Abstract
Background
Infant videofluoroscopic swallow studies (VFSSs) require clinicians to make determinations about swallowing deficits based on a limited number of fluoroscopically observed swallows. Although airway protection is known to decline throughout a bottle-feed, the paucity of data regarding the timing of this degradation has limited the development of procedural protocols that maximize diagnostic validity.
Objective
We tested the stability of key components of swallow physiology and airway protection at four standardized timepoints throughout the VFSS.
Materials and methods
Thirty bottle-fed infants with clinical signs of swallow dysfunction underwent VFSS. Fluoroscopy was turned on to allow visualization of five swallows at 0:00, 0:30, 1:30 and 2:30 (minutes:seconds [min:s]). We evaluated swallows for components of swallow physiology (oral bolus hold, initiation of pharyngeal swallow, timing of swallow initiation) and airway protection (penetration, aspiration). We used model-based linear contrasts to test differences in the percentage of swallows with low function component attributes.
Results
All components of swallow physiology exhibited a change throughout the VFSS (P≤0.0005). Changes were characterized by an increase in the number of sucks per swallow (P<0.0001), percentage of swallows with incomplete bolus hold (P=0.0005), delayed initiation of pharyngeal swallow (P<0.0001), delayed timing of swallow initiation (P=0.0004) and bolus airway entry (P<0.0001). These findings demonstrate that infants with dysphagia exhibit a change in swallow physiology throughout the videofluoroscopic swallow exam.
Conclusion
Fluoroscopic visualization that is confined to the initial swallows of the bottle feed limit the exam’s diagnostic validity. Developing evidence-based procedural guidelines for infant VFSS execution is crucial for maximizing the exam’s diagnostic and treatment yield.
Keywords: Dysphagia, Infants, Standardization, Swallowing, Videofluoroscopic swallow studies
Introduction
The videofluoroscopic swallow study (VFSS) is considered by many to be the gold standard oropharyngeal swallow assessment. A primary reason for this designation is its utility in providing visualization of the relationship between oropharyngeal structural movement and bolus flow as it transits through the upper aerodigestive tract [1, 2]. Unfortunately, fluoroscopy requires exposure to ionizing radiation. Infants are at increased susceptibility to radiation’s harmful carcinogenic effects when compared to their adult counterparts because of infants’ heightened cell division rates and longer remaining life expectancy [3]. Clinicians must therefore execute exams in a way that keeps radiation exposure as low as reasonably achievable by making determinations about an infant’s oropharyngeal swallowing deficits based on a limited number of fluoroscopically observed swallows [3–5].
Although generalizing about select captured swallows is valid if oropharyngeal swallowing physiology is stable throughout a feed, clinical evidence indicates this is not the case. Previous investigations studying non-dysphagic infants indicates that temporal changes in oropharyngeal physiology exist. The initiation of the bottle feed is characterized by the fastest sucking [6–8] and swallowing rates [9], where sucking and swallowing are frequently coupled at a 1-to-1 ratio [10]. Following this initial suck-burst, which is reported to last up to 30–40 s [9], infants use a down-regulated sucking and swallowing pattern [6–8, 10]. This is characterized by sucking and swallowing at higher suck-to-swallow ratios [10], lower sucking and swallowing rates [9, 10], and reduced intraoral suction pressures [6–8].
While these changes represent normal variants of function among infants with intact oropharyngeal physiology, observations by Newman et al. [11] in 2001 indicate that changes in physiology among dysphagic infants might exacerbate deficits and pose significant threats to airway protection. Videofluoroscopic observations of dysphagic infants indicate that bolus airway entry (penetration/aspiration) does not typically present until approximately 1 min into the exam [11] This work suggests that the timing of fluoroscopic visualization has an impact on the exam’s diagnostic validity. However, the failure to fluoroscopically evaluate for these changes at standardized timepoints across subjects has limited our understanding of the progression of this swallowing degradation and stifled the development of standardized fluoroscopic procedural protocols aimed at maximizing diagnostic yield. The aim of this pilot investigation was to test the stability of key components of oropharyngeal swallow physiology and airway protection among dysphagic bottle-fed infants at four standardized timepoints during the VFSS. Findings from this pilot investigation are to be used to guide the development of future investigations aimed at identifying the optimal timing of fluoroscopic visualization during the infant VFSS.
Materials and methods
Data collection
The research protocol was approved by the institutional review board of the Medical University of South Carolina and carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). Investigators conducted a cohort investigation in which VFSSs were sequentially conducted on bottle-fed infants referred for VFSS because of signs of oropharyngeal swallowing dysfunction. VFSSs were performed using a standardized protocol that was executed by a radiologist and speech-language pathologist. Infants were positioned in the lateral viewing plane with the fluoroscopic visualization field collimated to include the lips anteriorly, nasal cavity superiorly, cervical spinal column posteriorly, and the upper esophageal sphincter inferiorly to allow for full visualization of the oral cavity and the pharynx. Swallowing was evaluated using continuous fluoroscopy as the infants ingested standardized thin Varibar barium contrast agent (Bracco Diagnostics, Monroe Township, NJ). The exam was conducted using the infant’s typical bottle nipple, as identified by the child’s caregiver or primary medical team. Once the infant initiated sucking, fluoroscopy was turned on to allow for visualization of the first five suck–swallow sequences of the feed (0:00, min:s). Following their visualization fluoroscopy was turned off while the infant continued to feed. Fluoroscopy was then turned on and off following the described paradigm to visualize five additional suck–swallow sequences at 0:30, 1:30 and 2:30. Time points tested in this pilot investigation were determined based on previous reports of physiological change within the first 30 s of the bottle feed [9, 11], with subsequent time points (1:30 and 2:30) chosen as times that would enable further elucidation of swallowing stability without exposing infants to unnecessary elevations of radiation exposure, volumes of barium ingestion, or extended procedure times. No attempts were made to remove the bottle from the infant’s oral cavity or to provide compensatory interventions (i.e. pacing, viscosity, nipple) until the end of the standardized visualization period. The need for compensatory interventions and the method for their fluoroscopic visualization was left up to the discretion of the examining radiologist and speech-language pathologist. We excluded infants who could not maintain a latch for the first 1:30 s of the exam, required pacing throughout the exam to maintain clinical relevance in diagnostic yield (e.g., fragile premature infants), and who were found to be unsafe by the evaluating therapist following fluoroscopic observation to continue feeding for 1:30 without the provision of interventions (e.g., fluoroscopic observation of repeated aspiration prior to 1:30). All VFSSs were recorded at 30 frames/s on a high-resolution 1,024×1,024 pixel TIMS recording system (Foresight Imaging, Chelmsford, MA).
Upon completion of each exam fluoroscopy time was recorded and charts were reviewed for infant demographics, primary diagnosis, and VFSS indication. Primary diagnosis was selected from a list of six diagnostic categories (prematurity, airway malformation, gastrointestinal, neurologic, cardiovascular or pulmonary) based on the diagnostic category that was determined to pose the greatest detriment to swallowing. Likewise, VFSS indication was selected as the dysphagia sign that was determined to cause the infant the greatest functional impairment (coughing/choking with feeds, insufficient volume of milk ingestion, chronic respiratory morbidity [e.g., chronic respiratory infections], cardiopulmonary instability with feeds, fussiness with feeds, routine postsurgical evaluation or follow-up on previous impairment).
Data analysis
Videofluoroscopic exams were first independently analyzed by two speech-language pathologists, trained in scoring the swallowing components, who were blinded to patient and exam information using frame-by-frame review on a high-definition 2,560×1,440-pixel screen. Only swallows with complete fluoroscopic visualization of the entire sucking and swallowing sequence were included. Each swallow was evaluated for five attributes of oropharyngeal swallow physiology and airway protection that have been demonstrated to have inter-rater reliability >80%: number of sucks per swallow, oral bolus hold, bolus location at the initiation of pharyngeal swallow, timing of initiation of pharyngeal swallow, and bolus airway entry [12, 13]. See Table 1 for a full listing of variable definitions. These components were chosen because of their clinical significance and clinical observations of change throughout the VFSS. More specifically, number of sucks per swallow is of clinical significance because it reflects the infant’s ability to efficiently meet nutritional needs, with oral bolus hold and initiation of pharyngeal swallow indicative of an infant’s risk for bolus airway entry in a clinical context.
Table 1.
Oropharyngeal swallow component definitions
| Number of sucks per swallow |
Number of sucks, defined as the anterior-posterior lingual compression of the inferior nipple edge, exerted prior to the initiation of the pharyngeal swallow |
| Oral bolus hold | Containment of the bolus within the oral cavity prior to the initiation of the pharyngeal swallow. Physiology was categorized as low function if the bolus progressed beyond the soft palate to tongue base juncture prior to swallow initiation |
| Bolus location at initiation of swallow | Location of the bolus head at the time the soft palate exhibits its initial brisk superior contraction with pharyngeal contraction. Physiology was categorized as low function if initiation occurred below the level of the vallecula |
| Timing of swallow initiation | The duration of time (milliseconds) that the bolus resides in the location of initiation of pharyngeal swallow prior to swallow initiation |
| Bolus airway entry | Maximum extent that the bolus progresses into the airway. Progression was categorized as present if penetration, defined as bolus entry into the laryngeal vestibule without progression beyond the vocal folds, or aspiration, defined as progression of the bolus beyond the level of the vocal folds, was present |
We analyzed performance within each categorical attribute of swallowing physiology, using an operationally defined rank-ordered scale describing the integrity of each swallowing attribute (Baby Videofluoroscopic Swallow Study Impairment Profile-BaByVFSSImP) [13, 14]. To allow for statistical comparison of physiology at each time point, we dichotomized component scores into high-function and lowfunction groups based on their theoretical ability to facilitate complete airway protection [15] (Table 1). After each rater completed independent analysis, we monitored validity and reproducibility of results as per standard lab protocol by calculating inter-rater reliability for each component. We did this by calculating the percentage of swallows within each component that had complete concordance across raters. We then resolved all scoring discrepancies by consensus [13, 15].
Statistical considerations
Dichotomized categorical component scores were summarized for each time point by the percentage of low-function swallows and corresponding 95% confidence intervals (CIs), with standard errors used to construct CIs adjusted to account for the multiplicity of swallows from the same infant (i.e. clustering of observations within subjects) [16]. Data from subjects who did not have fluoroscopic swallow visualization at 2:30 were summarized for the first three timepoints and were not included in the last. We modeled the log odds of the probability of lowfunction swallow attributes as a function of evaluation time (treated as a nominal categorical variable) using generalized estimating equations (GEEs) [17, 18] with logit link and exchangeable working correlation structure. We performed comparisons between specific time points using model-based linear contrasts. We performed trend tests using the same GEE model but with time treated as a continuous rather than categorical variable. All inference was performed using robust variance estimation of model parameters. We performed a similar GEE analysis for the number of sucks per swallow, but used a log link function (as is appropriate for count data) to model the log suck rate as a function of time. Similarly, we used proportional hazards regression with robust variance estimation [19] to model the log hazard of swallowing as a function of time. We performed all analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Rating clinicians demonstrated ≥85% inter-rater concordance reliability in the assessment of all swallowing components. We included 30 infants (18 boys, 12 girls) in the investigation. Average postmenstrual age at time of VFSS was 49.5 (46.8–53.8) weeks. The average VFSS fluoroscopy time and dose including the protocol with subsequent clinician-determined compensatory interventions were 1.5 min (1.2–1.7 min) and 2.8 mGy (2.3–3.6 mGy), respectively. Primary reasons for VFSS referral included coughing/choking with feeds (36.7%), chronic respiratory morbidity (16.7%) and insufficient volume of milk ingestion (16.7%). Although all time points were visualized in the majority of infants (80.0%), swallowing was not observed at 2:30 in six infants secondary to repeated episodes of more than trace aspiration that warranted the provision of a compensatory intervention before the protocol could be completed. The average number of swallows available for analysis among the included infants was 18 (range 16.8–20.0) of the possible 20 swallows targeted. See Table 2 for subject and VFSS characteristics.
Table 2.
Patient demographics, primary diagnosis and videofluoroscopic swallow studies (VFSS) indication
| Demographics | Valuea |
|---|---|
| Gender | |
| Male | 18(60.0%) |
| Female | 12 (40.0%) |
| Race | |
| African-American | 7 (23.3%) |
| Caucasian | 21 (70.0%) |
| Other | 2 (6.7%) |
| Postmenstrual age in weeks | 49.5 (46.8–53.8) |
| Primary diagnosis | |
| Prematurity | 5 (16.7%) |
| Airway malformation | 6 (20.0%) |
| Gastrointestinal | 5 (16.7%) |
| Neurologic | 5 (16.7%) |
| Cardiovascular | 3 (10.0%) |
| Pulmonary | 6 (20.0%) |
| VFSS indication | |
| Coughing/choking with feeds | 11 (36.7%) |
| Insufficient volume of milk ingestion | 5 (16.7%) |
| Chronic respiratory morbidity | 5 (16.7%) |
| Follow-up on previous impairment | 3 (10.0%) |
| Cardiopulmonary instability with feeds | 2 (6.7%) |
| Fussiness with feeds | 2 (6.7%) |
| Routine postsurgical evaluation | 2 (6.7%) |
Values shown are number of infants (%) for all variables except postmenstrual age, for which the reported values are median and interquartile range. Percentages were rounded and might not add to 100%
All components of oropharyngeal swallow physiology were found to change throughout the VFSS (P≤0.0005; Table 3). The majority of these changes occurred incrementally between 0:00, 0:30 and 1:30. Specifically, the average number of sucks per swallow increased from 2.0 (1.7–2.4) sucks per swallow at 0:00 to 2.5 (2.1–3.0) sucks per swallow at 0:30 and 2.8 (2.4–3.3) sucks per swallow at 1:30 (P=0.004). Similar changes were observed in the percentage of swallows with incomplete bolus hold, delayed location of swallow initiation, and delayed timing of swallow initiation. The proportion of swallows with incomplete bolus hold increased from 41% (26–55) at 0:00 to 71% (59–84) and 86% (76–95) at 0:30 and 1:30, respectively (P≤0.003). Likewise, the proportion of swallows that initiated below the level of the valleculae increased from 37% (23–62) at 0:00 to 64% (53–76) at 0:30 and 78% (67–89) at 1:30 (P≤0.02). While swallows initiated immediately upon reaching the location of swallow initiation during the first five swallows of the exam, the bolus remained in the location of swallow initiation for longer durations as the feed progressed (0:30, 100 ms; 1:30, 280 ms; P≤0.04; Fig. 1).No changes in the proportion of swallows with bolus airway entry occurred between the initial five swallows and those at 0:30; however, changes were observed between 0:30 and 1:30. These were characterized by an elevation in the proportion of swallows with penetration or aspiration, which increased from 40% (29–51) at 0:30 to 63% (51–75) at 1:30 (P=0.0004; Fig. 2). No significant changes were found between 1:30 and 2:30 in any of the swallowing outcomes.
Table 3.
Summary of swallows with low function component attributes at each time interval across patients
| 0:00 n=30 | 0:30 n=30 | 1:30 n=30 | 2:30 n=24 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Valuea | 95% CI | Valuea | 95% CI | Valuea | 95% CI | Valuea | 95% CI | P-valueb | |
| Number of sucks per swallow | 2.0 | (1.7, 2.4) | 2.5 | (2.1, 3.0) | 2.8 | (2.4, 3.3) | 2.7 | (2.2, 3.2) | <0.0001 |
| Oral bolus hold: escape to pharynx (%) | 41 | (26, 55) | 71 | (59, 84) | 86 | (76, 95) | 83 | (70, 96) | 0.0005 |
| Initiation of pharyngeal swallow: below the valleculae (%) | 37 | (23, 52) | 64 | (53, 76) | 78 | (67, 89) | 76 | (65, 87) | <0.0001 |
| Timing of swallow initiation (ms) | 0 | (0, 140)c | 100 | (100, 190) | 280 | (190,370) | 230 | (900, 360) | 0.0004 |
| Bolus airway entry: penetration/aspiration (%) | 29 | (18, 40) | 40 | (29, 51) | 63 | (51,75) | 69 | (58, 80) | <0.0001 |
Values for oral bolus hold, initiation of pharyngeal swallow, and bolus airway entry are median percentage of swallows at each time point with identified function. Values for number of number of sucks per swallow are means, and values for time to swallow are medians
P-values based on trend tests (see “Statistical considerations” section)
Confidence interval (CI) lower bound truncated at 0
Fig. 1.
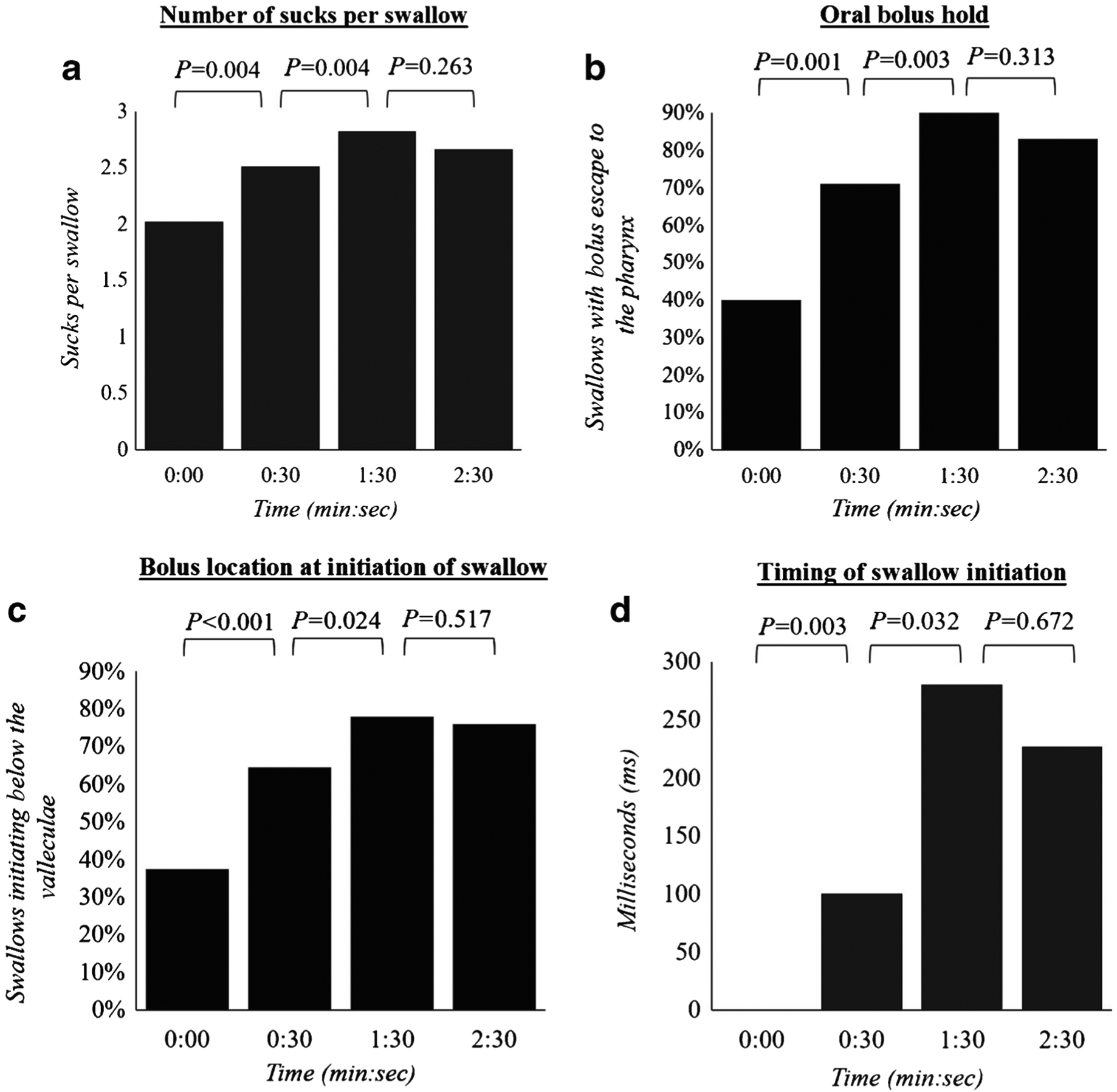
Change in oropharyngeal swallow physiology throughout the videofluoroscopic swallow exam. a–d Values represent median number of sucks per swallow (a), percentage of swallows with bolus escape to the pharynx (b), percentage of swallows with swallow initiation below the valleculae (c) and the mean timing of initiation of swallow among the five swallows visualized at each time point (d)
Fig. 2.

Change in bolus airway entry throughout the videofluoroscopic swallow exam. Values represent the median percentage of swallows among the five visualized swallows with penetration and aspiration at each time point
Discussion
We tested the stability of key components of oropharyngeal swallowing physiology and bolus flow throughout the initial 2:30 of bottle feeds among a sample of 30 dysphagic infants. Our primary findings indicate: (1) Components of swallowing physiology and bolus flow changed throughout the initial 1:30 of the videofluoroscopic swallow study; (2) Changes were characterized by incremental increases in sucks per swallow, proportion of swallows with incomplete bolus hold, delayed location of swallow initiation, and delayed timing of swallow initiation as the infant progressed from the first five swallows (0:00) to those at 0:30 and 1:30; (3) The proportion of swallows with bolus airway entry significantly increased from 0:30 to 1:30; and (4) No significant changes in swallowing were observed between 1:30 and 2:30 in any attribute of swallowing physiology or bolus flow.
The observed changes in sucking physiology that are consistent with findings from previous investigations examining feeding stability among non-dysphagic infants. Koenig et al. [10] in 1990 examined sucking stability among a mixed sample of non-dysphagic term and preterm infants 35–47 weeks’ postmenstrual age. Infants exhibited an initial period of rapid sucking lasting up to 2 min, during which the majority of sucks were immediately followed by a swallow (1:1 suck-toswallow) [10]. Following this initial 2 min of rapid sucking, however, suck-to-swallow ratio increased, with only one-third of the sucks followed immediately by a swallow [10]. Our results also demonstrated increases in the suck-to-swallow ratio throughout the initial 2 min of a bottle feed, with infants increasing from 2.0 to 2.7 sucks per swallow throughout the observation period. Interestingly, despite the similarity in sucking trends, it is notable that the average number of sucks per swallow was higher in our sample when compared to Koenig et al.
The number of sucks an infant exerts prior to initiating the pharyngeal swallow has been shown to increase throughout the first month of life [20]. Because the infants in the current investigation had an average postmenstrual age of 49.5 weeks, whereas those studied by Koenig et al. [10] had an average postmenstrual age of 41.0 weeks, it is possible this discrepancy was caused by maturational differences between our samples. Another source for this discrepancy might be differences in the testing conditions between the two investigations. Recent changes in bottle nipple manufacturing techniques have led to the production of bottle nipples that provide a greater restriction to fluid flow and require the infant to exert more sucks per swallow than those previously available clinically [21]. Likewise, slight differences in thin liquid viscosity between the thin formula used in Koenig’s investigation and the thin barium tested in the current investigation could yield a similar effect. Although both liquids are categorized as thin liquids, slight differences in viscosity between these liquids exist. It is possible that the barium used in the current investigation provided a greater restriction to flow through the nipple and thereby required the infant to exert more sucks to generate a sufficient bolus volume to swallow.
Of potentially greater clinical significance, however, is the increase in occurrences of bolus airway entry (penetration/aspiration) between 0:30 and 1:30. Our results revealed that 43% of the infants who exhibited penetration and 67% of the infants who exhibited aspiration did not do so until after the initial five swallows (0:00). These findings are consistent with those of Newman et al. [11], who in 2001 evaluated changes in airway protection among dysphagic infants under videofluoroscopy. In Newman et al.’s investigation, fluoroscopy was turned on to enable visualization of the initial swallows of the feed, with 1–2 swallows visualized every 15–30 s thereafter until aspiration was observed or 1–2 min had passed. Results revealed that bolus airway entry typically did not occur during the initial swallows of the feed. The average time to observe the initial episode of laryngeal penetration was 50.8 s, with even longer delays to observe the first occurrence of aspiration (65.4 s) [11]. Our work used standardized timepoints of evaluation to build on these findings and more precisely identify the timing and extent of swallowing degradation throughout the exam. Our results suggest that this degradation in swallowing is not a one-time event but instead a stepwise regression following the initiation of the feed. Bottle feeding not only requires sustained integrity within the movement of oropharyngeal musculature, but it also requires a sustained inhibition of the respiratory system to facilitate optimal respiratory–swallow coordination [9, 22, 23]. Deficits in the ability to sustain either of these functions can pose deleterious effects to an infant’s airway protection. While our ability to draw conclusions about the source for the observed reduction in airway protection is beyond the scope of the current investigation, future investigations using supplemental cross-systems measures capable of evaluating these origins are warranted.
Our results, though preliminary, highlight the importance of further research that supports the development of procedural guidelines that maximize diagnostic yield and minimize radiation exposure during infant videofluoroscopic swallow studies. The infant’s heightened susceptibility to fluoroscopy’s long-term carcinogenic effects necessitates that clinicians make determinations about the integrity of swallowing physiology based on a small sample of fluoroscopically observed swallows [4, 24, 25]. The absence of procedural guidelines, however, contributes to high variability in the manner that these swallows are fluoroscopically observed. The changes observed in the current investigation suggest this variability could greatly influence the exams’ diagnostic validity.
Timing of fluoroscopic visualization during the exam is certainly an area of importance; however, another element that requires further refinement is the identification of the minimum number of swallows that must be evaluated to capture clinically significant impairment. It is unclear whether the 20 swallows visualized in the current investigation is sufficient to adequately capture such impairments. Execution of the VFSS requires the clinician strike a delicate balance between minimizing radiation exposure and compromising the exam’s diagnostic yield. Identifying the minimum number of swallows that need to be fluoroscopically observed to gain valid diagnostic results requires further investigation of the significance of VFSS findings that extend beyond the presence of penetration and aspiration, and into variables such as the frequency of its occurrence, as well as other physiological components such as location and timing of swallow initiation. It is also important to note that the power of the VFSS is not isolated to identifying impairment; it can also be used to identify appropriate treatment regimens. Attention to the infant’s capacity for optimal function is of equal importance to identifying periods where it is suboptimal because this information could be used to guide shorter-duration therapeutic feeds. It is therefore suggested that the initial periods of visualization, where optimal function is likely to be observed, are of equal clinical significance to those later timepoints of suboptimal performance.
While the current investigation further elucidates the timing and stability of the associations between fluoroscopic visualization and swallowing physiology, the ability to draw larger conclusions is limited by several factors, the most significant of which is the small heterogeneous sample. It is likely that the stability of oropharyngeal swallowing differs based on underlying impairment, oral feeding experience, age, bottle nipple and other clinical factors. The small sample size in this pilot investigation also limited our ability to conduct more advanced statistical analysis to further refine specifications of how each component score changed. Future studies with larger sample sizes are necessary to further examine how swallowing stability differs based on these patient and clinical factors. Although the determination to limit the last time point of fluoroscopic visualization to 2:30 was done in an effort to maintain clinical relevance and feasibility in radiology workflow, this might have limited our ability to detect further changes that occurred later in the feed. Likewise, because more than trace aspiration was repeated within the initial 1:30, six infants were unable to undergo data collection at the last time point (2:30). It remains unclear whether the failure to detect significant changes between 1:30 and 2:30 is a result of swallowing stabilization or a consequence of small sample size and selection bias resulting in less severe infants at the last time point. Extensions of this work evaluating these attributes of swallowing stability are in further development.
Conclusion
In the current investigation, we tested the stability of key components of oropharyngeal swallow physiology and bolus flow during standard time intervals throughout the initial 2:30 of the VFSS. Findings indicate there are significant differences in swallowing physiology based on the timing of fluoroscopic visualization. Future work refining the optimal fluoroscopic procedural set is necessary to develop procedural guidelines that not only maximize diagnostic and therapeutic yield, but also do so while minimizing radiation exposure and continuing to allow for flexibility based on the clinical circumstances of the infant.
Acknowledgments
The research team would like to thank the Medical University of South Carolina Department of Radiology for its relentless support in improving pediatric dysphagia care. Without this support this investigation would not be possible. The team would also like to thank Dr. Maureen Lefton-Greif for her insight and contributions to this investigation. This work was supported by a Medical University of South Carolina foundation grant from the Mark and Evelyn Trammell Trust and the National Institute of Deafness and Other Communication Disorders (NIH, NIDCD, R01DC011290, CO-PI: Martin-Harris, Lefton-Greif).
Conflicts of interest Dr. Martin-Harris receives grant support from Bracco, is a paid consultant for phagogenesis, receives royalties from the Medical University of South Carolina Foundation for Research Development (Modified Barium Swallow Impairment Profile copyright royalties from Northern Speech Services through an agreement with Medical University of South Carolina), holds a patent for wireless medical sensors and methods, and receives speaker fees from Northern Speech Services.
References
- 1.Miller CK, Willging JP (2003) Advances in the evaluation and management of pediatric dysphagia. Curr Opin Otolaryngol Head Neck Surg 11:442–446 [DOI] [PubMed] [Google Scholar]
- 2.Dodrill P, Gosa M (2015) Pediatric dysphagia: physiology, assessment, and management. Ann Nutr Metab 66:24–31 [DOI] [PubMed] [Google Scholar]
- 3.ICRP Khong PL, Ringertz H et al. (2013) ICRP publication 121: radiological protection in paediatric diagnostic and interventional radiology. Ann ICRP 42:1–63 [DOI] [PubMed] [Google Scholar]
- 4.Weir K, McMahon S, Long G et al. (2007) Radiation doses to children during modified barium swallow studies. Pediatr Radiol 37:283–290 [DOI] [PubMed] [Google Scholar]
- 5.United States Nuclear Regulatory Commission (2007) Title 10 code of federal regulations, Part 20: standards for protection against radiation. http://www.nrc.gov/reading-rm/doc-collections/cfr/part020/. Accessed 13 Aug 2019
- 6.Lang W, Buist N, Geary ABS et al. (2011) Quantification of intraoral pressures during nutritive sucking: methods with normal infants. Dysphagia 26:277–286 [DOI] [PubMed] [Google Scholar]
- 7.Pollitt E, Consolazio B, Goodkin F (1981) Changes in nutritive sucking during a feed in two-dayand thirty-day-old infants. Early Hum Dev 5:201–210 [DOI] [PubMed] [Google Scholar]
- 8.Mathew P, Belan M, Thoppil C (1992) Sucking patterns of neonates during bottle feeding: comparison of different nipple units. Am J Perinatol 9:265–269 [DOI] [PubMed] [Google Scholar]
- 9.Bamford O, Taciak V, Gewolb IH (1992) The relationship between rhythmic swallowing and breathing during suckle feeding in term neonates. Pediatr Res 31:619–624 [DOI] [PubMed] [Google Scholar]
- 10.Koenig JS, Davies AM, Thach BT (1990) Coordination of breathing, sucking, and swallowing during bottle feeding in human infants. J Appl Physiol 69:1623–1629 [DOI] [PubMed] [Google Scholar]
- 11.Newman LA, Keckley C, Petersen MC, Hammer A (2001) Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatrics 108:E106. [DOI] [PubMed] [Google Scholar]
- 12.Weckmueller JE, Easterling C, Arvedson J (2011) Preliminary temporal measurement analysis of normal oropharyngeal swallowing in infants and young children. Dysphagia 26:135–143 [DOI] [PubMed] [Google Scholar]
- 13.Lefton-Greif MA, McGrattan KE, Carson KA et al. (2017) First steps towards development of an instrument for the reproducible quantification of oropharyngeal swallow physiology in bottle-fed children. Dysphagia 33:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Harris B, Carson KA, Pinto J, Lefton-Greif MA (2019) BaByVFSSImP© a novel measurement tool for videofluoroscopic assessment of swallowing impairment in bottle-fed babies: establishing a standard. Dysphagia. 10.1007/s00455-01910008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrattan KE, McGhee H, DeToma A et al. (2017) Dysphagia in infants with single ventricle anatomy following stage 1 palliation: physiologic correlates and response to treatment. Congenit Heart Dis 12:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochran WG (1977) Sampling techniques, 3rd edn. Wiley, New York [Google Scholar]
- 17.Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22 [Google Scholar]
- 18.Zeger SL, Lang KY, Albert PS (1988) Models for longitudinal data: a generalized estimating equation approach. Biometrics 44:1049–1060 [PubMed] [Google Scholar]
- 19.Lin DY, Wei LJ (1989) The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84:1074–1078 [Google Scholar]
- 20.Qureshi M, Vice F, Taciak V et al. (2002) Changes in rhythmic suckle feeding patterns in term infants in the first month of life. Dev Med Child Neurol 44:34–39 [DOI] [PubMed] [Google Scholar]
- 21.Pados BP, Park J, Dodrill P (2019) Know the flow: milk flow rates from bottle nipples used in the hospital and after discharge. Adv Neonatal Care 19:32–41 [DOI] [PubMed] [Google Scholar]
- 22.McGrattan KM, McFarland D, Dean J et al. (2017) Effect of singleuse, laser-cut, slow-flow nipples on respiration and milk ingestion in preterm infants. Am J Speech Lang Pathol 26:832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Sayed L, Schrank W, Thach B (1994) Ventilatory sparing strategies and swallowing pattern during bottle feeding in human infants. J Appl Physiol 77:78–83 [DOI] [PubMed] [Google Scholar]
- 24.Bonilha HSW, Wilmskoetter J, Tipnis SV et al. (2018) Estimating thyroid doses from modified barium swallow studies. Health Phys 115:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hersh C, Wentland C, Sally S et al. (2016) Radiation exposure from videofluoroscopic swallow studies in children with a Type 1 laryngeal cleft and pharyngeal dysphagia: a retrospective review. Int J Pediatr Otorhinolaryngol 89:92–96 [DOI] [PubMed] [Google Scholar]


