3T GE MR750 scanner with an eight-channel head coil. Two resting state whole-brain BOLD images were collected with T2*-weighted echo-planar imaging using the following parameters: repetition time (TR) = 2000ms, echo time (TE) = 25ms, field of view (FOV) = 240 × 240 mm2, flip angle = 90°, in-plane matrix size = 64 × 64, voxel size of 3.75 × 3.75 × 3.8 mm3, and 35 axial slices. Participants were instructed to keep their eyes open and observe a cross-hair displayed via projector on to a screen within the scanner bore. Each resting state scan had 180 volumes. T1-weighted structural images were acquired using a spoiled gradient echo sequence with the following parameters: TR = 8.156ms, TE = 3.18ms, FOV = 256 × 256 mm2, flip angle = 12°, in-plane matrix size = 256 × 256, voxel size of 1 × 1 × 1 mm3, number of axial slices = 166, inversion time = 450ms, and parallel acceleration factor = 2.
2.4. Preprocessing and Quality Control
The structural images were first visually checked to ensure full coverage and identify significant motion-related artifacts that could affect registration to functional images. Structural data were skull-stripped using FMRIB Software Library (FSL) Brain Extraction Toolbox (Smith, 2002). Structural images were registered to the 2-mm Montreal Neurological Institute standard-space template using FNIRT nonlinear registration (Andersson, Jenkinson, & Smith, 2007a, 2007b). Finally, the structural images were segmented into grey matter, white matter, and cerebrospinal fluid using FSL FAST (Zhang, Brady, & Smith, 2001).
Functional data were converted to LAS orientation and the first six volumes were excluded to allow the signal to reach a steady state. Motion correction was conducted using FSL’s MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Three translation and three rotational motion parameters were regressed out using rigid-body transformation. Slice-timing correction was then applied to the resting data. High-pass temporal filtering was performed using a Gaussian filter with a frequency of 0.01 Hz to remove low-frequency artifacts. Data were then smoothed with a kernel of 6mm using FEAT and then intensity was normalized across volumes (mean=1000). Motion correction, slice-timing correction, high-pass temporal filtering, and intensity normalization were all done using FSL’s FEAT (Smith et al., 2004). Additional denoising was conducted through ICA-AROMA, a data-driven method to identify and remove motion and physiological-related components from fMRI data (Pruim et al., 2015). Nuisance regressors consisting of mean signal within the white matter and cerebrospinal fluid were regressed out of the functional data. Finally, the functional data were registered to the T1-weighted anatomical slices with FLIRT (Jenkinson et al., 2002; Jenkinson & Smith, 2001) using a 12-parameter affine transformation
The two separate resting state runs were combined by first taking each of the registered functional runs and creating a binarized mask image for each. Each run was then demeaned utilizing the mask image and the two demeaned functional runs were concatenated.
A motion threshold of relative mean displacement > 0.3mm was utilized for each run. No individual runs were found to be in excess of this threshold. In-scanner motion, measured by absolute and relative root mean square displacement in the concatenated resting state data, did not differ between groups (absolute: F(3,75) = 0.15, p > 0.90, relative: F(3,75) = 0.75, p > 0.50). Absolute and relative root mean square displacement means (standard deviations) are as follows: absolute: 0.24 (0.12), relative: 0.06 (0.03)
2.5. Construction of Functional Connectivity Networks
We used an atlas comprised of 300 cortical, subcortical, and cerebellar regions (Seitzman et al., 2019) to extract timecourses of brain activity in each region for every participant. The timecourses consisted of the average activity within a region in every volume in both runs and were obtained using nilearn (Abraham et al., 2014). Regions were excluded from analyses if 25% of the voxels within the region had insufficient coverage for any participant. Using this criteria, no regions were excluded. Then for each participant, the timecourses for each region were correlated with timecourses for every other region to create 300 × 300 connectivity matrices. As the purpose of the analyses herein was to compare our results to the existing connectivity literature, and to lay the groundwork for future work investigating the synergistic effects of HIV and marijuana on functional connectivity, we established functional connectivity using Pearson correlation to be consistent with the broad brain network organization literature (e.g. Braun et al., 2012; Hayasaka & Laurienti, 2010; Liao et al., 2013; van den Heuvel et al., 2017). Future work will be necessary to explore other methods of establishing functional connectivity (e.g. DSouza, Abidin, Chockanathan, Schifitto, & Wismuller, 2018; DSouza, Abidin, Leistritz, & Wismuller, 2017; Guo, Seth, Kendrick, Zhou, & Feng, 2008; Lombardi et al., 2019). Connections between regions whose anatomical centers were less than 20mm apart were excluded from analyses as connectivity between anatomically close regions can be artificially inflated by motion (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Those matrices were then Fisher-transformed. The matrices were thresholded at densities ranging from 0.01–0.25, inclusive, in increments of 0.01, so that only the strongest X% of connections remained at each threshold. The matrices retained their edge weights.
2.6. Graph Theory Analysis
Graph theory analyses were performed using the Brain Connectivity Toolbox (Rubinov & Sporns, 2010). Metrics that can be obtained for each brain region separately (local efficiency, within-module degree, betweenness, strength, and participation coefficient) were averaged across every region in the brain. The graph metrics are defined below.
Basic concepts and notation: N is the set of all nodes in the network, and n is the number of nodes. L is the set of all links in the network, and l is the number of links. (i,j) is a link between nodes i and j, (i,j ∈ N). aij is the connection status between i and j. aij = 1 when link (i,j) exists (when i and j are neighbors); aij = 0 otherwise. Links (i,j) are associated with connection weights wij. lw is the sum of all weights in the network. The degree of a node i is and the weighted degree of a node i is .
2.6.1. Local Connectivity
2.6.1.1. Modularity
Modularity is the extent to which a graph can be divided into non-overlapping modules, or networks of regions. We used the Louvain community detection algorithm (Blondel, Guillaume, Lambiotte, & Lefebvre, 2008) to quantify modularity. The modules detected were used in participation coefficient and within-module degree calculations (see below).
2.6.1.2. Transitivity
Transitivity is the relative number of connected triangles in a graph compared to the total number of triplets
Where is the geometric mean of triangles around a node i, ,
2.6.1.3. Local Efficiency
Local efficiency is global efficiency (see below) calculated on a node’s neighborhood.
where is the shortest path length between nodes j and h.
2.6.1.4. Within-Module Degree
Within-module degree is the degree of a node calculated for nodes within its network.
where mi is the module containing node i (identified using the aforementioned Louvain community detection algorithm), is the within-module degree of i, meaning the sum of all links between i and other nodes within its module. We used weighted within-module degree.
2.6.2. Long-Range Connectivity
2.6.2.1. Global Efficiency
Global efficiency is the average inverse characteristic path length, which is the shortest path between two nodes, averaged across every pair of nodes.
where Ni is the number of nodes that are direct neighbors of node i and is the shortest path between nodes i and j.
2.6.2.2. Betweenness
Betweenness is the fraction of all shortest paths that contain a given node. Nodes with high betweenness participate in a large number of shortest paths. For node i in a network N:
is the total number of shortest paths from node h to node j, and is the shortest number of paths from node h to node j that pass through i.
2.6.2.3. Strength
Node strength is the sum of weights of links connected to the node.
where wij is the weight of the connections between nodes i and j.
2.6.2.4. Participation Coefficient
Participation coefficient is a measure of diversity of intermodular connections of individual nodes.
where M is the set of modules (identified using the aforementioned Louvain community detection algorithm), m is a single module, is the sum of all links between i and all nodes and ki(m) is the sum of all links between i and all nodes in module m.
2.7. Data Analysis
Demographic and clinical characteristics were compared between groups using one-way ANOVAs, t-tests, or chi-squared analyses, as appropriate, using SPSS 26. Significant ANOVAs were probed with Tukey’s honestly significant difference post hoc tests. We calculated the graph metrics at each threshold and then calculated the area under the curve (AUC) across all thresholds by integrating along the x-axis using the composite trapezoidal rule. The AUC for each metric was compared between groups in 2×2 ANCOVA (HIV x MJ). As a result of the significant group differences in age (in years), education (in years), and daily cigarette use (yes/no), graph theory metrics are analyzed controlling for these variables in ANCOVAs using SPSS. Significant ANCOVA results are reported at p < 0.05. Results surviving False Discovery Rate (FDR) correction for the comparison of eight graph metrics are indicated below.
We also examined the effects of suppressed viral load and CD4 count on graph metrics. To examine the effects of viral load suppression on functional connectivity in individuals with HIV, we performed separate ANCOVAs using each graph metric as the dependent variable and suppressed vs. unsuppressed viral load as the independent variable, with age, education, and daily cigarette use as covariates of no interest. To examine the effects of CD4 count on functional connectivity in individuals with HIV, we performed separate regression models using each graph metric as the dependent variable and recent and nadir CD4 counts as independent variables (in different models). Age, education, and smoking were included as covariates of no interest in all models.
3. RESULTS
3.1. Demographic and Clinical Characteristics
The final sample included 79 total participants: 20 co-occurring, 20 HIV-only, 17 MJ-only, and 22 controls. Table 1 summarizes the demographic and clinical characteristics of the sample. Across all groups, 73.42% identified as male and 63.29% identified as African American. Gender and racial distributions did not differ across groups. The average age was 36.92 (SD=9.52) years, and average years of education was 14.43 (SD=2.04) years. There were significant group differences on age and education (p’s < 0.001). Post-hoc tests revealed that the HIV-only group was older than the co-occurring and MJ-only groups, and that the control group had significantly more years of education than the co-occurring and MJ-only groups. The average premorbid verbal IQ was 96.71 (SD = 17.70). Despite the differences in education, there were no group differences (p > 0.05) in IQ.
Table 1:
Demographic and clinical characteristics (N = 79)
| Co-Occurring (N = 20) | HIV-only (N = 20) | MJ-only (N = 17) | Control (N = 22) | Statistic | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Male, % | 75.00% | 80.00% | 70.59% | 68.18% | χ2(3) = 0.85 |
| Age in years, M (SD) | 32.65 (9.93) | 43.35 (7.67) | 33.35 (6.90) | 37.73 (9.52) | F(3,75) = 6.29* |
| Race | χ2(6) = 9.66 | ||||
| African American, % | 65.00% | 70.00% | 70.59% | 50.00% | - |
| Caucasian, % | 35.00% | 30.00% | 11.76% | 36.36% | - |
| Other/Mixed, % | 0.00% | 0.00% | 17.65% | 13.64% | - |
| Education in years, M (SD) | 13.30 (1.84) | 14.50 (2.21) | 13.88 (1.54) | 15.82 (1.65) | F(3,75) = 7.25* |
| IQ, M (SD) | 93.40 (16.54) | 96.30 (21.87) | 93.35 (13.26) | 102.68 (17.10) | F(3,75) = 1.29 |
| HIV Characteristics | |||||
| Suppressed HIV viral load, % | 60.00% | 70.00% | - | - | χ2(1) = 0.44 |
| Most recent CD4 count, median (IQR) | 721 (395) | 721 (366) | - | - | t(38) = −0.01 |
| Lowest CD4 count, median (IQR) | 401 (480) | 292 (358) | - | - | t(38) = 1.36 |
| Years since HIV diagnosis, M (SD) | 8.25 (8.16) | 10.15 (6.66) | - | - | t(38) = −0.81 |
| Marijuana Use Characteristics | |||||
| Age of first regular use, M (SD) | 19.70 (6.49) | - | 18.35 (3.46) | - | t(35) = 0.77 |
| Days of use in past 90 days, M (SD) | 77.45 (20.55) | - | 81.35 (19.14) | - | t(35) = −0.59 |
| Hours high/day, M (SD) | 6.35 (5.32) | - | 6.81 (5.59) | - | t(34) = −0.25a |
| Years of regular marijuana use, M (SD) | 12.40 (8.22) | - | 12.06 (5.49) | - | t(35) = 0.15 |
| Current cannabis dependence, % | 50.00% | - | 52.94% | - | χ2(1) = 0.03 |
| Other Substance Use Characteristics | |||||
| Alcohol use in past 30 days, % | 70.00% | 65.00% | 70.59% | 50.00% | χ2(3) = 2.48 |
| Daily cigarette use in past 30 days, % | 60.00% | 15.00% | 52.94% | 9.09% | χ2(3) = 24.22* |
Abbreviations: HIV, human immunodeficiency virus; MJ, marijuana; M, mean; SD, standard deviation; IQR, interquartile range
p < 0.01
Data unavailable for one person in the MJ-only group
In the HIV-positive groups, there were no significant differences between the co-occurring and the HIV-only groups on HIV characteristics (p’s > 0.05). More than half (65.00%) had a suppressed viral load of <50 copies/mL. The mean number of years since HIV diagnosis was 9.20 (range: 1–29 years, SD = 7.41). Nadir CD4 count ranged from 3 to 1037 (median = 283.50, IQR = 345). Recent CD4 counts ranged from 77 to 1477, (median 725.50, IQR = 366).
Among marijuana users, there were no significant differences between the co-occurring and the MJ-only groups on marijuana use characteristics (p’s > 0.05). 51.35% endorsed criteria for marijuana dependence. The mean age of first regular use was 19.08 (range: 11–40, SD = 5.29), they used on 79.24 out of the past 90 days (range: 25–90, SD = 19.74), they were high an average of 6.56 hours/day (range: 0–20, SD = 5.37) when they used, and lifetime use in years was 12.24 years (range: 1–30, SD = 7.01 years).
Significantly fewer controls smoked cigarettes in the past 30 days compared to the other three groups, but there were no differences in past 30-day prevalence of alcohol use.
3.2. Graph Theory Metrics
There were no main effects of HIV or MJ for any of the graph metrics (all p’s > 0.05, Table 2). There were significant interactions for three local metrics (see Table 2): transitivity (F(1,72) = 9.28, p < 0.005), local efficiency (F(1,72) = 8.61, p < 0.005), and within-module degree (F(1,72) = 7.10, p < 0.01). There were also significant interactions for all long-range metrics (see Table 2): global efficiency (F(1,72) = 6.00, p < 0.05), strength (F(1,72) = 6.14, p < 0.005), betweenness (F(1,72) = 4.44, p < 0.05), and participation coefficient (F(1,72) = 4.15, p < 0.05). All three local metrics, and two of the long-range metrics, strength and global efficiency, survived FDR-correction for multiple comparisons.
Table 2.
HIV × MJ effects examining group differences in graph metrics. All degrees of freedom = 1, 72.
| Main Effect of HIV | Main Effect of MJ | HIV × MJ Interaction | |||||
|---|---|---|---|---|---|---|---|
| Co-Occurring, M (SE) | HIV-Only, M (SE) | MJ-Only, M (SE) | Control, M (SE) | F-statistic | F-statistic | F-statistic | |
| Local Connectivity | |||||||
| Modularity | 10.40 (0.47) | 10.30 (0.46) | 10.25 (0.49) | 10.40 (0.46) | 0.01 | 0.00 | 0.08 |
| Transitivity | 26.42 (2.41) | 31.39 (2.32) | 31.19 (2.47) | 22.67 (2.33) | 0.78 | 0.39 | 9.28*** |
| Local Efficiency | 17.78 (1.18) | 20.13 (1.14) | 20.02 (1.21) | 15.99 (1.14) | 0.75 | 0.36 | 8.61*** |
| Within-Module Degree | 970.53 (105.94) | 1175.50 (102.04) | 1140.88 (108.76) | 827.31 (102.19) | 0.82 | 0.19 | 7.10** |
| Global Connectivity | |||||||
| Global Efficiency | 6.13 (0.22) | 6.49 (0.21) | 6.40 (0.22) | 5.78 (0.21) | 1.21 | 0.26 | 6.00* |
| Strength | 1054.53 (97.85) | 1248.28 (94.25) | 1247.19 (100.46) | 995.66 (94.39) | 0.75 | 0.53 | 9.01*** |
| Betweenness | 5927.94 (856.73) | 5336.77 (825.20) | 4344.46 (879.52) | 7067.07 (826.40) | 0.01 | 1.34 | 4.43* |
| Participation Coefficient | 2.59 (0.47) | 1.49 (0.45) | 1.91 (0.48) | 2.57 (0.45) | 0.21 | 0.15 | 4.16* |
p < 0.005
p < 0.01
p < 0.05
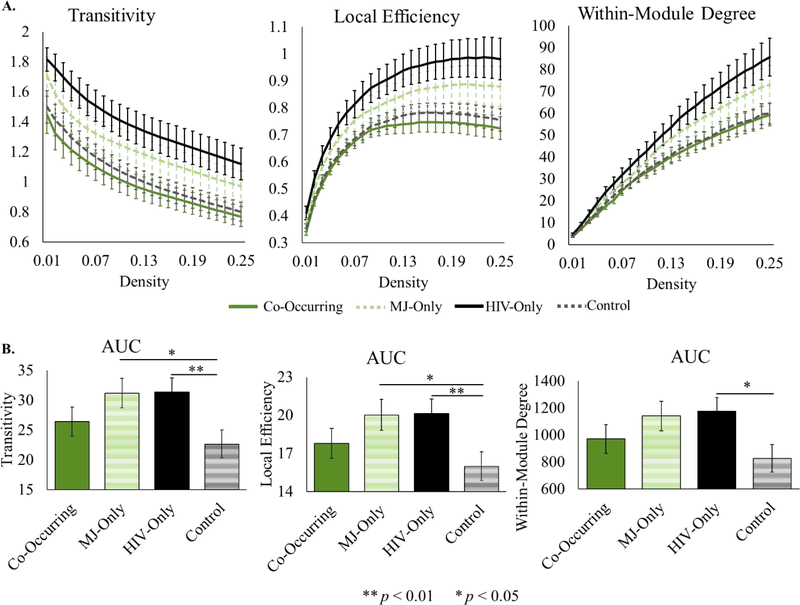
All local metrics showed similarity between the co-occurring and control groups. Additionally, there was increased local connectivity in the HIV-only and MJ-only groups compared to the co-occurring and control groups (see Figure 1). Simple effects testing revealed that the HIV-only group had significantly higher values than the control group for transitivity (F(1,72) = 7.74, p < 0.01, η2 = 0.10), local efficiency (F(1,72) = 7.24, p < 0.01, η2 = 0.09) and within-module degree (F(1,72) = 6.39, p < 0.05, η2 = 0.08). The MJ-only group also had significantly higher values than the control group for transitivity (F(1,72) = 5.49, p < 0.05, η2 = 0.07) and local efficiency (F(1,72) = 5.09, p < 0.05, η2 = 0.07), and there was a trend in the same direction for within-module degree (F(1,72) = 3.85, p < 0.06, η2 = 0.05).
Figure 1.
Local graph metrics showing a significant HIV x MJ effect. A. Local metrics plotted across all densities by group. Error bars represent standard error. B. Area under the curve for local metrics. Means adjusted for covariates (age, education, smoking) are displayed on the y-axis.
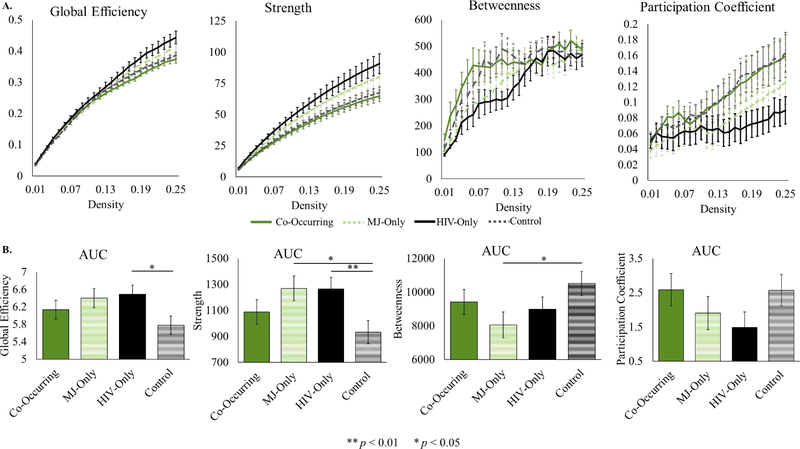
All long-range metrics also showed similar connectivity between the control group and the co-occurring group and similarity between the HIV-only and MJ-only groups (see Figure 2). While the HIV-only and MJ-only groups differed from the control group, the co-occurring group had values comparable to the control group. Similarly to the local metrics, the HIV-only and MJ-only groups had higher global efficiency and strength than the co-occurring and control groups. Simple effects testing revealed that the HIV-only group had a significantly higher global efficiency (F(1,72) = 6.33, p < 0.05, η2 = 0.08) and strength (F(1,72) = 7.49, p < 0.01, η2 = 0.09) than the control group. The MJ-only group had significantly higher strength (F(1,72) = 5.74, p < 0.05, η2 = 0.07) and showed a trend towards higher global efficiency (F(1,72) = 3.57, p < 0.07, η2 = 0.05) compared to the control group. In contrast, betweenness and participation coefficient were lower in the HIV-only and MJ-only groups than the co-occurring and control groups. Simple effects testing revealed that the MJ-only group had significantly lower betweenness than the control group (F(1,72) = 4.78, p < 0.05, η2 = 0.06) and that there was a trend towards significantly lower participation coefficient in the HIV-only group compared to the control group (F(1,72) = 3.13, p < 0.09, η2 = 0.04).
Figure 2.
Long-range graph metrics. A. Long-range metrics plotted across all densities tested, shown for illustrative purposes. Error bars represent standard error. Means are not adjusted for covariates. B. Area under the curve for long-range metrics. Means adjusted for covariates (age, education, smoking) are displayed on the y-axis
Analyses within the HIV group investigating the relationship between graph metrics and HIV characteristics including viral load suppression, recent CD4 count, and nadir CD4 count did not reveal any significant relationships between the graph metrics and HIV characteristics (all p’s > 0.1).
4. DISCUSSION
To our knowledge, this is the first study investigating the effects of marijuana on resting state brain function in individuals with HIV. Using graph theory metrics, we show that whole-brain network organization in HIV-only and MJ-only was different from controls but similar amongst themselves. In contrast, whole-brain network organization in co-occurring HIV and marijuana use is similar to that of controls, measured across multiple local and long-range graph metrics. Local connectivity was increased in both HIV-only and MJ-only, whereas long-range connectivity showed both increases and decreases, depending on the metric. Two of the long-range metrics, participation coefficient and betweenness, which both show decreased connectivity in the HIV-only and MJ-only groups, are less influenced by strong connectivity within local communities and may be a more pure measure of long-range connectivity than our other two measures of long-range connectivity, strength and global efficiency. We discuss this in greater detail below.
The similarity in brain network organization between controls and individuals with co-occurring HIV and marijuana-use suggests that marijuana offsets the disruptive effects of HIV on whole-brain network organization. This raises the question about whether this similarity reflects a benefit or detriment in functioning in co-occurring HIV and marijuana use compared to HIV- and MJ-only. The existing literature does not reach a consensus on the matter. On the one hand, marijuana may protect against the detrimental effects of HIV by reducing inflammation. Cells infected with HIV release viral proteins that are toxic for neurons (Aggoun-Zouaoui et al., 1996; Hazleton, Berman, & Eugenin, 2010; Meucci & Miller, 1996; Pu et al., 2003), which propagates a chronic inflammatory response and a disruption of the blood brain barrier (Hazleton et al., 2010). Marijuana may counteract this inflammatory milieu by enhancing anti-inflammatory activity in protective cells or by diminishing function in cells that cause the inflammatory response (for reviews, see: Di Marzo, Bifulco, & De Petrocellis, 2004; Klein, 2005; Maroon & Bost, 2018).
The notion that marijuana offers protection against harm caused by HIV is supported by work showing that marijuana also has a neuroprotective effect on multiple neurodegenerative disorders (for reviews, see: Centonze, Finazzi-Agro, Bernardi, & Maccarrone, 2007; Fernandez-Ruiz et al., 2013) in part due to its anti-inflammatory properties. In fact, recent studies have shown that activating the endocannabinoid system can counteract neuroinflammation and cognitive decline in neurodegenerative disorders with chronic inflammation, and can improve behavioral outcomes in animal models of neurocognitive impairment (for a review, see Wu, Zhang, Asher, & Thayer, 2019). Further, the cannabinoid receptors have increased expression in HIV-associated neurocognitive disorder, making HIV a strong potential target for cannabinoid-based treatments (Wu et al., 2019). If inflammation caused by HIV damages brain structures that are critical for facilitating the flow of information across the brain, then the anti-inflammatory properties of marijuana may normalize brain network organization such that it is similar to that of controls. The protective role of marijuana against the harmful effects of HIV could suggest a role for marijuana in treating HIV associated neurocognitive disorder. We note that we did not measure any mediators of inflammation, nor did we measure the cannabinoid content of the cannabis used by participants. As cannabinoids, and specifically cannabidiol, is believed to be responsible anti-inflammatory properties of marijuana (Booz, 2011), future research is needed to empirically test the anti-inflammatory hypotheses about our results.
It is interesting to note that the MJ-only group does not have the same reduction in whole-brain connectivity as the co-occurring group. This suggests that marijuana may not protect against disruption in brain function unless HIV is present. We speculate that this may be because marijuana’s anti-inflammatory protective effects are most evident when there is underlying inflammation. The anti-inflammatory and psychotropic effects of cannabis act through different mechanisms, binding primarily to different receptors (Corroon & Felice, 2019; Herkenham et al., 1990). The psychotropic effects likely underlie marijuana’s disruptive effects (Yucel et al., 2016). Without underlying inflammation, the harm caused by activation of the psychotropic system may become more apparent or in contrast, the activation of the anti-inflammatory system may cause harm. However, this is purely speculative and future work that includes inflammatory markers will be necessary to probe this question.
In contrast to the position that marijuana is generally protective against harm caused by HIV, work done on brain function in individuals with co-occurring HIV and marijuana use suggests that marijuana may exacerbate some aspects of neurocognitive dysfunction in HIV, like learning and memory. A review investigating the independent effects of HIV and marijuana on memory hypothesized that co-occurring marijuana use and HIV-infection strain neural resources, resulting in more pronounced impairment (Skalski, Towe, Sikkema, & Meade, 2016). Evidence in support of this idea comes from a study done using a cognitive interference task in which there is increased activity in co-occurring HIV and marijuana use compared to controls in a set of regions across the brain; this increase in activity only occurred in a subset of those regions in HIV- and MJ-only groups (Meade et al., 2019). This suggests that as neural insult grows, increases in brain activity, thought to compensate for inefficient neural function (Fornito, Zalesky, & Breakspear, 2015), become more widespread. Though the current work during resting state does not show this same pattern of increased connectivity in the co-occurring group compared to the control group, this may be due to the fact that we measured whole-brain connectivity rather than connectivity within specific regions or networks. If there was profound brain network reorganization across the brain, with increased connectivity in some regions countering decreases in others, whole-brain connectivity may appear unchanged. When neural insult is mild, as it may be in HIV-only or MJ-only, reorganization may be limited to increases or decreases in connectivity within a few regions that are not countered by connectivity changes in other regions and therefore, are apparent when measuring whole-brain network organization. Work done on the brain network organization in comatose patients has shown that whole-brain network organization does not change but that the distribution of hub regions (highly connected regions thought to be critical relay stations) radically changes (Achard et al., 2012). This supports the idea that a drastic reorganization of brain network organization can occur even when whole-brain network organization metrics are unchanged. Future work with a larger sample size that can detect subtle effects at the nodal level could examine the organization of hub regions in HIV and co-occurring marijuana use and shed light on this issue.
Turning to whole-brain network organization in the HIV-only group, we show increased local connectivity compared to controls, measured by transitivity, local efficiency, and within-module degree. The increase in local connectivity was unexpected because decreased connectivity is often shown in the HIV literature (Chaganti et al., 2017; du Plessis et al., 2017; Guha et al., 2016; Herting et al., 2015; Ortega, Brier, & Ances, 2015; Thomas, Brier, Ortega, Benzinger, & Ances, 2015; Thomas et al., 2013). We propose two possible explanations for why we find increased local connectivity. The first is that increases in functional connectivity may be difficult to detect when measuring connectivity at the level of individual regions but measuring across the whole brain, some of the small but consistent effects may become detectable. However, there are some instances of increased connectivity in HIV (Herting et al., 2015; Toich et al., 2018; H. J. Wang et al., 2018) and further, one study shows increases in local connectivity over time in HIV (Correa et al., 2017), and another shows increasing small-world properties (a measure of high local connectivity with few strong connections linking the local communities) with increasing cognitive impairment in HIV (Abidin et al., 2018). The second is that the mean age of our participants (~37 years) is lower than many neuroHIV samples. Since HIV is theorized to exacerbate the aging process in the brain (Holt, Kraft-Terry, & Chang, 2012), and longer HIV infection/higher age is associated with greater brain disruption (Cysique et al., 2017; Thomas et al., 2015) it is also possible that connectivity patterns reported in the literature in older individuals may differ from the patterns we report in younger individuals with HIV.
The MJ-only group has a similar pattern of disruption as the HIV-only group. Marijuana use, unlike HIV, is frequently associated with increased connectivity (Cheng et al., 2014; Filbey & Dunlop, 2014; Lopez-Larson et al., 2015; Manza, Tomasi, & Volkow, 2018; Pujol et al., 2014), though there are exceptions (C. Orr et al., 2013; Pujol et al., 2014). This suggests that increases in local connectivity may be more easily detectable at the level of individual regions than they are in HIV.
The direction of disruption in long-range metrics also differs in the HIV-only and MJ-only groups compared to controls, with higher global efficiency and strength and lower betweenness and participation coefficient. Though it is difficult to interpret the inconsistency between these long-range metrics, participation coefficient is unique among our long-range metrics in that it measures the diversity of communities to which a region is connected and therefore, it cannot be disproportionately inflated by a large or strongly connected local community (Power, Schlaggar, Lessov-Schlaggar, & Petersen, 2013). Using participation coefficient as a measure of inter-network connectivity, we find decreased inter-network connectivity and accompanying increases in local/intra-network connectivity. Betweenness is also thought to be a long-range metric that is less dependent on the size of the local community. These results may help to resolve some of the inconsistencies in the HIV and marijuana literature. The discrepancy in these literatures may be due to increased local connectivity compensating for decreased long-range connectivity. The pattern of decreased long-range connectivity with concomitant increases in local connectivity is also seen in other conditions during resting state. Tomasi and Volkow (2012) show decreased long-range connectivity and increased short-range connectivity associated with aging. Inflammation has also been associated with select increases in local connectivity and decreases in connectivity across the brain (Marsland et al., 2017; Yin et al., 2019). In fact, hub regions are often highly impacted by a variety of neurologic conditions (Crossley et al., 2014), which can disrupt the balance in long-range and local connectivity (Bertolero, Yeo, Bassett, & D’Esposito, 2018; Gratton, Nomura, Perez, & D’Esposito, 2012). In HIV, the long-range connectivity may be weakened, and the brain subsequently reorganizes to prioritize local connections.
Abnormal functional activity is likely to be driven in part by changes in underlying brain structure. The relationship between structural and functional abnormalities has been demonstrated in a variety of conditions that impact neurocognitive function, including healthy aging, Alzheimer’s Disease, schizophrenia, and attention-deficit/hyperactivity disorder (Hakun, Zhu, Brown, Johnson, & Gold, 2015; Schlosser et al., 2007; Z. Q. Wang et al., 2015). As HIV and marijuana use are both associated with abnormalities in brain structure (Ances, Ortega, Vaida, Heaps, & Paul, 2012; Ashtari et al., 2009; Becker et al., 2011; Filbey et al., 2014; Filippi, Ulug, Ryan, Ferrando, & van Gorp, 2001; J. M. Orr, Paschall, & Banich, 2016; Pomara, Crandall, Choi, Johnson, & Lim, 2001) future work should investigate the relationship between functional brain network organization and underlying structural integrity.
4.1. Limitations
There are many strengths in the current work, including the first investigation of the effects of marijuana on resting-state brain function in individuals with HIV, a novel application of a graph theory approach to quantifying brain network organization, and a well-characterized sample of heavy and chronic marijuana users. Nevertheless, this is a preliminary investigation with a relatively small sample size (group sizes ranging from 17–22 participants), and thus the results should be interpreted with caution. Due to this small sample size, we chose to focus on whole-brain measures of network organization rather than exploring effects at individual nodes. However, it is likely that the mechanisms underlying brain network organization alteration in HIV and marijuana use are subtle and require a nuanced investigation of differences at the network level or even the level of the interaction between the individual region and the rest of the brain. This work is also cross-sectional. Longitudinal designs are needed to ascertain the degree to which HIV and marijuana use are causal factors in disrupting brain network organization or whether differences in brain network organization exist prior to the contraction of HIV or onset of marijuana use. We also do not relate brain network organization to neurocognitive performance. However, variations in global topological properties have been associated with intellectual performance and they change over time as task cognitive performance improves (Douw et al., 2011; Langer, von Bastian, Wirz, Oberauer, & Jancke, 2013; van den Heuvel & Pol, 2010) so we expect that the brain network abnormalities observed in this study may reflect changes in cognition. Understanding how these abnormalities in brain network organization relate to neurocognitive performance will help clarify whether these abnormalities underlie enhancement or impairments in behavioral outcomes. Finally, two of our long-range metrics did not survive FDR correction for multiple comparisons. A larger sample could reduce measurement error and increase the effect size.
5. CONCLUSIONS
The present study indicates that the synergistic effect of marijuana use and HIV on brain network organization is distinct from the effects of either HIV or marijuana use alone. Whole brain network organization is similar between co-occurring HIV and marijuana use and controls whereas it differs between HIV-only and MJ-only and controls. It is unclear whether the similarity between co-occurring HIV/marijuana and controls is indicative of the protective effects of marijuana on brain function or of increased brain dysfunction in co-occurring HIV and marijuana use. This work lays important groundwork for future studies examining the relationship between brain network organization and behavioral outcomes in HIV and marijuana users.
Highlights.
We measured functional brain network organization in HIV and marijuana use
Organization is similar between co-occurring HIV and marijuana use and controls
HIV-only and marijuana use-only have increased local connectivity
Long-range connectivity disruptions in HIV and marijuana use vary across metrics
Acknowledgements
We would like to thank all participants for agreeing to take part in this research.
Funding: This work was supported by the National Institute on Drug Abuse of the National Institutes of Health [R01-DA045565, R03-DA035670]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abidin AZ, DSouza AM, Nagarajan MB, Wang L, Qiu X, Schifitto G, & Wismüller A (2018). Alteration of brain network topology in HIV-associated neurocognitive disorder: A novel functional connectivity perspective. NeuroImage: Clinical, 17, 768–777. doi: 10.1016/j.nicl.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, … Varoquaux G. (2014). Machine learning for neuroimaging with scikit-learn. Front Neuroinform, 8, 14. doi: 10.3389/fninf.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Delon-Martin C, Vertes PE, Renard F, Schenck M, Schneider F, … Bullmore ET. (2012). Hubs of brain functional networks are radically reorganized in comatose patients. Proceedings of the National Academy of Sciences of the United States of America, 109(50), 20608–20613. doi: 10.1073/pnas.1208933109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggoun-Zouaoui D, Charriaut-Marlangue C, Rivera S, Jorquera I, Ben-Ari Y, & Represa A (1996). The HIV-1 envelope protein gp120 induces neuronal apoptosis in hippocampal slices. Neuroreport, 7(2), 433–436. doi: 10.1097/00001756-199601310-00014 [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, & Paul R (2012). Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of Acquired Immune Deficiency Syndromes, 59(5), 469–477. doi: 10.1097/QAI.0b013e318249db17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, & Smith S (2007a). Non-linear optimisation FMRIB Technical Report TR07JA1. Retrieved from FMRIB Centre, Oxford, United Kingdom: [Google Scholar]
- Andersson JLR, Jenkinson M, & Smith S (2007b). Non-linear Registration, aka Spatial Normalisation. Retrieved from Oxford, United Kingdom: http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf
- Ann HW, Jun S, Shin NY, Han S, Ahn JY, Ahn MY, … Choi JY. (2016). Characteristics of resting-state functional connectivity in HIV-associated neurocognitive disorder. PLoS ONE, 11(4), e0153493. doi: 10.1371/journal.pone.0153493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, & Kumra S (2009). Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use (vol 43, pg 189, 2009). Journal of Psychiatric Research, 43(11), 1003–1003. doi: 10.1016/j.jpsychires.2009.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Maruca V, Kingsley L, Sanders J, Alger J, Barker P, … Study f. t. M. A. C. (2011). Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology, 1–9. doi: 10.1007/s00234-011-0854-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BTT, Bassett DS, & D’Esposito M (2018). A mechanistic model of connector hubs, modularity and cognition. Nature Human Behaviour, 2(10), 765–777. doi: 10.1038/s41562-018-0420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8524021 [DOI] [PubMed] [Google Scholar]
- Blondel VD, Guillaume JL, Lambiotte R, & Lefebvre E (2008). Fast unfolding of communities in large networks. Journal of Statistical Mechanics-Theory and Experiment. doi: 10.1088/1742-5468/2008/10/P10008 [DOI] [Google Scholar]
- Booz GW (2011). Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radical Biology and Medicine, 51(5), 1054–1061. doi: 10.1016/j.freeradbiomed.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Plichta MM, Esslinger C, Sauer C, Haddad L, Grimm O, … Meyer-Lindenberg A. (2012). Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. NeuroImage, 59(2), 1404–1412. doi: 10.1016/j.neuroimage.2011.08.044 [DOI] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agro A, Bernardi G, & Maccarrone M (2007). The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends in Pharmacological Sciences, 28(4), 180–187. doi: 10.1016/j.tips.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Chaganti JR, Heinecke A, Gates TM, Moffat KJ, & Brew BJ (2017). Functional connectivity in virally suppressed patients with HIV-associated neurocognitive disorder: A resting-state analysis. American Journal of Neuroradiology, 38(8), 1623–1629. doi: 10.3174/ajnr.A5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Skosnik PD, Pruce BJ, Brumbaugh MS, Vollmer JM, Fridberg DJ, … Newman SD. (2014). Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users - a multi-voxel pattern analysis. Journal of Psychopharmacology, 28(11), 1030–1040. doi: 10.1177/0269881114550354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockanathan U, DSouza AM, Abidin AZ, Schifitto G, & Wismuller A (2019). Automated diagnosis of HIV-associated neurocognitive disorders using large-scale Granger causality analysis of resting-state functional MRI. Computers in Biology and Medicine, 106, 24–30. doi: 10.1016/j.compbiomed.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, … Navia B. (2010). Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of Neurovirology, 16(1), 25–32. doi: 10.3109/13550280903552420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DG, Zimmermann N, Ventura N, Tukamoto G, Doring T, Leite SC, … Gasparetto EL. (2017). Longitudinal evaluation of resting-state connectivity, white matter integrity and cortical thickness in stable HIV infection: Preliminary results. The Neuroradiology Journal, 30(6), 535–545. doi: 10.1177/1971400917739273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, & Felice JF (2019). The Endocannabinoid System and its Modulation by Cannabidiol (CBD). Alternative Therapies in Health and Medicine, 25, 6–14. Retrieved from <Go to ISI>://WOS:000510159400002 [PubMed] [Google Scholar]
- Crane HM, McCaul ME, Chander G, Hutton H, Nance RM, Delaney JAC, … Kitahata MM. (2017). Prevalence and factors associated with hazardous alcohol use among persons living with HIV across the US in the current era of antiretroviral treatment. AIDS and Behavior, 21(7), 1914–1925. doi: 10.1007/s10461-017-1740-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiani SA, Pukay-Martin ND, & Bornstein RA (2004). Marijuana use and cognitive function in HIV-infected people. Journal of Neuropsychiatry and Clinical Neurosciences, 16(3), 330–335. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15377740 [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, & Bullmore ET (2014). The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain, 137(Pt 8), 2382–2395. doi: 10.1093/brain/awu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Soares JR, Geng GQ, Scarpetta M, Moffat K, Green M, … Rae, C. (2017). White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration (vol 23, pg 539, 2017). Journal of Neurovirology, 23(4), 548–549. doi: 10.1007/s13365-017-0542-z [DOI] [PubMed] [Google Scholar]
- D’Souza G, Matson P, Grady CD, Nahvi S, Merenstein D, Weber K, … Wilson TE. (2012). Medicinal and recreational marijuana use among HIV-infected women in the Women’s Interagency HIV Cohort (WIHS), 1994–2010. Journal of Acquired Immune Deficiency Syndromes, 61(5), 618–626. doi: 10.1097/QAI.0b013e318273ab3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, & Beckmann CF (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103(37), 13848–13853. doi: 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, & Thompson PM (2014). Functional Brain Connectivity Using fMRI in Aging and Alzheimer’s Disease. Neuropsychology Review, 24(1), 49–62. doi: 10.1007/s11065-014-9249-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, & De Petrocellis L (2004). The endocannabinoid system and its therapeutic exploitation. Nature Reviews. Drug Discovery, 3(9), 771–784. doi: 10.1038/nrd1495 [DOI] [PubMed] [Google Scholar]
- Douw L, Schoonheim MM, Landi D, van der Meer ML, Geurts JJG, Reijneveld JC, … Stam CJ. (2011). Cognition Is Related to Resting-State Small-World Network Topology: An Magnetoencephalographic Study. Neuroscience, 175, 169–177. doi: 10.1016/j.neuroscience.2010.11.039 [DOI] [PubMed] [Google Scholar]
- DSouza AM, Abidin AZ, Chockanathan U, Schifitto G, & Wismuller A (2018). Mutual connectivity analysis of resting-state functional MRI data with local models. NeuroImage, 178, 210–223. doi: 10.1016/j.neuroimage.2018.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSouza AM, Abidin AZ, Leistritz L, & Wismuller A (2017). Identifying HIV associated neurocognitive disorder using large-scale granger causality analysis on resting-state functional MRI. Proceedings of SPIE--the International Society for Optical Engineering, 10133. doi: 10.1117/12.2254690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis L, Paul RH, Hoare J, Stein DJ, Taylor PA, Meintjes EM, & Joska JA (2017). Resting-state functional magnetic resonance imaging in clade C HIV: within-group association with neurocognitive function. Journal of Neurovirology, 23(6), 875–885. doi: 10.1007/s13365-017-0581-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Sagredo O, Pazos MR, Garcia C, Pertwee R, Mechoulam R, & Martinez-Orgado J (2013). Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? British Journal of Clinical Pharmacology, 75(2), 323–333. doi: 10.1111/j.1365-2125.2012.04341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, & Segall J (2014). Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences of the United States of America, 111(47), 16913–16918. doi: 10.1073/pnas.1415297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, & Dunlop J (2014). Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug and Alcohol Dependence, 140, 101–111. doi: 10.1016/j.drugalcdep.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi CG, Ulug AM, Ryan E, Ferrando SJ, & van Gorp W (2001). Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. American Journal of Neuroradiology, 22(2), 277–283. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11156769 [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1996). Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient/Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fornito A, Zalesky A, & Breakspear M (2015). The connectomics of brain disorders. Natural Reviews Neuroscience, 16(3), 159–172. doi: 10.1038/nrn3901 [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, & Bullmore ET (2012). Schizophrenia, neuroimaging and connectomics. NeuroImage, 62(4), 2296–2314. doi: 10.1016/j.neuroimage.2011.12.090 [DOI] [PubMed] [Google Scholar]
- Gamarel KE, Brown L, Kahler CW, Fernandez MI, Bruce D, & Nichols S (2016). Prevalence and correlates of substance use among youth living with HIV in clinical settings. Drug and Alcohol Dependence, 169, 11–18. doi: 10.1016/j.drugalcdep.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Nomura EM, Perez F, & D’Esposito M (2012). Focal Brain Lesions to Critical Locations Cause Widespread Disruption of the Modular Organization of the Brain. Journal of Cognitive Neuroscience, 24(6), 1275–1285. doi:DOI 10.1162/jocn_a_00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. doi: 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, Wang L, Tanenbaum A, Esmaeili-Firidouni P, Wendelken LA, Busovaca E, … Valcour V. (2016). Intrinsic network connectivity abnormalities in HIV-infected individuals over age 60. Journal of Neurovirology, 22(1), 80–87. doi: 10.1007/s13365-015-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SX, Seth AK, Kendrick KM, Zhou C, & Feng JF (2008). Partial Granger causality - Eliminating exogenous inputs and latent variables. Journal of Neuroscience Methods, 172(1), 79–93. doi: 10.1016/j.jneumeth.2008.04.011 [DOI] [PubMed] [Google Scholar]
- Hakun JG, Zhu ZD, Brown CA, Johnson NF, & Gold BT (2015). Longitudinal alterations to brain function, structure, and cognitive performance in healthy older adults: A fMRI-DTI study. Neuropsychologia, 71, 225–235. doi: 10.1016/j.neuropsychologia.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Carlini BH, Newville H, Crane HM, Eron JJ, Geng EH, … Donovan DM. (2017). Identifying HIV care enrollees at-risk for cannabis use disorder. AIDS Care, 29(7), 846–850. doi: 10.1080/09540121.2016.1271393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, & Laurienti PJ (2010). Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. NeuroImage, 50(2), 499–508. doi: 10.1016/j.neuroimage.2009.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazleton JE, Berman JW, & Eugenin EA (2010). Novel mechanisms of central nervous system damage in HIV infection. HIV/AIDS - Research and Palliative Care, 2, 39–49. doi: 10.2147/hiv.s9186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, Decosta BR, & Rice KC (1990). Cannabinoid Receptor Localization in Brain. Proceedings of the National Academy of Sciences of the United States of America, 87(5), 1932–1936. doi:DOI 10.1073/pnas.87.5.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Uban KA, Williams PL, Gautam P, Huo Y, Malee K, … Sowell ER. (2015). Default mode connectivity in youth with perinatally acquired HIV. Medicine, 94(37), e1417. doi: 10.1097/MD.0000000000001417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JL, Kraft-Terry SD, & Chang L (2012). Neuroimaging studies of the aging HIV-1-infected brain. Journal of Neurovirology. doi: 10.1007/s13365-012-0114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. doi:S1053811902911328 [pii] [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. [DOI] [PubMed] [Google Scholar]
- Klein TW (2005). Cannabinoid-based drugs as anti-inflammatory therapeutics. Nature Reviews Immunology, 5(5), 400–411. doi: 10.1038/nri1602 [DOI] [PubMed] [Google Scholar]
- Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, & Obermann M (2011). Structural gray and white matter changes in patients with HIV. Journal of Neurology, 258(6), 1066–1075. doi: 10.1007/s00415-010-5883-y [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, … Fox PT. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. doi: 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, von Bastian CC, Wirz H, Oberauer K, & Jancke L (2013). The effects of working memory training on functional brain network efficiency. Cortex, 49(9), 2424–2438. doi: 10.1016/j.cortex.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Liao XH, Xia MR, Xu T, Dai ZJ, Cao XY, Niu HJ, … He Y. (2013). Functional brain hubs and their test-retest reliability: A multiband resting-state functional MRI study. NeuroImage, 83, 969–982. doi: 10.1016/j.neuroimage.2013.07.058 [DOI] [PubMed] [Google Scholar]
- Lombardi A, Guaragnella C, Amoroso N, Monaco A, Fazio L, Taurisano P, … Tangaro S. (2019). Modelling cognitive loads in schizophrenia by means of new functional dynamic indexes. NeuroImage, 195, 150–164. doi: 10.1016/j.neuroimage.2019.03.055 [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, & Yurgelun-Todd D (2015). Aberrant orbitofrontal connectivity in marijuana smoking adolescents. Developmental Cognitive Neuroscience, 16, 54–62. doi: 10.1016/j.dcn.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Tomasi D, & Volkow ND (2018). Subcortical Local Functional Hyperconnectivity in Cannabis Dependence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(3), 285–293. doi: 10.1016/j.bpsc.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroon J, & Bost J (2018). Review of the neurological benefits of phytocannabinoids. Surgical Neurology International, 9, 91. doi: 10.4103/sni.sni_45_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Kuan DC, Sheu LK, Krajina K, Kraynak TE, Manuck SB, & Gianaros PJ (2017). Systemic inflammation and resting state connectivity of the default mode network. Brain, Behavior, and Immunity. doi: 10.1016/j.bbi.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, & Nath A (2010). Human Immunodeficiency Virus-Associated Neurocognitive Disorders Mind the Gap. Annals of Neurology, 67(6), 699–714. doi: 10.1002/ana.22053 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Argeriou M. (1992). The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9(3), 199–213. doi: 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
- Meade CS, Bell RP, Towe SL, Chen NK, Hobkirk AL, & Huettel SA (2019). Synergistic effects of marijuana abuse and HIV infection on neural activation during a cognitive interference task. Addiction Biology, 24(6), 1235–1244. doi: 10.1111/adb.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, & Miller RJ (1996). gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. The Journal of Neuroscience, 16(13), 4080–4088. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8753870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, Reisner SL, Grasso C, Crane HM, Safren SA, Kitahata MM, … Mayer KH. (2013). Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. American Journal of Public Health, 103(8), 1457–1467. doi: 10.2105/ajph.2012.301162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor CN, Cook RL, Chen X, Surkan PJ, Becker JT, Shoptaw S, … Plankey MW. (2017). Trajectories of marijuana use among HIV-seropositive and HIV-seronegative MSM in the Multicenter AIDS Cohort Study (MACS), 1984–2013. AIDS and Behavior, 21(4), 1091–1104. doi: 10.1007/s10461-016-1445-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor CN, Zhou Z, Burrell LE, 2nd, Kelso NE, Whitehead NE, Harman JS, … Cook RL. (2017). Marijuana use and viral suppression in persons receiving medical care for HIV-infection. The American Journal of Drug and Alcohol Abuse, 43(1), 103–110. doi: 10.1080/00952990.2016.1191505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, … Garavan H. (2013). Altered resting-state connectivity in adolescent cannabis users. American Journal of Drug and Alcohol Abuse, 39(6), 372–381. doi: 10.3109/00952990.2013.848213 [DOI] [PubMed] [Google Scholar]
- Orr JM, Paschall CJ, & Banich MT (2016). Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. NeuroImage. Clinical., 12, 47–56. doi: 10.1016/j.nicl.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M, Brier MR, & Ances BM (2015). Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. AIDS, 29(6), 703–712. doi: 10.1097/QAD.0000000000000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, & Sullivan EV (2012). Regional Brain Structural Dysmorphology in Human Immunodeficiency Virus Infection: Effects of Acquired Immune Deficiency Syndrome, Alcoholism, and Age. Biological Psychiatry. doi: 10.1016/j.biopsych.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, & Emsley R (2014). HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS, 28(6), 803–811. doi: 10.1097/QAD.0000000000000151 [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, & Lim KO (2001). White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging, 106(1), 15–24. doi: 10.1016/S0925-4927(00)00082-2 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, & Petersen SE (2013). Evidence for Hubs in Human Functional Brain Networks. Neuron, 79(4), 798–813. doi: 10.1016/j.neuron.2013.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, & Beckmann CF (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. doi: 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, & Toborek M (2003). HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Molecular and Cellular Neuroscience, 24(1), 224–237. doi: 10.1016/S10447431(03)00171-4 [DOI] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, … Martin-Santos R. (2014). Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. Journal of Psychiatric Research, 51, 68–78. doi: 10.1016/j.jpsychires.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors, 28(1), 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage, 52(3), 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, & Bullmore ET (2005). Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral Cortex, 15(9), 1332–1342. doi: 10.1093/cercor/bhi016 [DOI] [PubMed] [Google Scholar]
- Samboju V, Philippi CL, Chan P, Cobigo Y, Fletcher JLK, Robb M, … Teams RP. (2018). Structural and functional brain imaging in acute HIV. Neuroimage-Clinical, 20, 327–335. doi: 10.1016/j.nicl.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Keller MA, Kumar R, Nielsen-Saines K, Michalik DE, … Thomas MA. (2014). Regional brain gray and white matter changes in perinatally HIV-infected adolescents. Neuroimage-Clinical, 4, 29–34. doi: 10.1016/j.nicl.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, … McArthur JC. (2016). HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nature Reviews Neurology, 12(5), 309. doi: 10.1038/nrneurol.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser RGM, Nenadic I, Wagner G, Gullmar D, von Consbruch K, Kohler S, … Sauer H. (2007). White matter abnormalities and brain activation in schizophrenia: A combined DTI and fMRI study. Schizophrenia Research, 89(1–3), 1–11. doi: 10.1016/j.schres.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Seitzman BA, Gratton C, Marek S, Raut RV, Dosenbach NUF, Schlaggar BL, … Greene DJ. (2019). A set of functionally-defined brain regions with improved representation of the subcortex and cerebellum. NeuroImage, 116290. doi: 10.1016/j.neuroimage.2019.116290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalski LM, Towe SL, Sikkema KJ, & Meade CS (2016). The impact of marijuana use on memory in HIV-infected patients: a comprehensive review of the HIV and marijuana literatures. Current Drug Abuse Reviews, 9, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Matthews PM. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23 Suppl 1, S208–219. doi: 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Stam CJ (2014). Modern network science of neurological disorders. Nature Reviews Neuroscience, 15(10), 683–695. doi: 10.1038/nrn3801 [DOI] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, & Hammond A (2017). Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV- adults. Drug and Alcohol Dependence, 170, 120–127. doi: 10.1016/j.drugalcdep.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, & Kuhn TP (2016). Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care, 28(5), 628–632. doi: 10.1080/09540121.2015.1124983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Ortega M, Benzinger TL, & Ances BM (2015). Weighted brain networks in disease: centrality and entropy in human immunodeficiency virus and aging. Neurobiology of Aging, 36(1), 401–412. doi: 10.1016/j.neurobiolaging.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, & Ances BM (2013). Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology, 80(13), 1186–1193. doi: 10.1212/WNL.0b013e318288792b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toich JTF, Taylor PA, Holmes MJ, Gohel S, Cotton MF, Dobbels E, … Meintjes EM. (2018). Functional Connectivity Alterations between Networks and Associations with Infant Immune Health within Networks in HIV Infected Children on Early Treatment: A Study at 7 Years. Frontiers in Human Neuroscience, 11. doi: 10.3389/fnhum.2017.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2012). Aging and functional brain networks. Molecular Psychiatry, 17(5), 471, 549–458. doi: 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2019). Fact sheet - Latest global and regional statistics on the status of the AIDS epidemic. Retrieved from Geneva: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- Valcour V, Sithinamsuwan P, Letendre S, & Ances B (2011). Pathogenesis of HIV in the central nervous system. Current HIV/AIDS Reports, 8(1), 54–61. doi: 10.1007/s11904-010-0070-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, de Lange SC, Zalesky A, Seguin C, Yeo BTT, & Schmidt R (2017). Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: Issues and recommendations. NeuroImage, 152, 437–449. doi: 10.1016/j.neuroimage.2017.02.005 [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, & Pol HEH (2010). Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519–534. doi: 10.1016/j.euroneuro.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Wang HJ, Li RL, Zhou YW, Wang YM, Cui J, Nguchu BA, … Li HJ. (2018). Altered cerebro-cerebellum resting-state functional connectivity in HIV-infected male patients. Journal of Neurovirology, 24(5), 587–596. doi: 10.1007/s13365-018-0649-x [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Wang JL, Zhang H, Mchugh R, Sun XY, Li KC, & Yang QX (2015). Interhemispheric Functional and Structural Disconnection in Alzheimer’s Disease: A Combined Resting-State fMRI and DTI Study. PLoS ONE, 10(5). doi: 10.1371/journal.pone.0126310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CWM, Paolillo EW, Morgan EE, Umlauf A, Sundermann RJE, Letendre S, … Grant I. (2020). Cannabis Exposure is Associated With a Lower Likelihood of Neurocognitive Impairment in People Living with HIV. Journal of Acquired Immune Deficiency Syndromes, 83(1), 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2001). Wechsler Test of Adult Reading (WTAR) Manual. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Wu MM, Zhang XW, Asher MJ, & Thayer SA (2019). Druggable targets of the endocannabinoid system: Implications for the treatment of HIV-associated neurocognitive disorder. Brain Research, 1724. doi: 10.1016/j.brainres.2019.146467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Ma ZM, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, … Satterthwaite TD. (2018). Linked dimensions of psychopathology and connectivity in functional brain networks. Nature Communications, 9. doi: 10.1038/s41467-018-05317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, … Chee MWL. (2015). Functional Specialization and Flexibility in Human Association Cortex. Cerebral Cortex, 25(10), 3654–3672. doi: 10.1093/cercor/bhu217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin LJ, Xu XD, Chen G, Mehta ND, Haroon E, Miller AH, … Felger JC. (2019). Inflammation and decreased functional connectivity in a widely-distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain Behavior and Immunity, 80, 657–666. doi: 10.1016/j.bbi.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lorenzetti V, Suo C, Zalesky A, Fornito A, Takagi MJ, … Solowij N. (2016). Hippocampal harms, protection and recovery following regular cannabis use. Translational Psychiatry, 6, e710. doi: 10.1038/tp.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. doi: 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]