Abstract
Obesity is a problem in captive chimpanzee colonies that can lead to increased risk for disease; therefore, implementation of effective weight management strategies is imperative. To properly implement a weight management program, captive managers should be able to noninvasively identify and assess overweight or obese individuals. Traditional means of categorizing obese individuals involve sedating the animals to obtain body weights or skin fold measurements. The current study aimed to validate a noninvasive, subjective body condition score (BCS) system for captive chimpanzees. The system utilizes a ten-point scale, with one rated as ‘emaciated’, five as ‘normal’, and 10 as ‘extremely obese’. Between 2013 and 2014, 158 chimpanzees were weighed and scored using this system 1) while sedated and 2) while awake in their social group within 1–3 days of sedation (“In-group” ratings). We found high inter-rater reliability between In-group raters, as well as between sedated and In-group scores. BCSs, which require observation only, were significantly positively correlated with weight (an objective measure of obesity often requiring anesthetization), supporting the scale’s validity. The BCS system identified 36 individuals as “overweight”, while the use of weights alone identified only 26 individuals as “overweight”. Furthermore, the BCS system was able to classify individuals of the same sex and weight as having different BCSs, ranging from normal to overweight. Lastly, using focal animal behavioral observations from 2016–2018 (N=120), we found that In-group BCS predicted individual levels of inactive behavior more than two years later, demonstrating the predictive validity of the scale. These results illustrate the utility of the BCS system as a noninvasive, reliable, and valid technique that may be more sensitive than traditional methods in identifying and quantifying obesity in chimpanzees. This system can be a useful tool for captive managers to monitor and manage the weight of chimpanzees and other nonhuman primates.
Keywords: Body Condition Scoring, Chimpanzee, Behavioral Management, Obesity, Welfare
Obesity is a frequent problem when managing captive chimpanzees (Lambeth et al., 2011; Reamer et al., 2014; Videan et al., 2007). In humans, obesity is a precursor to a variety of co-morbidities, including cardiovascular disease, hypertension, renal disease, and diabetes (Must et al., 1999). In chimpanzees, the connection between obesity and disease is not formally established. However, chimpanzees are afflicted with similar chronic co-morbidities (Denton et al., 1995; Eichberg & Shade, 1987; Hubbard et al., 1991; Nunamaker, Lee, & Lammey, 2012; Obanda et al., 2014; Reamer et al., 2014) and female chimpanzee blood pressure can increase with obesity (Ely et al., 2013). Furthermore, similar relationships have been observed among other primate species (cynomolgus macaques: Young et al., 2003; orangutans: Weisenberg et al., 1991). Therefore, as part of an overall wellness program for chimpanzees, it is imperative to develop weight management strategies that can accurately, frequently, and noninvasively identify and quantify weight changes for both overweight and underweight individuals.
Traditionally, body weight has been used to evaluate obesity in chimpanzees (Brent, 1995; Ely et al., 2013; Videan et al., 2007). However, body weight alone does not take into account overall body frame, body composition (e.g., muscle mass vs fat mass), or tumescent swellings in female chimpanzees. To correct for body size in humans, height is often used in conjunction with weight to calculate a body mass index (BMI) (Garrow & Webster, 1985). However, BMI as an indicator of obesity has several disadvantages. First, BMI does not account for overall body composition (particularly percentage of body fat) and lacks both sensitivity and specificity, as it fails to identify sex differences (Rothman, 2008) and changes due to aging (when muscle mass decreases). Second, the use of BMI to categorize obesity in humans can result in misclassification and subsequent bias in the estimation of obesity rates (Rothman, 2008). Finally, and perhaps most importantly, BMI has been shown to be a poor indicator of health/obesity in humans who have high muscle mass, such as body builders (Goh et al., 2004; Rothman, 2008). Among chimpanzees, particularly males, muscle mass is quite similar to human bodybuilders (Bauman, 1923). Combined, these factors suggest that BMI is unlikely to be a particularly useful indicator of health and body condition in chimpanzees (Obanda et al., 2014).
Magnetic resonance imaging (MRI) or dual-energy X-ray absorptiometry (DEXA) are the most accurate tools to measure overall body condition (Videan et al., 2007). However, these methods are expensive and labor-intensive. Furthermore, these techniques require that chimpanzees be anesthetized, which presents inherent risks (heightened by obesity) and is not practical for frequent assessments and monitoring of body condition. Another suggested alternative is the combination of weight, skinfold measurements, and/or crown-rump lengths, but, these measurements also require sedation of the chimpanzee (Videan et al., 2007). While the combination of these techniques may provide data that are superior to BMI, a need still exists for practical measurement techniques that noninvasively evaluate chimpanzee body condition, allowing frequent monitoring to assess the effectiveness of weight management strategies (Bridges et al., 2013; Lambeth et al., 2011).
The application of a body condition score (BCS) system has been used and validated to evaluate health and management programs in companion animals (cats: Kronfeld et al., 1994; Laflamme, 1997a; dogs: Donoghue et al., 1991, Laflamme, 1997b; cats/dogs: German et al., 2006), livestock (cattle: Domecq et al., 1995; Edmonson et al., 1989; Mathews et al., 2012; sheep: Russel, 1984; horses: Carroll & Huntington, 1988), and rodents (mice: Ullman-Cullere & Foltz 1999; rats: Hickman & Swan, 2010). Body condition scoring has also been validated for rhesus macaques in free-ranging (Berman & Schwartz, 1988), and research settings (Clingerman & Summers, 2012; Summers et al., 2012). Indeed, BCS systems used for rhesus macaques have been shown to be consistent with objective meaures of body condition, including weight and percentage of body fat using DEXA (mentioned above), and are reliable within and across raters (Berman & Schwartz, 1988; Clingerman & Summers, 2012; Summers et al., 2012). An obesity classification system that determines ‘overweight’ individuals using only body weight involves taking the mean weight of the population and classifying ‘overweight’ individuals as those who are systematically above the population’s mean body weight (e.g., a certain percentage or number of standard deviations above the mean). This is the major disadvantage of such systems: in an obese population, the mean body weight is elevated, thereby promoting misclassification of individuals (i.e., classifying an individual as not obese when they actually are obese). With a BCS system, this potential problem is eliminated, since each subject is assessed on standardized criteria that do not change based on the characteristics of the sample population.
Despite the widespread health consequences of obesity in chimpanzees, the behavioral consequences of obesity have remained unexplored. In humans, obesity is associated with anxiety, emotional dysregulation, sleep pattern disturbances, and exercise intolerance (Baker et al., 2017; Lewis et al., 2019; Simon et al., 2019). Obesity affects behavior, but behavior also affects obesity; that is, there is a circular pattern of causality that only compounds the obesity issue. However, the relationships between weight and/or body condition and activity in chimpanzees has not been explicitly demonstrated. If there is such a relationship, captive chimpanzee weight management programs should include both nutritional and behavioral interventions (i.e., exercise regimens).
It is important to note that obesity is not the only weight issue that can present in chimpanzees (although it is certainly the more common one). Geriatric (35 years and older, Neal Webb et al., 2019) and ill chimpanzees may experience weight loss due to chronic conditions, infection, severe stress, or simply as a function of aging (Davenport et al., 1996; Goodall, 1986; Terrio et al., 2011; Williams et al., 2008). This can be difficult to identify over extended periods, as caregivers and veterinarians likely see the individual often, making subtle changes difficult to distinguish. Implementing a BCS system would create a record for each individual that can be used 1) to monitor individual body condition over time, 2) in quality of life programs (Lambeth et al., 2013), and 3) to identify a change in weight early on, before it becomes a health issue.
The importance of weight management within captive chimpanzee colonies necessitates a noninvasive assessment tool that can be used to frequently assess obesity, as well as weight loss, in ill or geriatric chimpanzees. Our aim was to develop a reliable and valid species-relevant BCS system that could be used in the absence of sedation, while individuals are within their social groups. Therefore, we created an In-group BCS system for captive chimpanzees using a previously-developed BCS system for rhesus macaques (Clingerman & Summers, 2012; Summers et al., 2012). We collected and compared BCSs while awake in the social group (In-group BCS), and under sedation (Sedated BCS, which has been used previously by others). The goal was to examine the reliability and validity of the In-group BCS for easy, noninvasive assessment of body condition. We expected that In-group BCS would be positively correlated with more traditional methods of assessing obesity (body weight and Sedated BCS), as well as with behavior, including inactivity and locomotion. We also hypothesized that higher BCSs would be associated with chronic health conditions related to obesity, including hypertension, cardiac disease, arthritis, and diabetes. Lastly, we expected that Sedated and In-group BCS would meet established criteria characteristic of a population in which there is an increasing number of obese individuals (i.e., a positively skewed weight distribution). Specifically, we expected a successful (reliable and valid) BCS parameter to show:
A positively-skewed distribution of weight, and thus, of Sedated and In-group BCSs; Penman and Johnson (2006) found that when obesity is increasing in human populations, the weight distribution becomes more positively skewed. In the current population there are underweight, normal, overweight, and obese individuals, but we expected to see higher frequencies of heavier individuals compared to normal weight individuals since the current population has a known obesity issue, resulting in a positive skew.
High inter-observer reliability of scores between the In-group BCS raters.
High inter-observer reliability of scores between the In-group BCS raters and Sedated BCS rater. This would demonstrate reliability and validity via comparisons of a more traditional assessment of obesity (under sedation) to the less invasive alternative (body condition scoring within the social group).
A significant positive correlation between Sedated and In-group BCSs and body weight (the more traditional, objective measure of obesity).
Sex differences in BCSs. Chimpanzees are sexually dimorphic (Leigh & Shea, 1995) and most previous captive studies have shown significant variation between male and female weights, with females typically being overweight more often than males (Brent, 1995; Lambeth et al., 2011; Videan et al., 2007).
Method
Subjects were 158 (62M, 96F) chimpanzees housed at the National Center for Chimpanzee Care, Michale E. Keeling Center for Comparative Medicine and Research (KCCMR), of The University of Texas MD Anderson Cancer Center in Bastrop, TX. The KCCMR has been continuously accredited by AAALAC International since 1979. Chimpanzees were housed in Primadomes™ or corrals in social groups of between 2 and 10 individuals. BCS data were collected between January, 2013 and January, 2014. Animal ages ranged from 12–52 years at the time of data collection.
Body Condition Score (BCS)
To create the BCS system, our team (including a veterinarian, behavioral researcher, veterinary technician, and trainer) adapted a previously established BCS system for rhesus macaques (Clingerman & Summers, 2012; Summers et al., 2012) to accommodate chimpanzee morphology. The resulting system is shown in Figure 1, in which 1 represents “Emaciated”, 5 represents “Normal”, and 10 represents “Extremely Obese”. We obtained two types of scores using this system: a Sedated BCS and an In-group BCS. Each chimpanzee at the KCCMR is sedated annually for a physical examination as part of our health surveillance program. To obtain Sedated BCS, one rater (RJ) recorded body weight (kg) and also scored the body condition of the sedated chimpanzee in a prone position using observation of the right lateral top view (shown in Figure 1) during each physical examination (N=158). To obtain In-group BCS, a second rater (either ET or RH) rated the subject while the individual was awake in its social group within 1–3 days following sedation, using the ambulating view of the BCS chart described in Figure 1 (N=132). There were fewer In-group than Sedated BCSs due to issues with rater availability (e.g., the rater was unavailable to obtain an In-group BCS within 1–3 days following sedation) and individual animals with special restrictions pertaining to disease status (i.e., an animal received a Sedated BCS, but did not receive an In-group BCS due to observation restrictions). All BCSs were obtained using observation only, and ratings required less than five minutes per chimpanzee.
Figure 1.
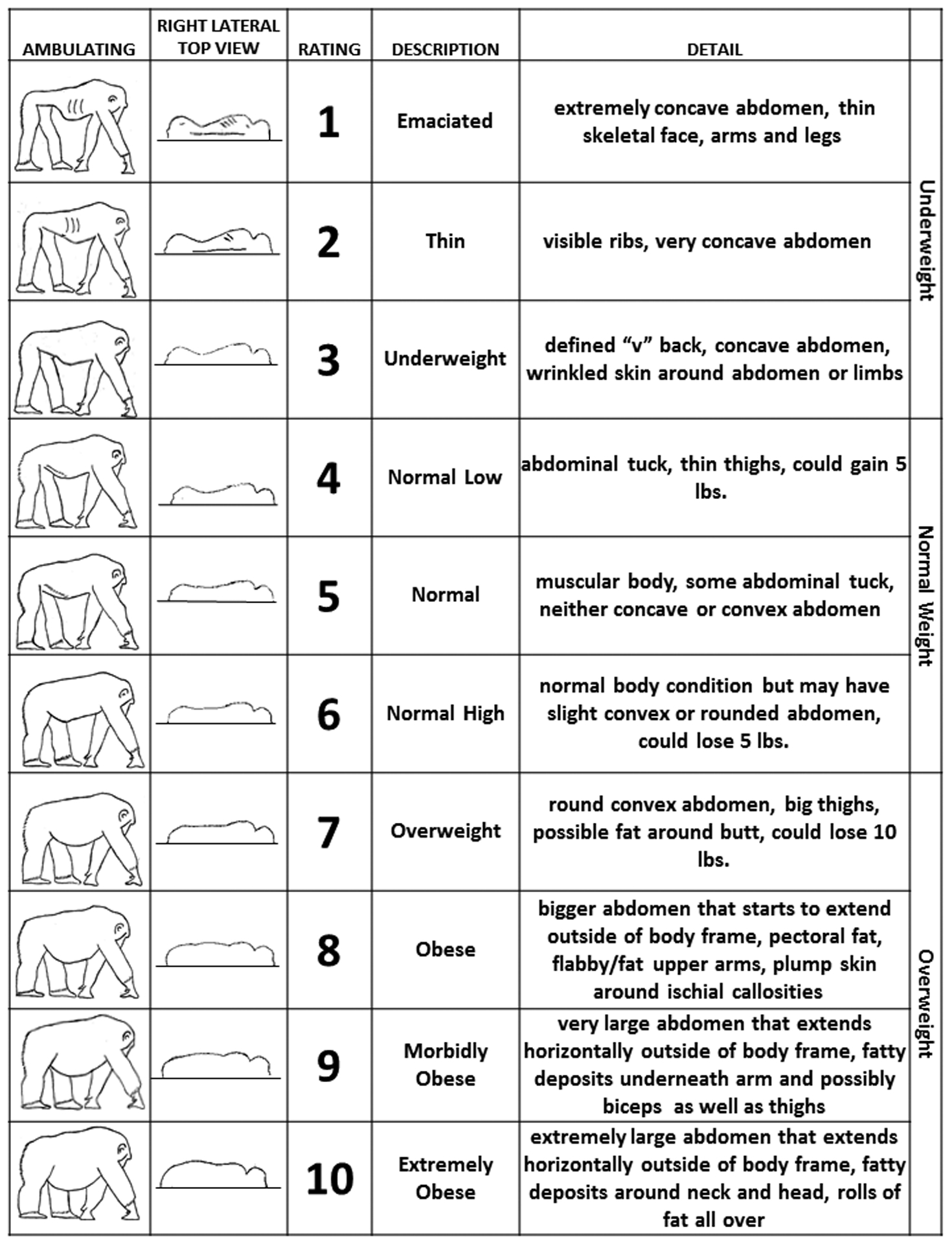
Chimpanzee Body Condition Score (BCS) chart used for Sedated (right lateral top view) and In-group (ambulating) BCS.
Obesity-related chronic health conditions
To examine whether higher BCSs were associated with higher frequency of obesity-related health conditions, we used archival data to determine whether each chimpanzee had been diagnosed with hypertension, diabetes, cardiac disease, and/or arthritis by 2014. We chose these particular conditions due to their relationship to obesity in chimpanzees and humans, and because of their prevalence in chimpanzees (Ely et al., 2013; Nunamaker et al., 2012; Van Raemdonck et al., 2018; Varki et al., 2009). Each chimpanzee was given a score of 0 (no disease present) or 1 (disease present), based on diagnosis by the veterinarian.
Behavior
Behavioral observations were collected between July 2016 and May 2018 (Neal Webb et al., 2018, 2019) for 120 of the 158 chimpanzees described above. Observations consisted of 15-minute, continuous focal-animal sampling. Each chimpanzee served as a focal subject in a minimum of 22 observations, although some chimpanzees were observed up to 31 times [see Neal Webb et al. (2018) for the 51-behavior ethogram used]. For the current study, only locomotive and inactive behaviors were used to examine predictive validity of the BCS system.
All research and protocols complied with the approved protocols of the UTMDACC Institutional Animal Care and Use Committee, and complied with the legal requirements of the United States and the ethical guidelines put forth by AALAS, the Animal Welfare Act, The Guide for the Care and Use of Laboratory Animals, and the ASP Principles for Ethical Treatment of Non-human Primates.
Analyses
We first examined descriptive statistics of BCSs and body weight, including mean, standard deviation (SD), and range of these measurements within our sample (N = 158). We then examined the skew of body weight, and both Sedated and In-group BCS distributions, using the Shapiro-Wilk W statistic.
To assess In-group rater consistency, we used Cohen’s Kappa to compare BCS from the two In-group BCS raters (Raters ET and RH) who scored the body condition of 59 individuals prior to data collection. To assess reliability between Sedated and In-group BCSs, Sedated BCSs (rated by RJ) were compared to In-group BCSs rated by ET (N=68) and In-group BCSs rated by RH (N=64) in two separate Cohen’s Kappa analyses.
We then examined the relationship between body weight and Sedated BCS (N = 158) and In-group BCS (N = 132) using a Spearman’s Rho correlation. To explore sex differences in body weight and BCS, we used an independent samples t-test or Mann-Whitney U test, as appropriate, based on the skew of the distributions. We report corrected t and df where Levene’s test for equality of variances is violated. To examine potential differences in In-group BCSs as a function of the presence or absence of an obesity-related health condition, we used a univariate ANCOVA with sex and disease status (obesity-related health condition present or absent) as the independent factors and age as a covariate (N = 132). This analysis was also repeated with Sedated BCS (N = 158) and weight (N = 158) as the dependent variables. Lastly, we examined the predictive validity of In-group and Sedated BCS on later inactive and locomotive behavior using linear regressions, while controlling for age and sex (N = 120 and N = 112, respectively, as there were subjects that had behavioral data from 2016–2018, but never received a Sedated BCS). We also used linear regressions to examine the relationship between inactive and locomotive behavior and Sedated BCS scores taken between 2016 and 2018 (that is, the BCSs taken during the time of behavioral observations, N=120). We did not have access to In-group BCSs taken between 2016 and 2018, and therefore, could not perform analyses with concurrent In-group BCSs. Alpha levels were set at p < 0.05 and all analyses were performed using SPSS 24 (IBM Corporation, Chicago, IL, USA). Data used for the current study are available from the corresponding author upon reasonable request.
Results
The mean weights and BCSs can be found in Table 1. Figure 2 shows the ranges of weight for each BCS in the sample. The distribution of body weight in the sample was normal (W=.992, df=158, p=0.572), whereas the distributions of Sedated and In-group BCSs were not (sedated: W=.83, df=158, p<0.001; In-group: W=.77, df=158, p<0.001; Figure 3). Therefore, all subsequent analyses for BCS were nonparametric.
Table 1.
Male and female body weight and BCS descriptive statistics.
| Range | Mean | SD | Overweight | Obese | |
|---|---|---|---|---|---|
| 1SD | 2SD | ||||
| Male | 48–88 | 62.51 | 8.11 | 70.6+ | 78.7+ |
| Female | 39–81 | 59.62 | 9.58 | 69.2+ | 78.8+ |
| Sedated BCS | |||||
| Male | 4–8 | 5.15 | 0.66 | 7+ | 8+ |
| Female | 4–9 | 6.12 | 1.19 | 7+ | 8+ |
| In-Group BCS | |||||
| Male | 5–7 | 5.34 | 0.65 | 7+ | 8+ |
| Female | 5–9 | 6.22 | 1.15 | 7+ | 8+ |
Figure 2.
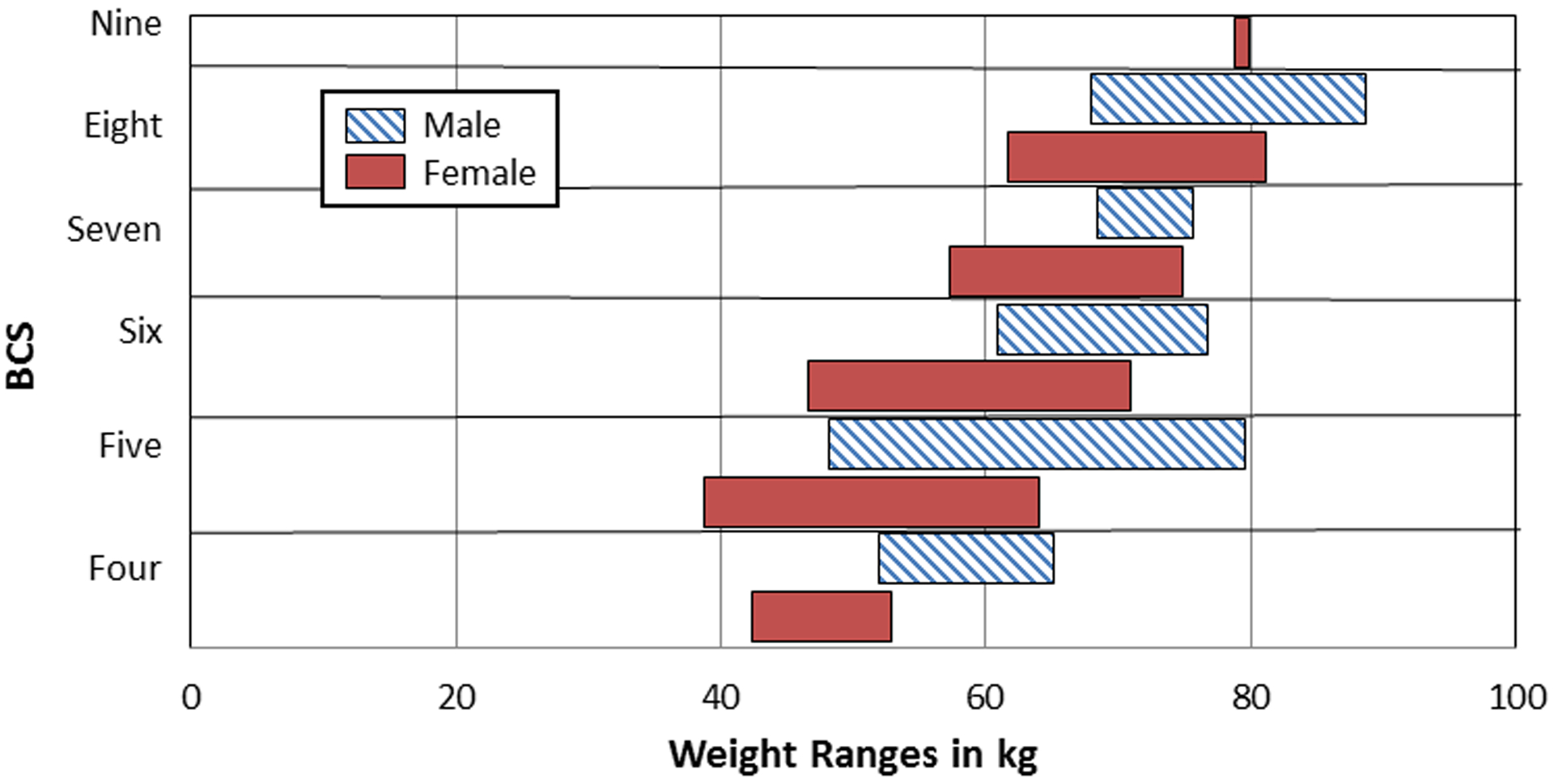
Body weight ranges according to Sedated Body Condition Score (BCS) of males and females.
Figure 3.
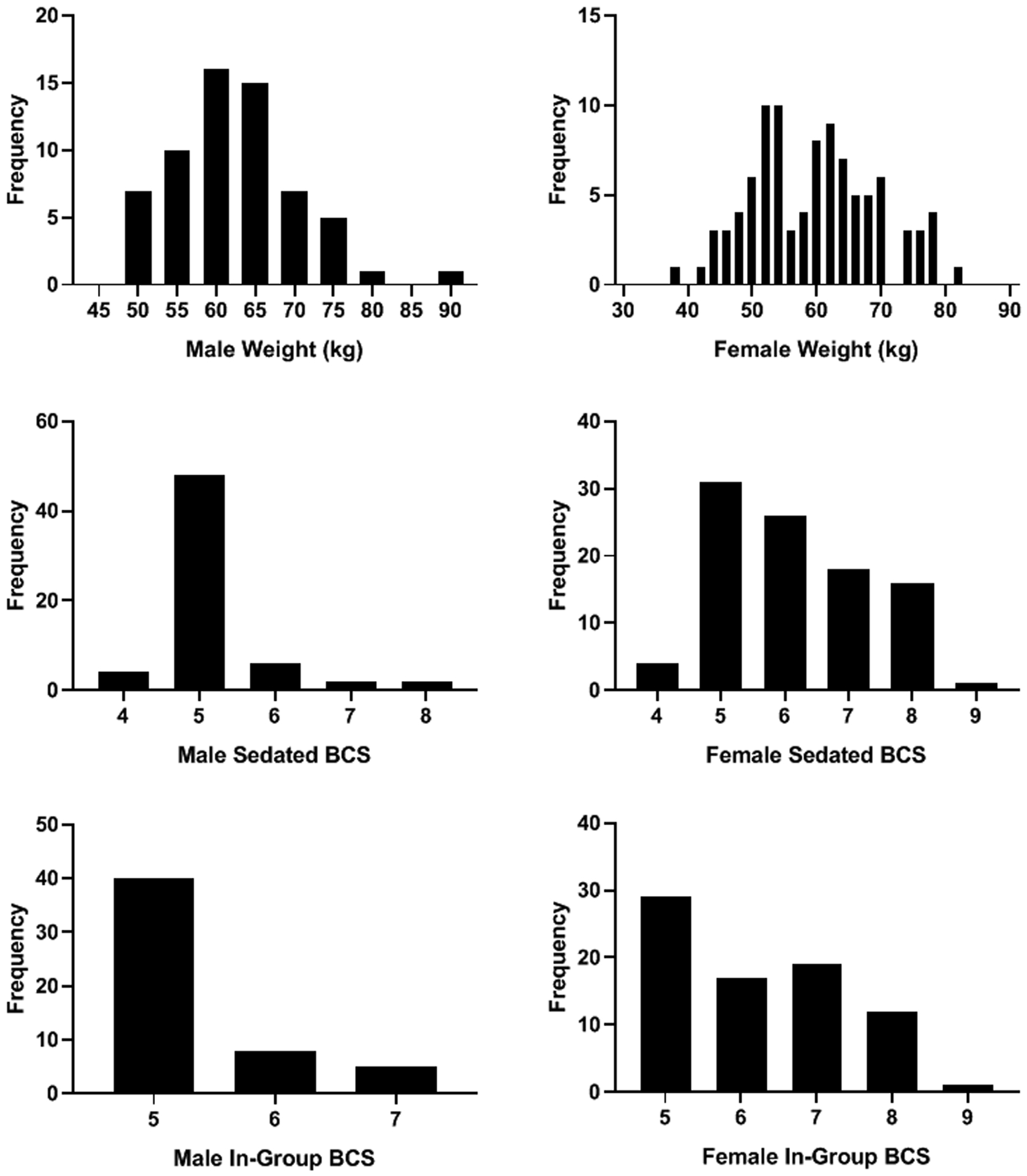
Frequency distributions of weight, Sedated, and In-group BCS for males and females.
Scores collected by the two In-group BCS raters prior to data collection were significantly positively correlated (Cohen’s Kappa =.860, N=59, p<0.001). Furthermore, there was significant agreement between Sedated BCSs and In-group BCSs by rater ET (Cohen’s Kappa =.455, N=68, p<0.001) and rater RH (Cohen’s Kappa=.662, N=64, p<0.001).
There were significant positive correlations between body weight and Sedated BCS (Rho=.54, N=158, p<0.001; Figure 4 top panel) and between body weight and In-group BCS (Rater ET: Rho=.62, N=68, p<0.001; Rater RH: Rho=.54, N=64, p<0.001; all In-group BCS: Rho=.58, N=132, p<0.0001); Figure 4 bottom panel). In order to examine sex differences in body weight, we used an independent samples t-test, which showed a significant difference in body weight between males (M = 62.51 kg, SE = 1.03) and females (M = 59.63 kg, SE = 0.98), t=2.03, df=144.94, p=0.04. There was also a significant difference between males and females in Sedated BCS (U=1546, N=158, p<0.001, male M = 5.19, SE = 0.09; female M = 6.15, SE = 0.12) and In-group BCS (U=1157, N=132, p<0.001, male M = 5.33, SE = 0.09; female M = 6.22, SE = 0.13), such that female BCSs were higher than male BCSs (Figure 4).
Figure 4.
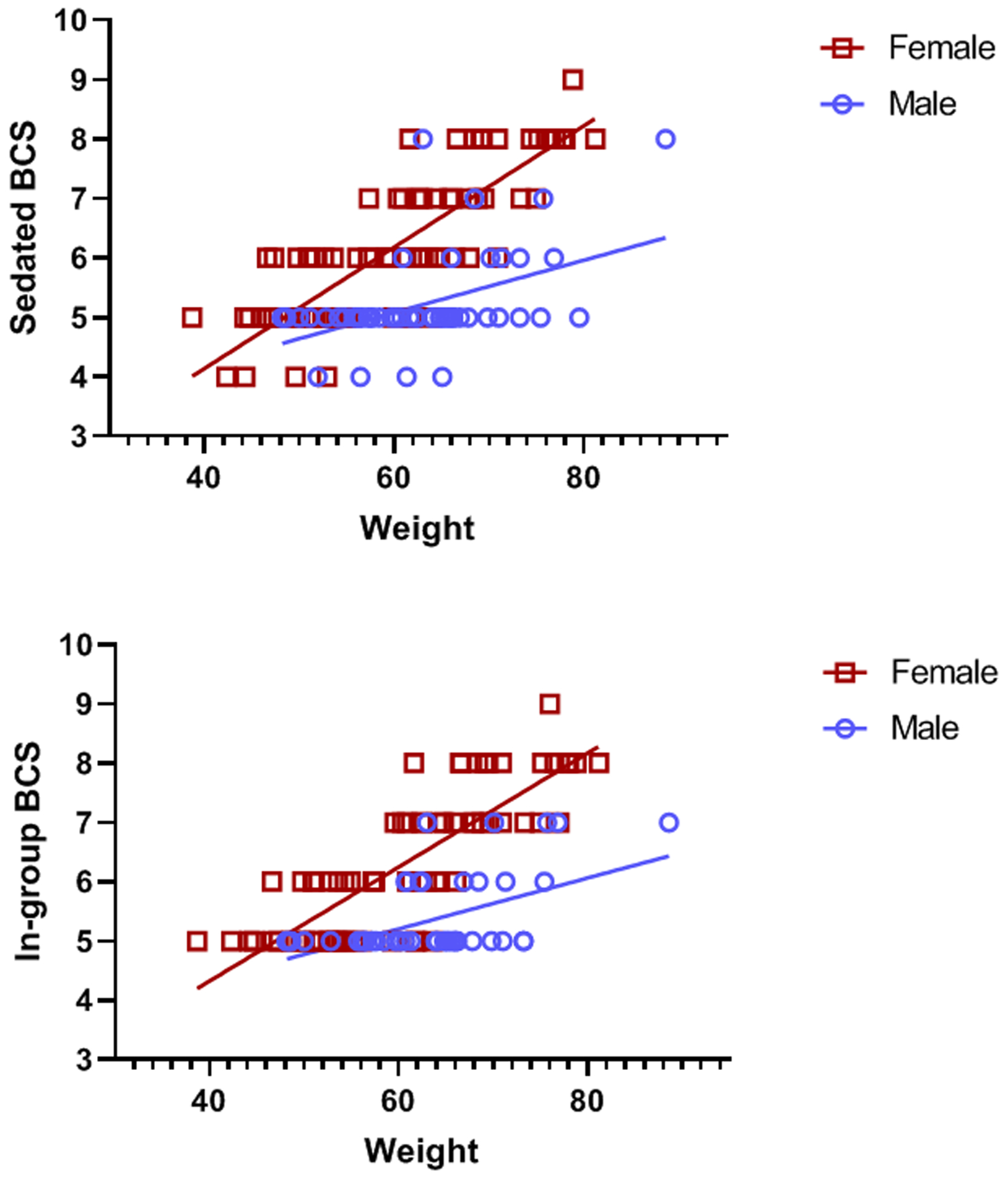
Relationship between male and female weight (kilograms) and Sedated BCS from 2013–2014 (top panel) and In-group BCS from 2013–2014 (bottom panel).
The ANCOVAs (controlling for age) showed that chimpanzees with an obesity-related health condition had significantly higher In-group BCSs [M = 6.20 ± SE = 0.21; F = 5.87, df = 1,126, p = 0.02] than those without such conditions (M = 5.62 ± SE = 0.09). Additionally, Figure 5 shows an uneven distribution of individuals with health conditions across BCSs: approximately 66% of chimpanzees with a score of 8 (obese) had a health condition, whereas only 7.2% of those with a BCS of 5 (normal) were diagnosed with a health condition. There were no differences in Sedated BCSs or weight as a function of disease presence (p >0.10).
Figure 5.
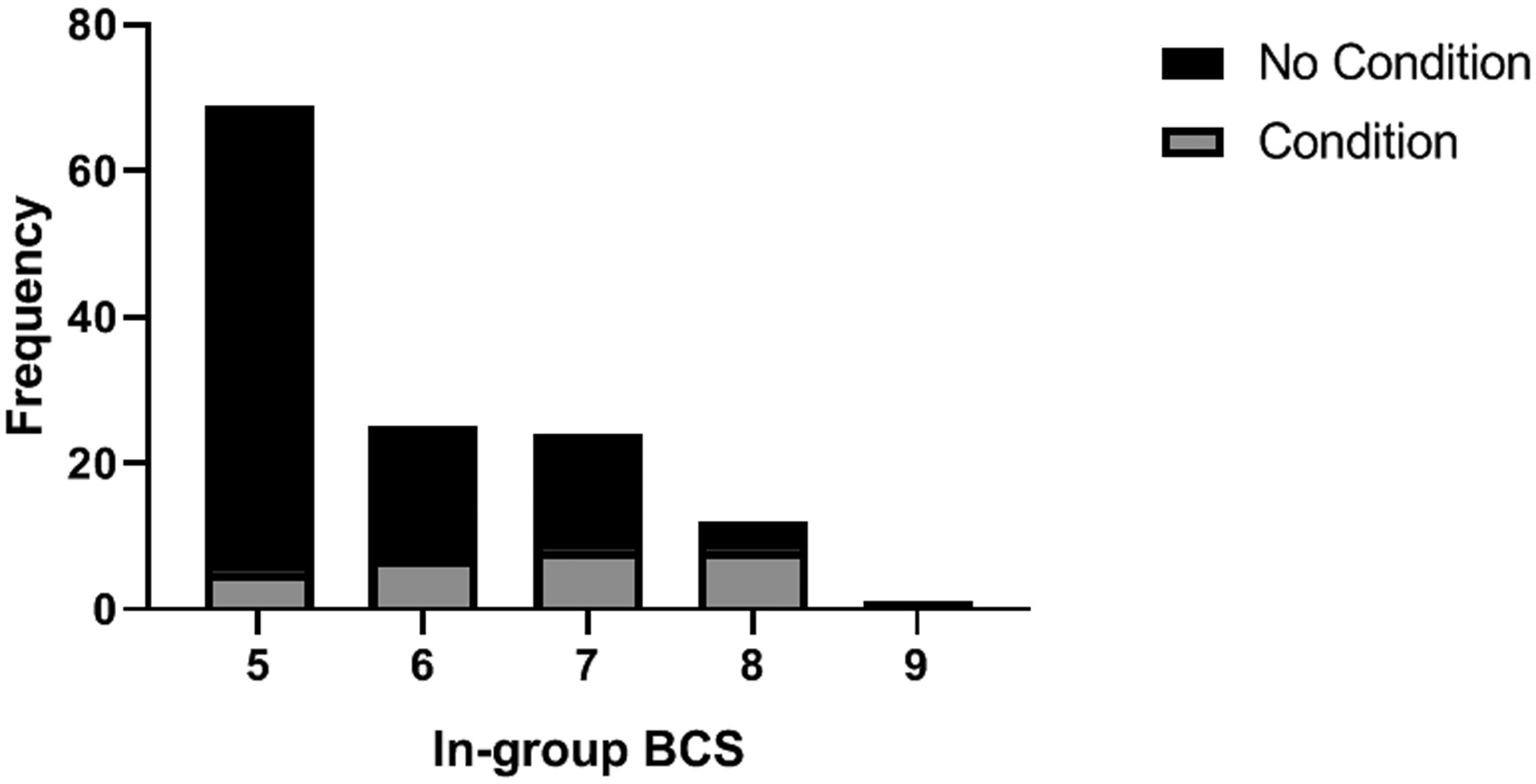
The number of chimpanzees with and without an obesity-related health condition in each BCS category.
Lastly, the linear regressions showed that 2013–2014 In-group and Sedated BCSs were not related to later levels of locomotion (2016–2018), p > 0.10. However, 2013–2014 In-group BCS was a significant predictor of later levels of inactivity (2016–2018), F=7.75, df = 3,116, p=0.0001, R2Adj=0.15. Specifically, with every one unit increase in BCS, there was a 2.83% increase in later inactivity (p=0.003; Table 2). However, this was sex-specific, as this increase occurred only in females (Figure 6 top panel). In-group BCS was also significantly associated with age and sex, such that higher BCSs were associated with older and female chimpanzees (Figure 6 middle panel). Almost identical results were found for the model predicting later inactivity with Sedated BCSs (2013–2014), F=7.68, df=3,109, p=0.0001, such that every one-unit increase in Sedated BCS resulted in a 2.66% increase in later inactivity (p=0.006). Additionally, the model predicting percentage of locomotion from concurrent Sedated BCS scores (collected between 2016 and 2018) was significant: F =2.59, df = 3,116, p = 0.05, R2Adj=0.04 (Table 2 and Figure 4 bottom panel). These Sedated BCSs were not related to concurrent levels of inactivity (p > 0.10).
Table 2:
Coefficients in the final model with In-Group BCS (2013–2014) predicting later levels of Inactivity, and Sedated BCS (2016–2017) predicting concurrent levels of locomotive behavior.
| Dependent Variable | Predictors | b | Beta | t | p |
|---|---|---|---|---|---|
| Inactivity (2016–2018) | Intercept | 12.133 | 1.873 | 0.064 | |
| Sex | 6.272 | 0.295 | 3.013 | 0.003 | |
| Age | 0.341 | 0.275 | 3.075 | 0.003 | |
| In-group BCS (2013–2014) | 2.390 | 0.248 | 2.493 | 0.014 | |
| Locomotion (2016–2018) | Intercept | 12.222 | 5.53 | 0.000 | |
| Sex | 0.099 | 0.014 | 0.138 | 0.891 | |
| Age | −0.062 | −0.146 | −1.612 | 0.110 | |
| Sedated BCS (2016–2017) | −0.524 | −0.190 | −1.918 | 0.058 |
Figure 6.
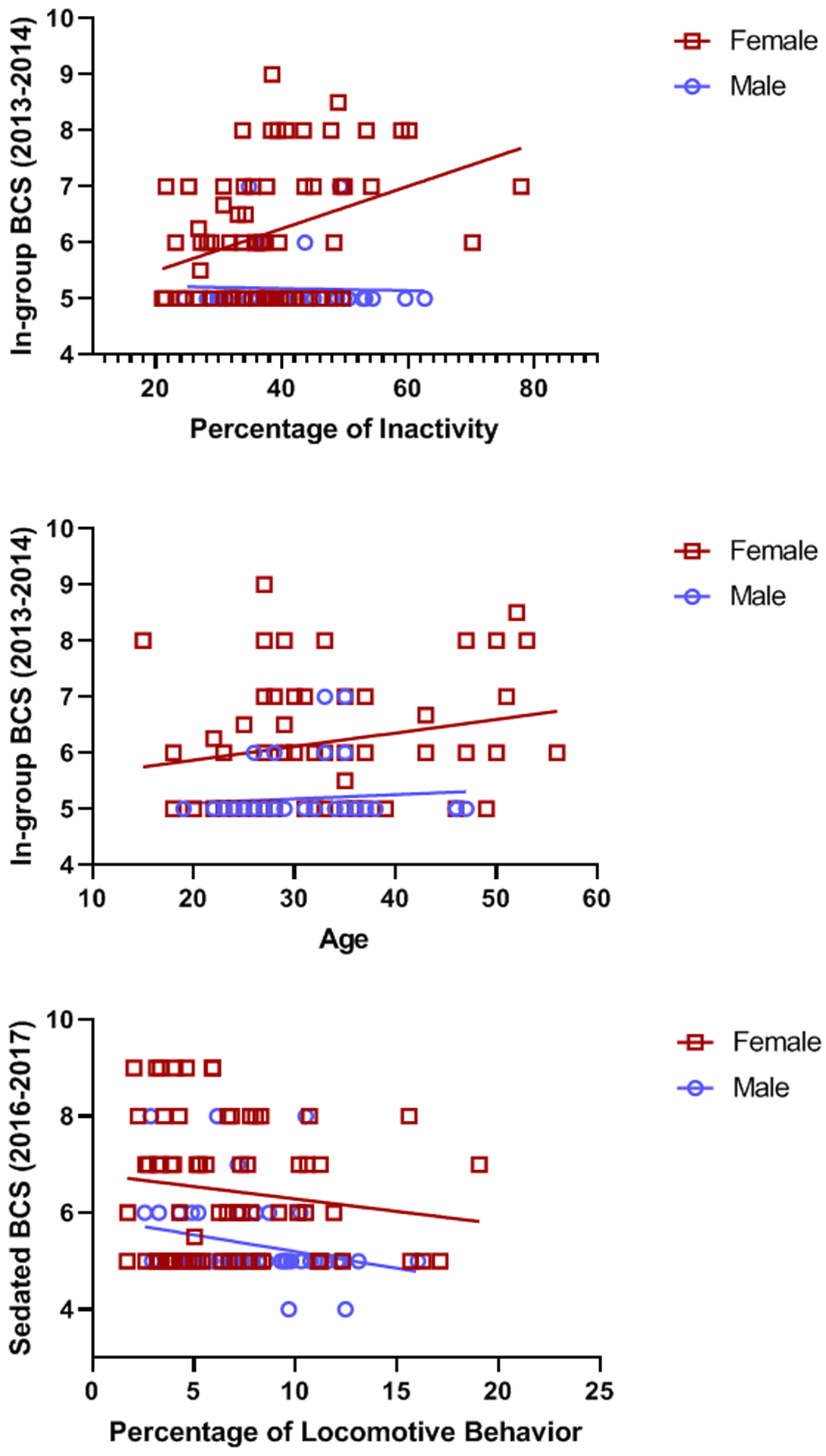
Results of the linear regressions showing that In-group BCS predicted inactivity in females, but not in males (top panel). Higher In-group BCSs are associated with older age in females, but not males (middle panel). Furthermore, Sedated BCSs collected in 2016–2017 were related to levels of locomotive behavior in both males and females (bottom panel).
Discussion
The new, noninvasive In-group BCS system proposed here was found to be both valid and reliable. First, significant inter-rater agreement between both raters demonstrated high reliability. Second, the In-group BCS system was correlated with both the Sedated BCS and the traditional criterion of body weight, demonstrating the validity of the In-group BCS. Third, we were able to demonstrate the predictive validity of the In-group BCS in terms of (later) inactivity, as well as the concurrent validity of Sedated BCS and locomotive behavior. Fourth, as predicted in an obese population (i.e., Penman & Johnson, 2006), In-group BCS was found to be significantly, positively skewed, and therefore, likely a better indicator than those related to measures of central tendency (mean/median). Lastly, two thirds of chimpanzees with a BCS of 8 or higher had an obesity-related health condition. This is perhaps not surprising given that other BCS systems have been validated in various species (Berman & Schwartz, 1988; Clingerman & Summers, 2012; Domecq et al., 1995; German et al., 2006). Most importantly, unlike many traditional measurements that are invasive and can increase health risks, the In-group BCS rating system is noninvasive, easy to use, and does not require sedation/anesthesia.
The distributions of the BCSs were positively skewed (hence an obese population, according to Penman & Johnson, 2006), whereas body weights were normally distributed. The fairly normal distribution of body weights found in the current study likely suggests that our population shows a distribution that is shifted entirely upward due to obesity. This makes it difficult to identify individuals that fall outside of the “normal” (most frequent) weight range. In contrast, individuals outside of the “normal” (healthy) range are immediately apparent in the BCS distribution. Knowing that obesity is an issue in the present population, this finding not only addresses validity, but also suggests that the BCS system is likely to be a more accurate measure of obesity than body weight alone.
Similarly, perhaps the most compelling argument for the use of a body condition scoring system is illustrated in Figure 2, where it is apparent that individuals with the same body weights can have different BCSs. For example, females weighing 60kg in the current study fall into three different body condition scoring categories: 5, 6 and 7. This demonstrates that the BCS system accounts for the animal’s overall body type, whereas body weight and BMI do not. For example, using the weights presented here and applying the broadest possible definition of overweight (one standard deviation above the mean), our sample included 26 individuals that were classified as ‘overweight’ (see Table 1 for specific weights). Yet, when employing the BCS system, our sample contained 36 individuals that were considered ‘overweight’ (scoring a 7 or higher). This shows that, in practice, if only body weights were used to categorize overweight individuals in this colony, 10 chimpanzees with weight issues might be misidentified as not needing weight management intervention.
To make this point further, if we assess the category of ‘obese’ (restricting the body weight criterion from one standard deviation above the mean to two standard deviations), the number of ‘obese’ individuals in our population decreases to just four chimpanzees. However, when categorizing obesity via BCS (scoring an 8 or higher), there are 17 obese individuals in the current population, suggesting that assessing obesity by simple body weight could leave 13 individuals without weight management-related intervention. This is particularly important given the finding that BCS predicts later inactivity. By failing to intervene with weight management strategies at early stages, captive managers may be propagating the circular obesity issue: overweight animals are less active, and lower activity leads to weight gain, which leads to less activity, and so on. Furthermore, although we are unable to determine the direction of causality, we found a disproportionate number of obesity-related conditions (including hypertension, diabetes, arthritis, and cardiac disease) in animals classified as “overweight” and “obese” (i.e., animals with scores of 7 or higher). Given that obesity is linked to several major health issues in chimpanzees, early identification of obesity using a more sensitive technique, such as the BCS system, is particularly important in captive chimpanzee management.
We found that higher In-group BCS were related to the presence of an obesity-related condition, as well as later inactivity, but Sedated BCS and weight showed no such relationship. Furthermore, Sedated BCSs were related to concurrent locomotion, whereas In-group BCS were not. This may suggest that Sedated and In-group BCSs have differential sensitivities in their relationships with behavior, and in determining certain outcomes. As shown in Figure 1, Sedated and In-group BCS have slightly different measurement criterion, with Sedated BCS measured while the chimpanzees is in a supine position, and In-group BCSs are measured while the chimpanzee is awake and mobile. It is possible that In-group BCSs allow for more nuanced observation of body condition than is possible while a chimpanzee is static and lying down under sedation. However, Sedated BCSs tended to show more extreme scores (a higher number of 4s and 8s), suggesting that Sedated BCS may be more sensitive to extremes. Therefore, while Sedated and In-group BCS have their own benefits, the use of both may provide the most complete information.
We found that older female chimpanzees seem to have the highest risk for becoming obese. This is consistent with previous reports that female chimpanzees are more likely than their male counterparts to be overweight or obese (Nunamaker et al., 2012; Lowenstine, McManamon, & Terio, 2016). Human females also have a higher risk for obesity and metabolic syndrome, and this risk increases with age (Kanter & Cabarello, 2012; Lowenstine et al., 2016). We could speculate that there may be both behavioral and physiological explanations for this pattern. For example, it is reasonable to assume that females are less active overall than males. However, our data do not seem to support this, as cursory analyses show that males and females in the current study do not exhibit differences in rates of inactivity or locomotion. It seems most likely that physiological factors offer an explanation for this pattern. Metabolism, energy-homeostasis, and fat stores are controlled by sex-specific hormones throughout development (Mauvais-Jarvis, 2015). In humans, males have a higher metabolic rate than females, and this difference increases with age (Ferraro et al., 1992). In chimpanzees, an early study showed that, before puberty, female energy metabolism was lower than that of males (Dale, Shanklin, Johnson, & Brown, 1967). As such, lower overall metabolic rate in females that also decreases with age, may contribute to the obesity seen in the current study. However, this question must be tested empirically.
Importantly, as a strictly noninvasive measurement tool, BCSs can also be utilized effectively for body condition assessments of sanctuary and wild chimpanzees. Weights are difficult to obtain in the field, and a BCS system could be used to quantitatively monitor changes in body condition resulting from a variety of influences in the environment, including tourism and farming. Additionally, a change in weight or body condition is often a key symptom of disease in wild chimpanzees, including respiratory and wasting diseases, serious wounds, infection, and epidemic illnesses, such as mange and polio (Williams et al., 2008). The BCS system could serve as an early diagnostic tool in health monitoring, and perhaps, facilitate prevention of disease progression of wild chimpanzees. Furthermore, the use of the BCS system can be adapted to other wild, sanctuary, and captive populations of NHPs. In the same way that we utilized a team of experts to adapt the BCS from rhesus macaques to chimpanzees, teams of experts of other NHP species may adapt the system to their species, and examine validity and reliability in ways similar to those described here.
In summary, we have demonstrated that the In-group BCS system, which uses observation only, can be used to obtain reliable and valid measures of obesity and body condition. We showed that In-group BCSs were positively correlated with Sedated BCSs and body weight, both more traditional measures of obesity. Furthermore, we believe this system is more accurate than weight measurements alone and may be a more sensitive tool for categorizing ‘obesity’, by consistently identifying more ‘at-risk’ individuals. Indeed, as shown in the current study, older females, in particular, seem to have the highest risk for becoming obese. The In-group BCS system permits captive managers to easily monitor every individual chimpanzee’s body condition, thereby allowing early identification of chimpanzees in need of weight management interventions. Importantly, because the In-group BCS eliminates the need for evaluations requiring anesthesia, it allows for frequent, noninvasive assessments during weight management (weight-loss or weight-gain) initiatives. While our focus has been on animals with obesity, BCS can also be used for ailing, geriatric, or underweight captive chimpanzees that require continued monitoring of body condition.
Acknowledgements
The authors would like to thank the chimpanzee care and management staffs at the KCCMR, as well as Dr. Stephanie Buchl, Elizabeth Lindemann, and Amanda Ott. Thank you also to Jennifer Bridges and Mary Catherine Mareno for assistance with this process. Cooperative agreement NIH/U42‐OD011197 supports the KCCMR chimpanzee colony. The authors have no conflicts of interest to declare.
References
- Baker KD, Loughman A, Spencer SJ, & Reichelt AC (2017). The impact of obesity and hypercaloric diet consumption on anxiety and emotional behavior across the lifespan. Neuroscience & Biobehavioral Reviews, 83, 173–182. 10.1016/j.neubiorev.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Bauman JE (1923). The strength of the chimpanzee and orang. The Scientific Monthly, 16(4), 432–439. [Google Scholar]
- Berman CM, & Schwartz S (1988). A nonintrusive method for determining relative body fat in free‐ranging monkeys. American Journal of Primatology, 14(1), 53–64. 10.1002/ajp.1350140105 [DOI] [PubMed] [Google Scholar]
- Brent L (1995). Feeding enrichment and body weight in captive chimpanzees. Journal of Medical Primatology, 24(1), 12–16. 10.1111/j.1600-0684.1995.tb00139.x [DOI] [PubMed] [Google Scholar]
- Bridges JP, Mocarski EC, Reamer LA, Lambeth SP, & Schapiro SJ (2013). Weight management in captive chimpanzees (Pan troglodytes) using a modified feeding device. American Journal of Primatology, 75, 51. [Google Scholar]
- Carroll CL, & Huntington PJ (1988). Body condition scoring and weight estimation of horses. Equine Veterinary Journal 20(1), 41–45. 10.1111/j.2042-3306.1988.tb01451.x [DOI] [PubMed] [Google Scholar]
- Clingerman KJ, & Summers L (2012). Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): Inter-and intrarater variability. Journal of the American Association for Laboratory Animal Science, 51(1), 31–36. [PMC free article] [PubMed] [Google Scholar]
- D’Eath RB, Tolkamp BJ, Kyriazakis I, & Lawrence AB (2009). ‘Freedom from hunger’ and preventing obesity: The animal welfare implications of reducing food quantity or quality. Animal Behaviour, 77(2), 275–288. 10.1016/j.anbehav.2008.10.028 [DOI] [Google Scholar]
- Dale HE, Shanklin MD, Johnson HD, & Brown WH (1967). Energy metabolism of the chimpanzee: a comparison of direct and indirect calorimetry. Journal of Applied Physiology, 22(4), 850–853. [DOI] [PubMed] [Google Scholar]
- Davenport RK, Menzel EW, & Rogers CM (1966). Effects of severe isolation on normal juvenile chimpanzees: Health, weight gain, and stereotyped behaviors. Archives of General Psychiatry, 14(2), 134–138. 10.1001/archpsyc.1966.01730080022004 [DOI] [PubMed] [Google Scholar]
- Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P, … & Paillard F (1995). The effect of increased salt intake on blood pressure of chimpanzees. Nature Medicine, 1(10), 1009 10.1038/nm1095-1009 [DOI] [PubMed] [Google Scholar]
- Domecq JJ, Skidmore AL, Lloyd JW, & Kaneene JB (1995). Validation of body condition scores with ultrasound measurements of subcutaneous fat of dairy cows. Journal of Dairy Science, 78(10), 2308–2313. 10.3168/jds.s0022-0302(95)76857-6 [DOI] [PubMed] [Google Scholar]
- Donoghue S, Khoo L, Glickman LT, & Kronfeld DS (1991). Body condition and diet of relatively healthy older dogs. The Journal of Nutrition, 121(11), S58–S59. 10.1093/jn/121.suppl_11.s58 [DOI] [PubMed] [Google Scholar]
- Edmonson AJ, Lean IJ, Weaver LD, Farver T, & Webster G (1989). A body condition scoring chart for Holstein dairy cows. Journal of Dairy Science, 72(1), 68–78. 10.3168/jds.s0022-0302(89)79081-0 [DOI] [Google Scholar]
- Eichberg JW, & Shade RE (1987). “Normal” blood pressure in chimpanzees. Journal of Medical Primatology, 16(5), 317–321. [PubMed] [Google Scholar]
- Ely JJ, Zavaskis T, & Lammey ML (2013). Hypertension increases with aging and obesity in chimpanzees (Pan troglodytes). Zoo Biology, 32(1), 79–87. 10.1002/zoo.21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro R, Lillioja S, Fontvieille AM, Rising R, Bogardus C, & Ravussin E (1992). Lower sedentary metabolic rate in women compared with men. The Journal of Clinical Investigation, 90(3), 780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrow JS, & Webster J (1985). Quetelet’s index (W/H2) as a measure of fatness. International Journal of Obesity, 9(2), 147–153. [PubMed] [Google Scholar]
- German AJ, Holden SL, Moxham GL, Holmes KL, Hackett RM, & Rawlings JM (2006). A simple, reliable tool for owners to assess the body condition of their dog or cat. The Journal of Nutrition, 136(7), 2031S–2033S. 10.1093/jn/136.7.2031s [DOI] [PubMed] [Google Scholar]
- Goodall J (1986). The chimpanzees of Gombe: Patterns of behavior. Cambridge: The Belknap Press of Harvard University Press. [Google Scholar]
- Goh VH, Tain CF, Tong TY, Mok HP, & Wong MT (2004). Are BMI and other anthropometric measures appropriate as indices for obesity? A study in an Asian population. Journal of Lipid Research, 45(10), 1892–1898. 10.1194/jlr.m400159-jlr200 [DOI] [PubMed] [Google Scholar]
- Hickman DL, & Swan M (2010). Use of a body condition score technique to assess health status in a rat model of polycystic kidney disease. Journal of the American Association for Laboratory Animal Science, 49(2), 155–159. [PMC free article] [PubMed] [Google Scholar]
- Hubbard GB, Lee DR, & Eichberg JW (1991). Diseases and pathology of chimpanzees at the Southwest Foundation for Biomedical Research. American Journal of Primatology, 24(3‐4), 273–282. 10.1002/ajp.1350240313 [DOI] [Google Scholar]
- Kanter R, & Caballero B (2012). Global gender disparities in obesity: A review. Advances in Nutrition, 3(4), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, … & Fairbanks L (2010). Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proceedings of the Royal Society B: Biological Sciences, 278(1712), 1626–1632. 10.1098/rspb.2010.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfeld DS, Donoghue S, & Glickman LT (1994). Body condition of cats. The Journal of Nutrition, 124(12), 2683S–2684S. 10.1093/jn/124.suppl_12.2683s [DOI] [PubMed] [Google Scholar]
- Laflamme D (1997a). Development and validation of a body condition score system for cats: A clinical tool. Feline Practice, 25,13–18. [Google Scholar]
- Laflamme DRPC (1997b). Development and validation of a body condition score system for dogs: A clinical tool. Canine Practice, 22, 10–15. [Google Scholar]
- Lambeth SP, Bernacky BJ, Hanley P, & Schapiro SJ (2011). Weight management in a captive colony of chimpanzees (Pan troglodytes). American Journal of Primatology, 73, 40–40. [Google Scholar]
- Leigh SR, & Shea BT (1995). Ontogeny and the evolution of adult body size dimorphism in apes. American Journal of Primatology, 36(1), 37–60. 10.1002/ajp.1350360104 [DOI] [PubMed] [Google Scholar]
- Lewis MT, Lujan HL, Tonson A, Wiseman RW, & DiCarlo SE (2019). Obesity and inactivity, not hyperglycemia, cause exercise intolerance in individuals with type 2 diabetes: Solving the obesity and inactivity versus hyperglycemia causality dilemma. Medical Hypotheses, 123, 110–114. 10.1016/j.mehy.2019.01.013 [DOI] [PubMed] [Google Scholar]
- Lowenstine LJ, McManamon R, & Terio KA (2016). Comparative pathology of aging great apes: Bonobos, chimpanzees, gorillas, and orangutans. Veterinary Pathology, 53(2), 250–276. [DOI] [PubMed] [Google Scholar]
- Matthews LR, Cameron C, Sheahan AJ, Kolver ES, & Roche JR (2012). Associations among dairy cow body condition and welfare-associated behavioral traits. Journal of Dairy Science, 95(5), 2595–2601. 10.3168/jds.2011-4889 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F (2015). Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of Sex Differences, 6(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, & Dietz WH (1999). The disease burden associated with overweight and obesity. JAMA, 282(16), 1523–1529. 10.1001/jama.282.16.1523 [DOI] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2018). Captive chimpanzee (Pan troglodytes) behavior as a function of space per animal and enclosure type. American Journal of Primatology, 80(3), e22749 10.1002/ajp.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, Lambeth SP, & Schapiro SJ (2019). Differences in behavior between elderly and nonelderly captive chimpanzees and the effects of the social environment. Journal of the American Association for Laboratory Animal Science, 58(6), 783–789. 10.30802/aalas-jaalas-19-000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunamaker EA, Lee DR, & Lammey ML (2012). Chronic diseases in captive geriatric female chimpanzees (Pan troglodytes). Comparative medicine, 62(2), 131–136. [PMC free article] [PubMed] [Google Scholar]
- Obanda V, Omondi GP, & Chiyo PI (2014). The influence of body mass index, age and sex on inflammatory disease risk in semi-captive chimpanzees. PloS ONE, 9(8). 10.1371/journal.pone.0104602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman AD, & Johnson WD (2006). The changing shape of the body mass index distribution curve in the population: Implications for public health policy to reduce the prevalence of adult obesity. Preventing Chronic Disease, 3(3), 1–4. [PMC free article] [PubMed] [Google Scholar]
- Reamer LA, Haller RL, Thiele EJ, Freeman HD, Lambeth SP, & Schapiro SJ (2014). Factors affecting initial training success of blood glucose testing in captive chimpanzees (Pan troglodytes). Zoo Biology, 33(3), 212–220. 10.1002/zoo.21123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ (2008). BMI-related errors in the measurement of obesity. International Journal of Obesity, 32(S3), S56 10.1038/ijo.2008.87 [DOI] [PubMed] [Google Scholar]
- Russel A (1984). Body condition scoring of sheep. In Practice 6, 91–93. 10.1136/inpract.6.3.91 [DOI] [PubMed] [Google Scholar]
- Simon SL, Diniz Behn C, Laikin A, Kaar JL, Rahat H, Cree-Green M, … & Nadeau KJ (2019). Sleep & circadian health are associated with mood & behavior in adolescents with overweight/obesity. Behavioral Sleep Medicine, 1–10. 10.1080/15402002.2019.1629444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers L, Clingerman KJ, & Yang X (2012). Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): Assessment of body composition by using dual-energy X-ray absorptiometry. Journal of the American Association for Laboratory Animal Science, 51(1), 88–93. [PMC free article] [PubMed] [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, Mlengeya T, Lipende I, Kirchhoff CA, … & Keele BF (2011). Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from Gombe National Park, Tanzania, 2004–2010. Journal of Zoo and Wildlife Medicine, 42(4), 597 10.1638/2010-0237.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman-Culleré MH, & Foltz CJ (1999). Body condition scoring: A rapid and accurate method for assessing health status in mice. Comparative Medicine, 49(3), 319–323. [PubMed] [Google Scholar]
- Van Raemdonck K, Umar S, Szekanecz Z, Zomorrodi RK, & Shahrara S (2018). Impact of obesity on autoimmune arthritis and its cardiovascular complications. Autoimmunity Reviews, 17(8), 821–835. 10.1016/j.autrev.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki N, Anderson D, Herndon JG, Pham T, Gregg CJ, Cheriyan M, … & Varki A (2009). Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evolutionary Applications, 2(1), 101–112. 10.1111/j.1752-4571.2008.00064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videan EN, Fritz J, & Murphy J (2007). Development of guidelines for assessing obesity in captive chimpanzees (Pan troglodytes). Zoo Biology, 26, 93–104. 10.1002/zoo.20122 [DOI] [PubMed] [Google Scholar]
- Weisenberg E, Snook S, & Letcher J (1991). Sudden death in an obese orangutan with hypertensive heart disease and a history of stroke. Proceedings of the American Association of Zoo Veterinarians, 172. [Google Scholar]
- Williams JM, Lonsdorf EV, Wilson ML, Schumacher‐Stankey J, Goodall J, & Pusey AE (2008). Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. American Journal of Primatology, 70(8), 766–777. 10.1002/ajp.20573 [DOI] [PubMed] [Google Scholar]
- Young SS, Skeans SM, Austin T, & Chapman RW (2003). The effects of body fat on pulmonary function and gas exchange in cynomolgus monkeys. Pulmonary Pharmacology & Therapeutics, 16(5), 313–319. 10.1016/s1094-5539(03)00073-7 [DOI] [PubMed] [Google Scholar]


