Abstract
Objective. Music has been shown to modulate pain, although the impact of music on specific aspects of nociceptive processing is less well understood. Using quantitative sensory testing (QST), we assessed the impact of a novel music app on specific aspects of nociceptive processing. Design. Within-subjects paired comparison of pain processing in control vs music condition. Setting. Human psychophysical laboratory. Subjects. Sixty healthy adult volunteers. Methods. Subjects were assessed for baseline anxiety, depression, and catastrophizing using validated questionnaires. QSTs measured included 1) pain threshold and tolerance to deep muscle pressure, 2) pain with mechanical pinprick, 3) temporal summation of pain (TSP) with a repeated pain stimulus, and 4) conditioned pain modulation (CPM) with a second painful stimulus. QSTs were performed in the absence and presence of music delivered through a music app. Results. We found an increase in pressure pain thresholds in both the forearm (P = 0.007) and trapezius (P = 0.002) with music, as well as a decrease in the amount of pinprick pain (P < 0.001) and TSP (P = 0.01) with music. Interestingly, CPM was also significantly diminished (P < 0.001) in the music condition. No significant difference in cold pain, anxiety, or situational catastrophizing was observed with music. Higher baseline pain catastrophizing scores were associated with less music-induced pressure pain reduction. Conclusions. Several measures of mechanical pain sensitivity were reduced with music. TSP, a measure of central sensitization, also decreased with music, but CPM, a measure of descending modulation of pain, was not further augmented by music.
Keywords: Quantitative Sensory Testing, Music, Pain, Temporal Summation
Introduction
Pain is a common sensation experienced by 20% of the US population on a daily basis [1]. Persistent pain or episodes of acute pain may be devastating to individuals and society [2] and are associated with poorer health outcomes, increased health care utilization, and higher rates of disability [3–5]. The experience of both acute and chronic pain is multifaceted, arising biologically from peripheral nociceptor activation after injury, inflammation of tissues, and further modulated by peripheral, spinal, and supraspinal input. Psychosocial factors [6,7], including anxiety, depression, and catastrophizing [8], also modulate pain. The treatment of pain, therefore, is complex and challenging. Although pharmacologic therapies aim to blunt the experience of pain through agonism at the mu opioid receptor (opioids), alteration of the inflammatory cascade (NSAIDs), or modulation of spinal neurotransmission (ketamine), behavioral therapies aim to influence the psychosocial modulation of pain, potentially altering individual thoughts about pain (cognitive behavioral therapy) or imparting skills to reframe pain in the moment (mindfulness) [9,10].
One potential intervention that may affect multiple components of pain processing is music. Previous investigations have described the phenomenon of music-induced analgesia (MIA), a subjective reduction of pain perception after listening to music [11]. Music may be administered in a clinical context both as simple music listening, facilitated by health care professionals using a music app (e.g., playing recorded music, using streaming service), and as more formal structured music therapy delivered by board-certified music therapists, where patients may also themselves participate in production of music [12,13]. Although certified music therapists are available only in limited care settings, simple music listening is potentially quite widely available. Listening to music has been demonstrated to confer MIA in acute experimental [14,15] and clinical [12,16–18] settings. A neurochemical basis for MIA has also been suggested from functional magnetic resonance imaging (fMRI) studies that show that MIA is associated with changes in brain activation [19]. Listening to pleasurable music also upregulates the dopaminergic and serotonergic pathways involved with reward in the nucleus accumbens [20–22] and is associated with “thrills” experienced while listening to music [23,24], perhaps indirectly modulating pain.
Quantitative sensory testing (QST) systematically characterizes psychophysical responses [25], and enhanced sensitivity on QST may be used to predict greater pain responses [26]. The extent to which music alters various aspects of pain perception and processing is not well characterized. In this investigation, we assessed the impact of music on the nociceptive processing of pain, as measured through a basic set of QST, while concurrently measuring its impact on anxiety and situational catastrophizing. We hypothesized that music would alter nociceptive processing and decrease ratings of anxiety and catastrophizing and that these effects would be related.
Methods
This observational study was approved by the Brigham and Women’s Hospital Institutional Review Board (IRB) and registered as NCT03692247. Volunteers over the age of 18, without a self-reported history of chronic opioid use or neuropathy, were recruited through social media. Participants meeting enrollment criteria attended a single study visit. Participants were instructed not to ingest nonsteroidal anti-inflammatory medications (NSAIDs) or acetaminophen 48 hours before their scheduled study day.
Participants were familiarized with the quantitative sensory testing equipment (through demonstration by the investigator on him/herself), after which they completed several questionnaires to assess psychosocial phenotype and any baseline pain, including the Brief Pain Inventory [27], Pain Catastrophizing Scale [28], and Patient-Reported Outcomes Measurement Information System (PROMIS) short forms for anxiety (seven items), depression (eight items), and sleep disturbance (eight items) [29]. This was followed by a 10-minute baseline QST session. They were then instructed in the use of a music app on a study iPhone and selected a preferred generative music theme, which was played throughout a second QST session that was identical to the baseline session. Each set of QSTs was immediately followed by a brief questionnaire assessing the extent of catastrophizing during the QST session (Situational Pain Catastrophizing Scale [SPCS]) (Figure 1). All QST sessions were performed by a single member of the study staff. Study data were collected and managed using REDCap, a secure web-based application to support data recording for research [30].
Figure 1.
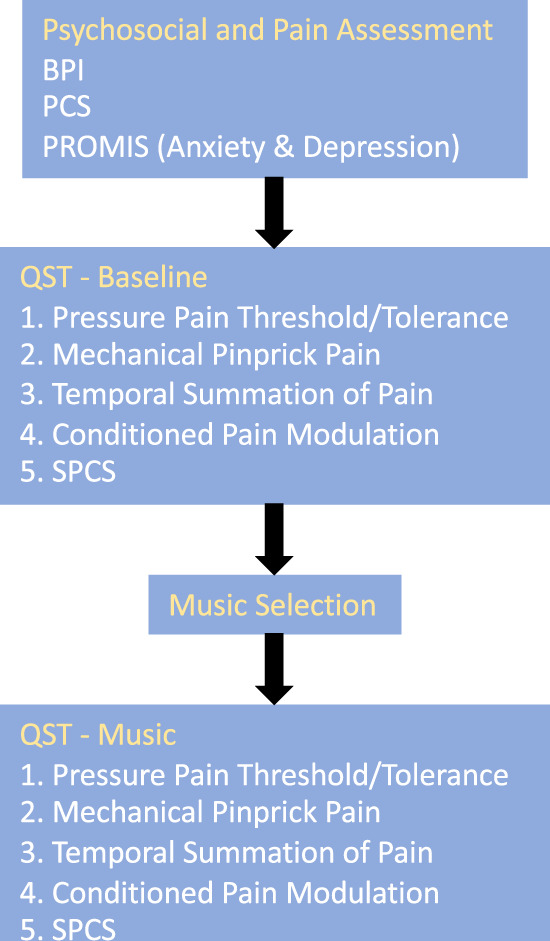
Study schema.
Quantitative Sensory Testing
Pain Sensitivity Testing: Pressure Pain Threshold and Tolerance
Pressure pain threshold and tolerance were assessed in a similar manner to our previous studies [31], using a digital pressure algometer (Wagner FDX, Greenwich, CT, USA) with a flat round transducer, probe area 0.785 cm2. Testing sites were bilateral on the dorsal aspect of the proximal forearm ∼3–4 cm distal to the elbow crease (extremity site) and over the trapezius muscle at the upper back ∼2–3 cm above the scapular spine, midway between the C7 prominence and humeral head (truncal site). For pressure pain, pressure was increased at a steady rate of ∼1 lb/sec (0.45 kg/sec), and the subject was asked to indicate when this pressure first became painful (threshold). The pressure was further increased, and the subject was asked to indicate when the pain from the stimulus was no longer tolerable (tolerance). Two trials were performed at each site, alternating from side to side and between extremity and truncal sites.
Pain Sensitivity Testing: Mechanical Pinprick Pain
Mechanical pinprick pain was assessed as in our previous studies [31,32], using standardized weighted pinprick applicators similar to those described by Rolke et al. [25]. A single stimulation of the lowest-force (128 mN) pinprick was applied to the dorsal aspect of the index finger between the first and second interphalangeal joints of the left hand while resting the palm facing downward on a flat surface. The subject then rated the pain intensity from the mechanical stimulus on a scale of 0–10. If this was rated as 0–1, the next highest-force probe was tested as a single application.
Temporal Summation of Pain and Painful Aftersensations
Calibrated force mechanical pinprick probes (128, 256, and 512 mN) were applied in increasing order to the dorsum of the left index finger, and the lowest-force probe was found to result in a mildly painful sensation with a single application selected [1–3]. After a break of at least 10 seconds after the single-stimulus testing, a train of 10 stimuli was applied at the same location, at a rate of one stimulation per second. The subject rated pain on a scale of 0–10 after the first, fifth, and 10th stimuli. After this train of stimuli, patients were asked to rate any pain remaining 15 seconds after cessation of the last stimulus (PAS) on a scale of 0–10. The same procedure was repeated on the right index finger and the middle finger of each hand, alternating sides of testing. Temporal summation of pain was calculated as the pain rating in response to the 10th stimulation minus the pain rating in response to the first stimulation. This was calculated for each of the four finger sites and averaged.
Conditioned Pain Modulation
Conditioned pain modulation (CPM) was measured using testing procedures as in previous studies [33]. Pressure pain threshold and tolerance were measured using an algometer over the nondominant trapezius muscle, as described above. Participants were then asked to submerge their dominant hand (up to the wrist) into an ice water bath, but they could remove it whenever it became intolerable. Ten seconds after hand submersion, pressure pain threshold and tolerance were again measured over the nondominant trapezius. CPMthreshold was calculated as the change in pressure pain threshold in the absence and presence of cold pain: (thresholdcold – thresholdbaseline/thresholdbaseline)*100. CPMtolerance was calculated as the change in pressure pain threshold in the absence and presence of cold pain: (tolerancecold – tolerancebaseline/tolerancebaseline)*100.
Music Intervention
We used a novel music web app, Unwind (Bose Corporation, Boston, MA, USA), which was designed to address pain and anxiety. The Unwind project sought to develop optimally relaxing tracks based on pleasurable musical genes (e.g., features of intonation and rhythm that are pleasurable) extracted using machine learning techniques. Unwind commissioned five instrumental tracks using these pleasurable music genes as a template. We have previously described preliminary acceptance of users to operate the Unwind app and whether they found it feasible to address anxiety and pain in the emergency department [34]. We chose to utilize Unwind in this investigation because we were interested in the potential for this machine-generated remix of human-composed music tracks to modulate nociceptive processing. Users were presented with a preview screen that allowed them to sample five different tracks and select their favorite among them. Each music listening session was standardized to 10 minutes.
Statistical Analysis
Subject characteristics were summarized using frequencies and percentages for categorical variables, and mean or median values with standard deviation or interquartile ranges (Q1–Q3) for continuous variables, according to normality of distribution. Comparison of QST parameters between baseline and with music was accomplished using a nonparametric paired test (related-samples Wilcoxon signed rank test). All statistical tests were two-tailed, and the level of significance was set at α = 0.05. All analyses were performed using SPSS 25.
Power Analysis
Sample size calculation was based on previous data estimating the effect of distraction on laboratory pain scores (pinprick pain) [35], in which temporal summation of pain scores (0–100 scale) was reduced by a mean of 15.2 with an SD of 21.8 (paired comparison). Setting the power to 0.9 and α to 0.05 and adjusting for the clusters in this previous sample, we calculated that a sample of ∼60 subjects would be required to allow observance of a similar reduction, and we planned to screen an additional 10–20% to account for ineligibility and dropout.
Results
Sample Characteristics
During the study period, 78 individuals were screened, with 77 qualifying after phone screening and 60 enrolling in the study, with the reason for nonparticipation being lack of schedule availability (normal business hours). The mean age of participants (range) was 26 ± 8.5 (20–73) years (Table 1). More women (N = 38, 63%) than men (N = 22, 37%) participated in the study. Participants reported minimal pain on the Brief Pain Inventory (BPI; 0.7 ± 0.7). Similarly, T scores for anxiety and depression (PROMIS Short Form) were near 50, suggesting scores comparable to population averages (Table 1). Pain Catastrophizing Scale and SPCS scores (measured after each QST session) were variable but were well below the scores typically reported by chronic pain patients. Although a decrease in situational catastrophizing was observed after the music session, this did not reach statistical significance.
Table 1.
Study subject characteristics
| Category | Mean ± SD or No. (%) |
|---|---|
| Age (20–73), y | 26.3 ± 8.5 |
| Males | 22 (37) |
| Females | 38 (63) |
| Brief Pain Inventory | 0.7 ± 0.7 |
| Pain Catastrophizing Scale | 6.4 ± 6.8 |
| PROMIS Anxiety t score | 50.3 ± 7.7 |
| PROMIS Depression t score | 46.0 ± 7.4 |
| Situational Pain Catastrophizing Scale–Baseline | 1.90 ± 2.5 |
| Situational Pain Catastrophizing Scale–Music | 1.45 ± 1.9 |
PROMIS = Patient-Reported Outcomes Measurement Information System.
Effect of Music on Pressure Pain
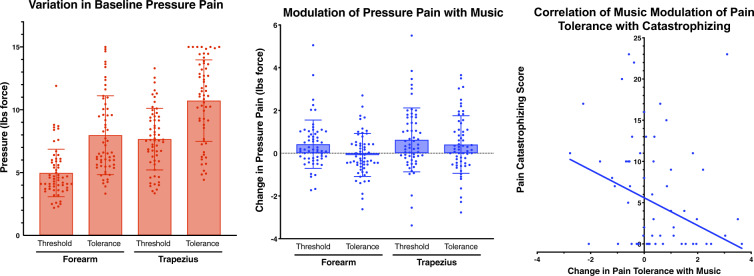
Pressure pain threshold and tolerance were variable between individuals (Figure 2A) but highly correlated within each subject across sites and conditions (Spearman’s rho = 0.7–0.9). Participants reported a significant increase in pressure pain thresholds in both the forearm and trapezius, as well as an increase in trapezius pressure pain tolerance, during the music condition compared with baseline (Figure 2B). No significant change was seen in FA pressure pain tolerance with music (Figure 2B). Interestingly, the degree of reduction in pressure pain tolerance with music was negatively correlated to pain catastrophizing (Spearman’s rho = –0.273, P = 0.035), such that those reporting higher baseline catastrophizing showed less increased (and in some cases decreased) pain threshold with music (Figure 2C).
Figure 2.
The effect of music on pressure pain threshold and tolerance.
Effect of Music on Temporal Summation of Pinprick Pain and Painful Aftersensations
Subjects were tested with weighted pinprick probes on the dorsum of the index and middle fingers, rating pain at the beginning (PP1), during (PP5), and end (PP10) of a 10-stimulus, 1-Hz frequency train, both in the absence and presence of music. Paired comparisons show reduced pinprick pain ratings on the first, fifth, and 10th stimuli with music (Figure 3). Temporal summation of pain (TSP), which is the tendency for a stimulus to become more painful with repeated applications, was defined as the increase in pain score between the first and 10th applications of the stimulus train (PP10–PP1). In the absence of music, participants showed varying degrees of TSP, with most reporting an increase in pain score over the course of the 10-stimulus train. However, lower TSP was observed in the music condition compared with baseline (P = 0.006, related-samples Wilcoxon signed rank). Additionally, 15 seconds after the 10-stimulus train, subjects were asked to rate any lingering pain in the area of stimulation (painful aftersensations [PAS]). PAS were also reduced in the music condition (P < 0.001).
Figure 3.
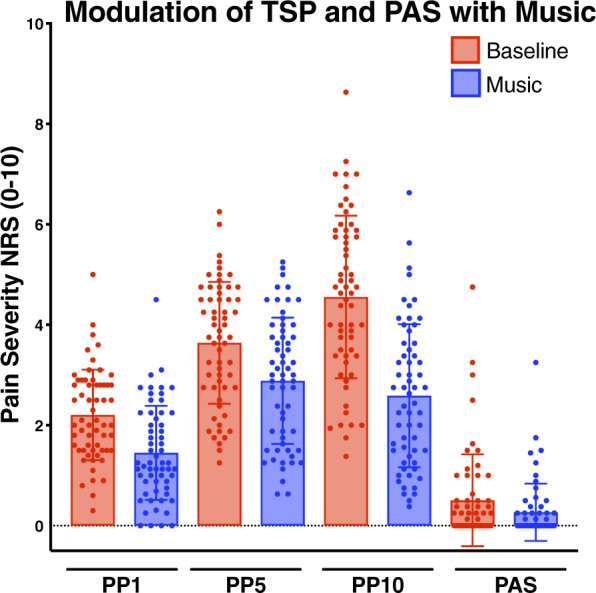
Modulation of temporal summation of pain and painful after-sensations before and after music.
Effect of Music on Conditioned Pain Modulation
To investigate conditioned pain modulation, we used a second potent conditioning painful stimulus (5ºC water bath) to modulate the response to a first pain stimulus (pressure pain threshold or tolerance at trapezius). Most subjects exhibited CPM, represented as an increase in pressure pain threshold and tolerance pain (reduced sensitivity) during the contralateral cold stimulus in the absence of music (Figure 4). We were interested in whether the presence of music impacted this process, potentially serving as a further modulator of pain. Interestingly, we in fact observed a significant decrease in CPM, whether measured as a pain threshold or pain tolerance in the music condition (P < 0.001) (Figure 4).
Figure 4.
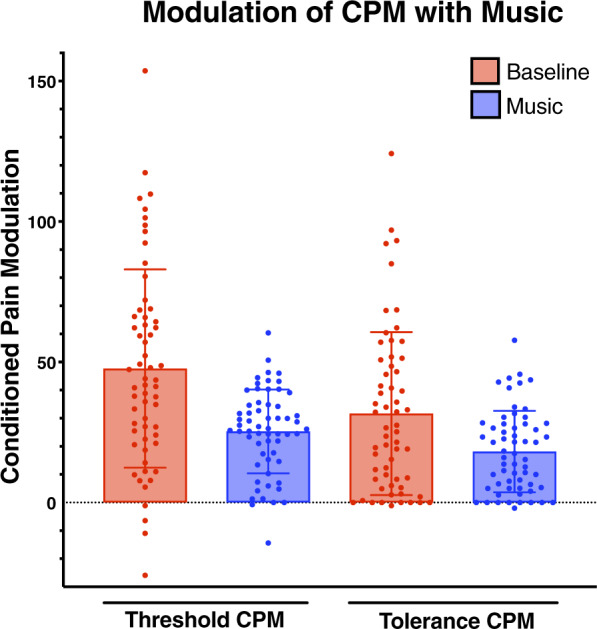
The effect of music on conditioned pain modulation.
Discussion
This study demonstrated a modest but significant modulation of pain processing while listening to music generated through an interactive app. Specifically, pressure pain threshold and tolerance at truncal and extremity sites increased, while reported pain with weighted mechanical pinprick probes decreased. Additionally, temporal summation of pain and painful aftersensations with a repeated stimulus decreased in response to music. Modulation of pain by a second painful stimulus (conditioned pain modulation), however, was not further augmented by music, but rather decreased by it. Taken together, these findings indicate that music may diminish pain sensitivity and dampen central facilitation of pain but cannot further enhance the descending inhibition induced by a second conditioning pain stimulus. These data from healthy volunteers provide insights into the mechanistic basis of modulation of normal nociception by music and lay the groundwork to understand how music interventions may benefit individuals living with chronic pain.
Temporal summation of pain (a measure of facilitation of pain) and CPM (a measure of endogenous inhibition of pain) are used to characterize the psychophysical profile of patients with chronic pain; TSP is frequently higher and CPM lower among chronic pain patients compared with pain-free controls [36–38]. Additionally, these tests predict the degree of postsurgical pain [31,33,39–49]. In particular, greater temporal summation of pain at several body sites has been associated with greater postsurgical pain [32,50–54] and opioid utilization [41]. Music did decrease TSP in this study, suggesting that it may be useful in dampening pain facilitation. On the other hand, music did not improve the ability to endogenously inhibit pain, measured by CPM. In fact, the calculated CPM was reduced in the music condition, whether measured as pain threshold or tolerance. This finding suggests that music cannot produce an additional effect in the presence of a second conditioning pain stimulus. It is possible that music may inhibit the perceived intensity or salience of the conditioning cold pain stimulus, thus interfering with its modulating effect [55].
Prior investigations have demonstrated a reduction of clinical pain and opioid use with the addition of music [56–59]. Our results support the use of music as an additive therapy that may blunt the sensation of pain and increase tolerance to pain. Music, in combination with pharmacotherapy, might be effective in managing acute episodes of pain where there is partial relief from a nonopioid analgesic. Instead of using opioid analgesics for unrelieved pain, clinicians could rely on music’s ability to increase an individual’s pain threshold and tolerance, obviating the need for additional opioids and thereby avoiding the risks of opioid exposure. Additionally, in individuals already on opioid therapy, music may prevent the need for increased opioid dosage in response to acutely worsening pain. The preliminary results from our group suggest that this music app is acceptable to patients in the emergency department who are experiencing pain [34].
Psychosocial variables such as negative affect and catastrophizing augment pain processing, both among healthy individuals and chronic pain patients, including fibromyalgia (FM) [53] and HIV-associated pain [60]. We did not observe a significant decrease in situational pain catastrophizing in the music condition among these healthy volunteers. This may arise from low baseline catastrophizing measures among this group of subjects. We did, however, observe a significant negative correlation between catastrophizing and music-induced analgesia in this group. This result is somewhat at odds with our previous findings that distraction produced more analgesia in high catastrophizers [35]. Although the precise cause of this discrepancy in findings is unclear, the latter studies included individuals who experienced chronic pain with an elevated degree of catastrophizing. Affect augments pain processing, particularly in individuals with chronic pain [5]. Further study is needed to understand whether the relationship between psychosocial variables and MIA differs between individuals with and without chronic pain. Additionally, music as an adjunct to pain management may need to be paired with interventions that address these psychosocial influences among individuals with greater catastrophizing. Interestingly, decreases in catastrophizing due to participation in a meditation/yoga intervention including self-guided visualization to distract from pain were associated with decreased pain among FM patients [61].
Although our study demonstrated a significant analgesic effect of music on threshold, tolerance, and facilitation of pain, we studied healthy volunteers who likely experience pain differently than many individuals with acute or chronic pain. We nonetheless believe these findings represent a first step toward understanding which aspects of nociception may be modulated by music, suggesting that healthy individuals with minimal psychosocial influencers may experience analgesic benefit from listening to music and indicating potential to serve in combination with other modalities to decrease pain in healthy individuals who experience acute pain or injury. Of note, we studied the nociceptive effects of listening to music. Individuals may also engage with music through various other modalities as well, including music-guided meditation and breathing exercises, generation of music by playing instruments, or participation in a structured music therapy session with a board-certified music therapist [62]. Further investigation will determine the manner in which music interventions should be integrated into real-world pain management regimens. In addition, future investigations can determine whether changing the threshold of pain actually leads to a decrease in clinical pain and need for opioid analgesics, either in the setting of a new injury/acute exacerbation of pain or in the management of chronic pain. Future study is also needed to determine whether these findings extend to individuals who experience chronic pain and likely have greater psychosocial modulation of pain.
Behavioral interventions often require substantial effort and are therefore expensive to sustain. For many clinicians, a faster, less costly solution to pain management is opioid-based therapy. Although opioids may be effective for acute and even chronic pain, their use is associated with considerable risk. Additionally, persistent opioid use can in some cases lead to nonmedical opioid use, including the transition from oral opioids to injection drug use and its associated comorbidities [63]. Even though pain is increasingly recognized as a complex, biopsychosocial process for which pharmacological treatment alone may be insufficient, pharmacological treatment of chronic pain is often chosen over behavioral interventions, many of which are time-consuming, labor-intensive, and difficult to scale. Listening to music is an appealing adjunctive therapy for pain because it is universally accessible. Unlike pharmacotherapy or complex behavioral therapies that require careful monitoring of medication use or access to experienced therapists, music listening is nonstigmatizing and can be accessed at home, used on demand, and highly personalized. This investigation describes a potential mechanistic effect of music on pain processing. Future studies should assess the feasibility of deploying music in populations that experience pain in order to understand the contextual basis in which music might be used, as well as the operational aspects of integrating a music intervention into existing pain management regimens.
This study has some limitations. First, the order of music vs baseline QST was not randomized, creating the possibility that participants were more comfortable with the QST sessions in the music condition. In future studies, randomizing the order of the music and nonmusic QST conditions would further separate out the differences between the two sessions. Second, participants were not blinded to experimental conditions. Third, participants, being young and healthy, may not reflect the demographics or psychosocial phenotypes of individuals with acute or chronic pain. Last, this study only used a single type of music delivered through an app; to what extent these findings generalize to other types of music is unclear. Having participants self-select personalized music from a wider range of genres may be more effective than preselected music choices in modulating nociception.
Conclusions
Music delivered through an app positively improves certain aspects of nociceptive processing, including pain threshold tolerance and temporal summation of pain. Music may partially interfere with other aspects of pain processing, including conditioned pain modulation in healthy volunteers. Further studies are needed to understand whether these findings extend to clinical settings, chronic pain patients, and other types of music.
Funding sources: NIDA K23DA044874 (PI: Chai), NIDA K24DA037109 (Boyer), NIH K23 GM110540 (PI: Schreiber).
Conflicts of interest: PRC is supported by research grants from Philips Biosensing, Gilead Sciences, and the Hans and Mavis Lopater Psychosocial Fund. The music intervention used in this investigation, along with technology support, was provided by Bose, Inc.
References
- 1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain among Adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13(8):715–24. [DOI] [PubMed] [Google Scholar]
- 3. Becker N, Thomsen AB, Olsen AK, Sjøgren P, Bech P, Eriksen J. Pain epidemiology and health related quality of life in chronic non-malignant pain patients referred to a Danish multidisciplinary pain center. Pain 1997;73(3):393–400. [DOI] [PubMed] [Google Scholar]
- 4. Elliott TE, Renier CM, Palcher JA. Chronic pain, depression, and quality of life: Correlations and predictive value of the SF-36. Pain Med 2003;4(4):331–9. [DOI] [PubMed] [Google Scholar]
- 5. Lerman SF, Rudich Z, Brill S, Shalev H, Shahar G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom Med 2015;77(3):333–41. [DOI] [PubMed] [Google Scholar]
- 6. Fillingim RB, Bruehl S, Dworkin RH, et al. The ACTTION-American Pain Society Pain Taxonomy (AAPT): An evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15(3):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kent ML, Tighe PJ, Belfer I, et al. The ACTTION-APS-AAPM Pain Taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. Pain Med 2017;18(5):947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 9. Khoo EL, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioural therapy for the treatment and management of chronic pain: A systematic review and network meta-analysis. Evid Based Ment Health 2019;22(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012;14(11):CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain 2008;134(1):140–7. [DOI] [PubMed] [Google Scholar]
- 12. Gutgsell KJ, Schluchter M, Margevicius S, et al. Music therapy reduces pain in palliative care patients: A randomized controlled trial. J Pain Symptom Manage 2013;45(5):822–31. [DOI] [PubMed] [Google Scholar]
- 13. Lee JH. The effects of music on pain: A meta-analysis. J Music Ther 2016;53(4):430–77. [DOI] [PubMed] [Google Scholar]
- 14. Guetin S, Ginies P, Siou DK, et al. The effects of music intervention in the management of chronic pain: A single-blind, randomized, controlled trial. Clin J Pain 2012;28(4):329–37. [DOI] [PubMed] [Google Scholar]
- 15. Matsota P, Christodoulopoulou T, Smyrnioti ME, et al. Music’s use for anesthesia and analgesia. J Altern Complement Med 2013;19(4):298–307. [DOI] [PubMed] [Google Scholar]
- 16. McCaffrey R, Freeman E. Effect of music on chronic osteoarthritis pain in older people. J Adv Nurs 2003;44(5):517–24. [DOI] [PubMed] [Google Scholar]
- 17. Onieva-Zafra MD, Castro-Sanchez AM, Mataran-Penarrocha GA, Moreno-Lorenzo C. Effect of music as nursing intervention for people diagnosed with fibromyalgia. Pain Manag Nurs 2013;14(2):e39–46. [DOI] [PubMed] [Google Scholar]
- 18. Yeo JK, Cho DY, Oh MM, Park SS, Park MG. Listening to music during cystoscopy decreases anxiety, pain, and dissatisfaction in patients: A pilot randomized controlled trial. J Endourol 2013;27(4):459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garza-Villarreal EA, Jiang Z, Vuust P, et al. Music reduces pain and increases resting state fMRI BOLD signal amplitude in the left angular gyrus in fibromyalgia patients. Front Psychol 2015;6:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chai PR, Carreiro S, Ranney ML, et al. Music as an adjunct to opioid-based analgesia. J Med Toxicol 2017;13(3):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage 2005;28(1):175–84. [DOI] [PubMed] [Google Scholar]
- 22. Ferreri L, Mas-Herrero E, Zatorre RJ, et al. Dopamine modulates the reward experiences elicited by music. Proc Natl Acad Sci U S A 2019;116(9):3793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panksepp J. The emotional sources of “chills” induced by music. Music Percept 1995;13(2):171–207. [Google Scholar]
- 24. Goldstein A. Thrills in response to music and other stimuli. Physiol Psychol 1980;8(1):126–9. [Google Scholar]
- 25. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006;123(3):231–43. [DOI] [PubMed] [Google Scholar]
- 26. Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology 2004;100(1):115–9; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 27. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain 2004;5(2):133–7. [DOI] [PubMed] [Google Scholar]
- 28. Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: Invariant factor structure across clinical and non-clinical populations. Pain 2002;96(3):319–24. [DOI] [PubMed] [Google Scholar]
- 29. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schreiber KL, Zinboonyahgoon N, Xu X, et al. Preoperative psychosocial and psychophysical phenotypes as predictors of acute pain outcomes after breast surgery. J Pain 2019;20(5):540–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edwards RR, Mensing G, Cahalan C, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: Influence of catastrophizing. J Pain Symptom Manage 2013;46(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abrecht CR, Cornelius M, Wu A, et al. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: Psychophysical and psychosocial factors. Pain Med 2019;20(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chai PR, Schreiber KL, Taylor SW, et al. The feasibility and acceptability of a smartphone-based music intervention for acute pain. Proc Annu Hawaii Int Conf Syst Sci 2019;2019:3917–25. [PMC free article] [PubMed] [Google Scholar]
- 35. Schreiber KL, Campbell C, Martel MO, et al. Distraction analgesia in chronic pain patients: The impact of catastrophizing. Anesthesiology 2014;121(6):1292–301. [DOI] [PubMed] [Google Scholar]
- 36. Petersen KK, McPhee ME, Hoegh MS, Graven-Nielsen T. Assessment of conditioned pain modulation in healthy participants and patients with chronic pain: Manifestations and implications for pain progression. Curr Opin Support Palliat Care 2019;13(2):99–106. [DOI] [PubMed] [Google Scholar]
- 37. O’Brien AT, Deitos A, Trinanes Pego Y, Fregni F, Carrillo-de-la-Pena MT. Defective endogenous pain modulation in fibromyalgia: A meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018;19(8):819–36. [DOI] [PubMed] [Google Scholar]
- 38. Arendt-Nielsen L. Central sensitization in humans: Assessment and pharmacology. Handb Exp Pharmacol 2015;227:79–102. [DOI] [PubMed] [Google Scholar]
- 39. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;138(1):22–8. [DOI] [PubMed] [Google Scholar]
- 40. Sangesland A, Storen C, Vaegter HB. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand J Pain 2017;15(1):44–52. [DOI] [PubMed] [Google Scholar]
- 41.Abrecht CR, Cornelius M, Wu A, et al. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: Psychophysical and psychosocial factors. Pain Med 2019;20(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andersen KG, Aasvang EK, Kroman N, Kehlet H. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiol Scand 2014;58(10):1240–8. [DOI] [PubMed] [Google Scholar]
- 43. Allison PD. Logistic Regression Using the SAS System: Theory and Application. Cary, North Carolina: Wiley-SAS; 2001. [Google Scholar]
- 44. Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain 2015;156(12):2413–22. [DOI] [PubMed] [Google Scholar]
- 45. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain 2011;12(7):725–46. [DOI] [PubMed] [Google Scholar]
- 46. Brennan TJ. Pathophysiology of postoperative pain. Pain 2011;152(Suppl):S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: A population-based cohort study. Pain 2014;155(2):232–43. [DOI] [PubMed] [Google Scholar]
- 48. Bruce J, Thornton AJ, Scott NW, et al. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer 2012;107(6):937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;156(1):55–61. [DOI] [PubMed] [Google Scholar]
- 51. Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain 2017;158(2):323–32. [DOI] [PubMed] [Google Scholar]
- 52. Ortner CM, Granot M, Richebe P, Cardoso M, Bollag L, Landau R. Preoperative scar hyperalgesia is associated with post-operative pain in women undergoing a repeat caesarean delivery. Eur J Pain 2013;17(1):111–23. [DOI] [PubMed] [Google Scholar]
- 53. Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in postmastectomy patients: Comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain 2013;154(5):660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weissman-Fogel I, Granovsky Y, Crispel Y, et al. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain 2009;10(6):628–36. [DOI] [PubMed] [Google Scholar]
- 55. Goubert D, Danneels L, Cagnie B, et al. Effect of pain induction or pain reduction on conditioned pain modulation in adults: A systematic review. Pain Pract 2015;15(8):765–77. [DOI] [PubMed] [Google Scholar]
- 56. Danhauer SC, Vishnevsky T, Campbell CR, et al. Music for patients with hematological malignancies undergoing bone marrow biopsy: A randomized controlled study of anxiety, perceived pain, and patient satisfaction. J Soc Integr Oncol 2010;8(4):140–7. [PMC free article] [PubMed] [Google Scholar]
- 57. Lee DW, Chan KW, Poon CM, et al. Relaxation music decreases the dose of patient-controlled sedation during colonoscopy: A prospective randomized controlled trial. Gastrointest Endosc 2002;55(1):33–6. [DOI] [PubMed] [Google Scholar]
- 58. Liu Y, Petrini MA. Effects of music therapy on pain, anxiety, and vital signs in patients after thoracic surgery. Complement Ther Med 2015;23(5):714–8. [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Tang H, Guo Q, et al. Effects of intravenous patient-controlled sufentanil analgesia and music therapy on pain and hemodynamics after surgery for lung cancer: A randomized parallel study. J Altern Complement Med 2015;21(11):667–72. [DOI] [PubMed] [Google Scholar]
- 60. Pillay P, Wadley AL, Cherry CL, Karstaedt AS, Kamerman PR. Psychological factors associated with painful versus non-painful HIV-associated sensory neuropathy. AIDS Behav 2018;22(5):1584–95. [DOI] [PubMed] [Google Scholar]
- 61. Lazaridou AKA, Devine J, Haack M, Jamison RN, Edwards RR, Schreiber KL. Impact of daily yoga-based exercise on pain, catastrophizing, and sleep amongst individuals with fibromyalgia. J Alt Compl Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodgers-Melnick SN, Matthie N, Jenerette C, et al. The effects of a single electronic music improvisation session on the pain of adults with sickle cell disease: A mixed methods pilot study. J Music Ther 2018;55(2):156–85. [DOI] [PubMed] [Google Scholar]
- 63. Marshall B, Bland MK, Hulla R, Gatchel RJ. Considerations in addressing the opioid epidemic and chronic pain within the USA. Pain Manag 2019;9(2):131–8. [DOI] [PubMed] [Google Scholar]