Abstract
Purpose of Review:
We review emerging evidence regarding the impact of gut microbes on anti-tumor immunity, and ongoing efforts to translate this in clinical trials.
Recent Findings:
Pre-clinical models and human cohort studies support a role for gut microbes in modulating overall immunity and immunotherapy response, and numerous trials are now underway exploring strategies to modulate gut microbes to enhance responses to cancer therapy. This includes the use of fecal microbiota transplant (FMT), which is being used to treat patients with Clostridium difficile infection among other non-cancer indications. The use of FMT is now being extended to modulate gut microbes in patients being treated with cancer immunotherapy, with the goal of enhancing responses and/or to ameliorate toxicity. However significant complexities exist with such an approach, and will be discussed herein.
Summary:
Data from ongoing studies of FMT in cancer will provide critical insights for optimization of this approach.
Keywords: fecal microbiota transplant, microbiome, immunotherapy, immune checkpoint blockade, cancer, clinical trials
Introduction
The development and approval of immune checkpoint blockade (ICB) has dramatically changed the landscape of cancer therapy and improved outcomes in many malignancies [1]. However, outcomes with immunotherapy are heterogeneous and there remains a critical need to identify predictors of response and resistance and develop synergistic strategies to enhance response. There is now strong evidence that the gut microbiome can shape response to immunotherapy. Importantly, unlike tumor genomics, the gut microbiome is modifiable, and thus, modulation of the gut microbiome to enhance response to immunotherapy is an attractive therapeutic strategy. Herein, we review the potential role for fecal microbiota transplant (FMT) in the context of immunotherapy, including ongoing clinical trial efforts.
Background and rationale for FMT in immunotherapy
The human microbiome refers to the trillions of microorganisms (and their genomes) that live on and in human bodies in a symbiotic relationship. The microbes that inhabit our gut aid in harvesting nutrients from our diet and maintaining gut mucosal integrity, among other functions. The gut microbiome has also been shown to play a key role in shaping the development of mucosal and systemic immunity [2]. There is considerable cross-talk between the gut microbiome and our immune system, with a homeostatic balance of tolerance of beneficial commensal microbes and defense against pathogenic bacteria.
Dysbiosis, i.e., an imbalance in the gut microbe, has been implicated in the pathogenesis of multiple diseases, including autoimmune diseases, gastrointestinal disorders, and luminal malignancies [3,4,5,6]. Initial evidence that the gut microbiome could play a role in therapeutic outcomes in cancer came from work in allogeneic hematopoietic stem cell transplant (HSCT) and graft-versus-host disease (GVHD) [7, 8, 9•]. In preclinical studies, it had been demonstrated that the gut microbiome can influence the immune response to chemotherapeutics such as cyclophosphamide [10].
A role for the gut microbiome in response to immunotherapy was first suggested in two preclinical studies published in Science in 2015 where it was shown that the gut microbiota influenced response to ICB in mice [11••, 12••]. Multiple studies in human cohorts subsequently demonstrated strong associations between the gut microbiome and response to ICB in various types of cancer [13••, 14••, 15••, 16, 17]. Importantly, it was further shown in preclinical models that the gut microbiome could be modulated to enhance therapeutic response [11••, 12••, 13••, 14••, 15••]. Thus, the gut microbiome is not only a biomarker of response to immunotherapy, but also an interventional target.
The gut microbiome can be targeted with a variety of modalities including via diet and the provision of putative beneficial organisms as either single-strain probiotics or bacterial consortia [18]. However, the most well-established method of microbiome modulation is through FMT, whereby a donor microbiome is transferred to a recipient in the form of a stool suspension given either endoscopically or in capsule format. FMT is a guideline-recommended therapy for recurrent Clostridium difficile infection, where it has been shown to correct the dysbiotic state and result in clinical resolution of symptoms in randomized clinical trials [19,20,21], and FMT is being investigated in multiple other diseases.
FMT is the most direct method of microbiome modulation and results in the transfer of the entire donor microbial ecosystem. This approach has two distinct advantages over the introduction of single putatively pro-immunotherapy response bacteria. First, colonization of single bacteria introduced into a complex host microbiome ecosystem with an established homeostasis can be challenging [22]. Second, the transfer of an entire ecosystem includes both the putative pro-response bacteria and the many other organisms which may either support or have redundant roles with these candidate bacteria.
Lessons learned from non-cancer FMT studies
Though investigations of FMT have only recently been initiated in the context of immunotherapy, FMT has been well-studied for other indications with important lessons to be learned from these investigations in terms of approach. The most established indication for FMT is for recurrent/refractory Clostridium difficile infection (CDI). CDI is a clear dysbiosis associated with antibiotic use disrupting the normal gut microbiome allowing outgrowth of this toxin-producing spore-forming obligate anaerobe [23]. CDI can be treated by metronidazole or oral vancomycin but recurrence is high, in part due to collateral damage to the commensal microbiota by these antibiotics [24]. Randomized clinical trials have established that FMT given as a single dose is a highly effective treatment for refractory CDI with systematic reviews demonstrating efficacy rate of 80–90%, and importantly a much lower rate of recurrence than standard-of-care, and consensus guidelines support the use of FMT for this indication [19,20,21]. Longitudinal microbiome profiling has demonstrated that in this setting, a single-dose colonoscopically delivered FMT from unselected healthy donors leads to rapid and reliable engraftment of donor stool, with normalization of community structure and diversity, and durability (at least up to 6 months which is longest time period which has been surveilled) [25]. In an effort to improve FMT accessibility and safety, more recent studies have utilized oral capsules of frozen donor stool specimens, with a randomized study demonstrating non-inferiority compared to colonoscopic FMT [26].
The success of FMT in CDI has prompted the investigation of this modality in multiple other indications, spanning from gastrointestinal to metabolic to neuropsychiatric disorders [27]. The most well-studied indication beyond CDI is inflammatory bowel disease (IBD), a disease in which the gut microbiome has also been implicated, though direct causality is less clear [28]. FMT within this context has shown significant promise, though results have been more mixed than in CDI with variability of engraftment and clinical response when single-dose unselected FMT is used in this setting [28, 29]. However, more recently, an RCT of an intensive FMT protocol in ulcerative colitis published in The Lancet demonstrated a significant improvement in steroid-free clinical remission rates for the FMT group vs placebo (26% vs 8%) [30]. In this study, patients received a single FMT by colonoscopic infusion followed by FMTs via enema, 5 times per week for 8 weeks. Interestingly, despite meeting the primary outcome of clinical response, there was no significant benefit detected in terms of quality of life which may reflect the intensity of the treatment regimen. While single infusion healthy donor FMTs have repeatedly been shown to be effective in CDI, these data suggest that less profoundly dysbiotic disease states/hosts may be more resistant to therapeutic microbiota modulation and require more intensive/ongoing interventions [31]. Donor selection may also be important, with “superdonor” patterns emerging in some trials in IBD where clinical response is enriched by specific donors [32]. Specific donor microbiota features associated with response have been described, including taxonomic and metabolic profiles, as well as overall richness and diversity emerging as a key characteristic [32,33,34,35]. Again, this is in contrast to CDI where response does not appear to vary by donor. As the optimal donor microbial characteristics in IBD are being established, others have instead pursued a donor “pooling” approach to minimize donor-dependent effects [30]; however, how pooling effects on the overall ecology of the infused stool and associated engraftment is unclear and there are potential risks associated with donor pooling, as well as issues in understanding the contribution of specific microbes/donor profiles to potential therapeutic response.
FMT has also been studied in metabolic syndrome bolstered by preclinical studies demonstrating that obesity and metabolic syndrome are transferable by FMT in mice [36]. Two clinical studies have suggested that insulin resistance can be improved by FMT from lean donors to patients with metabolic syndrome, though these responses were variable and not durable. Of note, these studies also employed a single FMT infusion and no preceding antibiotic ablation [37, 38]. In this setting, baseline fecal microbiota composition of the recipient predicted response to FMT, with those with lower baseline diversity exhibiting better response [38]. These findings along with those from the IBD studies have important potential implications for optimizing recipient and donor selection and FMT frequency and duration to promote engraftment into less dysbiotic and potentially more resistant host states. Studies in these other indications have shown that FMT is quite safe, with the most common adverse event being abdominal discomfort [19]. Over the many patients treated in trials and now in clinical practice with FMT, infection transmission has not been a substantial problem with only 4 cases of gram-negative bacteremia reported, three of which had alternative explanations [39]. However, recently, there were two cases (and one resulting death) reported of systemic infections with extended-spectrum beta-lactamase-producing (ESBL) Escherichia coli which was traced back to donor stool [39]. Obviously, this has placed renewed attention on the established guidelines to test donors for potential pathogens [40] and the Food and Drug Administration (FDA) issued a safety alert and has mandated additional screening following these events [41].
Overall, experience with FMT for recurrent CDI supports that FMT can be a highly effective and safe approach to modulate the microbiome, with more variable results in other indications suggesting that host and donor characteristics and timing and duration of intervention may influence therapeutic efficacy and are important considerations in the design of clinical trials in the immunotherapy space (Fig. 1)—as this patient population is more reminiscent of a highly heterogeneous group of individuals with relative dysbiosis (as opposed to the profound dysbiosis observed in CDI).
Figure 1.
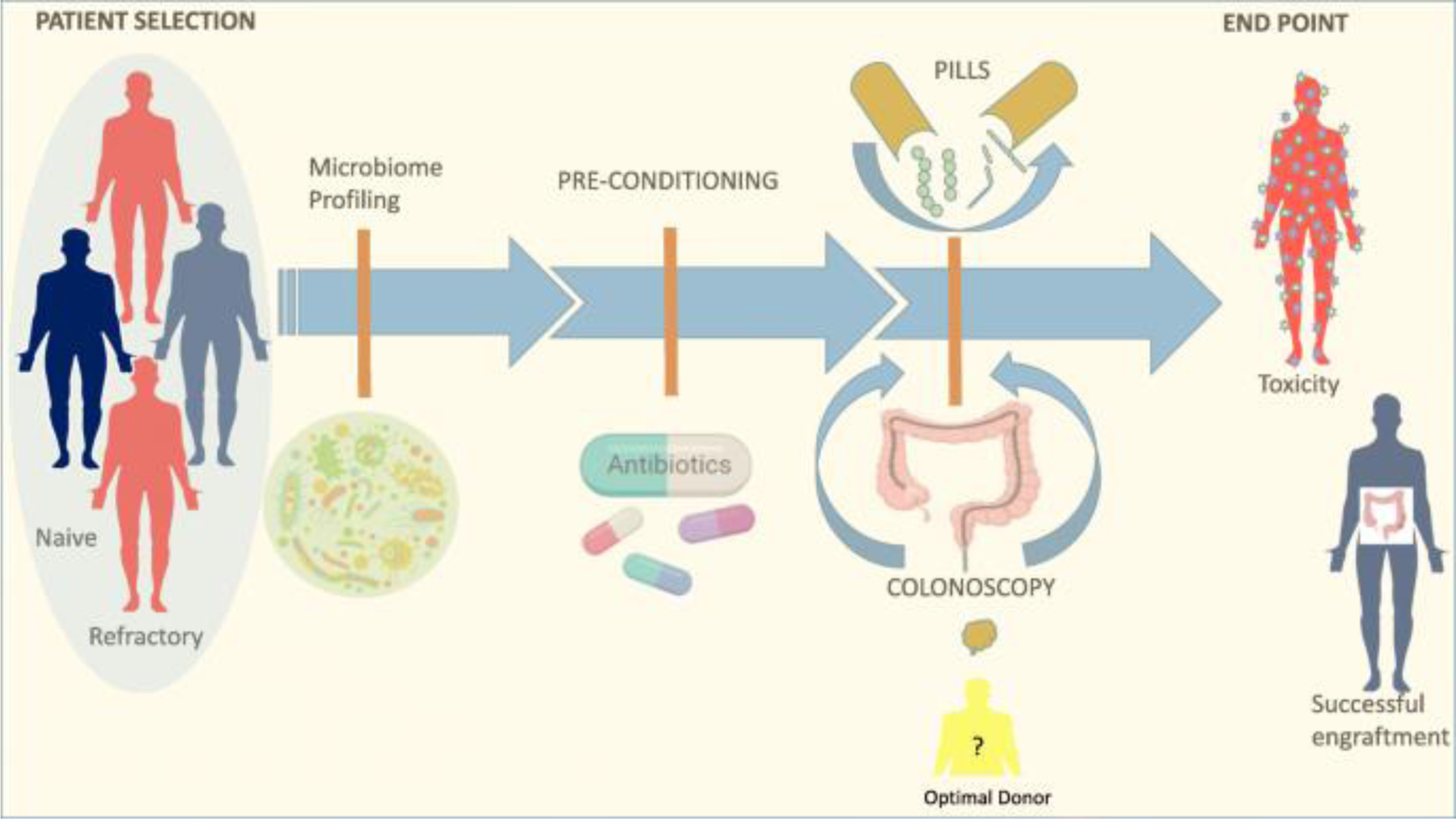
Fecal microbiota transplant to enhance immunotherapy in cancer patients: overview of the potential role of fecal microbiota transplant (FMT) as a therapeutic strategy to modulate the gut microbiome. Through FMT, donor microbiome is transferred to a recipient in the form of a stool suspension given either endoscopically or via pills. Key considerations in the design of FMT interventions include patient selection, donor profile, timing, FMT modality, and post-FMT follow-ups.
Current efforts of FMT in immunotherapy:
Knowledge gained from FMT for other indications as well as from studies of FMT combined with immunotherapy in the preclinical setting has informed the design of several clinical trials of FMT in the context of ICB in patients with cancer (Table 1). In a search of the U.S. National Library of Medicine Clinical Trial database, 8 clinical trials of FMT in patients receiving ICB were identified, with relevant features summarized in Table 1. Of these, six studies are aimed at using FMT as a strategy to improve response to ICB, while two are targeting ICB-induced colitis.
Table 1.
Trials of FMT in different cancer types
| NCT Number | Patient population | Patients (n) | Intervention | Study Phase | Treatment Setting (PD1 naïve vs Refractory) | Randomized vs Single Arm | FMT Donor (Healthy vs ICB responder) | FMT Donor (Pooled vs single donor) | FMT Modality (Route of Administration) | FMT Frequency | FMT Timing (in relation to PD1) | Primary endpoint | Secondary Endpoints | Study Start Date | Enrollment Status | Sites Enrolling |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03353402 | Anti-PD-1 refractory melanoma | 40 | FMT+ Pembrolizumab/Nivolumab | 1 | Refractory | Single arm | Responder | Pooled | Colonoscopy & Capsule | Not specified | Post | Safety Engraftment | Immune profile change | 11/30/2017 | Recruiting | Sheba Med Center, Tel HaShomer, Israel |
| NCT03341143 | Anti-PD-1 refractory melanoma | 20 | FMT + Pembrolizumab/Nivolumab | 2 | Refractory (non-response at 12 weeks) | Single arm | Responder | Not Specified | Colonoscopy | Single dose | Same day | ORR | Immune profile change Effect on the gut microbiome |
1/10/2018 | Recruiting | University of Pittsburgh, Pennsylvania, USA |
| NCT03772899 | Metastatic melanoma | 20 | FMT + Pembrolizumab/Nivolumab | 1 | Naive | Single arm | Healthy | Single | Capsule | Not specified | 1 week prior to | Safety of combination therapy | ORR Effect on the gut microbiome Peripheral Immune profile change Metabolomics |
3/27/2019 | Recruiting | Western University, Ontario Canada |
| NCT03817125 | Metastatic melanoma | 30 | FMT + Nivolumab | 1b | Naive | Randomized | Not specified | Single | Capsule | Capsule taken QOD x3 Then Qweekly x8 |
1 week prior to | Safety | Microbiome change ORR Immune profile change Survival |
1/28/2019 | Recruiting | MD Anderson Cancer Center, USA |
| NCT04130763 | Anti-PD1-refractory luminal GI cancer | 5 | FMT + Pembrolizumab/Nivolumab | 1 | Refractory | Single arm | Not specified | Not specified | Capsule | Capsule taken QOD x3 Then Qweekly x2 |
Prior to | ORR Safety | Immune profile change Effect on the gut microbiome |
10/1/2019 | Not yet recruiting | Peking University, Beijing, China |
| NCT04116775 | Metastatic castrate-resistant prostate cancer | 32 | FMT + Pembrolizumab + Enzalutamide + Androgen deprivation | 2 | Refractory CRPC patients initiated on Pembrolizumab + Enzalutatmide. who fail to respond at 12 weeks | Single arm | Responder | Pooled | Colonoscopy | Once | 12 weeks post 1st dose | PSA change | Radiographic response rate Progression free survival Overall Survival |
10/1/2019 | Not yet recruiting | VA Portal HealthCare System, Oregon USA |
| NCT04056026 | Metastatic mesothelioma | 1 | FMT+ Pembrolizumab/Nivolumab | 1 | Naïve | Single arm | Healthy | Pooled | Colonoscopy | Single dose | Prior to | Progression free survival | None | 9/18/2018 | Completed | ProgenaBiome, CA USA |
| FMT Directed | for Toxicity | |||||||||||||||
| NCT03819296 | Patients with melanoma or GU cancer who develop ICB-related colitis | 100 | FMT | 1 | ICI treated | Single arm | Healthy | Not specified | Colonoscopy | Not specified | Post | Safety | Change in stool microbiome | 11/15/2019 | Not yet recruiting | MD Anderson Cancer Center, USA |
| NCT04038619 | Patients with GU cancer who develop ICB-related colitis | 40 | FMT + Loperamide | 1 | Prior ICI therapy | Single arm | Healthy | Pooled | Colonoscopy | Once, 15 to 30 minutes | Post | Safety Clinical resolution of colitis | Colitis recurrence at 3 months Endoscopic and histologic remission |
1/1/2020 | Not yet recruiting | MD Anderson Cancer Center, USA |
PSA= Prostate specific antigen, RECIST= Response criteria in solid tumor, iRECIST= Immune-related response criteria in solid tumor, ORR= objective response rate, ICB= Immune checkpoint blockade, CTCAE= Common Terminology Criteria for Adverse Events version 5, CRCP= castrate-resistant prostate cancer, QOD= every other day, QWeekly= Every week, RCT= randomized control trial, FMT= fecal microbiota transplant.
As the malignancy with the most established indication for immunotherapy, melanoma is the most common cancer type for which FMT is being investigated. Other cancer types studied include gastrointestinal and genitourinary cancers. Notably, this field is still relatively nascent; thus, these studies are all relatively small phase I/II studies, and the primary endpoint of the majority of these trials is safety. Heterogeneity in the design of these studies reflects many ongoing questions in the optimal study population and intervention for FMT in the setting of ICB (Fig. 1) [18]. For example, the majority of studies are enrolling patients refractory to anti-PD1, while some are enrolling ICB-naïve patients. Of the studies enrolling patients in the anti-PD1-exposed setting, some studies are enrolling patients who are truly refractory to anti-PD1 (i.e., verified progressive disease), whereas others enrich for patients who do not achieve a response at 12 weeks. Methods of FMT delivery also vary, either via colonoscopy (as a one-time FMT) or frozen stool capsules which can be delivered over a longer period of time.
Another factor to be considered is effect of pretreatment antibiotic ablation. Peri-immunotherapy antibiotic use has been associated with diminished response to immunotherapy in retrospective cohorts [15••, 42]. However, antibiotic ablation seems to improve rates of engraftment in other indications, and the antibiotic used for this purpose (oral vancomycin) may spare the beneficial pro-response bacteria based on its coverage [43]. However, the impact of antibiotic use must be carefully evaluated in the context of treatment with FMT and immunotherapy, and it is likely that more targeted approaches may be prudent in optimizing such regimens in the future.
Early preliminary data from five patients from the Israeli study (NCT03353402) were presented at the 2019 AACR meeting [44], demonstrating safety and efficacy of this approach in a subset of treated patients. In this trial, patients with metastatic melanoma were considered eligible for enrollment if they progressed on at least one line of anti-PD-1 therapy—with BRAF-V600E-positive patients also having progressed on BRAF-targeted therapy. Several FMT donors were used in this trial and were chosen on the basis of a durable (> 1 year) complete response to anti-PD-1 monotherapy and also passed all standard FMT safety screening. Microbiome modulation was performed via colonoscopy (single dose) and oral capsules (every 2 weeks with anti-PD-1 administration) after a preparative regimen using oral antibiotics and polyethylene glycol orally prior to colonoscopy. The trial is not fully accrued yet; however, early results demonstrate at least 3 responses of 10 treated patients (with one complete response and 2 partial responses observed). The treatment thus far has been well-tolerated in the group with no severe adverse events related to FMT and no grade 2 or above immune-related adverse events. Microbiome analysis demonstrated a change in the microbiome of treated patients with increase in immune infiltrates in tumors of treated patients.
The Canadian group presented initial safety data from the first two patients treated on their clinical trial (NCT03772899) at The Society of Immunotherapy of Cancers (SITC) 34th Annual meeting in 2019 [45], with no AEs attributed to FMT and one patient with a mixed clinical response and one partial response, though of note this study is being conducted in PD1-naïve patients.
FMT is also being studied in the treatment of ICB-related colitis/diarrhea with two trials (NCT03819296, NCT04038919) evaluating safety, toxicities, and rate of diarrhea resolution (Table 1). ICB-colitis is a common immune-related adverse event, especially with anti-CTLA-4-based therapy. The standard treatment is steroids and other immunosuppressive medications, each of which has its own potential side effects, and some patients have recurrent/refractory colitis despite these treatments. Wang et al. recently published results from the first reported case series of two patients with refractory ICB-associated colitis successfully treated with FMT from healthy donors [46]. Both patients reportedly had complete resolution of clinical symptoms following treatment with FMT, as well as marked endoscopic and histologic improvement. Building on this experience, prospective clinical trials of FMT for refractory colitis are now being conducted.
Future of FMT: Influence on tumor microbiome
As treatment with ICB is being investigated and approved in more and more malignancies, there is a growing interest in investigating the effect of microbiota (and potential for microbiome modulation via FMT) in these cancers. Provocative recent data has further suggested that modulation of the gut microbiome may impact the intratumoral microbiome in gastrointestinal malignancies. Recent studies have demonstrated that, far from being sterile, there are intratumoral bacteria in the majority of pancreatic cancers (and not in adjacent normal pancreatic tissue) [47]. Studies in murine models have further suggested that this likely represents translocation of these bacteria from the gut [5]. In a recent Cell publication, long-term survivors of pancreatic adenocarcinoma were found to have distinct tumor microbiomes compared to short-term survivors, with an intratumoral microbiome signature highly predictive of survival [48]. Interestingly, FMT from humans to mice using fecal microbiome specimens from long-term vs short-term demonstrated that FMT could modulate not just the gut microbiome in the mice but also the tumor microbiome, tumor growth, and tumor immune infiltrate. This study provides a strong rationale to study the impact of FMT on tumor microbiome in patients.
Another malignancy where this is a strong rationale to examine the role of the intestinal (and tumor) microbiome and FMT is hepatocellular carcinoma. Studies have shown that the liver is exposed to intestinal microbiota through the portal vein which delivers gut-derived bacterial products or toxins, such as lipopolysaccharide and deoxycholic acid [49]. FMT has shown some initial promise in small studies in the treatment of chronic liver disease, including alcoholic hepatitis [50], hepatic encephalopathy [51], and viral hepatitis clearance [52]. Given the recent approval of ICB in HCC, the potential of FMT in patients with hepatocellular carcinoma deserves investigation.
Next Steps:
Valuable lessons will be gained from these initial trials of FMT in immunotherapy and will be used to further optimize this modality in the effort to improve therapeutic outcomes. Intensive characterization and analysis of the host and donor features predictive of benefit will be critical to efforts to further personalize microbiota modulation efforts. Early data has suggested both healthy donors and complete responder gut microbiota profiles differ in expression of pro-response signatures identified in retrospective cohorts suggesting that donor profiling may be an important consideration [53]. In studies that utilize multiple donors, it will thus be important to examine microbiota characteristics associated with better engraftment (though studies will be too small at this point to examine associations with therapeutic response). Similarly, though no studies at this point appear to be using baseline microbiota profile as an inclusion criteria, at least one study is stratifying patients based on previously identified pro-response microbiota signature (NCT03353402), and again, these early studies may suggest microbiota features to be used as selection criteria for future studies to enrich for patients most likely to benefit from FMT. Beyond donor and recipient microbiota characteristics, underlying genetic and immunological differences could influence both engraftment and downstream physiological and clinical outcomes. External factors may also be critical—medications can strongly influence the gut microbiota [54], and antibiotic use has been associated with impaired response to immunotherapy [15••]. Antibiotic ablation prior to FMT may be critical to allow engraftment of the donor microbiota; however, medication and antibiotic use (and relationship to microbiota profile) should be carefully tracked following FMT. Interestingly, many of the candidate pro-immunotherapy response bacteria have well-described dietary associations and known functions in the metabolism of specific nutrients such as fiber, a key prebiotic [18]. Diet is a key determinant of the gut microbiota, with nutrients ingested by the host providing the commensal microbes with substrates required for their proliferation and survival and the microbes in turn digesting nutrients otherwise indigestible to their hosts [55, 56]. The effects of pre- or post-FMT diet on engraftment has not been well-studied to date [57], but dietary assessment at baseline and after FMT would be a useful adjunct to understand this potential interaction which could plausibly inform future patient guidance on how to best sustain a donor microbiota. However, this also begs the question of how important maintenance of the donor microbiota is in this setting. Is an initial shift in the gut microbiota triggering an immune response sufficient or is sustainment of the transplanted gut microbiota necessary to sustain response? Longitudinal profiling of the fecal microbiota and integration with response and clinical variables from these early trials will provide rich data on these interactions.
Conclusions:
There is compelling evidence from preclinical models and human cohorts that the gut microbiota shapes response to immunotherapy. FMT is a proven method of modulating the gut microbiota and is currently being investigated in multiple clinical trials in the setting of immunotherapy to either enhance response or treat toxicity. Key considerations in the design of FMT interventions based on trials for other indications include patient selection, donor profile, timing, FMT modality, and post-FMT follow-up (Fig. 1). The results from these early studies, and analysis of correlative fecal, blood, and tissue biospecimens, will yield important insights into the mechanism whereby microbiome modulation might impact antitumor immunity, as well as help optimize interventional approaches.
Acknowledgments
Gabriel O. Ologun receives funding from the National Institutes of Health (NIH) grant T32 CA 09599 and the M.D. Anderson Cancer Center support grant P30 CA016672.
Jennifer A. Wargo receives research funding through grants from the American Association for Cancer Research Stand Up To Cancer, the NIH, and the Melanoma Research Alliance; has received clinical trial support from Bristol-Myers Squibb, GlaxoSmithKline, Roche/Genentech, and Novartis; has received speaker’s honoraria from Bristol-Myers Squibb, Illumina, Imedex, Omniprex, Gilead, PeerView, Physician Education Resource, Medimmune, and Exelixis; has received compensation for service as a consultant/clinical scientific advisor from Bristol-Myers Squibb, Microbiome DX, Biothera Pharma, Merck, and Dhome; has received compensation for participation on advisory boards from GlaxoSmithKline, Roche/Genentech, Novartis, and AstraZeneca; and has a patent application pending submitted by the University of Texas M.D. Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome.
Footnotes
Conflict of Interest
Jennifer L. McQuade has received compensation from Bristol-Myers Squibb and Merck for service as a consultant.
Reetakshi Arora declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References:
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. American Association for the Advancement of Science; 2018. [cited 2020 Feb 17];359:1350–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29567705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016. [cited 2020 Jan 8];535:75–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27383982. [DOI] [PubMed] [Google Scholar]
- 3.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Heal Dis Co-Action Publishing. 2015;26. [DOI] [PMC free article] [PubMed]
- 4.Garrett WS. Cancer and the microbiota. Science. 2015. p. 80–6. [DOI] [PMC free article] [PubMed]
- 5.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018. [cited 2019 Nov 26];8:403–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29567829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013. [cited 2020 Feb 17];13:800–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24132111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. Elsevier Inc. 2015;21:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016. [cited 2020 Jan 8];8:339ra71 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27194729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.•.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. American Society of Hematology; 2014;124:1174–82.This paper was the first to demonstrate a potential role of the gut microbiome in cancer outcomes in a human cohort.
- 10.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science (80-). 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.••.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015. [cited 2020 Jan 8];350:1079–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26541610.These papers were the first.
- 12.••.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy Science (80-). American Association for the Advancement of Science; 2015;350:1084–9.These papers were the first.
- 13.••.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018. [cited 2019 Sep 17];359:97–103. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29097493.These papers were the first.
- 14.••.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients Science (80-). American Association for the Advancement of Science; 2018;359:104–8.These papers were the first.
- 15.••.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors Science (80-). American Association for the Advancement of Science; 2018;359:91–7.These papers were the first.
- 16.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol Off J Eur Soc Med Oncol. 2017. [cited 2020 Jan 8];28:1368–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28368458. [DOI] [PubMed] [Google Scholar]
- 17.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients Neoplasia (US). Neoplasia Press, Inc. 2017;19:848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer Lancet Oncol. Lancet Publishing Group; 2019. p. e77–91. [DOI] [PubMed] [Google Scholar]
- 19.Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, De Vos WM, et al. Duodenal infusion of donor feces for recurrent clostridium difficile. N Engl J Med Massachussetts Medical Society. 2013;368:407–15. [DOI] [PubMed] [Google Scholar]
- 20.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. Oxford University Press; 2018:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faecal microbiota transplant for recurrent Clostridium difficile infection | Guidance | NICE [Internet]. NICE; [cited 2019 Oct 17]. Available from: https://www.nice.org.uk/guidance/ipg485 [Google Scholar]
- 22.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018. [cited 2020 Jan 8];174:1388–1405.e21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30193112. [DOI] [PubMed] [Google Scholar]
- 23.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence Nat Rev Microbiol. Nature Publishing Group; 2016. [cited 2020 Feb 17];14:609–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27573580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, et al. Loss of microbiota-mediated colonization resistance to Clostridium difficile infection with oral vancomycin compared with metronidazole J Infect Dis. Oxford University Press; 2015. [cited 2020 Feb 17];212:1656–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25920320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010. [cited 2020 Feb 17];44:551–61. Available from: https://insights.ovid.com/crossref?an=00004836-201009000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule– vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial JAMA. American Medical Association; 2017;318:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. Annual Reviews; 2019. [cited 2020 Feb 17];70:335–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30403550. [DOI] [PubMed] [Google Scholar]
- 28.Yalchin M, Segal JP, Mullish BH, Quraishi MN, Iqbal TH, Marchesi JR, et al. Gaps in knowledge and future directions for the use of faecal microbiota transplant in the treatment of inflammatory bowel disease Therap Adv Gastroenterol. SAGE Publications Ltd; 2019. [cited 2020 Feb 17];12:1756284819891038 Available from: http://www.ncbi.nlm.nih.gov/pubmed/31803254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanahan F, Quigley EMM. Manipulation of the microbiota for treatment of IBS and IBD—challenges and controversies Gastroenterology WB Saunders; 2014;146:1554–63. [DOI] [PubMed] [Google Scholar]
- 30.Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial Lancet Lancet Publishing Group; 2017;389:1218–28. [DOI] [PubMed] [Google Scholar]
- 31.Siegmund B Is intensity the solution for FMT in ulcerative colitis? Lancet (London, England). Lancet Publishing Group; 2017. [cited 2020 Feb 17];389:1170–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28214090. [DOI] [PubMed] [Google Scholar]
- 32.Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. 2019. [cited 2019 Oct 8];9:2 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30719428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kump P, Wurm P, Gröchenig HP, Wenzl H, Petritsch W, Halwachs B, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis Aliment Pharmacol Ther. Blackwell Publishing Ltd; 2018. [cited 2020 Feb 17];47:67–77. Available from: http://doi.wiley.com/10.1111/apt.14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial Gastroenterology. W.B. Saunders; 2015;149:102–109.e6. [DOI] [PubMed] [Google Scholar]
- 35.Vermeire S, Joossens M, Verbeke K, Wang J, Machiels K, Sabino J, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease J Crohns Colitis. Oxford University Press; 2016. [cited 2020 Feb 17];10:387–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26519463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 37.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome Gastroenterology. W.B. Saunders; 2012;143. [DOI] [PubMed] [Google Scholar]
- 38.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. Cell Press. 2017;26:611–619.e6. [DOI] [PubMed] [Google Scholar]
- 39.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019. [cited 2019 Nov 19];NEJMoa1910437. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1910437 [DOI] [PubMed]
- 40.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017. [cited 2019 Oct 8];66:569–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28087657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms | FDA. [cited 2019 Nov 19]. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse.
- 42.Huang X-Z, Gao P, Song Y-X, Xu Y, Sun J-X, Chen X-W, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019. [cited 2020 Jan 8];8:e1665973 Available from: http://www.ncbi.nlm.nih.gov/pubmed/31741763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freitag TL, Hartikainen A, Jouhten H, Sahl C, Meri S, Anttila V-J, et al. Minor effect of antibiotic pre-treatment on the engraftment of donor microbiota in fecal transplantation in mice. Front Microbiol. 2019;10:2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baruch E, Youngester YOR. Fecal microbiota transplantation (FMT) and re-induction of anti-PD-1 therapy in refractory metastatic melanoma patients—preliminary results from a phase I clinical trial (NCT03353402) AACR Annual Meeting; Atlanta, Georgia; 2019. [Google Scholar]
- 45.Maleki S, Lenehan JBJ. Combination of fecal microbiota transplantation from healthy donors with anti-PD1 immunotherapy in treatment-naïve advanced or metastatic melanoma patients. National Harbor, MD; 2019. [Google Scholar]
- 46.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis Nat Med Nature Publishing Group; 2018;24:1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine Science (80-). American Association for the Advancement of Science; 2017;357:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. Cell Press. 2019;178:795–806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells Science (80-). American Association for the Advancement of Science; 2018:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS, et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017. [cited 2019 Nov 26];15:600–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27816755. [DOI] [PubMed] [Google Scholar]
- 51.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial Hepatology. John Wiley and Sons Inc. 2017;66:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren Y-D, Ye Z-S, Yang L-Z, Jin L-X, Wei W-J, Deng Y-Y, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017. [cited 2019 Nov 26];65:1765–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28027582. [DOI] [PubMed] [Google Scholar]
- 53.Helmink B, Gopalakrishnan V, Khan A et al. Variation of the gut microbiome of complete responders to immune checkpoint blockade and healthy individuals—implications for clinical trial design SITC. 2018; National H:115. [Google Scholar]
- 54.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria Nature Nature Publishing Group; 2018;555:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho I, Blaser MJ. The human microbiome: AT the interface of health and disease. Nat Rev Genet. 2012:260–70. [DOI] [PMC free article] [PubMed]
- 56.McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, et al. American gut: an open platform for citizen science microbiome research mSystems. American Society for Microbiology; 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson S, Guetterman H, Taylor A, Bogner A, Martin D, Farrell JJ, et al. Dietary predictors of fecal microbiota transplantation success. J Acad Nutr Diet. Elsevier BV. 2016;116:A76. [Google Scholar]


