Abstract
Purpose:
To study the risk of metastatic prostate cancer development in men with Grade Group 2 disease being managed with active surveillance (AS) at Memorial Sloan Kettering Cancer Center.
Materials and Methods:
219 men with Grade Group 2 prostate cancer were managed with AS between 2000–2017. Biopsy was performed every 2–3 years or upon changes in magnetic resonance imaging, prostate-specific antigen (PSA) level, or digital rectal examination. The primary outcome was development of distant metastasis. The Kaplan-Meier method was used to estimate treatment-free survival.
Results:
The median age at diagnosis was 67 years (IQR 61, 72), the median PSA was 5 ng/mL (IQR 4, 7), and most patients (69%) had non-palpable disease. During follow-up, 64 men received treatment: 36/64 (56%) radical prostatectomy; 20/64 (31%) radiotherapy; 3/64 (5%) hormone therapy; and 5/64 (8%) focal therapy. Of the 36 patients who underwent radical prostatectomy, 32/36 (89%) had Grade Group 2 disease on pathology and 4/36 (11%) had Grade Group 3. Treatment-free survival was 61% (95% CI 52%–70%) at 5 years and 49% (95% CI 37%–60%) at 10 years. Three men experienced biochemical recurrence, no men developed distant metastasis, and no men died of prostate cancer during the follow-up. The median follow-up was 3.1 years (IQR 1.9, 4.9).
Conclusions:
AS appears to be a safe initial management strategy in the short term for carefully selected and closely monitored men with Grade Group 2 prostate cancer managed at a tertiary cancer center. Definitive conclusions await further follow-up.
INTRODUCTION
Most patients with favorable-risk prostate cancer do not need immediate treatment, and can instead be managed by active surveillance (AS) in an effort to avoid, or delay, the side effects of radical therapy.1 In the context of AS, patients are regularly monitored for signs of disease progression, which then prompts curative intervention. Originally, AS was used very restrictively around the world, for example, the group at Hopkins restricted their cohort to patients with low-risk prostate cancer, predominately a subgroup with very low-risk (defined as clinical stage T1c, PSA density < 0.15 ng/mL, biopsy Gleason score ≤ 6, ≤2 positive biopsy cores, and a maximum of 50% involvement of any biopsy core with cancer).2 The Toronto cohort also included some patients older than 70 with Gleason Score 3+4=7 (Grade Group 2).3 Over time, AS inclusion criteria have been relaxed in cohorts around the world to also include men with high-volume Gleason score 6 (Grade Group 1) and prostate-specific antigen (PSA) levels between 10–20 ng/mL as well as select men with intermediate-risk disease with Gleason score 3+4=7 (Grade Group 2).2–8
The strongest predictor of oncologic outcome among men with prostate cancer is the histopathologic grade group.9 All published prospective AS cohorts that have reported long-term outcomes thus far have either reported only on Grade Group 1 or a mixture of low- and intermediate risk disease (Grade Groups 1–3).2–8
The paucity of data on outcomes in men at the lower end of intermediate-risk prostate cancer managed with AS contributes to uncertainty for both physicians and patients engaging in shared decision making around prostate cancer management decisions. Here we report our experience of 219 men with Grade Group 2 (Gleason score 3+4) prostate cancer managed with AS at Memorial Sloan Kettering Cancer Center.
PATIENTS AND METHODS
This study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center. We queried our institutional database and identified 219 patients with Grade Group 2 prostate cancer who were managed with AS from 2000 to 2017. Patients were determined to be on AS if they were not treated within a year of diagnosis. Chart reviews were performed to assess if patients were followed on AS. Those who were followed on a watchful waiting basis (no biopsies, advanced age, hormonal treatment) were not included. Management strategies have evolved over this 17-year period; our contemporary management strategy is described here. Following diagnosis and confirmatory biopsy, patients underwent PSA testing and physical and digital rectal exams every 6 months. In addition, patients had multiparametric (T2-weighted, diffusion-weighted and/or dynamic contrast-enhanced) magnetic resonance imaging (MRI) performed every 18 months. Non-targeted systematic biopsy was performed every 2 to 3 years, with MRI/ultrasound fusion biopsy of suspicious lesions identified on MRI. Historically, patients may have been managed with yearly biopsies. If there was a change in PSA or MRI, a biopsy was performed prior to the 3-year period. Questionnaires were sent to patients to ascertain their treatment status. The majority of initial biopsies were performed at the institution. All but 15 biopsies (diagnostic and surveillance biopsies) were reviewed by subspecialty urologic pathologists at the institution.
Statistics
Our primary endpoint was the incidence of distant metastasis in patients managed with AS using the Kaplan-Meier method. Patients were censored at the last date of contact with the clinic. Secondary endpoints included treatment-free survival, overall survival, and radical prostatectomy outcomes. Grade progression was defined as an upgrade to Grade Group 3 (Gleason Score 3+4) or higher. Volume progression was defined by MRI or number of cores. Univariate Cox regression was used to determine if receiving treatment following active surveillance were associated with any diagnostic variable of interest including age at diagnosis, PSA level at diagnosis, number of positive cores, percent cancer and total mm of cancer at diagnosis. Statistical analyses were performed using Stata 15.0 (StataCorp, College Station, TX, USA).
RESULTS
Patient characteristics are displayed in Table 1. Among the 219 men with Grade Group 2 prostate cancer, the majority of patients enrolled in AS after 2010: 113/219 (52%) between 2010–2014 and 68/219 (31%) between 2015–2017. The median age at diagnosis was 67 years (IQR 61, 72), the median PSA was 5 ng/mL (IQR 4, 7) and the majority of patients (69%) had non-palpable disease. The median number of positive cores was 2 (IQR 1, 3), and the median total length of cancer on diagnostic biopsy was 4 mm (IQR 2, 6).
Table 1.
Patient characteristics for men with Grade Group 2 prostate cancer
Numbers represent median (interquartile range) or frequency (percentage).
| Characteristic | N=219 |
|---|---|
| Age at Diagnosis, years | 67 (61, 72) |
| Diagnostic PSA (ng/mL, N=201) | 5 (4, 7) |
| Number of Positive Cores at Diagnosis (N=213) | 2 (1, 3) |
| Number of Total Cores at Diagnosis (N=213) | 12 (7, 14) |
| Nomogram Risk of Locally Advanced Disease (N=201) | 50% (40%, 60%) |
| Total Cancer Length at Diagnosis (N=205), millimeters | 4 (2, 6) |
| Year of Diagnosis | |
| 2000–2004 | 7 (3.2%) |
| 2005–2009 | 31 (14%) |
| 2010–2014 | 113 (52%) |
| 2015–2017 | 68 (31%) |
| Clinical T Stage | |
| ≤T1C | 151 (69%) |
| T2A | 31 (14%) |
| T2B | 5 (2.3%) |
| T2C | 3 (1.4%) |
| T3A | 2 (0.9%) |
| Unknown | 27 (12%) |
| Charlson Comorbidity Index | |
| 0 | 158 (72%) |
| 1 | 26 (12%) |
| 2 | 26 (12%) |
| ≥3 | 9 (4.1%) |
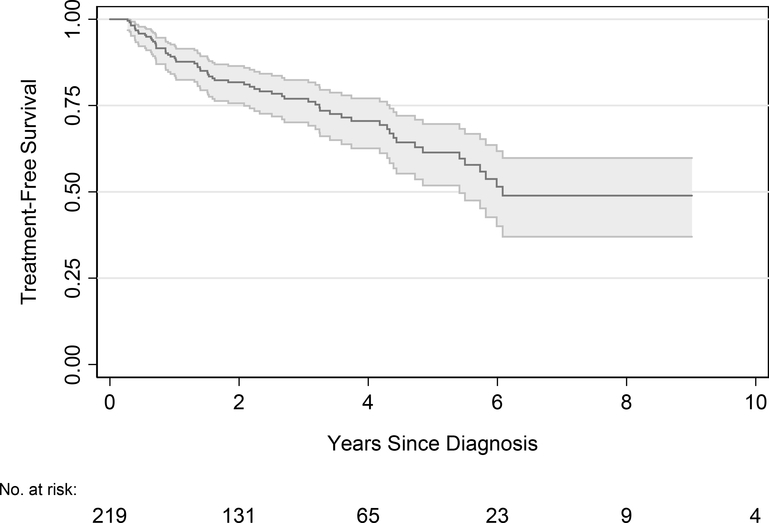
The median follow-up for those who did not die was 3.1 years (IQR 1.9, 4.9). There were 55 men followed for at least 5 years and 5 men followed for 10 years. During follow-up, 64 men received treatment: 36/64 (56%) radical prostatectomy; 20/64 (31%) radiotherapy; 3/64 (5%) hormone therapy; and 5/64 (8%) focal therapy. Treatment-free survival was 61% (95% CI 52%–70%) at 5 years and 49% (95% CI 37%–60%) at 10 years (Figure 1). The triggers for treatment were mainly patient preference and/or disease progression by grade or volume upgrade (Table 2). Of the 36 patients who underwent radical prostatectomy, 32/36 (89%) had Grade Group 2 disease on pathology and 4/36 (11%) had Grade Group 3. The majority of patients who underwent prostatectomy had organ-confined disease, 25/36 pT2, (69%) and 11/36 (31%) of patients had non-organ confined disease (pT3). Three men experienced biochemical recurrence after radical prostatectomy.
Figure 1.
Treatment-free survival
Table 2.
Triggers for treatment
Values represent frequency (percentage).
| Reason | N=64 |
|---|---|
| Patient Preference | 19 (30%) |
| Biopsy Volume Progression | 15 (23%) |
| Biopsy Grade Progression | 14 (22%) |
| MRI Result | 9 (14%) |
| Change in PSA Value or Digital Rectal Examination | 4 (6%) |
| Prolaris Result (tissue-based biomarker) | 3 (5%) |
Abbreviation: MRI=magnetic resonance imaging, PSA=prostate-specific antigen
Results from the univariable Cox regression analyses are displayed in Table 3. Diagnostic PSA level (HR=1.08; 95% CI 1.03–1.13, p=0.003) and total millimeters of cancer at diagnosis (HR=1.07; 95% CI 1.01–1.14, p=0.021) were significantly associated with receiving treatment for prostate cancer and discontinuing AS.
Table 3.
Factors associated with receiving treatment
Univariable Cox proportional hazards regression was used to determine if factors are associated with receiving treatment for prostate cancer.
| Predictor | HR | 95% CI | p-value |
|---|---|---|---|
| Age at Diagnosis (per 1 year increase) | 0.97 | 0.94, 1.00 | 0.053 |
| Year of Diagnosis (per 1 year increase) | 1.00 | 1.00, 1.00 | 0.003 |
| Diagnostic PSA (per 1 ng/mL increase) | 1.08 | 1.03, 1.13 | 0.003 |
| Number of Positive Cores at Diagnosis (per mm increase) | 1.03 | 0.93, 1.15 | 0.6 |
| Percent Cancer at Diagnosis (per % increase) | 1.01 | 1.00, 1.02 | 0.058 |
| Total mm Cancer at Diagnosis (per mm increase) | 1.07 | 1.01, 1.14 | 0.021 |
Abbreviations: HR=hazard ratio, CI=confidence interval
The overall survival for the entire cohort of 219 men was 97% (95% CI 93%–99%) at 5 years and 77% (95% CI 48%–92%) at 10 years after diagnosis. No men developed distant metastases and no men died of prostate cancer during the follow-up. Two men had lymph node metastasis during follow-up. The first patient had multiple comorbidities (chronic obstructive pulmonary disease, coronary artery disease, aortic aneurysm, and gastric cancer). He was followed on AS and developed lymph node metastasis but later died of cardiac disease. The second patient was on AS for 4 months before receiving focal therapy. He had 2 positive biopsies and had positive lymph nodes at radical prostatectomy 1.5 years later. He was further managed with hormonal therapy and is still alive today.
DISCUSSION
With the growing acceptance of AS, long-term studies are essential to reassure patients and physicians of the safety and efficacy of the program. Patient preferences for curative therapy vis-a-vis side effects from treatment must be considered when making individualized treatment decisions for the select group of men with low-intermediate-risk prostate cancer.
Here, we report one of the world’s largest cohorts of patients monitored by AS, which includes 219 men with intermediate-risk prostate cancer. Similar to prior reports,8, 10 and as hypothesized, we have demonstrated that carefully selected men with intermediate-risk features had a higher likelihood of receiving treatment than published estimates from cohorts of only Grade Group 1 patients. However, the chance of cure was not compromised, and only 2 men developed lymph node metastasis during follow-up, confirming the short-term safety of AS in carefully selected and monitored patients.
Current data suggest that men with Grade Group 2 (Gleason Score 3+4) prostate cancer may be candidates for initial management with AS if they have low-volume disease (fewer than 3 positive cores, <5% Gleason pattern 4); low PSA density (PSA/volume); favorable histology (no cribriform pattern); favorable genomic testing; and favorable mpMRI findings.8, 11–14 More research is needed to identify the optimal selection criteria, monitoring methods, and triggers for intervention for men with intermediate-risk prostate cancer on AS. Risk profiling may be improved by combining clinical risk assessment with novel biomarkers and advanced imaging technology. This includes mpMRI, an important component of an AS program that is used both to confirm eligibility and during surveillance; future work is necessary to determine the most efficient use.15 We used changes in volume of Grade Group 2 cores, upgrade to Grade 3 or higher and/or changes in MRI such as extraprostatic extension to trigger treatment. It is clear that the lack of predefined criteria for intervention explain the high rate of discontinuation of AS on the basis of anxiety (30%) in our cohort.
While most men with carefully selected Grade Group 2 prostate cancer do well on AS, men with intermediate-risk features considering AS as a primary option should be informed of the increased oncologic risk over time. In the Toronto cohort, men with intermediate-risk prostate cancer had a 2.7-fold higher risk for metastasis than men with low risk, despite selective delayed intervention. Given the increased risk, careful patient selection and careful surveillance for early signs of progression are necessary.16, 17 Gleason Score 7, PSA doubling time ≤ 3 years, and 3 or more positive biopsy cores were associated with development of metastases in the Toronto cohort. Among the Toronto patients with Gleason Score 3+4 who developed metastasis, all had ≥ 5% Gleason pattern 4 (in whom this information was available). In contrast, having a PSA level over 10 ng/mL and Gleason Score 6 in the Toronto cohort was not associated with increased risk for metastatic disease.16
A recent 2017 review identified 5 published AS series that have reported outcomes for men with intermediate-risk features (with varying definitions): Toronto (209 men), University of California San Francisco (90 men), Prostate Cancer Active Surveillance Study (115 men), European Randomized Study of Screening for Prostate Cancer (50 men), and Royal Marsden Hospital in Denmark (128 men).10 Despite using varying definitions of intermediate-risk disease, all studies found that men with Grade Group 2 prostate cancer had higher rates of clinical progression and were more likely to undergo treatment over time than men at lower risk. In contrast, a recent report on the AS experience at the Cleveland Clinic found that the 10-year metastasis-free survival was 97.4% for 514 men with very low/low-risk prostate cancer and 99.0% for 117 men with intermediate/high-risk with Grade Group 2 disease. There were no deaths from prostate cancer during follow-up.18
A limitation of the present study is that we could not calculate the total millimeters or percent of Gleason pattern 3 or 4 on diagnostic biopsy. We started reporting these calculations on all Grade Group 2 biopsies reviewed by our pathology department in 2015, so this data was only available for a small percentage of our cohort. As our cohort matures and we continue to monitor more patients with intermediate-risk prostate cancer, we will record these parameters and report on their significance. We have recently shown that quantitation of Gleason pattern 4 conveys a net benefit to clinical decision making beyond standard clinic-pathologic variables in a cohort of Grade Group 2 patients who went to radical prostatectomy.19 Additional limitations include the retrospective study design with evolution in the AS protocol over time, short median follow-up for the endpoints of metastasis and death from prostate cancer and lack of recording of reasons for patient preference to undergo treatment. Definitive conclusions will need to await further follow-up.
CONCLUSIONS
AS appears to be a safe initial management strategy at a tertiary cancer center for carefully selected men with Grade Group 2 prostate cancer who are monitored closely according to a well-defined AS program. Men with Grade Group 2 considering AS should be informed about the relative lack of evidence on long-term oncologic outcomes with this approach. Future studies that elucidate which patients with intermediate-risk prostate cancer have better long-term outcomes on AS are needed.
Acknowledgements:
This work was supported in part by funds from the Sidney Kimmel Center for Prostate and Urologic Cancers, a Specialized Programs of Research Excellence grant (P50 CA92629) from the National Cancer Institute, a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center and the David H. Koch prostate cancer research fund. Sigrid Carlsson is supported by a National Institutes of Health/National Cancer Institute Transition Career Development Award (K22-CA234400). The funding sources had no role in the design and execution of the current study, nor in the analysis, interpretation of the data or manuscript writing.
Conflict of interest: Sigrid Carlsson has received a lecture honorarium and travel support from Astellas Pharma (unrelated to current study). Andrew Vickers is named on a patent for a statistical method to detect prostate cancer that has been commercialized by OPKO Health. Andrew Vickers receives royalties from sales of the test and has stock options in OPKO Health.
Key abbreviations
- ADT
Androgen Deprivation Therapy
- AS
Active Surveillance
- CI
Confidence Interva
- DRE
Digital Rectal Examination
- MRI
Magnetic Resonance Imaging
- PSA
Prostate-Specific Antigen
- RP
Radical Prostatectomy
- RT
Radiotherapy
REFERENCES
- 1.Choo R, Klotz L, Danjoux C et al. : Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol, 167: 1664, 2002 [PubMed] [Google Scholar]
- 2.Tosoian JJ, Mamawala M, Epstein JI et al. : Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol, 33: 3379, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotz L, Vesprini D, Sethukavalan P et al. : Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol, 33: 272, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Klotz L: Active surveillance for low-risk prostate cancer. Curr Opin Urol, 27: 225, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Bul M, Zhu X, Valdagni R et al. : Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol, 63: 597, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Tosoian JJ, Carter HB, Lepor A et al. : Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol, 13: 205, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinsella N, Helleman J, Bruinsma S et al. : Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Androl Urol, 7: 83, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klotz L: Active Surveillance for Intermediate Risk Prostate Cancer. Curr Urol Rep, 18: 80, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Epstein JI, Zelefsky MJ, Sjoberg DD et al. : A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol, 69: 428, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dall’Era MA, Klotz L: Active surveillance for intermediate-risk prostate cancer. Prostate Cancer Prostatic Dis, 20: 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welty CJ, Cowan JE, Nguyen H et al. : Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol, 193: 807, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Kweldam CF, Wildhagen MF, Steyerberg EW et al. : Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol, 28: 457, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Truong M, Frye T, Messing E et al. : Historical and contemporary perspectives on cribriform morphology in prostate cancer. Nat Rev Urol, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Gondo T, Hricak H, Sala E et al. : Multiparametric 3T MRI for the prediction of pathological downgrading after radical prostatectomy in patients with biopsy-proven Gleason score 3 + 4 prostate cancer. Eur Radiol, 24: 3161, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Giganti F, Moore CM: Magnetic resonance imaging in active surveillance-a modern approach. Transl Androl Urol, 7: 116, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto T, Musunuru B, Vesprini D et al. : Metastatic Prostate Cancer in Men Initially Treated with Active Surveillance. J Urol, 195: 1409, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Musunuru HB, Yamamoto T, Klotz L et al. : Active Surveillance for Intermediate Risk Prostate Cancer: Survival Outcomes in the Sunnybrook Experience. J Urol, 196: 1651, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Nyame YA, Almassi N, Haywood SC et al. : Intermediate-Term Outcomes for Men with Very Low/Low and Intermediate/High Risk Prostate Cancer Managed by Active Surveillance. J Urol, 198: 591, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Dean LW, Assel M, Sjoberg DD et al. : Clinical Usefulness of Total Length of Gleason Pattern 4 on Biopsy in Men with Grade Group 2 Prostate Cancer. J Urol, 201: 77, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]