Abstract
TERT promoter mutations are commonly associated with 1p/19q codeletion in IDH-mutated gliomas. However, whether these mutations have an impact on patient survival independent of 1p/19q codeletion is unknown. In this study, we investigated the impact of TERT promoter mutations on survival in IDH-mutated glioma cases. Detailed clinical information and molecular status data were collected for a cohort of 560 adult patients with IDH-mutated gliomas. Among these patients, 279 had both TERT promoter mutation and 1p/19q codeletion, while 30 had either TERT promoter mutation (n = 24) or 1p/19q codeletion (n = 6) alone. A univariable Cox proportional hazard analysis for survival using clinical and genetic factors indicated that a Karnofsky performance status score (KPS) of 90 or 100, WHO grade II or III, TERT promoter mutation, 1p/19q codeletion, radiation therapy, and extent of resection (90–100%) were associated with favorable prognosis (p < 0.05). A multivariable Cox regression model revealed that TERT promoter mutation had a significantly favorable prognostic impact (hazard ratio = 0.421, p = 0.049), while 1p/19q codeletion did not have a significant impact (hazard ratio = 0.648, p = 0.349). Analyses incorporating patient clinical and genetic information were further conducted to identify subgroups showing the favorable prognostic impact of TERT promoter mutation. Among the grade II-III glioma patients with a KPS score of 90 or 100, those with IDH-TERT co-mutation and intact 1p/19q (n = 17) showed significantly longer survival than those with IDH mutation, wild-type TERT, and intact 1p/19q (n = 185) (5-year overall survival, 94% and 77%, respectively; p = 0.032). Our results demonstrate that TERT promoter mutation predicts favorable prognosis independent of 1p/19q codeletion in IDH-mutated gliomas. Combined with its adverse effect on survival among IDH-wild glioma cases, the bivalent prognostic impact of TERT promoter mutation may help further refine the molecular diagnosis and prognostication of diffuse gliomas.
Electronic supplementary material
The online version of this article (10.1186/s40478-020-01078-2) contains supplementary material, which is available to authorized users.
Keywords: IDH1/2, TERT, 1p/19q codeletion, CDKN2A, Glioma
Introduction
Recent advances in molecular genetics over the last decade have facilitated the integration of molecular markers into the diagnosis of brain tumors. The revised 4th edition of the World Health Organization (WHO) classification of Tumours of the Central Nervous System (the CNS WHO 2016) incorporated molecular diagnosis in the diagnostic criteria for the first time in its history [17]. The IDH1/2 (IDH) status plays a crucial role in defining adult diffuse gliomas in the current diagnostic system. IDH mutation and 1p/19q codeletion are necessary and sufficient to make the diagnosis of oligodendrogliomas regardless of the histology. The 1p/19q codeletion is the key diagnostic marker to delineate oligodendrogliomas and distinguish them from astrocytomas in IDH-mutated tumors. Although the consortium to inform molecular and practical approaches to CNS tumor taxonomy-not official WHO (cIMPACT-NOW) recommended a practical diagnostic scheme for diffuse gliomas based on the results of ATRX/p53 immunohistochemistry [16], the ATRX status is only a surrogate and sometimes inconclusive [24].
TERT promoter mutations are common in oligodendrogliomas and glioblastomas [4]. We and others have shown that TERT promoter mutations are frequently observed (> 90%) in oligodendrogliomas with mutant IDH and 1p/19q codeletion, and that the presence of TERT promoter mutations is associated with favorable outcomes in IDH-mutated gliomas [6, 14, 15]. These findings strongly suggest that TERT promoter mutations may serve as an alternative diagnostic marker for oligodendrogliomas when combined with the IDH status. Another aspect of TERT promoter mutation is that this alteration without accompanying IDH mutation suggests clinically and biologically aggressive characteristics comparable with those of glioblastomas when found in histologically diagnosed as diffuse gliomas [6]. The presence of the TERT promoter mutation indicates the underestimation of the tumor grades when observed in grade II–III diffuse gliomas without IDH mutation. cIMPACT-NOW Update 3 recommended TERT promoter mutations as one of the three criteria (the other two being either EGFR amplification or combined whole chromosome 7 gain/chromosome 10 loss) to diagnosis “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” [8]. Thus, TERT promoter mutations serve as a diagnostic marker to delineate histologically verified IDH-wild diffuse astrocytomas with poor outcome comparable with glioblastomas. Evaluation of this marker is becoming an essential part of the routine diagnosis for diffuse astrocytic tumors with wildtype IDH. The bivalent impact of TERT promoter mutations on glioma biology depends on the IDH status, as such, we have previously proposed a molecular classification based on the IDH and TERT status, which can efficiently identify diffuse astrocytomas and oligodendrogliomas [6].
In this study, in order to further understand the diagnostic and prognostic value of TERT promoter mutation, we examined the impact of TERT promoter mutations on survival in a series of IDH-mutated glioma cases using a large retrospective tumor cohort. Our results showed that TERT promoter mutations predict favorable prognosis regardless of 1p/19q status in IDH-mutated gliomas. We propose that TERT promoter mutations are bivalent diagnostic and prognostic markers for adult diffuse gliomas.
Materials and methods
Patient cohorts
Two cohorts were integrated for this retrospective study: one that was analyzed in our previous study [6] and the other was newly collected for this study. The inclusion criteria for both cohorts were as follows: histological diagnosis of IDH1/2-mutated diffuse glioma, 18 years of age or older, clinical data obtained for survival analysis, and availability of genomic DNA extracted from frozen tissues at the time of initial surgery before chemoradiation. Out of the 951 cases analyzed in the previous study, 286 cases with IDH mutations from 13 institutions were enrolled in this study, and their clinical data were updated. The new cohort included 274 cases from 8 institutions. Thus, in total, 560 cases of IDH-mutated diffuse glioma were analyzed in the present study.
Clinical data and histology
Detailed clinical information including patient age, preoperative Karnofsky Performance status (KPS) score, tumor location, extent of resection (EOR), and adjuvant therapy following the initial surgery was obtained from patient medical records. Local histological diagnosis made at each institution was obtained. The majority of tumors (540/560 cases, 96%) were operated on before May 2016; thus, the histopathological diagnosis was almost entirely made according to the CNS WHO 2007 in each center. In this study, an integrated diagnosis was determined by incorporating molecular data and histological diagnosis, which made the diagnosis compatible with the CNS WHO 2016. WHO grade IV tumors with IDH mutation and 1p/19q codeletion were reclassified as grade III based on the current diagnostic criteria which classifies these as anaplastic oligodendrogliomas with IDH mutation and 1p/19q codeletion. The histological diagnosis of the original data is also provided in Additional file 1: Table S1 to show the relationship between molecular features and microscopic findings. For survival analysis, patients were subdivided into two groups based on age (≤ 50 or > 50 years) and preoperative KPS score (< 90 or ≥ 90%). These cutoffs were based on the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System, established and validated by a multi-institutional outcome analysis of cohorts consisting of low-grade gliomas [9, 10]. The EOR was based on the report made by the surgeons in the operation record of the initial surgery.
Molecular analysis
Genomic DNA from frozen tumor tissues was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Tokyo, Japan), according to the manufacturer’s protocol. Molecular testing was performed as previously described. Briefly, the mutational status of IDH1/2 and TERT promoter was tested by Sanger sequencing and/or pyrosequencing [5, 6]. The 1p/19q status was examined by a multiplex ligation-dependent probe amplification (MLPA) [6], microsatellite analysis [23, 27], or microarray-based comparative genomic hybridization [1, 4]. The results of fluorescence in situ hybridization were not included to avoid ambiguity of judgment that could be caused by partial deletions in 1p and/or 19q [13]. The copy number of the CDKN2A locus was also determined by MLPA [6].
Statistical analysis
Categorized data were compared between molecular groups using a Chi square test. Survival was estimated by the Kaplan–Meier method and compared using a log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox regression model in patients with complete clinical information (n = 557). Overall survival (OS) was defined as the duration from the date of initial surgery to that of either death or the last follow-up, with a censoring cutoff date of 30 September 2017. Patients alive at the last follow-up were considered censored during the survival analysis. Differences were considered significant if the p value was < 0.05. All statistical analyses were performed using JMP Pro version 14 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 560 diffuse glioma patients with confirmed IDH mutations were analyzed in the present study. The mean age of all patients was 43.5 years (range 18–82 years). Most patients were diagnosed with lower grade gliomas (527 cases, 94.1%) based on CNS WHO 2016. Approximately 82% (460 cases) of patients had only minor symptoms or no complaints (KPS score 90 or 100). The median follow-up period was 64.7 months (range; 0.85 to 208 months). TERT promoter mutation and 1p/19q codeletion were found in 303 (54.1%) and 285 (50.9%) cases, respectively. Among them, 279 cases harbored both TERT mutation and 1p/19q codeletion, while 30 cases had either TERT mutation (n = 24) or 1p/19q codeletion (n = 6). The remaining 251 IDH-mutant cases had neither of them. Infratentorial tumors with IDH mutation were extremely rare (n = 3) and harbored neither of TERT promoter mutation nor 1p/19q codeletion. The patients’ clinical background and molecular status are summarized in Table 1, and detailed information for each case is provided in Additional file 1: Table S1.
Table 1.
Patient characteristics (n = 560)
| IDH | All | mut | mut | mut | mut |
|---|---|---|---|---|---|
| TERT | mut | mut | wt | wt | |
| 1p/19q | codel | intact | codel | intact | |
| Total (n) | 560 | 279 | 24 | 6 | 251 |
| Mean age (y.o.) | 43.5 | 46.4 | 41.8 | 48.2 | 40.3 |
| − 50 | 399 | 178 | 18 | 3 | 200 |
| > 50 | 161 | 101 | 6 | 3 | 51 |
| M/F | 317/243 | 162/117 | 14/10 | 5/1 | 136/115 |
| WHO gradea | |||||
| II | 287 | 145 | 13 | 4 | 125 |
| III | 240 | 134 | 6 | 2 | 98 |
| IV | 33 | 0 | 5 | 0 | 28 |
| Integrated diagnosisa | |||||
| DA | 138 | 0 | 13 | 0 | 125 |
| AA | 104 | 0 | 6 | 0 | 98 |
| OL | 149 | 145 | 0 | 4 | 0 |
| AO | 136 | 134 | 0 | 2 | 0 |
| GBM | 33 | 0 | 5 | 0 | 28 |
| KPS | |||||
| 90–100 | 460 | 238 | 20 | 6 | 196 |
| < 90 | 98 | 41 | 4 | 0 | 53 |
| nd | 2 | 0 | 0 | 0 | 2 |
| Location | |||||
| Supratentorial | 557 | 279 | 24 | 6 | 248 |
| Infratentorial | 3 | 0 | 0 | 0 | 3 |
| CDKN2A | |||||
| Homo Del | 19 | 3 | 1 | 1 | 14 |
| Non-Del | 365 | 187 | 17 | 3 | 158 |
| nd | 176 | 89 | 6 | 2 | 79 |
| RT | |||||
| (+) | 318 | 137 | 17 | 3 | 161 |
| (−) | 241 | 141 | 7 | 3 | 90 |
| nd | 1 | 1 | 0 | 0 | 0 |
| Chemo | |||||
| (+) | 379 | 210 | 16 | 3 | 150 |
| (−) | 180 | 68 | 8 | 3 | 101 |
| nd | 1 | 1 | 0 | 0 | 0 |
| EOR | |||||
| 90–100% | 329 | 179 | 15 | 2 | 133 |
| < 90% | 231 | 100 | 9 | 4 | 118 |
AA, anaplastic astrocytoma, IDH-mutant; AO, anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted; Chemo, Chemotherapy; codel, codeleted; DA, diffuse astrocytoma, IDH-mutant; Del, Deletion; EOR, extent of resection; F, female; GBM, glioblastoma, IDH-mutant; Homo, Homozygous; nd, no data; KPS, Karnofsky Performance Status; M, male; mut, mutated; OL, oligodendrolioma, IDH-mutant and 1p/19q-codeleted; RT, radiation therapy; y.o., years old; wt, wild-type
aDiagnosis based on CNS WHO2016
TERT promoter mutation has a favorable impact on survival, independent of 1p/19q status
The results of a univariable Cox proportional hazard analysis for survival using each clinical and genetic factor are shown in Table 2. KPS score, WHO grade, TERT promoter status, 1p/19q status, adjuvant radiation therapy, and EOR were significantly associated with survival. The Kaplan–Meier survival curves also showed that both TERT promoter mutation and 1p/19q codeletion were strongly associated with a favorable prognosis in IDH-mutated gliomas (Additional file 2: Fig. S1A and B).
Table 2.
Univariable and multivariable Cox regression analysis for survival (n = 557)
| n | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% C.I. | p value | HR | 95% C.I. | p value | ||
| Sex | |||||||
| M | 315 | 0.832 | 0.592–1.169 | 0.289 | 1.076 | 0.759–1.527 | 0.680 |
| F | 242 | Ref | Ref | ||||
| Age | |||||||
| − 50 | 397 | Ref | Ref | ||||
| > 50 | 160 | 1.361 | 0.945–1.960 | 0.098 | 1.555 | 1.051–2.300 | 0.027 |
| KPS | |||||||
| 90–100 | 459 | Ref | Ref | ||||
| < 90 | 98 | 2.833 | 1.971–4.074 | < 0.0001 | 1.706 | 1.136–2.562 | 0.010 |
| WHO gradea | |||||||
| II | 285 | Ref | Ref | ||||
| III | 239 | 1.167 | 0.808–1.687 | 0.410 | 1.198 | 0.778–1.845 | 0.413 |
| IV | 33 | 8.646 | 5.135–14.558 | < 0.0001 | 5.761 | 2.978–11.145 | < 0.0001 |
| TERT | |||||||
| wt | 255 | Ref | Ref | ||||
| mut | 302 | 0.278 | 0.191–0.404 | < 0.0001 | 0.421 | 0.178–0.998 | 0.0494 |
| 1p/19q | |||||||
| Non-codel | 273 | Ref | Ref | ||||
| Codel | 284 | 0.286 | 0.195–0.419 | < 0.0001 | 0.648 | 0.262–1.604 | 0.349 |
| RT | |||||||
| (−) | 240 | Ref | Ref | ||||
| (+) | 317 | 1.555 | 1.084–2.232 | 0.017 | 0.847 | 0.531–1.349 | 0.484 |
| Chemo | |||||||
| (−) | 179 | Ref | Ref | ||||
| (+) | 378 | 1.252 | 0.857–1.829 | 0.246 | 1.299 | 0.769–2.196 | 0.329 |
| EOR | |||||||
| 90–100% | 326 | 0.487 | 0.346–0.685 | < 0.0001 | 0.513 | 0.359–0.735 | 0.0003 |
| < 90% | 231 | Ref | Ref | ||||
Chemo, Chemotherapy; C.I., Coefficient interval; codel, codeleted; EOR, extent of resection; F, female; HR, hazard ratio; KPS, Karnofsky Performance Status; M, male; mut, mutant; Ref, Reference; RT, radiation therapy; wt, wild-type
aDiagnosis based on CNS WHO2016
We further conducted a multivariable analysis using the Cox regression model for survival incorporating clinical factors and treatments (Table 2). TERT promoter mutation had a survival benefit with an HR of 0.421 (95% CI: 0.178–0.998, p = 0.0494), whereas the impact of 1p/19q status was not apparent (HR 0.648; 95% CI 0.262–1.604; p = 0.349). To elucidate the subgroup with a benefit or disadvantage from the TERT promoter mutation, we evaluated the HR of TERT promoter mutation by subgroup analysis in 1p/19q codeleted and intact groups, respectively. For this subgroup analysis, we performed multivariable analysis of the clinical factors that were considered to be significant in the initial multivariable analysis of all cases. The point estimates of HR consistently indicated the favorable impact of TERT promoter mutation regardless of the combination of clinical factors in both the 1p/19q codeleted and intact groups (Additional file 1: Table S2A-L and S3A-L).
The prognosis of the IDH-TERT co-mutated-1p/19q intact group was comparable to that of the IDH-TERT co-mutated-1p/19q codeleted group among WHO grade II-III cases
For the purpose of investigating the impact of TERT promoter mutation and 1p/19q codeletion on survival in IDH-mutated glioma cases, the patient cohort was divided into four groups dictated by TERT and 1p/19q statuses. The patient details of each group are shown in Table 1. The original histological diagnoses of the TERT-mutated-1p/19q intact (IDH-mutated) group included various histological types and contained fewer pure oligodendroglial tumors (9 out of 24 cases), while most TERT-wildtype-1p/19q codeleted tumors were histologically diagnosed as pure oligodendroglial tumors (5 out of 6 cases) (Additional file 1: Table S1).
The TERT-mutated-1p/19q intact group, including all histological grades, showed intermediate survival between that of the TERT-mutated-1p/19q codeleted and TERT-wildtype-1p/19q intact groups; however, the differences were not statistically significant (p = 0.17 and 0.13, respectively) (Fig. 1).
Fig. 1.
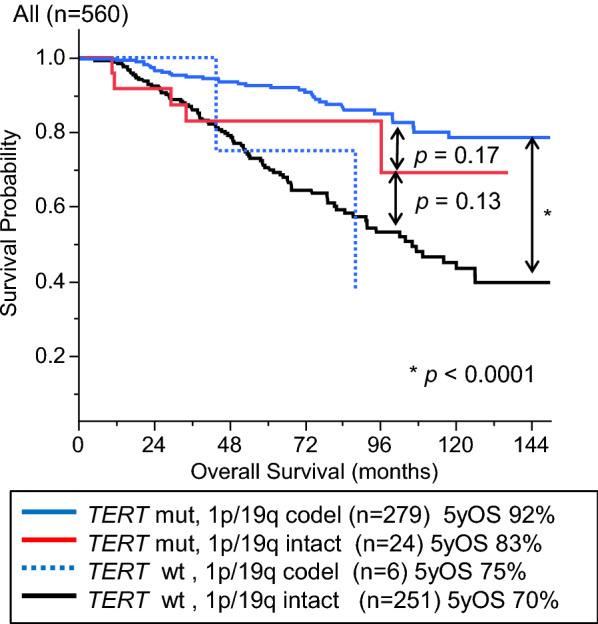
Kaplan-Meier analysis for OS in IDH-mutated gliomas when stratified by TERT and 1p/19q status (n = 560). TERT-mutated-1p/19q intact group showed an intermediate survival curve between TERT-mutated-1p/19q codeleted and TERT-wildtype-1p/19q intact groups, although the differences were not statistically significant. codel, codeleted; OS, overall survival; and 5yOS, 5-year overall survival
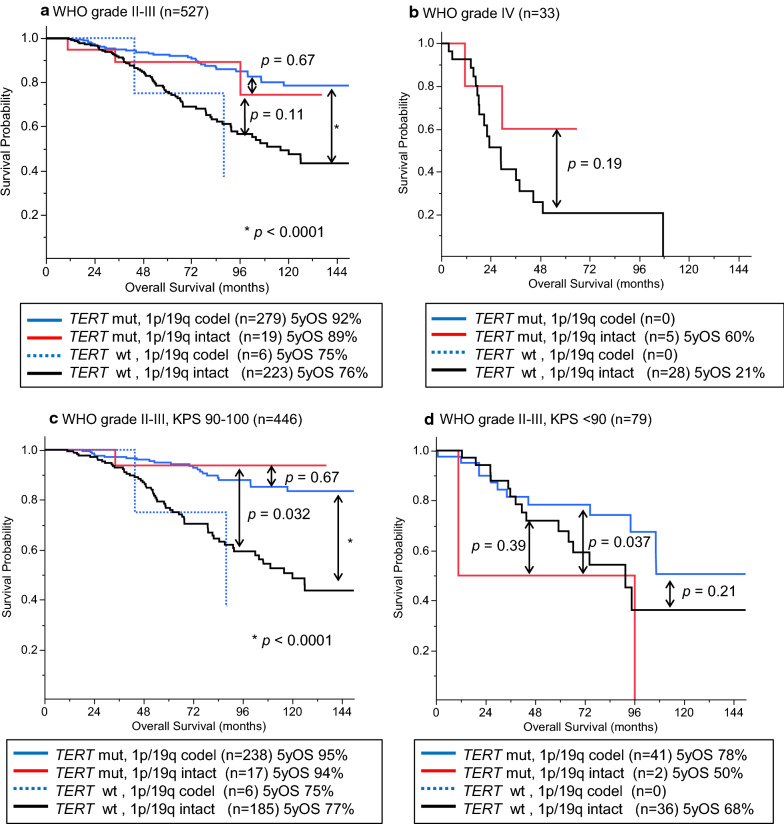
Further subgroup analysis was performed in the groups of grades II–III and IV because the Kaplan–Meier and Cox proportional hazard analyses demonstrated apparent differences between these grade groups (Additional file 2: Fig. S2 and Table 2). In the grade II-III glioma group, there was a significant difference in survival between the TERT-mutated-1p/19q codeleted group and TERT-wildtype-1p/19q intact group (p < 0.0001) (Fig. 2a). The survival curve of the TERT-mutated-1p/19q intact group was close to that of the TERT-mutated-1p/19q codeleted group. The TERT-mutated-1p/19q intact group showed a tendency towards longer survival than that of the TERT-wildtype-1p/19q intact group, although the difference was not statistically significant (p = 0.11); this is probably because of the limited number of these rare cases in the cohort (Fig. 2a). The survival curve of the TERT-wildtype-1p/19q codeleted group was close to that of the TERT-wildtype-1p/19q intact group (Fig. 2a). In the grade IV tumor group, the TERT-mutated-1p/19q intact group showed a tendency towards longer survival compared with that of the TERT-wildtype-1p/19q intact group, although the difference was not statistically significant (p = 0.19) (Fig. 2b). Thus, TERT promoter mutations without 1p/19q codeletion seemed to have a favorable impact on survival.
Fig. 2.
Survival impact of TERT and 1p/19q statuses in grade II–III gliomas when stratified by Karnofsky Performance Status (KPS) scores. a OS of WHO grade II–III cases (n = 527) stratified by the TERT and 1p/19q statuses. Survival curves of TERT-mutated-1p/19q intact group and TERT-mutated-1p/19q codeleted group were mostly overlapping. b OS of WHO grade IV cases (n = 33) stratified by the TERT and 1p/19q statuses. TERT-mutated-1p/19q intact group showed a tendency towards longer survival compared with that of the TERT-wildtype-1p/19q intact group although the difference was not significant. c TERT-mutated-1p/19q intact cases with a high KPS score (90 or 100) had a favorable prognosis comparable to that of the TERT-mutated-1p/19q codeleted cases. D. When analyzed in the population with a KPS score below 90, TERT mutation without 1p/19q codeletion did not show survival benefit. codel, codeleted; KPS, Karnofsky Performance Status; OS, overall survival.; 5yOS, 5-year overall survival
The favorable prognosis associated with TERT promoter mutations independent of 1p/19q codeletion was seen in grade II–III IDH-mutated cases with higher KPS scores (≥ 90)
We further analyzed the effect of TERT promoter mutation in IDH-mutated grade II-III tumors with respect to prognosis after stratification by KPS scores. KPS score was a significant prognostic factor among pretreatment parameters (sex, age, and KPS score) in the univariable and multivariable Cox proportional hazard analyses (Table 2). Moreover, when the grade II-III group was subdivided by KPS scores, cases with a good performance status (KPS score of 90-100) showed favorable prognosis compared to those with a KPS score under 90 (p = 0.0002) (Additional file 2 Fig. S3). When comparing molecular subgroups, the TERT-mutated groups with patients with grade II–III tumors and higher KPS scores (90–100) showed longer survival regardless of 1p/19q status (Fig. 2c). The TERT-mutated-1p/19q intact group showed significantly longer survival than that of the group with neither TERT promoter mutation nor 1p/19q codeletion (p = 0.032), and the survival of the former was comparable with that of the TERT-mutated-1p/19q codeleted group (Fig. 2c). The survival curve of the cases with higher KPS scores in the TERT-wildtype-1p/19q codeleted group was close to that of the TERT-wildtype-1p/19q intact group (Fig. 2c). On the other hand, neither 1p/19q codeletion nor TERT promoter mutation was associated with a favorable prognosis in subgroups with a lower KPS score (< 90) (Fig. 2d). Only two TERT-mutated-1p/19q intact cases were included in the analysis for low KPS score.
Histological grade was associated with survival in 1p/19q intact cases but not in 1p/19q codeleted cases
We investigated the difference in the prognostic impact of histological grade on survival between 1p/19q intact and codeleted cases. When subdivided by 1p/19q status, histological grade was not associated with survival in the 1p/19q codeleted cases but was significantly associated with prognosis in the 1p/19q intact IDH-mutated cases (Additional file 2: Fig. S4 A and B).
CDKN2A homozygous deletion was associated with shorter survival and higher histological grade among IDH-mutated-1p/19q intact tumors
We also analyzed the prognostic relevance of CDKN2A in IDH-mutated tumor cases. CDKN2A status was available for 385 patients. CDKN2A homozygous deletion was observed in all molecular groups; however, the majority of deletions were found in those with TERT-wildtype-1p/19q intact tumors (Table 1). This alteration was associated with a higher grade (p < 0.0001) and a lower KPS score (p < 0.0001) compared to those of cases without this alteration. Tumors with CDKN2A homozygous deletion and 1p/19q codeletion were rare (4 cases), and as such, their effect on prognosis could not be evaluated (Additional file 2: Fig. S5A). In 1p/19q intact tumors, cases with CDKN2A homozygous deletion (n = 15) showed significantly shorter survival than those without this copy number alteration (n = 175) (p < 0.0001) (Additional file 2 Fig. S5B). Most of the CDKN2A deleted tumors without 1p/19q codeletion showed a higher histological grade (grade II, one case; grade III, five cases; and grade IV, nine cases). When confined to the cases for which CDKN2A status was available, an unfavorable prognosis for WHO grade IV cases was retained even after excluding cases with CDKN2A homozygous deletion (Additional file 2: Fig. S5C).
Discussion
In this study, we investigated the survival impact of TERT promoter mutations in a large cohort of 560 IDH-mutated glioma cases with detailed patient data. We confirmed that majority of the TERT promoter mutations coincided with 1p/19q codeletion in IDH-mutated gliomas. However, there were notable exceptions, that is, 24 IDH-mutated tumors had TERT promoter mutations but not 1p/19q codeletion, whereas six tumors had 1p/19q codeletion without TERT promoter mutations. Multivariable analysis incorporating clinical background revealed that the prognostic impact of TERT promoter mutations was independent from that of 1p/19q codeletion (Table 2). In the subgroup analyses of grade II-III cases, the TERT-mutated-1p/19q intact group showed a favorable prognosis comparable to that of the TERT-mutated-1p/19q codeleted group, while the survival curve of the TERT-wildtype-1p/19q codeleted group was consistent with that of the TERT-wildtype-1p/19q intact group (Fig. 2a and c). These results of the subgroup analyses support the findings of the multivariable analyses.
A favorable prognostic impact of TERT promoter mutation in lower grade gliomas with an IDH mutation has been reported in several studies [6, 12, 14, 15]. However, whether TERT promoter mutations have an impact on patient survival independent of 1p/19q codeletion has not been fully investigated. We addressed this point by performing a multivariable analysis, first incorporating clinical factors. Our study also analyzed the prognosis of tumors with the “atypical” genotype of co-mutation in IDH and TERT without 1p/19q codeletion. The result of a large-scale retrospective study by Eckel-Passow et al. [12] indicated that this group of tumors had good prognosis comparable to that of triple-positive tumors, i.e., those with concurrent IDH mutation, TERT mutation, and 1p/19q codeletion. On the other hand, a follow-up of this study reported that TERT promoter mutation was a prognostic factor in 1p/19q codeleted cases, while the impact of TERT promoter mutation was not significant in 1p/19q intact cases [19]. However, in these studies, TERT mRNA expression was used as a surrogate for TERT mutational status in a considerable number of cases and, therefore, were not conclusive in their evaluation of the value of TERT promoter mutation as an independent prognostic marker in IDH-mutated gliomas [12, 19]. Another study involving over 300 IDH-mutated glioma cases also reported that survival of patients with IDH-TERT co-mutated tumors and grade II-III histology did not differ according to 1p/19q status [15]. Our results showed that TERT promoter mutations in IDH-mutated gliomas predict favorable prognosis regardless of 1p/19q status, highlighting the significant role of TERT promoter mutations as a prognostic marker. Significantly longer overall survival was seen in the TERT-mutated, 1p/19q intact, and IDH-mutated cases than in the TERT-wildtype, 1p/19q intact, and IDH-mutated cases, among patients with a high KPS score (90-100) in our study. Considering that even 1p/19q codeletion was not a prognostic indicator among patients with a low KPS (< 90), it appears that the relevance of molecular prognostic markers depends on the patient’s clinical factors. This needs to be considered in future studies investigating molecular markers.
Although the TERT-mutated, 1p/19q intact, and IDH-mutated cases showed comparable survival with that of the triple-positive cases, the histology of the former varied. Whether the definition of oligodendroglioma depends on the tumor’s histology or biological behavior anticipated by genotype, which is reflected in patient survival, is a matter for future debate. The current definition of oligodendroglial tumors in the CNS WHO 2016 prefers the latter [17]. On the other hand, the WHO classification is rapidly shifting from conventional morphology-based diagnosis to molecularly driven disease definition. Recognizing the significant impact of IDH mutation on the biology of astrocytic gliomas, cIMPACT-NOW update 5 has recently recommended a terminology “astrocytoma, IDH-mutated, grade 4” for the IDH-mutated diffuse astrocytic gliomas with histological/molecular features of glioblastoma, histological diagnosis over-ridden by molecular features [7]. A diagnosis should reflect the biology of the tumor, the natural course of disease, and/or response to therapy. The present study and other studies have reported that 1p/19q codeletion without accompanying TERT promoter mutations does not have prognostic benefit [19]. Of note, all cases with such genotype were histologically diagnosed as oligodendroglial tumors in our series. The combination of TERT promoter mutations and IDH mutations is a highly specific biomarker. Considering that very few single genetic alterations can sufficiently define a tumor type (even 1p/19q codeletion needs to be used in combination with IDH status), TERT promoter mutation may deserve recognition as a diagnostic marker as well.
The prognostic relevance of WHO grading in IDH-mutated gliomas is controversial, although it is associated with tumor aggressiveness in their wildtype counterparts [7, 18, 22, 26]. Our results showed that the survival of patients with IDH-mutated 1p/19q codeleted gliomas did not differ between WHO grade II and III cases (Additional file 2: Fig. S4A). The prognostic significance of WHO grading in molecularly proved oligodendrogliomas remains controversial; our result is comparable to another study [22] but in contrast with others [19]. As a nature of retrospective study, the differences in treatment variations including chemotherapy and radiation between WHO grading may have an impact on patient outcome. Future studies on oligodendroglial cases with controlled treatment background is warranted to assess this issue [19]. On the other hand, our results showed that the survival of patients with IDH-mutated astrocytomas differed among grade II, III, and IV tumors (Additional file 2: Fig. S4B); this result is comparable to those of some previous studies [25] but contrasts with others [18, 20]. Currently, the diagnosis of WHO grade II and III is essentially based on the mitotic index determined by microscopic observation of diffuse astrocytomas, and this has remained the same in the CNS WHO 2016 classification. Attempts to molecularly define the aggressive type of diffuse astrocytomas have suggested several genetic markers such as RB1 pathway alterations (e.g., CDKN2A/B homozygous deletion or CDK4 amplification), PIK3R1 mutation, PDGFRA amplification, or G-CIMP low type in the methylation cluster [2, 3, 7, 11, 21, 25]. Of these, the CDKN2A homozygous deletion has been proposed as a strong prognostic factor in IDH-mutated astrocytomas [3, 21, 25]. In our series, high histological grade and CDKN2A homozygous deletion were adverse prognostic factors in IDH-mutated-1p/19q intact gliomas. This is in line with previous reports [21]. However, the frequency of this copy number change was relatively low and strongly correlated with high histological grades. IDH-mutated glioblastomas without CDKN2A homozygous deletion still showed poorer prognosis compared with that of lower grade astrocytomas (Additional file 2: Fig. S5C). WHO grade IV was an independent risk factor for survival in the multivariable analysis for all cases (Table 2) and the subsequent subgroup analysis for 1p/19q intact tumors (Additional file 1: Table S3L). Thus, histologically defined grade IV tumors may have a fundamentally different biology from grade II–III tumors [7]. Further exploration of molecular markers indicating aggressive IDH-mutated astrocytomas is warranted. In the meantime, histologically defined WHO grading still appears to have an impact on the delineation of biologically and clinically malignant astrocytomas with IDH mutation.
Conclusions
Our results provide strong evidence that TERT promoter mutation confers a favorable prognosis regardless of the 1p/19q status in IDH-mutated gliomas. This observation was most evident in grade II–III gliomas as evidenced by subgroup analyses. TERT promoter mutations may not serve as diagnostic markers on their own as there are other types of IDH-wildtype glial neoplasms that may harbor TERT promoter mutations, including pleomorphic xanthoastrocytoma, ganglioglioma, anaplastic glioma with piloid features, and ependymoma [8]. However, very few molecular markers serve as standalone diagnostic markers for gliomas. Even IDH mutations or 1p/19q codeletion has to be used in combination to define a single entity of diffuse glioma [16, 20]. In line with this, it has been shown that TERT promoter mutations when combined with IDH mutation status serve as a very powerful prognostic predictor in diffuse gliomas. Given the current trend of using molecular and biological markers for diagnosis, it is worthwhile to consider TERT promoter mutation as a diagnostic as well as prognostic marker.
Supplementary information
Additional file 1: Table S1 Detailed information of patients registered in the present study. Table S2 Cox regression analysis for survival in 1p/19q codeleted cases (n=284). Table S3 Cox regression analysis for survival in 1p/19q intact cases (n=273).
Additional file 2: Fig. S1. OS of all cases (n=560) stratified by TERT (A) or 1p/19q (B) status. Both TERT promoter mutation (A) and 1p/19q codeletion (B) were strongly associated with favorable prognosis in IDH-mutated gliomas. codel, codeleted; OS, overall survival; and 5yOS, 5-year-overall survival. Fig. S2 Kaplan-Meier analysis for OS in IDH-mutated gliomas. Overall survival (OS) of all cases (n=560) stratified by histological grade. Fig. S3. Kaplan-Meier analysis for overall survival (OS) stratified by KPS score in histological grade II-III cases. When the grade II-III cohort was subdivided by the KPS score, cases with a good performance status (with a KPS score of 90-100) showed a more favorable prognosis than those with a KPS score under 90. codel, codeleted; KPS, Karnofsky Performance Status; OS, overall survival; and 5yOS, 5-year-overall survival. Fig. S4. Kaplan-Meier analysis for OS stratified by histological grade. A. OS of 1p/19q codeleted cases (n=285) stratified by histological grade. B. OS of cases without 1p/19q codeletion (n=275) stratified by histological grade. codel, codeleted; OS, overall survival; and 5yOS, 5-year-overall survival. Fig. S5. Kaplan-Meier analysis for overall survival stratified by CDKN2A status in IDH-mutated gliomas without -1p/19q codeletion (A) and those with codeletion tumors (B). A. The prognostic impact of CDKN2A homozygous deletion (n=4) was not apparent in the group with 1p/19q codeletion, although the number of cases was very small. B. In 1p/19q intact tumors, cases with CDKN2A homozygous deletion (n=15) showed significantly shorter survival than those without this copy number alteration. C. In 1p/19q intact cases, WHO grade IV cases showed unfavorable prognosis even when analyzing cases without CDKN2A homozygous deletion. codel, codeleted; KPS, Karnofsky Performance Status; OS, overall survival; and 5yOS, 5-year-overall survival.
Acknowledgements
The authors thank all clinicians who took care of the patients and made a great contribution to this study by providing specimens and clinical information.
Abbreviations
- CI
Confidence interval
- cIMPACT-NOW
Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy-not official WHO
- CNS WHO 2016
The revised 4th edition of the World Health Organization (WHO) classification of Tumours of the Central Nervous System
- EOR
Extent of resection
- KPS
Karnofsky performance status
- HR
Hazard ratio
- MLPA
Multiplex ligation-dependent probe amplification
- OS
Overall survival
Author’s contribution
HA, AK, KI: Study design. YMa, KY, NH, MO, SY, TSas, STan, FH, TI, KS, MK, KM, YMi, KTam, STam, TN, TU, YO, JF, DS, YH, ESP, RH, YI, YMi, KTan, STak, RO, TSak, KKo, RS, KKu, TSh, MNo, HS, MS, TK, HS, MM, HK, MNa, YS, TT, MNa, RN, YK, YN: Data collection. HA, RM: Data analysis. HA, RM, AK, YN, KI: Interpretation. HA, RM, AK, KI: Manuscript writing. All authors read and approved the final manuscript.
Funding
This investigation was supported by JSPS KAKENHI 16 K20006 (H.A.) and the Research award of the Osaka Cancer Society (H.A.).
Availability of data and materials
The anonymized datasets analyzed in the present study are provided in supplementary information.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board (IRB) of the National Cancer Center (No. 2013–042) and the corresponding local IRB of the participating centers.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest in association with this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hideyuki Arita, Email: neuro.husky@gmail.com.
Yuko Matsushita, Email: ymatsush@ncc.go.jp.
Ryunosuke Machida, Email: rmachida@ncc.go.jp.
Kai Yamasaki, Email: ka-yamasaki@med.osakacity-hp.or.jp.
Nobuhiro Hata, Email: hatanobu@ns.med.kyushu-u.ac.jp.
Makoto Ohno, Email: mohno@ncc.go.jp.
Shigeru Yamaguchi, Email: yama-shu@med.hokudai.ac.jp.
Takashi Sasayama, Email: takasasa@med.kobe-u.ac.jp.
Shota Tanaka, Email: tanakas-tky@umin.ac.jp.
Fumi Higuchi, Email: fhiguchi@dokkyomed.ac.jp.
Toshihiko Iuchi, Email: tiuchi@me.com.
Kuniaki Saito, Email: kusaito-tky@umin.ac.jp.
Masayuki Kanamori, Email: mkanamori@med.tohoku.ac.jp.
Ken-ichiro Matsuda, Email: matsuk@med.id.yamagata-u.ac.jp.
Yohei Miyake, Email: ymiyaken@gmail.com.
Kaoru Tamura, Email: tamura.nsrg@tmd.ac.jp.
Sho Tamai, Email: sho.tamai@med.kanazawa-u.ac.jp.
Taishi Nakamura, Email: n_taishi@yokohama-cu.ac.jp.
Takehiro Uda, Email: uda@med.osaka-cu.ac.jp.
Yoshiko Okita, Email: yokita4246@gmail.com.
Junya Fukai, Email: junfukai@wakayama-med.ac.jp.
Daisuke Sakamoto, Email: d.sakamoto.ns@gmail.com.
Yasuhiko Hattori, Email: yahiko_h@yahoo.co.jp.
Eriel Sandika Pareira, Email: erielsandika@yahoo.com.
Ryusuke Hatae, Email: ryhatae@ns.med.kyushu-u.ac.jp.
Yukitomo Ishi, Email: ishi-y@huhp.hokudai.ac.jp.
Yasuji Miyakita, Email: ymiyakit@ncc.go.jp.
Kazuhiro Tanaka, Email: kazutana@med.kobe-u.ac.jp.
Shunsaku Takayanagi, Email: takayanagi-nsu@umin.ac.jp.
Ryohei Otani, Email: ryouhei-ohtani@umin.ac.jp.
Tsukasa Sakaida, Email: tsakaida@chiba-cc.jp.
Keiichi Kobayashi, Email: kekobayashi@kki.biglobe.ne.jp.
Ryuta Saito, Email: ryuta@nsg.med.tohoku.ac.jp.
Kazuhiko Kurozumi, Email: kurozu20@hama-med.ac.jp.
Tomoko Shofuda, Email: shofuda.tomoko.td@mail.hosp.go.jp.
Masahiro Nonaka, Email: nonakam@hirakata.kmu.ac.jp.
Hiroyoshi Suzuki, Email: hirosuzsnh@gmail.com.
Makoto Shibuya, Email: shibuya@tokyo-med.ac.jp.
Takashi Komori, Email: komori-tk@igakuken.or.jp.
Hikaru Sasaki, Email: hsasaki@keio.jp.
Masahiro Mizoguchi, Email: mmizoguc@ns.med.kyushu-u.ac.jp.
Haruhiko Kishima, Email: hkishima@nsurg.med.osaka-u.ac.jp.
Mitsutoshi Nakada, Email: mnakada@med.kanazawa-u.ac.jp.
Yukihiko Sonoda, Email: ysonoda@med.id.yamagata-u.ac.jp.
Teiji Tominaga, Email: tomi@nsg.med.tohoku.ac.jp.
Motoo Nagane, Email: mnagane@ks.kyorin-u.ac.jp.
Ryo Nishikawa, Email: rnishika@saitama-med.ac.jp.
Yonehiro Kanemura, Email: kanemura.yonehiro.hk@mail.hosp.go.jp.
Aya Kuchiba, Email: akuchiba@ncc.go.jp.
Yoshitaka Narita, Email: yonarita@ncc.go.jp.
Koichi Ichimura, Email: kichimur@ncc.go.jp.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40478-020-01078-2.
References
- 1.Aihara K, Mukasa A, Nagae G, Nomura M, Yamamoto S, Ueda H, Tatsuno K, Shibahara J, Takahashi M, Momose T, et al. Genetic and epigenetic stability of oligodendrogliomas at recurrence. Acta Neuropathol Commun. 2017;5:18. doi: 10.1186/s40478-017-0422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki K, Nakamura H, Suzuki H, Matsuo K, Kataoka K, Shimamura T, Motomura K, Ohka F, Shiina S, Yamamoto T, et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20:66–77. doi: 10.1093/neuonc/nox132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appay R, Dehais C, Maurage CA, Alentorn A, Carpentier C, Colin C, Ducray F, Escande F, Idbaih A, Kamoun A, et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019;21:1519–1528. doi: 10.1093/neuonc/noz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N, et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126:267–276. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
- 5.Arita H, Narita Y, Matsushita Y, Fukushima S, Yoshida A, Takami H, Miyakita Y, Ohno M, Shibui S, Ichimura K. Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Brain Tumor Pathol. 2015;32:22–30. doi: 10.1007/s10014-014-0186-0. [DOI] [PubMed] [Google Scholar]
- 6.Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H, Tanaka S, Mukasa A, Shirahata M, Shimizu S, et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016;4:79. doi: 10.1186/s40478-016-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, Holland EC, Jenkins RB, Kleinschmidt-DeMasters B, Komori T, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139:603–608. doi: 10.1007/s00401-020-02127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136:805–810. doi: 10.1007/s00401-018-1913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang EF, Clark A, Jensen RL, Bernstein M, Guha A, Carrabba G, Mukhopadhyay D, Kim W, Liau LM, Chang SM, et al. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 10.Chang EF, Smith JS, Chang SM, Lamborn KR, Prados MD, Butowski N, Barbaro NM, Parsa AT, Berger MS, McDermott MM. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109:817–824. doi: 10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 11.de Souza CF, Sabedot TS, Malta TM, Stetson L, Morozova O, Sokolov A, Laird PW, Wiznerowicz M, Iavarone A, Snyder J, et al. A distinct DNA methylation shift in a subset of glioma CpG island methylator phenotypes during tumor recurrence. Cell Rep. 2018;23:637–651. doi: 10.1016/j.celrep.2018.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichimura K, Vogazianou AP, Liu L, Pearson DM, Backlund LM, Plant K, Baird K, Langford CF, Gregory SG, Collins VP. 1p36 is a preferential target of chromosome 1 deletions in astrocytic tumours and homozygously deleted in a subset of glioblastomas. Oncogene. 2008;27:2097–2108. doi: 10.1038/sj.onc.1210848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5:1515–1525. doi: 10.18632/oncotarget.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labussiere M, Di Stefano AL, Gleize V, Boisselier B, Giry M, Mangesius S, Bruno A, Paterra R, Marie Y, Rahimian A, et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br J Cancer. 2014;111:2024–2032. doi: 10.1038/bjc.2014.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, Batchelor TT, Cairncross JG, van den Bent M, Wick W, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018;135:639–642. doi: 10.1007/s00401-018-1826-y. [DOI] [PubMed] [Google Scholar]
- 17.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A (2016) World Health Organization histological classification of tumours of the central nervous system. International Agency for Research on Cancer, City [DOI] [PubMed]
- 18.Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, Armstrong TS, Sulman EP, Cahill DP, Vera-Bolanos E, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. 2015;129:585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pekmezci M, Rice T, Molinaro AM, Walsh KM, Decker PA, Hansen H, Sicotte H, Kollmeyer TM, McCoy LS, Sarkar G, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. 2017;133:1001–1016. doi: 10.1007/s00401-017-1690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, Sahm F, Koelsche C, Korshunov A, Olar A, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129:867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, Koelsche C, Wefers A, Reinhardt A, Huang K, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. doi: 10.1007/s00401-018-1849-4. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, Shimamura T, Niida A, Motomura K, Ohka F, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47:458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 23.Ueki K, Nishikawa R, Nakazato Y, Hirose T, Hirato J, Funada N, Fujimaki T, Hojo S, Kubo O, Ide T, et al. Correlation of histology and molecular genetic analysis of 1p, 19q, 10q, TP53, EGFR, CDK4, and CDKN2A in 91 astrocytic and oligodendroglial tumors. Clin Cancer Res. 2002;8:196–201. [PubMed] [Google Scholar]
- 24.Yamamichi A, Ohka F, Aoki K, Suzuki H, Kato A, Hirano M, Motomura K, Tanahashi K, Chalise L, Maeda S, et al. Immunohistochemical ATRX expression is not a surrogate for 1p19q codeletion. Brain Tumor Pathol. 2018;35:106–113. doi: 10.1007/s10014-018-0312-5. [DOI] [PubMed] [Google Scholar]
- 25.Yang RR, Shi ZF, Zhang ZY, Chan AK, Aibaidula A, Wang WW, Kwan JSH, Poon WS, Chen H, Li WC, et al. IDH mutant lower grade (WHO Grades II/III) astrocytomas can be stratified for risk by CDKN2A, CDK4 and PDGFRA copy number alterations. Brain Pathol. 2019 doi: 10.1111/bpa.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoda RA, Marxen T, Longo L, Ene C, Wirsching HG, Keene CD, Holland EC, Cimino PJ. Mitotic index thresholds do not predict clinical outcome for IDH-mutant astrocytoma. J Neuropathol Exp Neurol. 2019;78:1002–1010. doi: 10.1093/jnen/nlz082. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto K, Iwaki T, Inamura T, Fukui M, Tahira T, Hayashi K. Multiplexed analysis of post-PCR fluorescence-labeled microsatellite alleles and statistical evaluation of their imbalance in brain tumors. Jpn J Cancer Res. 2002;93:284–290. doi: 10.1111/j.1349-7006.2002.tb02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Detailed information of patients registered in the present study. Table S2 Cox regression analysis for survival in 1p/19q codeleted cases (n=284). Table S3 Cox regression analysis for survival in 1p/19q intact cases (n=273).
Additional file 2: Fig. S1. OS of all cases (n=560) stratified by TERT (A) or 1p/19q (B) status. Both TERT promoter mutation (A) and 1p/19q codeletion (B) were strongly associated with favorable prognosis in IDH-mutated gliomas. codel, codeleted; OS, overall survival; and 5yOS, 5-year-overall survival. Fig. S2 Kaplan-Meier analysis for OS in IDH-mutated gliomas. Overall survival (OS) of all cases (n=560) stratified by histological grade. Fig. S3. Kaplan-Meier analysis for overall survival (OS) stratified by KPS score in histological grade II-III cases. When the grade II-III cohort was subdivided by the KPS score, cases with a good performance status (with a KPS score of 90-100) showed a more favorable prognosis than those with a KPS score under 90. codel, codeleted; KPS, Karnofsky Performance Status; OS, overall survival; and 5yOS, 5-year-overall survival. Fig. S4. Kaplan-Meier analysis for OS stratified by histological grade. A. OS of 1p/19q codeleted cases (n=285) stratified by histological grade. B. OS of cases without 1p/19q codeletion (n=275) stratified by histological grade. codel, codeleted; OS, overall survival; and 5yOS, 5-year-overall survival. Fig. S5. Kaplan-Meier analysis for overall survival stratified by CDKN2A status in IDH-mutated gliomas without -1p/19q codeletion (A) and those with codeletion tumors (B). A. The prognostic impact of CDKN2A homozygous deletion (n=4) was not apparent in the group with 1p/19q codeletion, although the number of cases was very small. B. In 1p/19q intact tumors, cases with CDKN2A homozygous deletion (n=15) showed significantly shorter survival than those without this copy number alteration. C. In 1p/19q intact cases, WHO grade IV cases showed unfavorable prognosis even when analyzing cases without CDKN2A homozygous deletion. codel, codeleted; KPS, Karnofsky Performance Status; OS, overall survival; and 5yOS, 5-year-overall survival.
Data Availability Statement
The anonymized datasets analyzed in the present study are provided in supplementary information.