Vibrio is a large and diverse genus of bacteria, of which most are nonpathogenic species found in the aquatic environment. However, a subset of the Vibrio genus includes several species that are highly pathogenic, either to humans or to aquatic animals. In recent years, Danio rerio, commonly known as the zebrafish, has emerged as a major animal model used for studying nearly every aspect of biology, including infectious diseases. Zebrafish are especially useful because the embryos are transparent, larvae are small and facilitate imaging studies, and numerous transgenic fish strains have been constructed.
KEYWORDS: Vibrio, animal models, bacterial pathogenesis, zebrafish
ABSTRACT
Vibrio is a large and diverse genus of bacteria, of which most are nonpathogenic species found in the aquatic environment. However, a subset of the Vibrio genus includes several species that are highly pathogenic, either to humans or to aquatic animals. In recent years, Danio rerio, commonly known as the zebrafish, has emerged as a major animal model used for studying nearly every aspect of biology, including infectious diseases. Zebrafish are especially useful because the embryos are transparent, larvae are small and facilitate imaging studies, and numerous transgenic fish strains have been constructed. Zebrafish models for several pathogenic Vibrio species have been described, and indeed a fish model is highly relevant for the study of aquatic bacterial pathogens. Here, we summarize the zebrafish models that have been used to study pathogenic Vibrio species to date.
INTRODUCTION
Members of the Vibrio genus are Gram-negative, rod-shaped bacteria exclusively found in aquatic environments of various salinity, with different species isolated from freshwater, brackish conditions, and saltwater environments (1). Human infection distribution with various Vibrio species is typically aligned to seasonal changes, with the majority of cases occurring in warmer months (2). These bacteria prefer warm temperatures, as is typical of the regions in which they are endemic—southeast Asia, Africa, and Latin America—but can also be found in much cooler climates, such as the northwestern United States (3–5). Recently, concern for the climate change-enabled spread of both environmental and pathogenic vibrios has sparked studies that positively correlate rising seawater levels and temperatures with an increased range of both Vibrio parahaemolyticus and Vibrio vulnificus (6, 7).
Infection with pathogenic Vibrio species has threatened human health to various degrees for centuries (8, 9). Outbreaks of cholera and vibriosis have caused chaos in nearly every region of the world. While these situations are obvious sources for concern, not all Vibrio species—in fact relatively few—cause disease in humans, and even fewer subtypes are capable of causing pandemic outbreaks. Over 100 species of Vibrio have been identified, of which 12 cause disease in humans (2, 10). With some overlap, 16 species have been determined to cause pathogenesis in aquatic organisms which can, in turn, have significant impacts on animal health and aquaculture (11–13).
COMMONALITIES AMONG VIBRIO SPECIES
All Vibrio species, pathogenic or nonpathogenic, share a few general genotypic similarities. Generally, the Vibrio genome is composed of two circular chromosomes. Chromosome 1 is fairly conserved across species at about 3 Mb long and encodes most of the essential proteins (14). Most genomic variation between Vibrio species is located on chromosome 2, which encodes primarily accessory proteins, varies in size among different species, and is hypothesized to have evolved from plasmids (14). As an exception, two clinical Vibrio cholerae isolates have been found to contain only a single, fused chromosome and challenge the general rule that all naturally occurring V. cholerae strains contain two chromosomes (15–18). The processes of recombination and horizontal gene transfer are considered major contributors to the evolution of various Vibrio species.
Vibrios are generally considered to be halophiles, although different species have different tolerances for salinity, and this is an important factor when considering an aquatic animal model. V. cholerae can survive in freshwater, brackish water, and marine water. Zebrafish are freshwater fish, such that they are normally housed in water that has salinity between 0.1 and 1 g/liter. This low salinity apparently has no ill effects on V. cholerae survival, and we have observed that V. cholerae remains viable in freshwater over a span of several days. The bacteria are likely not replicating during this span due to nutrient limitations. Seawater salinity, on the other hand, is approximately 35 g/liter on average, and marine Vibrio species tend to prefer such higher salinity. For example, Vibrio vulnificus rapidly dies in low salt conditions normally used for zebrafish (D. Runft, M. N. Neely, and J. H. Withey, unpublished results). However, if the salinity is changed gradually, zebrafish can acclimate to significantly higher salinity, such as would be required for studies with V. vulnificus. One study found that zebrafish hatched and developed better at 2 g/liter salinity than under the normally recommended conditions (19). However, the upper range of salinity that would be tolerated by zebrafish is not clear, and exposure to high salt is likely to induce a stress response.
Temperature is another important factor in bacterial infections, and zebrafish can also tolerate a wide temperature range. Normally, fish are housed at a recommended temperature of 28°C but in the wild can survive at temperatures ranging from 6°C in winter to 38°C in summer. Studies on the maximum temperature range tolerated by zebrafish indicated they can survive from 6.2°C to 41.7°C (20). Rapid alterations in temperature can cause extreme stress or death, so if an altered temperature is required for an experiment, the fish should be slowly acclimated. However, at extreme temperatures, there is likely to be a metabolic cost that could affect susceptibility to a pathogen.
EPIDEMIOLOGY AND DISEASE
Infection with Vibrio bacteria can result in two types of illness, namely, vibriosis and cholera. Any noncholera illness caused by Vibrio infection is classified as vibriosis. The exact number of cases of Vibrio infection is difficult to determine due to several structural shortcomings, including underreporting or failure to report by countries in which infections occur, poor surveillance systems and a complete lack of a global surveillance framework, and general discrepancies in reporting procedures. Generally, cholera is considered a reportable disease internationally; however, vibriosis is not widely considered to be a reportable disease and has been surveilled only in select countries (21). Depending on the species, humans become exposed to Vibrio either orally or by open wound exposure (2).
Vibriosis can manifest as gastroenteritis, localized wound infections, tissue necrosis (V. vulnificus), or septicemia (2). Non-O1/O139 serogroup V. cholerae strains, Vibrio parahemolyticus, V. vulnificus, and Vibrio anguillarum can infect a human host by consumption of raw or undercooked seafood or by exposure to an open wound, while Vibrio alginolyticus exposure occurs only through open wounds (22). Wound infections by V. vulnificus are extremely severe (23). Septic cases are generally limited to hosts with chronic liver disease but can rapidly progress to death in less than 48 hours (23–25).
Cholera is a severe diarrheal disease caused by O1 and O139 serogroups V. cholerae. Human exposure to these pandemic V. cholerae strains occurs via consumption of contaminated water or food, and the bacteria are highly transmissible from person to person via the fecal-oral route especially within a household. Cholera is up to 50% lethal if untreated, but therapy with an oral rehydration solution reduces mortality to below 2%. Exposure to O1/O139 V. cholerae strains occurs mainly in areas of the world with poor infrastructure and sanitation, where safe drinking water is scarce (26). It is estimated between 1.4 and 4 million cases of cholera occur globally each year, resulting in about 140,000 deaths, of which at least half occur in children under 5 years old (3, 26).
Genetic analysis suggests the introduction of O1/O139 V. cholerae strains to regions where it is nonendemic is almost exclusively due to human movement (27–29). For example, V. cholerae was introduced to Haiti following an influx of international aid workers who responded to the devastating earthquake in 2010 (30–35). Cholera has since become endemic to this previously unexposed region.
The need to study these human-pathogenic vibrios should be evident, as is the need to study their nonpathogenic Vibrio counterparts. As all vibrios are aquatic bacteria, they are found associated with different forms of aquatic life in the environment, including teleost fish. Here, we summarize pathogenic Vibrio models established in the best-studied teleost fish, Danio rerio, commonly known as zebrafish.
ZEBRAFISH AS A NATURAL HOST MODEL
For more than a century, scientists have been using animal models to mimic human infection with Vibrio species. Until recently, the majority of this work was conducted in mammalian models, such as the mouse or rabbit, as described below (36–43). These models have been sufficient to study select aspects of infection, i.e., intestinal colonization and regulation of toxin production or other virulence activity, but have failed to provide a complete picture of the infectious cycle, which runs from exposure to colonization, to pathogenesis and disease, to escape from the host and transmission, and finally to infection clearance.
An alternative animal model, the zebrafish, was proposed in the past decade as a natural Vibrio host model that allows for a more well-rounded understanding of the infection process in its entirety (44, 45). Research teams have made significant discoveries since the development of zebrafish models in aspects of the Vibrio life cycle that had previously been impossible to investigate due to the limitations of mammalian models that are not natural Vibrio hosts. For the purpose of this review, we highlight the techniques and critical findings that have resulted from the use of the zebrafish natural host model with the following five major human Vibrio pathogens: Vibrio cholerae, V. parahaemolyticus, V. vulnificus, V. anguillarum, and V. alginolyticus. The different models that have been described and are reviewed here are summarized in Table 1.
TABLE 1.
Zebrafish models for vibrios
| Vibrio sp. | Zebrafish model | Route of administration | Reference |
|---|---|---|---|
| V. cholerae | Adult, 6–9 mo; larvae, 5 dpf | Oral gavage; immersion | Mitchell and Withey (45) |
| Adult, 6–9 mo; larvae, 5 dpf | Oral gavage; immersion | Runft et al. (44) | |
| Adult, age not specified | Immersion | Nag et al. (64) | |
| Adult, age not specified | Immersion | Nag et al. (62) | |
| Larvae, 5 dpf | Immersion | Manneh-Roussel et al. (66) | |
| Adult, age not specified | Immersion | DeAngelis et al. (67) | |
| V. parahaemolyticus | Adult, 5–6 mo | Intraperitoneal injection | Paranjpye et al. (77) |
| Adult, 7–8 mo | Immersion; intramuscular injection; intraperitoneal injection | Zhang et al. (82) | |
| Adult, age not specified | Intraperitoneal injection | Peng et al. (83) | |
| Larvae, 3 dpf | Immersion | Zhang et al. (84) | |
| Larvae, 3 dpf | Immersion | Ji et al. (85) | |
| Larvae, 3 dpf | Microinjection | Ji et al. (86) | |
| V. vulnificus | Adult, age not specified | Intraperitoneal injection | Pan et al. (91) |
| Adult, age not specified | Caudal peduncle injection | Pan et al. (92) | |
| Adult, age not specified | Intraperitoneal injection | Jheng et al. (93) | |
| Adult, age not specified | Intraperitoneal injection | Cheng et al. (94) | |
| Adult, age not specified | Intraperitoneal injection | Faikoh et al. (95) | |
| V. anguillarum | Adult, 12 mo | Intraperitoneal injection; foodborne | Randazzo et al. (100) |
| Larvae, 5 dpf | Immersion | Oyarbide et al. (101) | |
| Larvae, 6–8 dpf | Immersion | O'Toole et al. (102) | |
| Adult, 6+ mo | Immersion; intraperitoneal injection | Schmidt et al. (103) | |
| Adult, age not specified | Immersion | Zhang et al. (104) | |
| Adult, age not specified | Immersion | Zhang et al. (105) | |
| Adult, 6 mo | Immersion | Zhang et al. (106) | |
| Adult, 6 mo | Immersion | Liu et al. (107) | |
| Adult, 6 mo | Immersion | Liu et al. (108) | |
| Adult, age not specified | Immersion | Khansari et al. (110) | |
| Larvae, 5 dpf | Immersion | Caruffo et al. (111) | |
| V. alginolyticus | Adult, 3 mo | Intraperitoneal injection | Yang et al. (112) |
| Adult, age not specified | Intraperitoneal injection | Jiang et al. (113) |
ZEBRAFISH MODEL FOR V. CHOLERAE
Zebrafish have been used to study numerous bacterial pathogens, including members of the genera Aeromonas (46), Salmonella (47), and Mycobacterium (48), to name a few, but this model has also begun to make valuable contributions to the field of V. cholerae as well as other Vibrio spp. One reason zebrafish are becoming more widely used is the general relative ease of zebrafish models. Animal housing consists of tank systems capable of housing hundreds to thousands of fish within one confined area with minimal general upkeep and maintenance required. When zebrafish are infected by immersion, V. cholerae inoculum can be pipetted directly into beakers containing 4 or 5 fish (45), bypassing complicated and tedious infection procedures needed for other animal models. Once the inoculum is added, the zebrafish ingest the bacteria orally. When infected, the fish then produce the same mucus-laden, profuse, watery diarrhea seen in human disease and are able to infect other fish that then ingest the bacterium via the fecal-oral route, replicating the infection cycle seen in humans (44, 45). This process of infection allows researchers the ability to draw direct transmission parallels to human modes of transmission. This route of infection also avoids manipulation of the animal through stomach acid neutralization, use of antibiotics, or anesthesia, which may alter infection dynamics by changing the physiological environment through pH shifts, the lack of an intact microbiota, significant alteration to the immune system, or effects on intestinal motility.
The use of antibiotics in mammalian models depletes the intestinal tract of the resident microbiome, and this is likely required because V. cholerae did not evolve to compete with mouse or rabbit microbiota. The zebrafish model allows for examination of the relationship between V. cholerae infection and the host’s mature, intact intestinal microbiome, as aquatic V. cholerae can readily compete with the intestinal microbiota in fish, with many teleost species being natural hosts (49, 50). Although the microbiota of the zebrafish may differ greatly from that of humans, it is nonetheless complex and diverse. Microbial community shifts are observed in the zebrafish intestine throughout development, environmental changes, and dietary changes. As zebrafish age, their intestinal microbiota becomes increasingly unique, revealing an actively evolving community made up of hundreds of microbial species (51). Further solidifying the relevance of the microbiota in the zebrafish model, those microbial communities present in both domesticated and wild zebrafish share striking similarities and consist of specific core bacterial taxa (52).
The absence of manipulation in infecting zebrafish also excludes the need for anesthesia, such as ketamine-xylazine, which leads to immune suppression and decreased intestinal motility (53). Common mammalian V. cholerae models, such as infant mice and rabbits, have yet to develop mature immune systems, and germfree mice have been shown to have altered immune function (54). With its intact and fully functioning immune system during infection, the adult zebrafish is an ideal model to study host immune responses to V. cholerae or other vibrios. Furthermore, the immune system is evolutionarily well conserved across many animal species, and adult zebrafish possess innate and adaptive arms similar to those of humans (55). Due to the importance of zebrafish in developmental biology, much work has been done to characterize their basic biology, particularly in embryos and larvae, which have also been shown to be colonized by V. cholerae (44).
Evidence further solidifying the relationship between zebrafish and V. cholerae is plainly obvious in the geographical overlap between the two organisms in the environment. The natural habitat of zebrafish is broadly throughout southern Asia, overlapping areas where cholera is endemic for centuries, and vibrios and V. cholerae have been identified in the microbiota of zebrafish (56–58). However, the interaction between V. cholerae and fish is not limited to Danio rerio. Senderovich et al. found V. cholerae colonizing the intestinal tract of 10 wild-caught teleost species (59). V. cholerae has also been found in wild fish in other areas of the world, including Central America and Africa (49, 50). Aside from potentially being a fish commensal in some cases, V. cholerae may be using fish species as a vector for population expansion or for long-distance transport (59), as shown in the work of Runft et al., where infected zebrafish were able to transmit V. cholerae to naive uninfected zebrafish (44). This natural relationship between V. cholerae and fish may provide opportunities to understand the natural life cycle of Vibrio cholerae in ways other models cannot, due to their artificial nature.
Finally, the economical researcher needs look no further than the cost-effective nature of the zebrafish. Once housing systems are in place, maintaining and repopulating the zebrafish are very low cost. General system upkeep consists of regular tank cleaning and monitoring of water conditions. Zebrafish husbandry is simple and often produces large numbers of offspring. Once zebrafish are established, thousands of individuals may be kept within the confines of a single room. The use of rabbit or mouse models, on the other hand, may be extremely costly in the procurement and housing of animals over extended periods of time and may be a limiting factor in the ability of some laboratories to perform relevant research within the field.
Despite its advantages over other V. cholerae models, the zebrafish does present its own inherent set of unique disadvantages. Chiefly among them are comparisons that must be drawn between infections in teleost and human species. As previously discussed, the immune systems of the zebrafish and humans are similar in composition and function. However, there are differences in the immunoglobulins present in humans and fish, as fish lack IgG (60). The zebrafish gastrointestinal tract, although somewhat physiologically different and lacking a stomach, resembles much of the human small intestine, where V. cholerae infection takes place (61). However, some of the mammalian signals that induce virulence gene expression in humans are absent in fish. Indeed, the major human virulence factors produced by V. cholerae, namely, cholera toxin (CT) and toxin-coregulated pilus (TCP), are not required for zebrafish colonization or pathogenesis. The small size of zebrafish (2 to 4 cm) can present minor difficulties in handling and extracting specific tissues, such as the gastrointestinal tract, which can be vital for a localized infection, such as that by V. cholerae, or in immunological work. Meanwhile, a lack of commercially available reagents (e.g., antibodies and kits) makes performing select experiments such as enzyme-linked immunosorbent assay (ELISA) and Western blotting difficult or not feasible at this time.
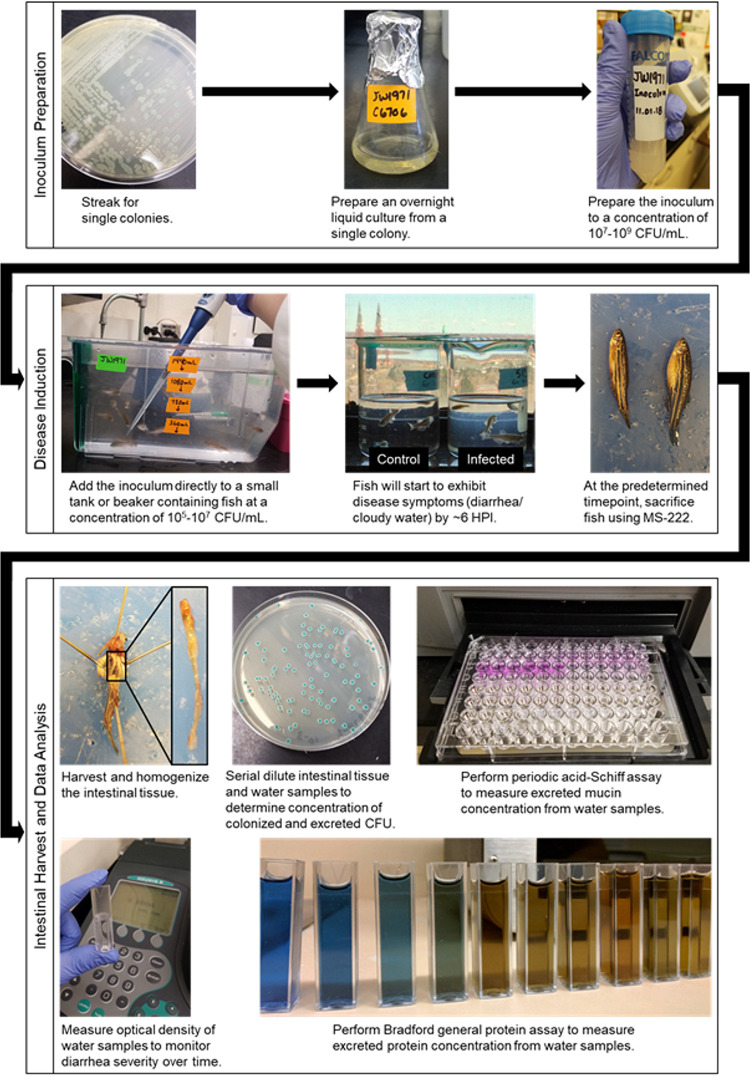
Another drawback of using the zebrafish model for V. cholerae is the inherent difficulty of controlling parameters surrounding the infection. Because bacterial inoculum is added directly to beakers containing tank water instead of being administered by oral gavage or intestinal inoculation, there is no guarantee that an identical amount of bacteria is ingested by each fish, creating potential discrepancies in the levels of inoculum they received. Once fish are infected, levels of colonization may vary over time with the exact duration of infection currently unclear. This point is underscored by the fact that some Vibrio spp. are commensals in fish (50, 62), potentially leading to extended continuous shedding of the bacteria. However, zebrafish do eventually clear V. cholerae infections (M. G. Walton and J. H. Withey, unpublished data.) Moreover, difficulties may arise in bath inoculation leading to inconsistent infections. V. cholerae is especially sensitive to pH below 7 and may die in the water before it is able to infect the fish if the pH of the water is not properly managed (63). Lastly, it is unknown if the diarrhea produced by zebrafish into the beaker water leads to a continuous cycle of reinfection, thereby muddying results from the original infection. Current models mitigate the chance for this to occur by performing regular water changes. The procedure for performing V. cholerae infections of adult zebrafish is summarized in Fig. 1.
FIG 1.
General procedure for inoculation of zebrafish with V. cholerae by passive immersion to model disease in a natural host. Initially described by Runft et al. (44) and further developed by Mitchell et al. (45) and Nag et al. (64).
CURRENT FINDINGS FROM THE V. CHOLERAE ZEBRAFISH MODEL
The use of adult zebrafish as a model for V. cholerae was first established by Runft et al. in 2014 (44). They found that both El Tor and classical strains of V. cholerae could colonize zebrafish adults or larvae; however, El Tor colonized for longer periods of time, and as mentioned above, neither CT nor TCP appeared to be required for colonization. Histological examination also showed an infection that closely mimics infections in mammals, taking place in clumps close to the epithelial surface (44). Mitchell et al. (45) later showed that the bacterial burden of V. cholerae infection in zebrafish, as well as associated pathology, could be quantified using a combination of optical density of the water, protein and mucin measurements, and bacterial CFU counts from infected zebrafish tank water. Infected fish had increased mucin production that aligned with an increase in filled goblet cells by 6 h after V. cholerae exposure. Furthermore, V. cholerae El Tor (E7946) colonized zebrafish for at least 6 days, when experiments were terminated, highlighting the need for further investigation into the length of colonization and potential shedding thereafter (64). The observation that classical (O395) and El Tor (N16961) biotypes exhibited differences in the duration of fish colonization suggests that genetic differences between these biotypes may be responsible. Potential therapeutic approaches are being investigated in the zebrafish model as well; Nag et al. have shown that administering probiotic Escherichia coli along with a glucose-based oral rehydration solution may lower gut pH, thereby inhibiting V. cholerae colonization (62). The zebrafish model has also been used to investigate the role of the type 6 secretion system (T6SS) in competition between V. cholerae and an intestinal commensal (65). Immobilized, germfree zebrafish larvae were seeded with a commensal Aeromonas species, and the effects of the T6SS on competition between V. cholerae and the commensal were assessed. That study intriguingly found, using sophisticated imaging, that the T6SS increased intestinal motility to eliminate the competitor, rather than directly attacking the competitor bacteria, implicating a previously unknown mechanism for T6SS.
In studying lifestyle switching of V. cholerae, Manneh-Roussel et al. (66) utilized larval zebrafish to assess gene expression and transcription factors necessary for intestinal colonization. This work mapped the binding of the cAMP receptor protein (CRP) across the genome, ultimately controlling lifestyle switching when entering the host from aquatic environments. Targets of CRP included rtxHCA and rtxBDE, two operons that are involved in the cytotoxic activity of V. cholerae (66). More recently, DeAngelis et al. (67) highlighted differences in colonization of the infant mouse and the adult zebrafish in regard to the phage shock protein (Psp) system, which senses and responds to inner membrane damage. In this study, the authors found that a strain lacking the Psp system was able to colonize the infant mouse intestine but had defects in zebrafish colonization, leading the authors to conclude the Psp response may contribute to disease transmission in the aquatic environment and may have a larger role in survival during environmental stress (67). V. cholerae isolates imaged in zebrafish were also demonstrated to be using similar techniques as human isolates where swimming motility and chemotaxis are used to counter intestinal flow, thereby determining spatial organization and aiding in persistence (68).
ZEBRAFISH MODELS FOR VIBRIO PARAHAEMOLYTICUS
Vibrio parahaemolyticus is a Gram-negative pathogen common in the environment that is responsible for most bacterial infections due to consumption of seafood (21, 69). Consumption of raw or undercooked shellfish, particularly oysters, is the most common way to acquire V. parahaemolyticus. The disease can cause a severe, but self-limiting, watery diarrhea often accompanied by abdominal cramping, nausea, vomiting, fever, and chills. Symptoms typically appear within 24 hours of ingestion, with illness lasting approximately 3 to 4 days (70, 71). V. parahaemolyticus is very diverse and complex and shows evidence of significant strain-specific differences in addition to the presence or absence of the known virulence determinants tdh (thermostable direct hemolysin [TDH] gene), trh (TDH-related hemolysin [TRH] gene), and type 3 secretion system 2 (T3SS2) and the pathogenicity islands, such as VPA1, that may contribute to virulence (72–76).
Experimental zebrafish can be infected intraperitoneally (i.p.) in a dose-dependent manner by both environmental and clinically isolated V. parahaemolyticus strains. The bacteria were lethal to zebrafish i.p. inoculated with an 50% lethal dose (LD50) of 5.7 × 105 CFU for the tdh+ trh mutant clinical strain (77). Differences in zebrafish survival after infection were observed regardless of the presence or absence of the known hemolysins TDH and TRH. This model could also be useful to evaluate the potential presence and/or contribution of additional unknown virulence factors in V. parahaemolyticus strains. This model may also be used to identify the unknown factors that affect erythrocytes, as changes in erythrocytes were observed in different isolates of V. parahaemolyticus regardless of the presence or absence of TDH and TRH. The zebrafish model is also valuable for comparing the virulence of wild-type strains to that of isogenic mutants and showed differences in the survivability of fish infected with a wild-type strain or isogenic ΔpilA and ΔmshA pilin mutants and a ΔgbpA mutant (78).
In another study, Zhang et al. (82) showed a parallel pathogenicity of fish-isolated V. parahaemolyticus 1.2164 and human-pathogenic V. parahaemolyticus 17 in the zebrafish model by four routes of infection, i.e., exposure by immersion only (78), exposure by immersion following dermal abrasion (77, 79), exposure by intramuscular injection (80, 81), and exposure by intraperitoneal (i.p.) injection (79). The survivability, histopathology, and innate immune-related cytokines induced by these two strains were compared in zebrafish, and the results suggested that the characteristics of pathogenicity were more obvious in V. parahaemolyticus 1.2164 (fish isolate)-infected zebrafish than in V. parahaemolyticus 17 (human isolate)-infected fish. Infection kinetics data showed that both V. parahaemolyticus strains cause a higher mortality rate with increasing dose and that i.p. injection had more significant effects than the other infection routes. The infection trend was confirmed by hematoxylin-eosin staining of liver and kidney, and intestine sections showed that histological lesions and proinflammatory cytokines were elevated following infection, namely, interleukin 1β (il1b product), interferon phi 1 (ifnϕ1 product), and tumor necrosis factor α (tnfα product) from tissues (82).
Adult zebrafish are also a useful model for V. parahaemolyticus vaccine studies. Peng et al. (80) showed that active or passive (rabbit antiserum against outer membrane proteins) immunization with outer membrane proteins (OMPs) yields protection against V. parahaemolyticus infection. VP2309 encodes outer membrane protein OmpH, VP0887 and VPA0548 are hypothetical proteins, and VP1019 is a known outer membrane protein of V. parahaemolyticus. OMP-immunized zebrafish demonstrated immunogenicity and cross-protective efficacy against infections by Aeromonas hydrophila or/and Pseudomonas fluorescens. When the passively immunized zebrafish were challenged with V. parahaemolyticus 3 h postimmunization, a survival rate greater than 70% was observed, whereas in case of cross-infection, the survival rate was between 25% and 85% compared with the control nonimmunized group. Actively immunized zebrafish showed a more than 60% survival rate when challenged with V. parahaemolyticus and a more than 50% survival rate in cross-infections compared with the control nonimmunized group (83).
Zebrafish larvae are also a beneficial model to study the transcriptome of V. parahaemolyticus. To describe the mechanism of the innate immune response in the zebrafish larvae infected by V. parahaemolyticus (infection by immersion), Zhang et al. (84) analyzed the transcriptomic profile of zebrafish larvae at 3 days postfertilization (dpf) immersed in a V. parahaemolyticus 13 (Vp13) strain suspension for 2 h. A total of 175 (29.07%) genes were upregulated, and 427 (70.93%) genes were downregulated. Among the latter were genes encoding complement and coagulation cascade factors, chemokines, TNF signaling pathway, nuclear factor kappa B (NF-κB) signaling pathway, and JAK-STAT signaling pathway (84). In another study, Ji et al. (85) analyzed the transcriptome of microRNAs (miRNAs) and mRNAs in zebrafish larvae infected with V. parahaemolyticus 13 (infection by immersion) to examine the regulation of innate immune responses. Thirty-seven known zebrafish miRNAs were differentially expressed in the infection group. Further study indicated that the miRNA regulation of the innate immune response was mainly driven by complex networks involving dre-miR-205-3p, dre-miR-141-5p, dre-miR-200a-5p, dre-miR-92a-2-5p, dre-miR-192, and dre-miR-1788 (85). Ji et al. later showed the effect of Notch signaling of zebrafish larvae (3 dpf) on V. parahaemolyticus 13 infection. notch1a−/− zebrafish were generated using CRISPR/Cas9. V. parahaemolyticus caused significantly higher lethality in notch1a−/− zebrafish larvae than that in wild type (WT). In transcriptome analysis, differentially expressed genes included TNF, complement, NF-κB, cathepsin, interleukin (IL), chemokines, serpin peptidase inhibitor, matrix metallopeptidase, innate immune cells, and pattern recognition receptor. In total, 246 significantly downregulated genes and 113 significantly upregulated genes were found in WT infected groups compared with WT control groups. In contrast, 82 genes were significantly downregulated, and 904 genes were significantly upregulated in notch1a−/−-infected groups compared with those of notch1a−/− control groups (86).
An advantage of the zebrafish model for V. parahaemolyticus is that V. parahaemolyticus can cause death upon infection, and survival studies are among the simplest and most informative to describe the pathogenicity and immunogenicity of bacteria in animal models. Both adult and larval zebrafish are useful for V. parahaemolyticus studies, and zebrafish can serve as a complete animal model for V. parahaemolyticus in different studies, including infection, pathogenesis, immunogenicity, and transcriptomic analysis. A potential challenge for V. parahaemolyticus studies is that many strains within this species are generally adapted to growth in higher salinity than the zebrafish host, which could be a confounding factor and a potential explanation as to why immersion infection seems to be less efficient for this pathogen than for V. cholerae.
ZEBRAFISH MODEL FOR VIBRIO VULNIFICUS
Vibrio vulnificus is a halophilic, pathogenic bacterial species that thrives in warm, tropical environments (87) and may be found in aquaculture farms during the period of highest water temperatures (88). This bacterium is commonly found as a member of natural microbiota of coastal marine environments worldwide, and as a result, it has been isolated from water, sediments, and a variety of seafood, including shrimp, fish, oysters, and clams (87). Beside those wild aquatic animals, V. vulnificus has a large effect on farm aquatic animals which is directly related to human health and sometimes can be lethal (89). The most common cause of death is septicemia caused by V. vulnificus, with an average mortality rate exceeding 50% in humans (24, 90). The consumption of seafood (primarily raw oysters) containing V. vulnificus can result in a severe systemic infection. According to the U.S. FDA, of 180 available cases between 2002 to 2007, 92.8% of patients had consumed raw oysters prior to the onset of symptoms (87).
The zebrafish is a useful model in which to study the V. vulnificus infection, where the infectivity of the bacteria can be assessed by survival studies of infected zebrafish either with or without the presence of protective or inducing agents. Pan et al. (91) showed the protective effect of pretreatment, cotreatment, and posttreatment with grouper (Epinephelus coioides) antimicrobial peptide epinecidin-1 on acute V. vulnificus infection in zebrafish. Intraperitoneal injection was selected for zebrafish infection and epinecidin-1 treatment. The study showed that cotreatment of zebrafish with epinecidin-1 and V. vulnificus achieved 78% to 97% survival rates after 30 days, whereas pretreatment and posttreatment with epinecidin-1 showed survival rates of 57% and 60%, respectively. When epinecidin-1 and V. vulnificus were coinjected and zebrafish were rechallenged after 30 days, zebrafish survival rates ranged from 22% to 47%. Using a microarray and quantitative PCR (qPCR) approach, the researchers showed that epinecidin-1 modulated the expression of immune-responsive genes like IL-10, IL-1β, tumor necrosis factor α, and interferon γ, which aided protection (91). In a parallel study, this group showed oral administration of recombinant epinecidin-1 (in E. coli BL21 containing the pET28a-epinecidin-1-dsRed plasmid) with food for 30 days significantly enhanced the expression of several immune-related genes, such as TNF-1 in grouper and Toll-like receptor 4 (TLR4), IL-1β, nitric oxide synthase 2 (NOS2), and NF-κB in zebrafish. After fish were challenged with V. vulnificus (injected into the abdominal cavity) for 24, 48, 72, or 96 h, the mortality was significantly reduced in recombinant epinecidin-1-treated grouper and zebrafish (92). This same research group also introduced plasmid DNA encoding an enhanced green fluorescent protein (EGFP)–epinecidin-1 fusion protein under the control of the cytomegalovirus (CMV) promoter into decapsulated Artemia (brine shrimp) cysts and used transgenic Artemia with commercial food for feeding zebrafish. The immune-responsive genes, including hepcidin and defbl2, were overexpressed, and the survival rate was enhanced by over 70% at 7, 14, and 21 days postinfection (dpi) in transgenic, Artemia-fed zebrafish compared with the control Artemia-fed zebrafish (93).
In another study, two transgenic zebrafish strains with strong liver-specific expression of Atlantic salmon’s Fadsd6, which catalyzes the production of docosahexaenoic acid, and Elvol5a, which catalyzes the production of eicosapentaenoic acid, driven by the zebrafish Fabp10 promoter were constructed using the Tol2 system and subjected to V. vulnificus infection (i.p.). Synthesis of n-3 polyunsaturated fatty acids (PUFAs) in these strains was increased by 2.5-fold compared with that of wild-type (WT) fish. The survival rate 24 h following challenge with V. vulnificus was 20% in WT but 70% in the transgenic fish. The expression levels of proinflammatory genes, such as TNF-α, IL-1β, and NF-κB, were suppressed between 9 and 12 hours after challenge, which increased the survival of the transgenic fish (94). Liposome-encapsulated cinnamaldehyde, a natural product isolated from cinnamon, enhanced zebrafish immunity and survival against V. vulnificus infection (95). These studies suggest that zebrafish are a good model for V. vulnificus infection in the presence of immunomodulatory and antibacterial agents.
ZEBRAFISH MODELS FOR VIBRIO ANGUILLARUM AND VIBRIO ALGINOLYTICUS
Vibrio anguillarum is responsible for hemorrhagic septicemia in several fish species, notably edible fish, including Pacific and Atlantic salmon, rainbow trout, turbot, European seabass, gilthead sea bream, striped bass, cod, and Japanese and European eel (96–99). In humans, the bacterium adheres, colonizes, and proliferates in the gut and then moves through the intestinal epithelium by endocytosis, followed by release of the bacteria in the lamina propria. Then, the pathogen enters the bloodstream resulting in septicemia or spreading to different organs, such as liver, spleen, and kidney (100).
Zebrafish served as a model for two studies with V. anguillarum, namely, a pathogenesis study and a vaccine study. As V. anguillarum is mainly a fish pathogen, the zebrafish is a very relevant small animal model for larger commercially relevant species. Randazzo et al. (100) demonstrated that zebrafish are very sensitive to i.p. challenge with V. anguillarum serotype O1 and showed severe histopathological changes in kidney hematopoietic tissue and in the intestine within 48 hpi. The fish died within 7 days. When V. anguillarum was ingested orally via infected Artemia nauplii (brine shrimp), the severity of the disease was mild with a moderate degree of immune cell infiltration of the mucosa, partial recovery at 12 dpi, and no mortality (101). Oyarbide et al. (101) used gnotobiotic zebrafish larvae (120 hpf) to investigate V. anguillarum pathogenesis. Colonization, mortality, malformations, and developmental delays were assessed at 3, 24, 48, and 72 hpi in gnotobiotic and nongnotobiotic larvae after infection by immersion with GFP-tagged V. anguillarum. Suppression of several innate immune genes, including nfκB, il1β, tlr4, mpx, and trf during the first 3 hpi was observed in both larval groups (101). In another study, O’Toole et al. showed the colonization, localization, and motility of GFP-expressing V. anguillarum in zebrafish larvae (102). Schmidt et al. compared the infection dynamics of V. anguillarum in adult zebrafish by two different administration routes, namely, i.p. injection (103) and bath inoculation (1.25 × 109 CFU), and found 50% mortality by 4 dpi in both cases. Systemic infection was detected after 24 hours of bath inoculation and immediately after i.p. injection (103).
Zhang et al. utilized zebrafish as a model for vaccine efficacy against V. anguillarum. Adult zebrafish were vaccinated with 1 × 108 CFU/ml live attenuated V. anguillarum by immersion in 3% saline for 8 min and challenged with wild-type pathogenic V. anguillarum (1 × 106 CFU/ml) by bath inoculation on day 28 after vaccination. Remarkable protection by a live attenuated strain was observed with a high relative protection survival (RPS) of about 90%. Moreover, the vaccination changed the expression of several immune-related genes in the spleens and livers of zebrafish, i.e., proinflammatory factors such as IL-1, IL-8, and major histocompatibility complex class II (MHC II) (104). The immense inflammatory response triggered by infection in nonvaccinated zebrafish was controlled in vaccinated zebrafish, and the expression levels of adaptive immune-related genes were increased in vaccinated fish after challenge compared with that in the nonvaccinated fish (105). In a microarray study, genes for iron metabolism related to the innate immunity and the signaling pathways and genes for adaptive immunity, including the genes associated with B and T cell activation, proliferation, and expansion, were highly expressed in the spleens of zebrafish vaccinated with the live attenuated V. anguillarum. Results also revealed that genes for Th17-related transcription factors, cytokines, and cytokine receptors 35 days postvaccination were highly expressed, indicating that Th17 cells were activated in bath-vaccinated zebrafish (106).
The live attenuated V. anguillarum vaccine induced notable mucosal immune responses by upregulation of TLR5, triggering a MyD88-dependent signaling pathway, antigen recognition by macrophages and neutrophils, and inflammation caused by immediate recruitment of lymphocytes in the intestine (107). The relationship between the local and systemic immune responses was described by the migration of immune cells from immune organs to antigen contact tissues for protection, such as Th1, Th2, Th17, and Treg-like responses. The production of IgM, IgZ1, and IgZ2 (the zebrafish analog for IgA) was observed in the kidney, spleen, intestine, and skin (108).
Finally, zebrafish were used to study marine Pseudomonas sp.-derived phenazine-1-carboxylic acid (PCA), which had antagonistic activities against V. anguillarum (109), stress and immune-related transcript outcomes triggered by V. anguillarum (110), and protective efficacy of probiotic yeasts against V. anguillarum challenge (111).
Zebrafish have also been used as an infection model for Vibrio alginolyticus, a waterborne pathogen that infects a wide variety of hosts, including fish and humans. Outbreaks of this pathogen can cause a huge economic loss in aquaculture. The increase in tricarboxylic acid cycle metabolites in zebrafish helps to protect them against V. alginolyticus infection (112). In another study, hepcidin (conserved in humans), an antimicrobial protein, showed innate antimicrobial activity against V. alginolyticus in a zebrafish model (113).
CONCLUDING REMARKS
Over the past 20 years, zebrafish have become a common animal model for nearly every aspect of biology, including many infectious diseases. The transparency of zebrafish embryos, relative ease of genetic manipulation, availability of transgenic animals, and relatively low cost make them an attractive model for many labs. Studies in zebrafish involving members of the Vibrio genus are especially relevant, given that vibrios are all aquatic bacteria, of which many naturally interact with fish in the environment. In a short period, the field of Vibrio microbiology in zebrafish has advanced significantly from the basic mechanistic understanding of infection, pathogenesis, immunogenicity, and transcriptomics to practical applications for therapeutics and vaccine studies, as described in this review. Future applications of the zebrafish as a host model offer an exciting opportunity to tackle the questions surrounding Vibrio species in a natural, holistic system and offer hope to the populations most affected by the diseases these bacteria entail. It is likely that this small fish will continue to generate new insights into the biology and infectious cycles of vibrios in coming years.
ACKNOWLEDGMENTS
We are grateful to the other members of the Withey lab for helpful discussions.
This work was supported by Public Health Service grant R01AI127390 (to J.H.W.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Baker-Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A, Martinez-Urtaza J. 2013. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat Clim Chang 3:73–77. doi: 10.1038/nclimate1628. [DOI] [Google Scholar]
- 2.Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J. 2018. Vibrio spp. infections. Nat Rev Dis Primers 4:8. doi: 10.1038/s41572-018-0005-8. [DOI] [PubMed] [Google Scholar]
- 3.Ali M, Nelson AR, Lopez AL, Sack DA. 2015. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters—Pacific Northwest, 1997. MMWR Morb Mortal Wkly Rep 47:457–462. [PubMed] [Google Scholar]
- 5.Nilsson WB, Paranjpye RN, Hamel OS, Hard C, Strom MS. 2019. Vibrio parahaemolyticus risk assessment in the Pacific Northwest: it’s not what’s in the water. FEMS Microbiol Ecol 95:fiz027. [DOI] [PubMed] [Google Scholar]
- 6.Vezzulli L, Grande C, Reid PC, Hélaouët P, Edwards M, Höfle MG, Bretta I, Colwell RR, Pruzzo C. 2016. Climate influence on Vibrio and associated human disease during the past half-century in the coastal North Atlantic. Proc Natl Acad Sci U S A 113:E5062–E5071. doi: 10.1073/pnas.1609157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeb R, Tufford D, Scott GI, Moore JG, Dow K. 2018. Impact of climate change on Vibrio vulnificus abundance and exposure risk. Estuaries Coast 41:2289–2303. doi: 10.1007/s12237-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddique AK, Cash R. 2013. Cholera outbreaks in the classical biotype era In Nair G, Takeda Y (ed), Cholera outbreaks. Current topics in microbiology and immunology, vol 379 Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 9.Mukhopadhyay AK, Takeda Y, Balakrish NG. 2014. Cholera outbreaks in the El Tor biotype era and the impact of the new El Tor variants In Nair G, Takeda Y (ed), Cholera outbreaks. Current topics in microbiology and immunology, vol 379 Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 10.Austin B. 2005. Bacterial pathogens of marine fish In Belkin S, Colwell RR (ed), Oceans and health: pathogens in the marine environment. Springer, Boston, MA. [Google Scholar]
- 11.Chatterjee S, Haldar S. 2012. Vibrio related diseases in aquaculture and development of rapid and accurate identification methods. J Marine Sci Res Develop S1:002. [Google Scholar]
- 12.Noriega-Orozco L, Acedo-Félix E, Higuera-Ciapara I, Jiménez-Flores R, Cano R. 2007. Pathogenic and non pathogenic Vibrio species in aquaculture shrimp ponds. Rev Latinoam Microbiol 49:60–67. [Google Scholar]
- 13.Novriadi R. 2016. Vibriosis in aquaculture. OmniAkuatika 12:1–12. [Google Scholar]
- 14.Okada K, Iida T, Kita-Tsukamoto K, Honda T. 2005. Vibrios commonly possess two chromosomes. J Bacteriol 187:752–757. doi: 10.1128/JB.187.2.752-757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada T, Arakawa E, Itoh K, Okitsu T, Matsushima A, Asai Y, Yamai S, Nakazato T, Nair GM, Albert MJ, Takeda Y. 1994. Extended serotyping scheme for Vibrio cholerae. Curr Microbiol 28:175–178. doi: 10.1007/BF01571061. [DOI] [Google Scholar]
- 16.Sozhamannan S, Waldminghaus T. 2020. Exception to the exception rule: synthetic and naturally occurring single chromosome Vibrio cholerae. Environ Microbiol doi: 10.1111/1462-2920.15002.. [DOI] [PubMed] [Google Scholar]
- 17.Chapman C, Henry M, Bishop-Lilly KA, Awosika J, Briska A, Ptashkin RN, Wagner T, Rajanna C, Tsang H, Johnson SL, Mokashi VP, Chain PSG, Sozhamannan S. 2015. Scanning the landscape of genome architecture of non-O1 and non-O139 Vibrio cholerae by whole genome mapping reveals extensive population genetic diversity. PLoS One 10:e0120311. doi: 10.1371/journal.pone.0120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson SL, Khiani A, Bishop-Lilly KA, Chapman C, Patel M, Verratti K, Teshima H, Munk AC, Bruce DC, Han CS, Xie G, Davenport KW, Chain P, Sozhamannan S. 2015. Complete genome assemblies for two single-chromosome Vibrio cholerae isolates, strains 1154-74 (serogroup O49) and 10432-62 (serogroup O27). Genome Announc 3:e00462-15. doi: 10.1128/genomeA.00462-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabrowski K, Miller M. 2018. Contested paradigm in raising zebrafish (Danio rerio). Zebrafish 15:295–309. doi: 10.1089/zeb.2017.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortemeglia C, Beitinger TL. 2005. Temperature tolerances of wild-type and red transgenic zebra Danios. Trans Am Fish Soc 134:1431–1437. doi: 10.1577/T04-197.1. [DOI] [Google Scholar]
- 21.Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996-2010: review of surveillance data from 2 systems. Clin Infect Dis 54:391–395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dechet AM, Yu PA, Koram N, Painter J. 2008. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis 46:970–976. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- 23.Rippey SR. 1994. Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev 7:419–425. doi: 10.1128/cmr.7.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hlady WG, Klontz KC. 1996. The epidemiology of Vibrio infections in Florida, 1981 to 1993. J Infect Dis 173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 25.Mitra AK. 2004. Vibrio vulnificus infection: epidemiology, clinical presentations, and prevention. South Med J 97:118–119. doi: 10.1097/01.SMJ.0000092520.47509.C2. [DOI] [PubMed] [Google Scholar]
- 26.Global Task Force on Cholera Control. 2017. Ending cholera—a global roadmap to 2030. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 27.Domman D, Quilici M-L, Dorman MJ, Njamkepo E, Mutreja A, Mather AE, Delgado G, Morales-Espinosa R, Grimont PAD, Lizárraga-Partida ML, Bouchier C, Aanensen DM, Kuri-Morales P, Tarr CL, Dougan G, Parkhill J, Campos J, Cravioto A, Weill F-X, Thomson NR. 2017. Integrated view of Vibrio cholerae in the Americas. Science 358:789–793. doi: 10.1126/science.aao2136. [DOI] [PubMed] [Google Scholar]
- 28.Weill F-X, Domman D, Njamkepo E, Tarr C, Rauzier J, Fawal N, Keddy KH, Salje H, Moore S, Mukhopadhyay AK, Bercion R, Luquero FJ, Ngandjio A, Dosso M, Monakhova E, Garin B, Bouchier C, Pazzani C, Mutreja A, Grunow R, Sidikou F, Bonte L, Breurec S, Damian M, Njanpop-Lafourcade B-M, Sapriel G, Page A-L, Hamze M, Henkens M, Chowdhury G, Mengel M, Koeck J-L, Fournier J-M, Dougan G, Grimont PAD, Parkhill J, Holt KE, Piarroux R, Ramamurthy T, Quilici M-L, Thomson NR. 2017. Genomic history of the seventh pandemic of cholera in Africa. Science 358:785–789. doi: 10.1126/science.aad5901. [DOI] [PubMed] [Google Scholar]
- 29.Weill F-X, Domman D, Njamkepo E, Almesbahi AA, Naji M, Nasher SS, Rakesh A, Assiri AM, Sharma NC, Kariuki S, Pourshafie MR, Rauzier J, Abubakar A, Carter JY, Wamala JF, Seguin C, Bouchier C, Malliavin T, Bakhshi B, Abulmaali HHN, Kumar D, Njoroge SM, Malik MR, Kiiru J, Luquero FJ, Azman AS, Ramamurthy T, Thomson NR, Quilici M-L. 2019. Genomic insights into the 2016-2017 Yemeni cholera epidemic. Nature 565:230–233. doi: 10.1038/s41586-018-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orata FD, Keim PS, Boucher Y. 2014. The 2010 cholera outbreak in Haiti: how science solved a controversy. PLoS Pathog 10:e1003967. doi: 10.1371/journal.ppat.1003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz LS, Petkau A, Beaulaurier J, Tyler S, Antonova ES, Turnsek MA, Guo Y, Wang S, Paxinos EE, Orata F, Gladney LM, Stroika S, Folster JP, Rowe L, Freeman MM, Knox N, Frace M, Boncy J, Graham M, Hammer BK, Boucher Y, Bashir A, Hanage WP, Domselaar GV, Tarr CL. 2013. Evolutionary dynamics of Vibrio cholerae O1 following a single-source introduction to Haiti. mBio 4:e00398-13. doi: 10.1128/mBio.00398-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. 2011. The origin of the Haitian cholera outbreak strain. N Engl J Med 364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimer AR, Van Domselaar G, Stroika S, Walker M, Kent H, Tarr C, Talkington D, Rowe L, Olsen-Rasmussen M, Frace M, Sammons S, Dahourou GA, Boncy J, Smith AM, Mabon P, Petkau A, Graham M, Gilmour MW, Gerner-Smidt P, Vibrio cholerae Outbreak Genomics Task Force. 2011. Comparative genomics of Vibrio cholerae from Haiti, Asia, and Africa. Emerg Infect Dis 17:2113–2121. doi: 10.3201/eid1711.110794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frerichs RR, Keim PS, Barrais R, Piarroux R. 2012. Nepalese origin of cholera epidemic in Haiti. Clin Microbiol Infect 18:E158–E163. doi: 10.1111/j.1469-0691.2012.03841.x. [DOI] [PubMed] [Google Scholar]
- 35.Hendriksen RS, Price LB, Schupp JM, Gillece JD, Kaas RS, Engelthaler DM, Bortolaia V, Pearson T, Waters AE, Upadhyay BP, Shrestha SD, Adhikari S, Shakya G, Keim PS, Aarestrup FM. 2011. Population genetics of Vibrio cholerae from Nepal in 2010: evidence on the origin of the Haitian outbreak. mBio 2:e00157-11. doi: 10.1128/mBio.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritchie JM, Rui HP, Bronson RT, Waldor MK. 2010. Back to the future: studying cholera pathogenesis using infant rabbits. mBio 1:e00047-10. doi: 10.1128/mBio.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klose KE. 2000. The suckling mouse model of cholera. Trends Microbiol 8:189–191. doi: 10.1016/s0966-842x(00)01721-2. [DOI] [PubMed] [Google Scholar]
- 38.Formal SB, Schneider H, Sprinz H, Kunev N, Kundel D. 1961. Studies with Vibrio cholerae in ligated loop of rabbit intestine. Br J Exp Pathol 42:504–510. [PMC free article] [PubMed] [Google Scholar]
- 39.Spira WM, Sack RB, Froehlich JL. 1981. Simple adult-rabbit model for Vibrio cholerae and entero-toxigenic Escherichia coli diarrhea. Infect Immun 32:739–747. doi: 10.1128/IAI.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole MD, Oliver JD. 1978. Experimental pathogenicity and mortality in ligated ileal loop studies of newly reported halophilic lactose-positive Vibrio sp. Infect Immun 20:126–129. doi: 10.1128/IAI.20.1.126-129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoashi K, Ogata K, Taniguchi H, Yamashita H, Tsuji K, Mizuguchi Y, Ohtomo N. 1990. Pathogenesis of Vibrio parahaemolyticus: intraperitoneal and orogastic challenge experiments in mice. Microbiol Immunol 34:355–366. doi: 10.1111/j.1348-0421.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 42.Calia FM, Johnson DE. 1975. Bacteremia in suckling rabbits after oral challenge with Vibrio parahaemolyticus. Infect Immun 11:1222–1225. doi: 10.1128/IAI.11.6.1222-1225.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown DF, Spaulding PL, Twedt RM. 1977. Enteropathogenicity of Vibrio parahaemolyticus in ligated rabbit ileum. Appl Environ Microbiol 33:10–14. doi: 10.1128/AEM.33.1.10-14.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. 2014. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol 80:1710–1717. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell KC, Withey JH. 2018. Danio rerio as a native host model for understanding pathophysiology of Vibrio cholerae, p 97–102. In Sikora AE. (ed), Vibrio cholerae: methods and protocols, vol 1839 Humana Press Inc, Totowa, NJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ran C, Qin C, Xie M, Zhang J, Li J, Xie Y, Wang Y, Li S, Liu L, Fu X, Lin Q, Li N, Liles MR, Zhou Z. 2018. Aeromonas veronii and aerolysin are important for the pathogenesis of motile aeromonad septicemia in cyprinid fish. Environ Microbiol 20:3442–3456. doi: 10.1111/1462-2920.14390. [DOI] [PubMed] [Google Scholar]
- 47.Howlader DR, Sinha R, Nag D, Majumder N, Mukherjee P, Bhaumik U, Maiti S, Withey JH, Koley H. 2016. Zebrafish as a novel model for non-typhoidal Salmonella pathogenesis, transmission and vaccine efficacy. Vaccine 34:5099–5106. doi: 10.1016/j.vaccine.2016.08.077. [DOI] [PubMed] [Google Scholar]
- 48.Prouty MG, Correa NE, Barker LP, Jagadeeswaran P, Klose KE. 2003. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol Lett 225:177–182. doi: 10.1016/S0378-1097(03)00446-4. [DOI] [PubMed] [Google Scholar]
- 49.Traoré O, Martikainen O, Siitonen A, Traoré AS, Barro N, Haukka K. 2014. Occurrence of Vibrio cholerae in fish and water from a reservoir and a neighboring channel in Ouagadougou, Burkina Faso. J Infect Dev Ctries 8:1334–1338. doi: 10.3855/jidc.3946. [DOI] [PubMed] [Google Scholar]
- 50.Kiiyukia C, Nakajima A, Nakai T, Muroga K, Kawakami H, Hashimoto H. 1992. Vibrio cholerae non-O1 isolated from ayu fish (Plecoglossus altivelis) in Japan. Appl Environ Microbiol 58:3078–3082. doi: 10.1128/AEM.58.9.3078-3082.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJ. 2016. The composition of the zebrafish intestinal microbial community varies across development. ISME J 10:644–654. doi: 10.1038/ismej.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. 2011. Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olivier V, Salzman NH, Satchell KJF. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect Immun 75:5043–5051. doi: 10.1128/IAI.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hapfelmeier S, Lawson MAE, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, Curtiss R, McCoy KD, Macpherson AJ. 2010. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brugman S. 2016. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol 64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Engeszer RE, Patterson LB, Rao AA, Parichy DM. 2007. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 57.Breen P, Winters AD, Nag D, Ahmad MM, Theis KR, Withey JH. 2019. Internal versus external pressures: effect of housing systems on the zebrafish microbiome. Zebrafish 16:388–400. doi: 10.1089/zeb.2018.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiles TJ, Jemielita M, Baker RP, Schlomann BH, Logan SL, Ganz J, Melancon E, Eisen JS, Guillemin K, Parthasarathy R. 2016. Host gut motility promotes competitive exclusion within a model intestinal microbiota. PLoS Biol 14:e1002517. doi: 10.1371/journal.pbio.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Senderovich Y, Izhaki I, Halpern M. 2010. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mashoof S, Criscitiello MF. 2016. Fish immunoglobulins. Biology 5:45. doi: 10.3390/biology5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Du J, Lam SH, Mathavan S, Matsudaira P, Gong Z. 2010. Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 11:392. doi: 10.1186/1471-2164-11-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nag D, Breen P, Raychaudhuri S, Withey JH. 2018. Glucose metabolism by Escherichia coli inhibits Vibrio cholerae intestinal colonization of zebrafish. Infect Immun 86:e00486-18. doi: 10.1128/IAI.00486-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Gu J. 2005. Influence of temperature, salinity and pH on the growth of environmental Aeromonas and Vibrio species isolated from Mai Po and the Inner Deep Bay Nature Reserve Ramsar Site of Hong Kong. J Basic Microbiol 45:83–93. doi: 10.1002/jobm.200410446. [DOI] [PubMed] [Google Scholar]
- 64.Nag D, Mitchell K, Breen P, Withey JH. 2018. Quantifying Vibrio cholerae colonization and diarrhea in the adult zebrafish model. J Vis Exp 12:57767. doi: 10.3791/57767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logan SL, Thomas J, Yan J, Baker RP, Shields DS, Xavier JB, Hammer BK, Parthasarathy R. 2018. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc Natl Acad Sci U S A 115:E3779–E3787. doi: 10.1073/pnas.1720133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manneh-Roussel J, Haycocks JR, Magán A, Perez-Soto N, Voelz K, Camilli A, Krachler AM, Grainger DC. 2018. cAMP receptor protein controls Vibrio cholerae gene expression in response to host colonization. mBio 9:e00966-18. doi: 10.1128/mBio.00966-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeAngelis CM, Nag D, Withey JH, Matson JS. 2019. Characterization of the Vibrio cholerae phage shock protein response. J Bacteriol 201:e00761-18. doi: 10.1128/JB.00761-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiles TJ, Schlomann BH, Wall ES, Betancourt R, Parthasarathy R, Guillemin K. 2020. Swimming motility of a gut bacterial symbiont promotes resistance to intestinal expulsion and enhances inflammation. PLoS Biol 18:e3000661. doi: 10.1371/journal.pbio.3000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerg Infect Dis 5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson DE, Weinberg L, Ciarkowski J, West P, Colwell RR. 1984. Wound infection caused by Kanagawa-negative Vibrio parahaemolyticus. J Clin Microbiol 20:811–812. doi: 10.1128/JCM.20.4.811-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weis KE, Hammond RM, Hutchinson R, Blackmore CGM. 2011. Vibrio illness in Florida, 1998-2007. Epidemiol Infect 139:591–598. doi: 10.1017/S0950268810001354. [DOI] [PubMed] [Google Scholar]
- 72.Hurley CC, Quirke A, Reen FJ, Boyd EF. 2006. Four genomic islands that mark post-1995 pandemic Vibrio parahaemolyticus isolates. BMC Genomics 7:104–123. doi: 10.1186/1471-2164-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Izutsu K, Kurokawa K, Tashiro K, Kuhara S, Hayashi T, Honda T, Iida T. 2008. Comparative genomic analysis using microarray demonstrates a strong correlation between the presence of the 80-kilobase pathogenicity island and pathogenicity in Kanagawa phenomenon positive Vibrio parahaemolyticus strains. Infect Immun 76:1016–1023. doi: 10.1128/IAI.01535-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kodama T, Hiyoshi H, Gotoh K, Akeda Y, Matsuda S, Park KS, Cantarelli VV, Iida T, Honda T. 2008. Identification of two translocon proteins of Vibrio parahaemolyticus type III secretion system 2. Infect Immun 76:4282–4289. doi: 10.1128/IAI.01738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y, Kimura S, Yasunaga T, Honda T, Shinagawa H, Hattori M, Iida T. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet 361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 76.Piñeyro P, Zhou X, Orfe LH, Friel PJ, Lahmers K, Call DR. 2010. Development of two animal models to study the function of Vibrio parahaemolyticus type III secretion systems. Infect Immun 78:4551–4559. doi: 10.1128/IAI.00461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paranjpye RN, Myers MS, Yount EC, Thompson JL. 2013. Zebrafish as a model for Vibrio parahaemolyticus virulence. Microbiology 159:2605–2615. doi: 10.1099/mic.0.067637-0. [DOI] [PubMed] [Google Scholar]
- 78.Frischkorn KR, Stojanovski A, Paranjpye R. 2013. Vibrio parahaemolyticus type IV pili mediate interactions with diatom-derived chitin and point to an unexplored mechanism of environmental persistence. Environ Microbiol 15:1416–1427. doi: 10.1111/1462-2920.12093. [DOI] [PubMed] [Google Scholar]
- 79.Neely MN, Pfeifer JD, Caparon M. 2002. Streptococcus-zebrafish model of bacterial pathogenesis. Infect Immun 70:3904–3914. doi: 10.1128/iai.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moyer TR, Hunnicutt DW. 2007. Susceptibility of zebra fish Danio rerio to infection by Flavobacterium columnare and F. johnsoniae. Dis Aquat Organ 76:39–44. doi: 10.3354/dao076039. [DOI] [PubMed] [Google Scholar]
- 81.Phelps HA, Runft DL, Neely MN. 2009. Adult zebrafish model of streptococcal infection. Curr Protoc Microbiol Chapter 9: Unit 9D.1. doi: 10.1002/9780471729259.mc09d01s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Q, Dong X, Chen B, Zhang Y, Zu Y, Li W. 2016. Zebrafish as a useful model for zoonotic Vibrio parahaemolyticus pathogenicity in fish and human. Dev Comp Immunol 55:159–168. doi: 10.1016/j.dci.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Peng B, Ye JZ, Han Y, Zeng L, Zhang JY, Li H. 2016. Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish Immunol 54:204–210. doi: 10.1016/j.fsi.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Q, Ji C, Ren J, Zhang Q, Dong X, Zu Y, Jia L, Li W. 2018. Differential transcriptome analysis of zebrafish (Danio rerio) larvae challenged by Vibrio parahaemolyticus. J Fish Dis 41:1049–1062. doi: 10.1111/jfd.12796. [DOI] [PubMed] [Google Scholar]
- 85.Ji C, Guo X, Ren J, Zu Y, Li W, Zhang Q. 2019. Transcriptomic analysis of microRNAs-mRNAs regulating innate immune response of zebrafish larvae against Vibrio parahaemolyticus infection. Fish Shellfish Immunol 91:333–342. doi: 10.1016/j.fsi.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 86.Ji C, Guo X, Dong X, Ren J, Zu Y, Li W, Zhang Q. 2019. Notch1a can widely mediate innate immune responses in zebrafish larvae infected with Vibrio parahaemolyticus. Fish Shellfish Immunol 92:680–689. doi: 10.1016/j.fsi.2019.06.058. [DOI] [PubMed] [Google Scholar]
- 87.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723–1733. doi: 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pedersen K, Skall HF, Lassen-Nielsen AM, Nielsen TF, Henriksen NH, Olesen NJ. 2008. Surveillance of health status on eight marine rainbow trout, Oncorhynchus mykiss (Walbaum), farms in Denmark in 2006. J Fish Dis 31:659–667. doi: 10.1111/j.1365-2761.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 89.Amaro C, Biosca EG, Fouz B, Alcaide E, Esteve C. 1995. Evidence that water transmits Vibrio vulnificus biotype 2 infections to eels. Appl Environ Microbiol 61:1133e7. doi: 10.1128/AEM.61.3.1133-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldhusen F. 2000. The role of seafood in bacterial foodborne diseases. Microbes Infect 2:1651–1660. doi: 10.1016/S1286-4579(00)01321-6. [DOI] [PubMed] [Google Scholar]
- 91.Pan CY, Wu JL, Hui CF, Lin CH, Chen JY. 2011. Insights into the antibacterial and immunomodulatory functions of the antimicrobial peptide, epinecidin-1, against Vibrio vulnificus infection in zebrafish. Fish Shellfish Immunol 31:1019–1025. doi: 10.1016/j.fsi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Pan CY, Huang TC, Wang YD, Yeh YC, Hui CF, Chen JY. 2012. Oral administration of recombinant epinecidin-1 protected grouper (Epinephelus coioides) and zebrafish (Danio rerio) from Vibrio vulnificus infection and enhanced immune-related gene expressions. Fish Shellfish Immunol 32:947–957. doi: 10.1016/j.fsi.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 93.Jheng YH, Lee LH, Ting CH, Pan CY, Hui CF, Chen JY. 2015. Zebrafish fed on recombinant Artemia expressing epinecidin-1 exhibit increased survival and altered expression of immunomodulatory genes upon Vibrio vulnificus infection. Fish Shellfish Immunol 42:1–15. doi: 10.1016/j.fsi.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 94.Cheng C-L, Huang S-J, Wu C-L, Gong H-Y, Ken C-F, Hu S-Y, Wu J-L. 2015. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J Biomed Sci 22:103. doi: 10.1186/s12929-015-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faikoh EN, Hong Y-H, Hu S-Y. 2014. Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol 38:15–24. doi: 10.1016/j.fsi.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 96.Toranzo AE, Barja JL. 1990. A review of the taxonomy and seroepizootiology of Vibrio anguillarum, with special reference to aquaculture in the northwest of Spain. Dis Aquat Org 9:73–82. doi: 10.3354/dao009073. [DOI] [Google Scholar]
- 97.Toranzo AE, Barja JL. 1993. Virulence factors of bacteria pathogenic for cold water fish. Annu Rev Fish Dis 3:5–36. doi: 10.1016/0959-8030(93)90027-9. [DOI] [Google Scholar]
- 98.Actis LA, Tolmasky ME, Crosa JH. 1999. Vibriosis, p 523–558. In Woo P, Bruno D (ed), Fish diseases and disorders: viral, bacterial fungal infections. CAB International Publishing, Wallingford, United Kingdom. [Google Scholar]
- 99.Toranzo AE, Magariños B, Romalde JL. 2005. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 246:37–61. doi: 10.1016/j.aquaculture.2005.01.002. [DOI] [Google Scholar]
- 100.Randazzo B, Abbate F, Marino F, Mancuso M, Guerrera MC, Muglia U, Navarra M, Germanà A. 2015. Induction of mild enterocolitis in zebrafish Danio rerio via ingestion of Vibrio anguillarum serovar O1. Dis Aquat Organ 115:47–55. doi: 10.3354/dao02864. [DOI] [PubMed] [Google Scholar]
- 101.Oyarbide U, Iturria I, Rainieri S, Pardo MA. 2015. Use of gnotobiotic zebrafish to study Vibrio anguillarum pathogenicity. Zebrafish 12:71–80. doi: 10.1089/zeb.2014.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Toole R, Von Hofsten J, Rosqvist R, Olsson PE, Wolf-Watz H. 2004. Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb Pathog 37:41–46. doi: 10.1016/j.micpath.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt JG, Korbut R, Ohtani M, Jørgensen LVG. 2017. Zebrafish (Danio rerio) as a model to visualize infection dynamics of Vibrio anguillarum following intraperitoneal injection and bath exposure. Fish Shellfish Immunol 67:692–697. doi: 10.1016/j.fsi.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Z, Wu H, Xiao J, Wang Q, Liu Q, Zhang Y. 2012. Immune responses of zebrafish (Danio rerio) induced by bath-vaccination with a live attenuated Vibrio anguillarum vaccine candidate. Fish Shellfish Immunol 33:36–41. doi: 10.1016/j.fsi.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z, Wu H, Xiao J, Wang Q, Liu Q, Zhang Y. 2013. Immune responses evoked by infection with Vibrio anguillarum in zebrafish bath-vaccinated with a live attenuated strain. Vet Immunol Immunopathol 154:138–144. doi: 10.1016/j.vetimm.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H, Fei C, Wu H, Yang M, Liu Q, Wang Q, Zhang Y. 2013. Transcriptome profiling reveals Th17-like immune responses induced in zebrafish bath-vaccinated with a live attenuated Vibrio anguillarum. PLoS One 8:e73871. doi: 10.1371/journal.pone.0073871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X, Wu H, Chang X, Tang Y, Liu Q, Zhang Y. 2014. Notable mucosal immune responses induced in the intestine of zebrafish (Danio rerio) bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol 40:99–108. doi: 10.1016/j.fsi.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 108.Liu X, Wu H, Liu Q, Wang Q, Xiao J, Chang X, Zhang Y. 2015. Profiling immune response in zebrafish intestine, skin, spleen and kidney bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol 45:342–345. doi: 10.1016/j.fsi.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L, Tian X, Kuang S, Liu G, Zhang C, Sun C. 2017. Antagonistic activity and mode of action of phenazine-1-carboxylic acid, produced by marine bacterium Pseudomonas aeruginosa PA31x, against Vibrio anguillarum in vitro and in a zebrafish in vivo model. Front Microbiol 8:289. doi: 10.3389/fmicb.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khansari AR, Balasch JC, Vallejos-Vidal E, Teles M, Fierro-Castro C, Tort L, Reyes-López FE. 2019. Comparative study of stress and immune-related transcript outcomes triggered by Vibrio anguillarum bacterin and air exposure stress in liver and spleen of gilthead seabream (Sparus aurata), zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 86:436–448. doi: 10.1016/j.fsi.2018.11.063. [DOI] [PubMed] [Google Scholar]
- 111.Caruffo M, Navarrete N, Salgado O, Diaz A, Lopez P, Garcia K, Feijoo CG, Navarrete P. 2015. Potential probiotic yeasts isolated from the fish gut protect zebrafish (Danio rerio) from a Vibrio anguillarum challenge. Front Microbiol 6:1093. doi: 10.3389/fmicb.2015.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang M-J, Cheng Z-X, Jiang M, Zeng Z-H, Peng B, Peng X-X, Li H. 2018. Boosted TCA cycle enhances survival of zebrafish to Vibrio alginolyticus infection. Virulence 9:634–644. doi: 10.1080/21505594.2017.1423188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang X-F, Liu Z-F, Lin A-F, Xiang L-X, Shao J-Z. 2017. Coordination of bactericidal and iron regulatory functions of hepcidin in innate antimicrobial immunity in a zebrafish model. Sci Rep 7:4265. doi: 10.1038/s41598-017-04069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]