Oral cholera vaccines (OCVs) are being deployed to combat cholera, but current killed OCVs require multiple doses and show little efficacy in young children. Live OCVs have the potential to overcome these limitations, but small-animal models for testing OCVs have shortcomings. We used an antibiotic treatment protocol for conventional adult mice to study the effects of short-term colonization by a single dose of HaitiV, a live-OCV candidate. Vaccinated mice developed vibriocidal antibodies against V. cholerae and delivered pups that were resistant to cholera, whereas mice vaccinated with inactivated HaitiV did not. These findings demonstrate HaitiV’s immunogenicity and suggest that this antibiotic treatment protocol will be useful for evaluating the efficacy of live OCVs.
KEYWORDS: cholera, live vaccine, oral cholera vaccine, streptomycin
ABSTRACT
Current mouse models for evaluating the efficacy of live oral cholera vaccines (OCVs) have important limitations. Conventionally raised adult mice are resistant to intestinal colonization by Vibrio cholerae, but germfree mice can be colonized and have been used to study OCV immunogenicity. However, germfree animals have impaired immune systems and intestinal physiology; also, live OCVs colonize germfree mice for many months, which does not mimic the clearance kinetics of live OCVs in humans. In this study, we leveraged antibiotic-treated, conventionally raised adult mice to study the effects of transient intestinal colonization by a live OCV V. cholerae strain. In a single-dose vaccination regimen, we found that HaitiV, a live-attenuated OCV candidate, was cleared by streptomycin-treated adult mice within 2 weeks after oral inoculation. This transient colonization elicited far stronger adaptive immune correlates of protection against cholera than did inactivated whole-cell HaitiV. Infant mice from HaitiV-vaccinated dams were also significantly more protected from choleric disease than pups from inactivated-HaitiV-vaccinated dams. Our findings establish the benefits of antibiotic-treated mice for live-OCV studies as well as their limitations and underscore the immunogenicity of HaitiV.
IMPORTANCE Oral cholera vaccines (OCVs) are being deployed to combat cholera, but current killed OCVs require multiple doses and show little efficacy in young children. Live OCVs have the potential to overcome these limitations, but small-animal models for testing OCVs have shortcomings. We used an antibiotic treatment protocol for conventional adult mice to study the effects of short-term colonization by a single dose of HaitiV, a live-OCV candidate. Vaccinated mice developed vibriocidal antibodies against V. cholerae and delivered pups that were resistant to cholera, whereas mice vaccinated with inactivated HaitiV did not. These findings demonstrate HaitiV’s immunogenicity and suggest that this antibiotic treatment protocol will be useful for evaluating the efficacy of live OCVs.
INTRODUCTION
Vibrio cholerae is the cause of cholera, and following ingestion of water or food contaminated with this Gram-negative rod, humans can develop the severe and sometimes lethal dehydrating diarrhea that characterizes cholera. Cholera remains a major threat to global public health, with approximately 2.9 million cases and 95,000 deaths reported annually (1). Serologic classification of V. cholerae is based on the structure and chemistry of the abundant lipopolysaccharide (LPS) O antigen, and the O1 serogroup of V. cholerae has given rise to all cholera pandemics. The O1 serogroup is further subdivided into the Ogawa and Inaba serotypes, which differ by the presence or absence of methylation of the terminal perosamine on their respective O antigens (2). Current pandemic cholera is predominantly caused by an O1 variant El Tor biotype strain, such as the strain responsible for the Haitian epidemic in 2010 (3).
Prior exposure to V. cholerae can elicit long-lived protective O-antigen-specific responses against V. cholerae (4, 5), suggesting that vaccination has the potential to elicit protective immunity if vaccines can safely mimic elements of natural infection. Consequently, several vaccination strategies for cholera have been developed over the decades. Among these, oral cholera vaccines (OCVs) are attractive options, as they stimulate immunity at the intestinal mucosal surface, the site of infection, and because of their ease of administration. Killed whole-cell OCVs, such as Shanchol, are being increasingly adopted as frontline public health tools in regions of endemicity (6) as well as to limit outbreaks (7). Live-attenuated OCVs have also been developed and have theoretical advantages over killed OCVs, including in vivo replication, which enables continuous presentation of in vivo-induced antigens at the intestinal mucosal surface (8). In contrast to killed OCVs, live vaccines will likely offer single-dose efficacy, a particularly important feature for reactive vaccination campaigns during epidemics. Furthermore, live OCVs appear to be more effective in children less than 5 years of age (9, 10), a group that is highly susceptible to death from cholera and that is not adequately protected by killed OCVs. In volunteer studies, live vaccines have shown great promise (11, 12), but none are approved for use in regions where cholera is endemic.
The development of both inactivated and live OCVs has been hampered by the lack of a small-animal model that closely recapitulates cholera pathogenesis in the setting of normal immune reactivity. V. cholerae readily colonizes the intestines of infant mice and infant rabbits (reviewed in reference 13), in which cholera-like disease can be observed, but these models lack mature immune systems. Conventionally raised adult mice cannot be orally colonized by V. cholerae, likely due to their resident gut microbiota (14). Germfree (GF) adult mice, which lack a microbiota, can be colonized by V. cholerae and have been used to profile OCV immunogenicity, but immune and intestinal physiological development is aberrant in these animals (15). Nonetheless, vaccinated GF mice develop immune correlates of clinical protection against toxigenic V. cholerae, including circulating vibriocidal antibodies and antigen-specific antibody responses (14, 16, 17). Another limitation of the GF adult mouse model is that following oral administration of a live OCV, the mice remain consistently colonized by V. cholerae for periods exceeding 3 months (14, 17), making single-dose live-OCV regimens difficult to interpret; moreover, the prolonged colonization in this model precludes challenging vaccinated animals with virulent V. cholerae. The consequence and significance of long-term monocolonization with a live OCV in GF mice also remain unknown.
Many studies have shown that oral administration of broad-spectrum antibiotics to mice depletes the gut microbiota and enables intestinal colonization by diverse bacteria (18). This is beneficial, as antibiotic-treated mice are conventionally raised and do not display the immunological and developmental defects that characterize GF mice. Antibiotic treatments can enable wild-type V. cholerae intestinal colonization for similar durations, typically 5 to 7 days, that humans are colonized by live OCVs (19, 20). We sought to leverage this model to profile HaitiV, a live-attenuated OCV derived from HaitiWT, a virulent V. cholerae O1 Ogawa clinical strain isolated during the Haitian cholera outbreak (21). HaitiV is nontoxigenic and highly engineered to address biosafety concerns. In infant rabbits, HaitiV provides unprecedented rapid protection against virulent V. cholerae, within 24 h of administration (21); furthermore, we showed that this vaccine is immunogenic in GF mice (17).
In this study, we adopted a streptomycin (Sm)-treated adult mouse model of V. cholerae (22) to profile HaitiV’s immunogenicity. Furthermore, we modified this model to assess the protective efficacy of immune responses in immunized female mice, by challenging their pups with HaitiWT. Our findings demonstrate the ease and utility of this approach for studies of live-attenuated OCVs.
RESULTS
Vaccine inoculation protocol and colonization kinetics.
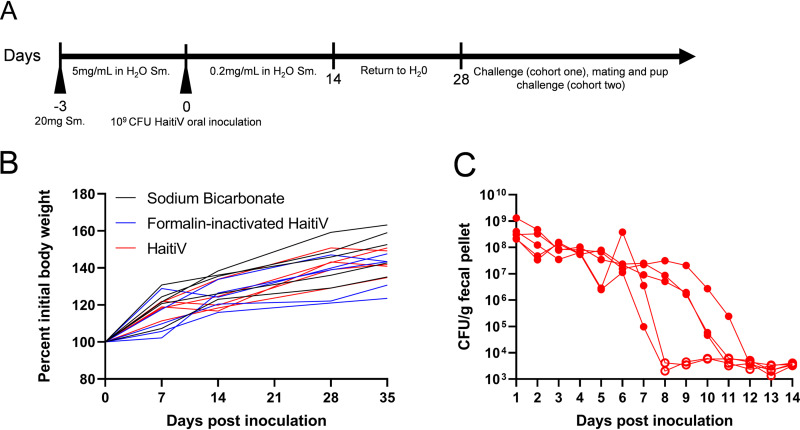
We modified the protocol presented by Bueno et al. (22) and orally treated two cohorts of 4-week-old C57BL/6 female mice with streptomycin (Sm) to deplete their intestinal microbiota to enable a longitudinal study of HaitiV intestinal colonization and immunogenicity (Fig. 1A). Three days after initiation of Sm treatment, mice were administered by oral gavage either a single dose of 109 CFU of HaitiV, an equivalent dose of formalin-inactivated HaitiV (FI-HaitiV) to mimic oral vaccination with an inactivated OCV, or sodium bicarbonate as a vehicle control. All mice across both cohorts gained weight over the duration of all experiments (Fig. 1B; see also Fig. S1A in the supplemental material) and did not display adverse effects from the antibiotic treatment or HaitiV colonization.
FIG 1.
Transient intestinal colonization of HaitiV in adult mice following treatment with streptomycin. (A) Schematic of streptomycin treatment and single oral inoculation with HaitiV. Arrowheads indicate peroral treatment with either streptomycin in sodium bicarbonate or oral gavage with bacteria. Three groups of female C57BL/6 mice (n = 5) were inoculated with either 109 CFU of HaitiV, 109 CFU of formalin-inactivated HaitiV, or a vehicle (sodium bicarbonate) control and kept in conventional housing with streptomycin-supplemented drinking water for the indicated time. Fresh fecal pellets were collected daily from all mice, and blood draws were conducted weekly starting on the day of inoculation. (B) Body weights of mice from cohort 1 over the course of this study. (C) Fecal shedding of HaitiV from mice inoculated with HaitiV. Open symbols depict CFU levels below the limit of detection. No CFU of HaitiV could be recovered from fecal pellets from mice inoculated with formalin-inactivated HaitiV or sodium bicarbonate.
Plating of fresh fecal pellets (FPs) from all animals inoculated with HaitiV revealed ∼108 CFU/g of FP for 5 to 7 days postinoculation (dpi), suggesting that initially the vaccine robustly colonized the intestines of Sm-treated adult mice. However, HaitiV was no longer detectable in FPs by 8 to 12 dpi (Fig. 1C; Fig. S1B), indicating clearance of the vaccine strain. No HaitiV CFU were recovered from Sm-treated mice that had been orally inoculated with FI-HaitiV or buffer control. The clearance kinetics of HaitiV from these mice resembles that previously charted by Nygren et al. (19) for wild-type cholera toxin-producing V. cholerae isolates, suggesting that cholera toxin does not play a substantial role in intestinal colonization in this model.
Transient HaitiV colonization elicits antibodies targeting both V. cholerae serotypes.
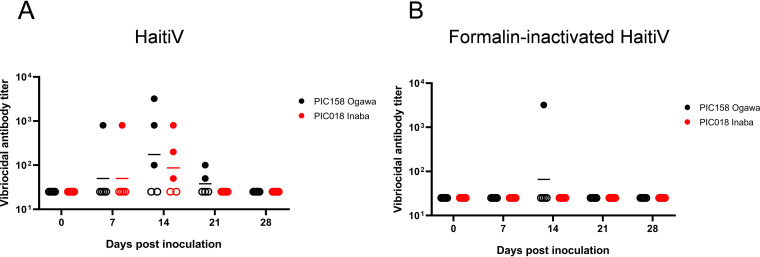
Sera from all mice were individually assayed to quantify the circulating vibriocidal antibody titers (VATs) targeting Ogawa and Inaba V. cholerae strains. By 7 dpi, most mice did not have detectable VATs, but by 14 dpi, when HaitiV was no longer detectable in FPs, most (3/5 mice) from cohort 1 seroconverted and developed high circulating VATs against both serotyped-matched (Ogawa) and serotype-mismatched (Inaba) V. cholerae (Fig. 2A), though the highest geometric mean VATs were directed against serotype-matched (Ogawa) isolates (Fig. 2A). Only one mouse in the FI-HaitiV group developed circulating VATs against Ogawa serotype V. cholerae, and no mice in this group developed vibriocidal antibodies against Inaba V. cholerae (Fig. 2B). Mice from cohort 2 (used for mating and subsequent infant mouse studies) displayed similar trends, with 4/5 HaitiV mice developing VATs against Ogawa V. cholerae and 2/5 HaitiV mice developing VATs against Inaba V. cholerae by 14 dpi (Fig. S1C). None of the Sm-treated mice that received sodium bicarbonate developed detectable vibriocidal antibodies. Thus, transient colonization by HaitiV is sufficient to elicit the generation of V. cholerae-specific circulating markers of immunity.
FIG 2.
Serum vibriocidal antibody titers detected in cohort 1 of Sm-treated mice immunized with HaitiV (n = 5) (A) or formalin-inactivated HaitiV (n = 5) (B). Blood was obtained weekly from all mice and assayed to quantify circulating vibriocidal antibodies. Circles indicate the lowest dilutions at which specific vibriocidal activity was detected, and the heights of the bars represent geometric mean titers in each group. Ogawa V. cholerae PIC158 was used to measure Ogawa serotype-specific antibodies, and Inaba V. cholerae PIC018 was used to measure Inaba serotype-specific antibodies. Circulating serum antibodies below the limit of detection are indicated by open symbols. No vibriocidal antibody titers were detected in mice inoculated with the sodium bicarbonate vehicle.
Streptomycin-treated adult mice resist recolonization by V. cholerae.
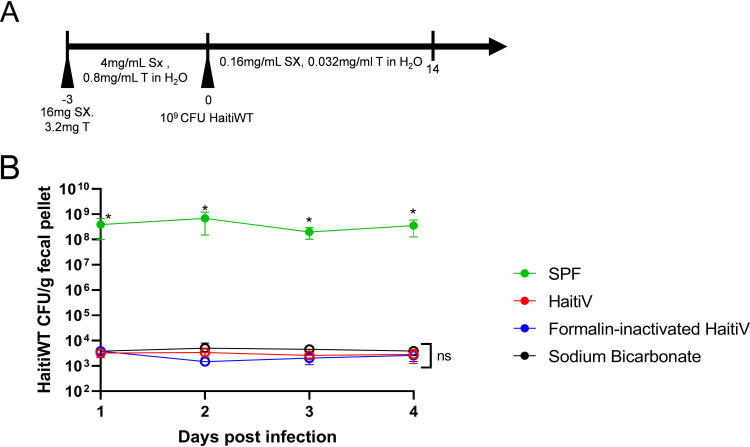
Administration of live OCVs to GF mice leads to long-term intestinal colonization (14, 17), precluding the possibility of challenge studies with wild-type V. cholerae. Since the Sm-treated mice cleared HaitiV, we investigated whether animals immunized with this OCV could be successfully challenged with HaitiWT. At 28 dpi, when all mice had stopped shedding HaitiV, they (live HaitiV, FI-HaitiV, and vehicle control) were orally treated with sulfamethoxazole-trimethoprim (SXT) to reduce the intestinal microbiota (Fig. 3A); unlike HaitiV, HaitiWT is resistant to SXT. All three groups of mice described above and another group, specific-pathogen-free (SPF) mice that had not been Sm treated or exposed to HaitiV, were included in this experiment. All 4 groups of mice were then administered 109 CFU of HaitiWT by oral gavage, and their FPs were monitored for HaitiWT colonization. Unexpectedly, the three groups of mice (HaitiV, FI-HaitiV, and sodium bicarbonate) that had been previously treated with Sm failed to be colonized by HaitiWT (Fig. 3B). In contrast, the SXT-treated SPF mice were robustly colonized by HaitiWT (∼108 CFU/g of FP) (Fig. 3B), indicating that although SXT treatment renders mice susceptible to V. cholerae colonization, the prior Sm treatment of the vaccinated animals rendered them resistant to recolonization with V. cholerae.
FIG 3.
SXT treatment and recolonization of Sm-treated adult mice by V. cholerae. SPF C57BL/6 female mice (n = 5), and the cohort 1 mice inoculated with HaitiV, formalin-inactivated HaitiV, and sodium bicarbonate, were treated with sulfamethoxazole and trimethoprim (SXT) by oral gavage and in their drinking water for 3 days to enable colonization with HaitiWT, the virulent parent strain HaitiV was derived from. All mice were dosed with 109 CFU of HaitiWT on day 0, and fecal pellets were collected daily. Open symbols represent fecal pellets from which no CFU of HaitiWT could be detected. An asterisk indicates differences with a P value of <0.05 as determined by a Mann-Whitney U test. ns, not significant.
Although the FPs of the previously Sm-treated and HaitiWT-challenged mice lacked detectable HaitiWT on the Sm agar plates used to detect V. cholerae, these plates contained high numbers of colonies of non-V. cholerae bacteria. 16S rRNA sequencing of Sm-resistant small colonies showed that these colonies corresponded to either Escherichia coli or SXT-resistant Lactobacillus murinus, which together were present at ∼108 CFU/g of FP. These observations suggest that a bloom of Sm-resistant organisms and other changes in the gut microbiota associated with prior oral Sm treatment render mice resistant to V. cholerae colonization.
Offspring of vaccinated dams are protected from virulent V. cholerae.
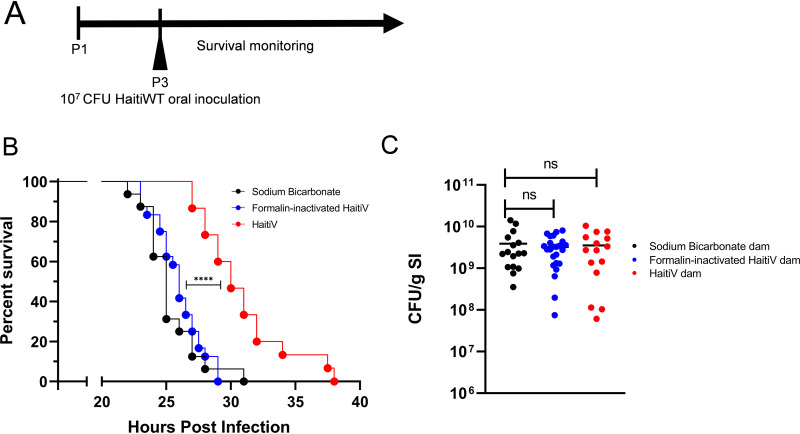
As challenge studies could not be performed with Sm-treated adult mice, we turned to the suckling mouse model of cholera to assess the protective efficacy of the immune response that was elicited by the transient HaitiV intestinal colonization. We recently found that the survival of suckling mice in this lethal-challenge model can be used to gauge OCV efficacy (17). Therefore, animals from HaitiV, FI-HaitiV, and sodium bicarbonate groups were mated with SPF males and their neonatal pups were infected with a lethal dose of HaitiWT (Fig. 4A).
FIG 4.
Transient colonization by HaitiV is protective in an infant mouse model of cholera. (A) Monitoring schematic for infant mouse studies of pups born from dams immunized with a single dose of HaitiV, formalin-inactivated HaitiV (FI-HaitiV), or a vehicle control (cohort 2). Infant mice were infected approximately 72 h postbirth (P3) and monitored closely. (B) P3 pups born to and suckled by dams transiently colonized by HaitiV (n = 15) were protected from a lethal HaitiWT challenge compared with pups born to dams immunized with FI-HaitiV (n = 24) (P = 0.0013 [****] by the Mantel-Cox test) or dams treated with a vehicle buffer (n = 16). (C) HaitiWT CFU recovered from the small intestines (SI) of the neonatal mice at the time they were moribund. Differences in the survival curves were determined by a log rank (Mantel Cox) test, and differences in CFU burdens were determined by a Mann-Whitney U test.
Pups from HaitiV-immunized dams survived significantly longer (median, 30 h postinoculation [hpi]) than pups from dams treated with FI-HaitiV or the sodium bicarbonate control (median, ∼25 hpi) (Fig. 4B). Despite the increased survival in the offspring of HaitiV-immunized dams, at time of death, there were no differences in the burdens of HaitiWT CFU recovered from the small intestines of the 3 groups (Fig. 4C). Thus, oral immunization and transient colonization of Sm-treated mice with HaitiV induce immune responses that significantly protect against choleric disease in mice.
DISCUSSION
The wide availability of diverse mutant mice and reagents for their study makes mice a preferred mammal model for studies of human disease and therapeutics. However, adult mice are not susceptible to intestinal colonization with V. cholerae, confounding evaluation of OCVs. In this study, we found that Sm-treated adult mice can be used to investigate the immunogenicity as well as the protective efficacy of live-attenuated OCVs. Sm-treated adult mice orally inoculated with HaitiV were colonized by this live-attenuated vaccine for 5 to 7 days, and this transient colonization was sufficient to elicit vibriocidal antibodies against both V. cholerae serotypes. Furthermore, pups born to HaitiV-immunized Sm-treated mice exhibited prolonged survival following lethal challenge with HaitiWT compared to that of pups born to dams immunized with FI-HaitiV or dams treated with the vehicle control. Together, these findings suggest that this model should be valuable for further studies of the efficacy and protective mechanisms of live OCVs.
Antibiotic treatment of adult mice limits the role that the resident intestinal microbiota plays in inhibiting live-OCV colonization. Unexpectedly, we found that oral Sm treatment enables the expansion of Sm- and SXT-resistant microbes that also inhibit V. cholerae colonization (Fig. 3) by mechanisms unknown to us. Detailed analyses of the compositions of the microbiota that are initially killed by oral Sm administration and those that bloom after the antibiotic is withdrawn would offer valuable clues to which bacteria inhibit V. cholerae colonization. Though SXT treatment did not enable HaitiWT colonization of previously Sm-treated animals (Fig. 3), it is possible that different antibiotic cocktails would facilitate rechallenge studies; for example, clindamycin was recently found to enable V. cholerae intestinal colonization of adult mice (23). Establishing conditions for rechallenge studies would simplify the model, bypassing the requirement for challenge studies with the offspring of immunized mice; however, only intestinal colonization resistance could be assayed in adult rechallenge studies since unlike suckling mice, adult mice are resistant to choleric diarrhea.
Although pups from HaitiV-immunized Sm-treated dams survived longer than control animals, there was no difference in the CFU burdens of HaitiWT in pups from immunized and control dams at the time they became moribund (Fig. 4). It is possible that vaccination partially delayed the replication of HaitiWT or that vaccination elicited antibodies that antagonize toxic factors, such as cholera toxin, that promote disease but do not directly modulate bacterial colonization. HaitiV ectopically expresses the GM1-binding B subunit of cholera toxin, and immune responses to this nontoxic component of cholera toxin are linked to short-term protection against cholera and enterotoxigenic E. coli (24), suggesting that the delayed death caused by cholera toxin produced by HaitiWT could be explained by antibodies to the B subunit of cholera toxin.
In contrast to GF mice, which were colonized by HaitiV for many months in our previous study (17), Sm-treated mice were colonized by HaitiV only for several days (Fig. 1). This is similar to the duration that live OCVs colonize the human intestine (20, 25, 26), suggesting that Sm-treated mice may provide a more physiologically relevant model to gauge OCV immunogenicity. However, it is important to note that although Sm-treated mice display HaitiV clearance kinetics that mimic human OCV clearance, mice are not natural hosts for V. cholerae. V. cholerae intestinal colonization in susceptible adult mice occurs primarily in the colon and does not rely on the V. cholerae toxin coregulated pilus (TCP), a critical factor for colonization of the small intestine in humans as well as in infant mice and rabbits (13, 19). Nonetheless, since adaptive protective immune responses are stimulated by HaitiV in both Sm-treated and GF mice, these models have value for testing vaccine immunogenicity.
We adopted the strategy that Sit et al. (17) used for studying the protective efficacy of OCVs in immunized GF mice and coupled the suckling mouse model of cholera with the Sm-treated adult model of OCV immunogenicity. In contrast to findings in the GF mouse model where immunized GF mice constantly shed V. cholerae in their feces and maintain elevated VATs, Sm HaitiV dams had undetectable HaitiV CFU in their feces and VATs in their sera at the time their pups were inoculated with HaitiWT (Fig. 1 and 4); these conditions more closely mimic those that will be present when immunized humans are exposed to V. cholerae. Despite the absence of circulating VATs, which are known to be relatively short-lived (27), the offspring of HaitiV-immunized dams exhibited significantly delayed death due to cholera-like disease, but all succumbed (Fig. 4). In contrast, the offspring of HaitiV-immunized GF dams were completely protected from challenge with HaitiWT (17), demonstrating the increased potency of HaitiV immunization in GF mice. The greater effectiveness of HaitiV vaccination in GF mice is likely attributable to the constant stimulation of the intestinal mucosa by HaitiV in the persistently monocolonized GF animals and could be consistent with multiple-dose live-OCV regimens. Thus, even though GF mice have immune defects, the model may still overestimate the potency of HaitiV and other live OCVs.
Killed whole-cell vaccines like Shanchol have ∼50% efficacy in single-dose trials (28). Notably, we found in the Sm-treated mice model that in marked contrast to HaitiV, a single dose of FI-HaitiV, which is similar to current killed whole-cell vaccines, did not elicit protective immunity, consistent with the idea that live-attenuated HaitiV is far more immunogenic than killed vaccines, at least when administered as a single dose. It is possible that greater rates of seroconversion would be observed in adult mice given multiple doses of HaitiV or FI-HaitiV, but for live OCVs, such multidose regimens will require additional modifications of the adult mouse model.
In summary, we have described an adult mouse model for the investigation of the protective efficacy of live OCVs. Using this model, we found that a single oral dose of HaitiV and transient colonization by the vaccine can elicit vibriocidal antibody titers and protective immune responses. Future work should enable refinement of this model to allow rechallenge of immunized animals and further characterization of the protective immune responses. Furthermore, given the availability of genetically defined mutant C57BL/6 mice, the model described here should be a valuable approach for unraveling the molecular determinants of live-vaccine-mediated protection against cholera and other mucosal pathogens.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Vibrio cholerae strains were grown in Luria-Bertani (LB) broth supplemented with relevant antibiotics: streptomycin (Sm) at 200 μg/ml and sulfamethoxazole-trimethoprim (SXT) at 80 μg/ml and 16 μg/ml, respectively, at 37°C, with continuous shaking at 220 rpm. For LB agar plates 1.5% agar was used and was supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at 60 μg/ml. Bacteria were kept as −80°C stocks in LB broth with 40% glycerol.
Oral immunization scheme.
Four-week-old C57BL/6 female mice were purchased (Charles River) and housed in a biosafety level 2 (BSL2) facility under conventional rearing conditions for the duration of the studies. On day 0, all mice were briefly anesthetized with isoflurane and treated by oral gavage with 20 mg of streptomycin in 100 μl of sterilized water. Mice were then provided drinking water supplemented with 5 mg/ml of Sm for 72 h (22). After this, mice were then administered by gavage 109 CFU of an overnight culture of either HaitiV (n = 5) or HaitiV inactivated by a 15-min treatment with 10% formalin (FI-HaitiV) (n = 5) and then resuspended in 2.5% Na2CO3. Control mice were also treated by gavage with 100 μl of 2.5% Na2CO3 alone (n = 5). Mice were then provided drinking water supplemented with 200 μg/ml of Sm for 14 days before being returned to unsupplemented water. All mice were weighed weekly, and blood samples were retrieved from each mouse by tail vein incision at each weighing. Blood samples were clotted for 45 min at room temperature and centrifuged at 20,000 × g for 10 min, and the serum was stored at −20°C for future analysis. A second cohort of mice was then established with the same antibiotic treatment protocol and immunization regimen, except that these mice were used for future mating and infant mouse studies.
Fresh fecal pellets were collected daily from each mouse, weighed, and plated in serial dilutions on LB–Sm–X-Gal agar to determine the colonization of each mouse by HaitiV. HaitiV colonies are white due to a disrupted lacZ gene. The limit of detection of this assay represents the lowest CFU count that could be detected for a fecal pellet of that weight.
Quantification of vibriocidal antibody titers.
Circulating titers of vibriocidal antibodies were quantified by determining the minimal serum dilution required to lyse PIC158 (Ogawa) or PIC018 (Inaba) V. cholerae as described previously (29, 30), with minor modifications. Briefly, serial dilutions of serum were incubated with guinea pig complement (Sigma) and the target strain and then allowed to grow in brain heart infusion (BHI) medium. Reported titers are the dilution of serum that caused more than 50% reduction in target strain optical density compared to normal saline control wells. A mouse monoclonal antibody, 432A.1G8.G1.H12, targeting V. cholerae O1 OSP was a positive control for the assay. The limit of detection of this assay represents the lowest serum dilution at which no inhibition of growth could be detected.
Colonization of immunized mice with toxigenic V. cholerae.
Mice from all three groups (HaitiV, FI-HaitiV, and a vehicle control) as well as 6-week-old female SPF mice (Charles River) were anesthetized with isoflurane and treated by gavage with 16 mg of sulfamethoxazole and 3.2 mg of trimethoprim (SXT) in 100 μl of water and provided drinking water supplemented with 4 mg/ml of sulfamethoxazole (SX) and 0.8 mg/ml of trimethoprim (T) for 72 h. All mice were then administered by gavage 109 CFU of an overnight culture of HaitiWT and switched to 0.16 mg/ml of SX and 0.032 mg/ml of T in their drinking water. Fecal pellets were collected daily and plated on both SXT agar and Sm agar under aerobic conditions. Non-V. cholerae colonies were isolated by streaking on fresh Sm plates, and 16S V1/V2 DNA sequences were amplified by colony PCR using primers F341 (TCG TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG CCT ACG GGN GGC WGC AG) and R805 (GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GGA CTA CHV GGG TAT CTA ATC C) to generate an ∼500-bp amplicon that was sequenced by Sanger sequencing. Identification of the bacteria was performed using BLAST (NCBI). Only results with >95% identity matched to >90% of the query were considered.
Infant mouse survival assay.
The infant mouse survival assay was adapted from a previous report (17). Female mice were mated with age-matched SPF male mice (Charles River) and singly housed at embryonic day 18 (E18) for delivery. At the third day of life (P3), pups were administered by oral gavage 107 CFU of HaitiWT and returned to their dams. Pups were monitored every 6 h until the first signs, typically including diarrhea and dehydration, were evident. At this point, monitoring was performed every 30 min until pups were moribund. Pups were then removed from the nest, euthanized with isoflurane, and decapitated and dissected. The small intestines of each pup were excised, homogenized, and plated on LB agar with Sm and X-Gal.
Statistical analysis.
All statistical analyses were performed with Prism 8 (GraphPad). Infant mouse survival curves were analyzed with a log rank (Mantel Cox) test, and CFU data were analyzed with a Mann-Whitney U test.
Animal use statement.
All experiments in this study were performed as approved by the Brigham and Women’s Hospital IACUC (protocol 2016N000416) and in compliance with the Guide for the Care and Use of Laboratory Animals (31).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an NIH grant (R01-AI-042347-24) and HHMI. B.S. was supported by an NSERC PGS-D fellowship (PGSD3-487259-2016).
B.F., B.S., and M.K.W. designed the experiments. B.F. and B.S. performed the experiments. B.F. and M.K.W. analyzed the data. B.F., B.S., and M.K.W. wrote the manuscript. All authors reviewed and approved the manuscript.
We are grateful to Edward Ryan for providing the monoclonal antibody used for the serum assays. We thank the M.K.W. laboratory members for helpful discussions.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ali M, Nelson AR, Lopez AL, Sack DA. 2015. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. 1993. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Commun 190:302–307. doi: 10.1006/bbrc.1993.1046. [DOI] [PubMed] [Google Scholar]
- 3.Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. 2011. The origin of the Haitian cholera outbreak strain. N Engl J Med 364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. 1981. Duration of infection-derived immunity to cholera. J Infect Dis 143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 5.Albert MJ, Alam K, Ansaruzzaman M, Qadri F, Sack RB. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 (Bengal strain) after oral immunization of rabbits with V. cholerae O1 vaccine strain CVD103-HgR. J Infect Dis 169:230–231. doi: 10.1093/infdis/169.1.230. [DOI] [PubMed] [Google Scholar]
- 6.Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, Qadri F, Digilio L, Sack DA, Ali M, Lessler J, Luquero FJ, Azman AS, Cavailler P, Date K, Sreenivasan N, Matzger H, Luquero F, Grais R, Wiesner L, Ko M, Rouzier V, Peak C, Qadri F, Landegger J, Lynch J, Azman A, Sack D, Henkens M, Ciglenecki I, Ivers L, Diggle E, Weiss M, Hinman A, Maina K, Mirza I, Gimeno G, Levine M. 2017. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 17:1080–1088. doi: 10.1016/S1473-3099(17)30359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker LA, Rumunu J, Jamet C, Kenyi Y, Lino RL, Wamala JF, Mpairwe AM, Muller V, Llosa AE, Uzzeni F, Luquero FJ, Ciglenecki I, Azman AS. 2017. Neighborhood-targeted and case-triggered use of a single dose of oral cholera vaccine in an urban setting: feasibility and vaccine coverage. PLoS Negl Trop Dis 11:e0005652. doi: 10.1371/journal.pntd.0005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lycke N. 2012. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol 12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 9.Qadri F, PXV Study Group, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, Saha A, Al Tarique A, Seidlein LV, Park E, Killeen KP, Mekalanos JJ, Clemens JD, Sack DA. 2007. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 25:231–238. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Van NT, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 11.Islam K, Hossain M, Kelly M, Mayo Smith LM, Charles RC, Bhuiyan TR, Kováč P, Xu P, LaRocque RC, Calderwood SB, Simon JK, Chen WH, Haney D, Lock M, Lyon CE, Kirkpatrick BD, Cohen M, Levine MM, Gurwith M, Harris JB, Qadri F, Ryan ET. 2018. Anti-O-specific polysaccharide (OSP) immune responses following vaccination with oral cholera vaccine CVD 103-HgR correlate with protection against cholera after infection with wild-type Vibrio cholerae O1 El Tor Inaba in North American volunteers. PLoS Negl Trop Dis 12:e0006376. doi: 10.1371/journal.pntd.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WH, Garza J, Choquette M, Hawkins J, Hoeper A, Bernstein DI, Cohen MB. 2015. Safety and immunogenicity of escalating dosages of a single oral administration of Peru-15 pCTB, a candidate live, attenuated vaccine against enterotoxigenic Escherichia coli and Vibrio cholerae. Clin Vaccine Immunol 22:129–135. doi: 10.1128/CVI.00560-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie JM, Waldor MK. 2009. Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies. Curr Top Microbiol Immunol 337:37–59. doi: 10.1007/978-3-642-01846-6_2. [DOI] [PubMed] [Google Scholar]
- 14.Butterton JR, Ryan ET, Shahin RA, Calderwood SB. 1996. Development of a germfree mouse model of Vibrio cholerae infection. Infect Immun 64:4373–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crean TI, John M, Calderwood SB, Ryan ET. 2000. Optimizing the germfree mouse model for in vivo evaluation of oral Vibrio cholerae vaccine and vector strains. Infect Immun 68:977–981. doi: 10.1128/iai.68.2.977-981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sit B, Zhang T, Fakoya B, Akter A, Biswas R, Ryan ET, Waldor MK. 2019. Oral immunization with a probiotic cholera vaccine induces broad protective immunity against Vibrio cholerae colonization and disease in mice. PLoS Negl Trop Dis 13:e0007417. doi: 10.1371/journal.pntd.0007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy EA, King KY, Baldridge MT. 2018. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol 9:1534. doi: 10.3389/fphys.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nygren E, Li BL, Holmgren J, Attridge SR. 2009. Establishment of an adult mouse model for direct evaluation of the efficacy of vaccines against Vibrio cholerae. Infect Immun 77:3475–3484. doi: 10.1128/IAI.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotuzzo E, Butron B, Seas C, Penny M, Ruiz R, Losonsky G, Lanata CF, Wasserman SS, Salazar E, Kaper JB, Cryz S, Levine MM. 1993. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun 61:3994–3997. doi: 10.1128/IAI.61.9.3994-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard TP, Billings G, Dörr T, Sit B, Warr AR, Kuehl CJ, Kim M, Delgado F, Mekalanos JJ, Lewnard JA, Waldor MK. 2018. A live vaccine rapidly protects against cholera in an infant rabbit model. Sci Transl Med 10:eaap8423. doi: 10.1126/scitranslmed.aap8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno E, Sit B, Waldor MK, Cava F. 2018. Anaerobic nitrate reduction divergently governs population expansion of the enteropathogen Vibrio cholerae. Nat Microbiol 3:1346–1353. doi: 10.1038/s41564-018-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You JS, Yong JH, Kim GH, Moon S, Nam KT, Ryu JH, Yoon MY, Yoon SS. 2019. Commensal-derived metabolites govern Vibrio cholerae pathogenesis in host intestine. Microbiome 7:132. doi: 10.1186/s40168-019-0746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kauffman RC, Bhuiyan TR, Nakajima R, Mayo-Smith LM, Rashu R, Hoq MR, Chowdhury F, Khan AI, Rahman A, Bhaumik SK, Harris L, O’Neal JT, Trost JF, Alam NH, Jasinskas A, Dotsey E, Kelly M, Charles RC, Xu P, Kováč P, Calderwood SB, Ryan ET, Felgner PL, Qadri F, Wrammert J, Harris JB. 2016. Single-cell analysis of the plasmablast response to Vibrio cholerae demonstrates expansion of cross-reactive memory B cells. mBio 7:e02021-16. doi: 10.1128/mBio.02021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagos R, San Martin O, Wasserman SS, Prado V, Losonsky GA, Bustamante C, Levine MM. 1999. Palatability, reactogenicity and immunogenicity of engineered live oral cholera vaccine CVD 103-HgR in Chilean infants and toddlers. Pediatr Infect Dis J 18:624–630. doi: 10.1097/00006454-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, Hall RH, Kessler RA, Lock M, Haney D, Lyon CE, Pasetti MF, Simon JK, Szabo F, Tennant S, Levine MM. 2016. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis 62:1329–1335. doi: 10.1093/cid/ciw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosley WH, Ahmad S, Benenson AS, Ahmed A. 1968. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull World Health Organ 38:777–785. [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri F, Ali M, Lynch J, Chowdhury F, Khan AI, Wierzba TF, Excler JL, Saha A, Islam MT, Begum YA, Bhuiyan TR, Khanam F, Chowdhury MI, Khan IA, Kabir A, Riaz BK, Akter A, Khan A, Asaduzzaman M, Kim DR, Siddik AU, Saha NC, Cravioto A, Singh AP, Clemens JD. 2018. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis 18:666–674. doi: 10.1016/S1473-3099(18)30108-7. [DOI] [PubMed] [Google Scholar]
- 29.Son MS, Taylor RK. 2011. Vibriocidal assays to determine the antibody titer of patient sera samples. Curr Protoc Microbiol Chapter 6:Unit 6A.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayeed MA, Bufano MK, Xu P, Eckhoff G, Charles RC, Alam MM, Sultana T, Rashu MR, Berger A, Escobedo GG, Mandlik A, Bhuiyan TR, Leung DT, LaRocque RC, Harris JB, Calderwood SB, Qadri F, Vann WF, Kováč P, Ryan ET. 2015. A cholera conjugate vaccine containing O specific polysaccharide (OSP) of V. cholerae O1 Inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHC) induces serum, memory and lamina proprial responses against OSP and is protective in mice. PLoS Negl Trop Dis 9:e0003881. doi: 10.1371/journal.pntd.0003881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.