Abstract
Objective/Subjects
To determine the autonomic effects of suboccipital release (SOR) during experimentally induced pain, 16 healthy subjects (eight women, eight men) experienced ischemic (forearm postexercise muscle ischemia [PEMI]) and cold (cold pressor test [CPT]) pain.
Design
Beat-to-beat heart rate (electrocardiogram), mean arterial blood pressure (finger photoplethysmography), baroreflex sensitivity (transfer function analysis), and pain perception were measured. SOR or a sham (modified yaw; 30 cycles/min) was performed in minute 2 of pain.
Results
PEMI increased blood pressure by 23 ± 2 and 20 ± 2 mmHg; no differences occurred between SOR or yaw. PEMI modestly elevated heart rate during ischemia, followed by significant reduction from baseline with SOR (–3 ± 2 bpm) and yaw (−4 ± 2 bpm); no differences were observed between treatments. CPT increased blood pressure (SOR = 11 ± 1, yaw = 9 ± 2 mmHg) and heart rate (SOR = 10 ± 2, yaw = 8 ± 3 bpm) before SOR and yaw. Neither treatment nor sham blunted blood pressure increases (SOR = 25 ± 2, yaw = 22 ± 2 mmHg) during CPT; both decreased heart rate (SOR = −3 ± 2, yaw = −2 ± 2 bpm) from baseline. PEMI and CPT caused increased pain without treatment modulation. Following pain and manual intervention, SOR increased baroreflex sensitivity in the 0.15–0.35 Hz range and decreased R-R interval power spectral density in the 0.03–0.5 Hz range compared with yaw. To probe potential mechanisms and interactions between manual treatment and a prototypic analgesic, oral aspirin (967 mg) was given 60 minutes before testing to reduce prostaglandin synthesis. Aspirin slightly attenuated pain but neither altered cardiovascular changes to PEMI nor interacted with SOR or yaw.
Conclusions
SOR has the capacity to modulate pain-induced autonomic control and regulation.
Keywords: Cold Pressor Test, Postexercise Muscle Ischemia, Baroreflex Sensitivity, Manipulative Treatment, Nonsteroidal Anti-inflammatory Drugs
Introduction
In 2012, ∼19 million adults in the United States used some form of manipulative treatment as part of their overall health care [1]. Suboccipital release (SOR) is a commonly used manual medicine technique of the head and neck. Other names and related techniques include suboccipital depression, CV4, basilar decompression, condylar decompression, and cervical myofascial release. Suboccipital myofascial release is used to promote joint and soft tissue mobility and decrease pain and tenderness [2]. SOR and related techniques are also used to treat pain from tension-type and migraine headaches [3,4]. The clinical effects of SOR are purported to be mediated via the autonomic nervous system [5], but similar to many manual techniques, the neurophysiological data in this area are sparse. Thus, the effects and mechanisms of SOR are unclear and not well understood.
A few reports have identified links between SOR-related techniques and autonomic activity. Cutler et al. [6] observed a decrease in direct measurements of muscle sympathetic nerve activity after the CV4 manual therapy technique but not after a sham procedure, indicating that cranial and spinal manual therapy can modulate the vasomotor component of sympathetic outflow to the muscle. Thus, arterial blood pressure and central mediators of cardiovascular function may be affected. Additionally, skin blood flow changes have been observed with cranial and spinal manual techniques [7–10]. As most blood vessels in the body are controlled by the sympathetic noradrenergic system, these data indicate that these manual techniques may have the capacity to modulate the sympathetic nervous system. The parasympathetic nervous system has also been implicated with SOR-related techniques, primarily via changes in heart rate variability [11–13], which is defined as variation in electrocardiogram-derived R-R intervals expressed in the time or frequency domain [14]. Despite these suggestive findings, clear autonomic nervous system evidence does not exist for SOR-related techniques.
Perturbations that alter autonomic nervous system effects are often difficult to observe in a healthy population in basal conditions. It is possible that SOR is most efficacious during nonhomeostatic conditions such as during strong sympathetic nervous system engagement. Henley et al. [15] used a 50º head-up tilt to engage sympathoexcitatory postural reflexes [16], which increased the ratio of normalized low-frequency to normalized high-frequency power using fast Fourier transform of R-R intervals to analyze heart rate variability. SOR attenuated this increase, with no effect observed in supine baseline or with a sham control [15]. These data indicate a clear potential for an autonomic effect. However, the effects with a more clinical sympathetic engagement, such as pain, and in other indices of autonomic function, such as vasoconstriction and arterial blood pressure, are less clear. Additionally, analysis of autonomic effects using more complex analytical methodology (fast Fourier transform of both the R-R interval and systolic blood pressure and transfer function analysis of their cross-spectra) can provide insight into autonomic control and regulation with or without changes in absolute values of end organs. This type of comprehensive approach has not yet been performed in an SOR experimental paradigm.
This study aimed to determine the effects of SOR on the cardiovascular system during cold pain and ischemic pain. Both cold pain via the cold pressor test (CPT) [17,18] and ischemic pain via postexercise muscle ischemia (PEMI) [19,20] have been identified to increase sympathetic activity. We hypothesized that cardiovascular reflex responses to acute perception of ischemic pain and cold pain would be reduced with SOR compared with a sham procedure (modified yaw head movements; previously identified not to alter sympathetic outflow to muscle and skin [21,22]). Many people who receive manual therapy also take nonprescription cyclooxygenase inhibitors such as aspirin and other nonsteroidal anti-inflammatory drugs for pain and inflammation management. We also aimed to determine whether inhibition of prostaglandin synthesis and SOR have a synergistic effect on lessening cardiovascular responses to ischemic pain. We hypothesized that if SOR works in part via a prostaglandin mechanism, then prostaglandin inhibition would mimic or potentiate SOR effects.
Methods
Subjects
Sixteen healthy subjects (eight male and eight nonpregnant female, age 23 ± 3 years, height 170 ± 9 cm, weight 73 ± 17 kg) participated in the study. Subject health was assessed via a health history and physical exam that included a 12-lead electrocardiogram. Females were also given a urine pregnancy test. All subjects’ arterial blood pressure was <140/90 mmHg and body mass index was <30 kg/m2. In addition, all subjects were nonsmokers and did not report history of disease or current medication usage that would affect the neurological, muscular, or cardiovascular systems. Individuals familiar with manual therapy were excluded from study participation. The protocol and procedures were approved by the Ohio University Biomedical Institutional Review Board and complied with the tenets and ethical principles for medical research outlined in the Declaration of Helsinki. All subjects gave written and verbal informed consent before participation in these studies.
Measurements
Heart rate and R-R interval were measured via standard limb lead I or II electrocardiogram (ECG100C, Biopac Systems Inc., Goleta, CA, USA) routed through a cardiotachometer (CT-1000, CWE Inc., Ardmore, PA, USA). Beat-by-beat arterial blood pressure was obtained from finger photoplethysmography (Finometer Pro, FMS, Amsterdam, the Netherlands) [23]. Beat-by-beat stroke volume was estimated from the arterial waveform via the Modelflow method (Beatscope, FMS). Skin blood flow was indexed from red blood cell flux via laser-Doppler flowmetry using integrative optic probes (MoorLab, Moor Instruments, Devon, UK). Venous occlusion plethysmography was used to determine calf blood flow (EC6, Hokanson, Bellevue, WA, USA) as previously described [24]. Respiration rate was monitored using a piezoelectric transducer connected to an elastic belt (Pneumotrace II, UFI, Morro Bay, CA, USA) to ensure that participants did not hold their breath during exercise or pain stimulus. Skin temperature was measured using a thermistor (400 series, YSI, Yellow Springs, OH, USA), and handgrip force was measured via a load cell device (TSD121C, Biopac), both of which were routed through amplifiers (SKT100C and DA100C, Biopac) into the data acquisition system (MP150, Biopac). Immediately after each recovery period, subjects were asked to verbally rate their pain during the preceding condition (0 = no pain, 10 = worst pain) [25].
Protocol
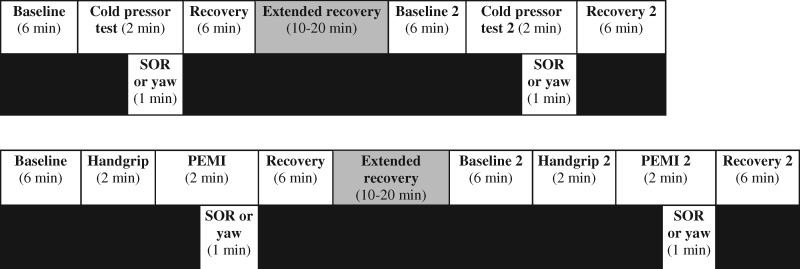
Subjects were tested in a supine position on three separate days. The research design consisted of testing the autonomic and cardiovascular effects of SOR (as previously described [2]) vs a hands-on sham control (yaw) during ischemic and cold pain perturbations. The sham was a modified yaw head movement, which consisted of passive rotation (30 cycles/min, timed by counting rotations per time frame) [21,22]. Each technique was performed during the full second minute of the painful stimulus. To standardize the time frame of the techniques, the investigator maintained the hand position for the SOR if release occurred before the 60-second time point. A single clinically trained investigator performed all treatments. Subjects were not informed which procedure was the sham. The order of the SOR and yaw treatments and experimental days was determined in a randomized, balanced design. Thus, the ischemic or cold pain perturbations were performed twice each experimental day to complete both treatments, separated with a recovery period that was extended until subjects’ heart rate and arterial blood pressure returned to baseline levels (Figure 1).
Figure 1.
Cold pressor test (CPT; top) and postexercise muscle ischemia (PEMI; bottom) timelines.
Cold Pressor Test
On the CPT experimental day, baseline recordings were taken for six minutes, followed by two minutes of CPT, consisting of immersion of one hand into a 4°C ice slurry up to the wrist. During the second minute of the CPT, either SOR or sham was performed. There was a six-minute recovery period, followed by another extended recovery period, allowing subjects’ heart rate and arterial blood pressure to return to baseline levels. The protocol was then repeated with the alternate treatment (i.e., SOR or sham).
Postexercise Muscle Ischemia
On the PEMI experimental day, maximal voluntary contraction was first assessed with a minimum of three attempts. Baseline measurements were taken for six minutes, followed by two minutes of handgrip at 25% of maximal voluntary contraction and two minutes of PEMI, as previously described [20]. During the second minute of PEMI, either SOR or sham was performed, followed by a six-minute recovery and an extended recovery, allowing for subjects’ heart rate and arterial blood pressure to return to baseline levels. The protocol was then repeated with the alternate treatment (i.e., SOR or yaw).
Postexercise Muscle Ischemia with Aspirin
On the PEMI with the cyclooxygenase inhibitor acetylsalicylic acid (aspirin) experimental day, aspirin (975 mg with a glass of water, witnessed and supervised by an investigator) was taken one hour before the protocol began, as aspirin’s duration of action is six to 12 hours [26]. The remainder of the protocol was identical to the PEMI day. Subjects quietly read or listened to music in the seated position as they waited for the drug to take effect.
Data Analysis
Data were sampled at 1,000 Hz via a data acquisition system (Biopac). Cardiac output was calculated as stroke volume × heart rate, and systemic vascular resistance was calculated as mean arterial blood pressure/cardiac output. Peripheral blood flow measurements were expressed as conductance to account for the pain-induced changes in arterial blood pressure. Vascular conductance is preferred over vascular resistance because its relationship is linear across physiological conditions [27]. Therefore, cutaneous vascular conductance was calculated as flux/mean arterial blood pressure, and calf vascular conductance as calf blood flow/mean arterial blood pressure. Baroreflex sensitivity was assessed by transfer function analysis by dividing the cross-spectra of R-R interval and systolic blood pressure by the power spectra of the input signal and expressing values as the magnitude of the gain in both the low-frequency (0.05–0.15 Hz) and high-frequency (0.15–0.35 Hz) ranges [28]. The cross-spectra were obtained as previously described [28]. To normalize individual differences in absolute power spectral density, the low-frequency and high-frequency ranges were divided by total spectral power (0.03–0.5 Hz). The 0.00–0.03 Hz frequency was removed because of the duration of the recordings and because this range contains the direct current component of the signal [29]. The R-R interval was also presented as the ratio of normalized low-frequency to high-frequency spectral power, as this can give insight into sympathetic and parasympathetic influences [14]. Fast Fourier transforms and transfer function analysis require five to six minutes of sustained (i.e., steady state) values and thus could only be completed during baseline and recovery. Differences in hand temperature and handgrip force were analyzed via paired t test. Differences in the mean values of the cold pressor test were analyzed after repeated-measures one-way analysis of variance (ANOVA). Differences in the mean values of handgrip PEMI were analyzed after repeated-measures two-way ANOVA. Pain perception and baroreflex sensitivity-related variables were analyzed across experimental pain and manual treatment via a 2 × 3 repeated-measures ANOVA. Because the magnitude of the effect of absolute values for baroreflex sensitivity-related variables is more difficult to assess, partial eta squared (ηp2) [30] was also calculated when significant P values were obtained for these variables. Student Newman Keuls post hoc analysis was performed when significant main effects were observed in ANOVAs. All values are reported as mean ± SE, and P values of <0.05 were considered statistically significant.
Results
Cold Pain
Participants experienced similar temperatures and pain responses during both treatment protocols. Baseline dorsal hand skin temperature was not different between groups (P = 0.680) and decreased (P < 0.001) to similar temperatures during the CPT (P = 0.495) (Table 1). Dorsal hand skin temperature decreased further during treatment (P = 0.001), but there was no difference between SOR and sham (P = 0.352) (Table 1). CPT caused similar pain in all participants (SOR = 6.02 ± 0.41, yaw = 5.91 ± 0.38).
Table 1.
Central and peripheral responses to cold and ischemic pain protocols
| A) Cold pressor test | |||||||
|---|---|---|---|---|---|---|---|
| Cardiac Output, L/min | Systemic Vascular Resistance, RU | Calf Vascular Conductance, mL/100 mL Tissue/min/mmHg | Cutaneous Vascular Conductance: Probe #1, Flux/mmHg | Cutaneous Vascular Conductance: Probe #2, Flux/mmHg | Dorsal Hand Skin Temperature, °C | ||
| SOR | Baseline | 5.2 ± 0.3 | 1,278 ± 45 | 3.0 ± 0.6 | 8.7 ± 2.7 | 10.9 ± 2.2 | 29.7 ± 0.9 |
| CPT | 6.2 ± 0.6 | 1,470 ± 77 | 2.2 ± 0.5 | 6.7 ± 1.3 | 9.4 ± 1.1 | 15.5 ± 0.7* | |
| Treatment | 4.9 ± 0.5 | 1,396 ± 74 | 1.8 ± 0.2* | 7.6 ± 1.3 | 11.0 ± 1.6 | 11.5 ± 1.0† | |
| Recovery | 5.0 ± 0.4 | 1,248 ± 63 | 2.3 ± 0.3 | 4.5 ± 0.5 | 8.6 ± 1.5 | n.d. | |
| Yaw | Baseline | 5.2 ± 0.3 | 1,272 ± 36 | 2.2 ± 0.3 | 6.2 ± 1.4 | 8.8 ± 1.1 | 29.1 ± 0.9 |
| CPT | 5.9 ± 0.6 | 1,428 ± 61 | 2.0 ± 0.4 | 5.2 ± 0.5 | 7.6 ± 0.8 | 16.4 ± 1.6* | |
| Treatment | 4.9 ± 0.5 | 1,355 ± 65 | 1.4 ± 0.2* | 6.2 ± 1.0 | 10.1 ± 1.6 | 12.7 ± 1.7† | |
| Recovery | 5.2 ± 0.4 | 1,278 ± 49 | 1.9 ± 0.2 | 4.3 ± 0.5 | 7.4 ± 0.8 | n.d. | |
| B) Postexercise muscle ischemia | ||||||
|---|---|---|---|---|---|---|
| Cardiac Output, L/min | Systemic Vascular Resistance, RU | Calf Vascular Conductance, mL/100 mL Tissue/min/mmHg | Cutaneous Vascular Conductance: Probe #1, Flux/mmHg | Cutaneous Vascular Conductance: Probe #2, Flux/mmHg | ||
| SOR | Baseline | 5.4 ± 0.4 | 1,395 ± 73 | 2.1 ± 0.2 | 9.5 ± 2.9 | 11.5 ± 3.8 |
| Exercise | 6.2 ± 0.5 | 1,789 ± 93 | n.d. | 10.0 ± 3.1 | 11.9 ± 2.2 | |
| PEMI | 5.4 ± 0.5 | 1,491 ± 112 | 2.2 ± 0.4 | 10.2 ± 3.1 | 12.1 ± 1.6 | |
| Treatment | 5.0 ± 0.4 | 1,493 ± 143 | 1.7 ± 0.3 | 8.0 ± 2.7 | 9.6 ± 1.7 | |
| Recovery | 5.2 ± 0.4 | 1,325 ± 75 | 2.0 ± 0.3 | 5.0 ± 1.2 | 8.0 ± 1.4 | |
| Yaw | Baseline | 5.2 ± 0.4 | 1,386 ± 66 | 2.3 ± 0.2 | 7.2 ± 1.6 | 8.1 ± 1.2 |
| Exercise | 6.1 ± 0.5 | 1,720 ± 87 | n.d. | 8.8 ± 2.2 | 11.2 ± 1.8 | |
| PEMI | 5.3 ± 0.5 | 1,444 ± 93 | 2.3 ± 0.3 | 11.1 ± 3.3 | 11.8 ± 2.1 | |
| Treatment | 5.0 ± 0.5 | 1,428 ± 134 | 2.0 ± 0.3 | 8.1 ± 2.7 | 9.9 ± 2.8 | |
| Recovery | 5.2 ± 0.4 | 1,340 ± 75 | 2.1 ± 0.2 | 4.3 ± 0.7 | 6.9 ± 1.2 | |
| C) Postexercise muscle ischemia + aspirin | ||||||
|---|---|---|---|---|---|---|
| Cardiac Output, L/min | Systemic Vascular Resistance, RU | Calf Vascular Conductance, mL/100 mL Tissue/min/mmHg | Cutaneous Vascular Conductance: Probe #1, Flux/mmHg | Cutaneous Vascular Conductance: Probe #2, Flux/mmHg | ||
| SOR | Baseline | 4.6 ± 0.2 | 1,219 ± 48 | 2.8 ± 0.7 | 5.3 ± 1.0 | 7.2 ± 0.9 |
| Exercise | 6.0 ± 0.3 | 1,535 ± 66 | n.d. | 8.7 ± 2.3 | 10.2 ± 1.7 | |
| PEMI | 4.8 ± 0.3 | 1,282 ± 50 | 2.6 ± 0.5 | 9.1 ± 3.3 | 11.0 ± 1.9 | |
| Treatment | 4.4 ± 0.3 | 1,200 ± 58 | 1.9 ± 0.4 | 4.2 ± 0.6 | 7.9 ± 1.1 | |
| Recovery | 4.6 ± 0.2 | 1,197 ± 49 | 2.2 ± 0.5 | 4.0 ± 1.0 | 5.4 ± 0.4 | |
| Yaw | Baseline | 4.6 ± 0.2 | 1,228 ± 38 | 1.9 ± 0.3 | 5.8 ± 0.8 | 10.1 ± 2.3 |
| Exercise | 5.6 ± 0.3 | 1,571 ± 74 | n.d. | 9.1 ± 2.2 | 12.2 ± 3.0 | |
| PEMI | 4.7 ± 0.3 | 1,396 ± 157 | 2.2 ± 0.5 | 7.0 ± 1.0 | 14.3 ± 3.0 | |
| Treatment | 4.4 ± 0.3 | 1,284 ± 145 | 2.2 ± 0.6 | 4.4 ± 0.5 | 9.8 ± 1.7 | |
| Recovery | 4.5 ± 0.2 | 1,229 ± 55 | 1.8 ± 0.3 | 4.0 ± 0.5 | 7.8 ± 2.0 | |
|
| ||||||
CPT = cold pressor test; n.d. = no data; PEMI = postexercise muscle ischemia; RU = vascular resistance units; SOR = suboccipital release.
Denotes difference compared with baseline (P ≤ 0.05).
Denotes difference compared with CPT.
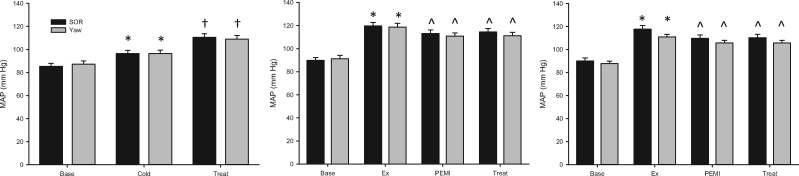
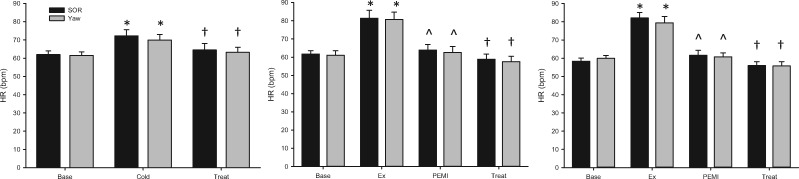
Cardiovascular system responses to CPT included increased mean arterial blood pressure (Figure 2) and heart rate (Figure 3), with no change in stroke volume before SOR and yaw. Neither SOR nor sham blunted the CPT-induced increases in mean arterial blood pressure (P < 0.001) or decreases in heart rate (P = 0.001) or stroke volume (P < 0.001). Calf vascular conductance decreased during both SOR and sham compared with baseline (P = 0.049); however, there were no differences between the SOR and sham conditions (P = 0.294) (Table 1). Additional cardiovascular variables are provided in Table 1.
Figure 2.
Change in mean arterial blood pressure (MAP) across the CPT (left), PEMI (center), and PEMI + aspirin (right) protocols, where Base = baseline, Treat = SOR or yaw, Ex = handgrip. *Denotes change from baseline. ^Denotes change from exercise. †Denotes change from pain condition (all P ≤ 0.05).
Figure 3.
Change in heart rate (HR) across the CPT (left), PEMI (center), and PEMI + aspirin (right) protocols, where Base = baseline, Treat = SOR or yaw, Ex = handgrip. *Denotes change from baseline. ^Denotes change from exercise. †Denotes change from pain condition (all P ≤ 0.05).
Ischemic Pain
Participants experienced similar handgrip exercise and pain responses during both treatment protocols. Participants were able to maintain the target handgrip force at similar levels before PEMI (SOR = 25 ± 2 and sham = 24 ± 3%, P = 0.793). Participants did not hold their breath or perform Valsalva maneuvers during exercise. Handgrip increased mean arterial blood pressure (Figure 2) and heart rate (Figure 3) and decreased stroke volume. PEMI caused similar pain in all participants (SOR = 5.55 ± 0.43, yaw = 5.44 ± 0.44).
Cardiovascular system responses to PEMI included a modest fall from the exercise-elevated mean arterial blood pressure (P < 0.001) (Figure 2) and heart rate (P < 0.001) (Figure 3) and a modest increase from the exercise-lowered stroke volume (P < 0.001). Only heart rate changed during treatment, with significant reductions (P = 0.015) occurring during both SOR and sham (Figure 3). No differences were observed between treatment and sham in mean arterial blood pressure (P = 0.103), heart rate (P = 0.738), or stroke volume (P = 0.206). Calf vascular conductance did not change throughout the protocol (P = 0.253) (Table 1). Additional cardiovascular variables are provided in Table 1.
Ischemic Pain + Aspirin
Similarly, during the PEMI + aspirin protocol, participants were able to maintain the target handgrip force at similar levels before PEMI (SOR = 24 ± 1 and sham = 23 ± 3%, P = 0.846) and did not hold their breath or perform Valsalva maneuvers during exercise. Handgrip increased mean arterial blood pressure (Figure 2) and heart rate (Figure 3) and decreased stroke volume. Pain perception was slightly decreased compared with the non-cyclooxygenase-inhibited treatments (P = 0.002), but no differences were observed between SOR and sham (SOR = 4.77 ± 0.44, yaw = 4.62 ± 0.48, P = 0.155).
Cardiovascular system responses to PEMI during this protocol included a modest fall from the exercise-elevated mean arterial blood pressure (P = 0.006) (Figure 2) and heart rate (P < 0.001) (Figure 3) and a modest increase from the exercise-lowered stroke volume. Only heart rate changed during treatment, with significant reductions (P = 0.005) occurring during both SOR and sham (Figure 3). No differences were observed between treatment and sham in mean arterial blood pressure (P = 0.312), heart rate (P = 0.302), or stroke volume (P = 0.936). Calf vascular conductance did not change throughout the PEMI + aspirin protocol (P = 0.336) (Table 1). Additional cardiovascular variables are provided in Table 1.
Pre–Post Pain Alterations in the Baroreflexes
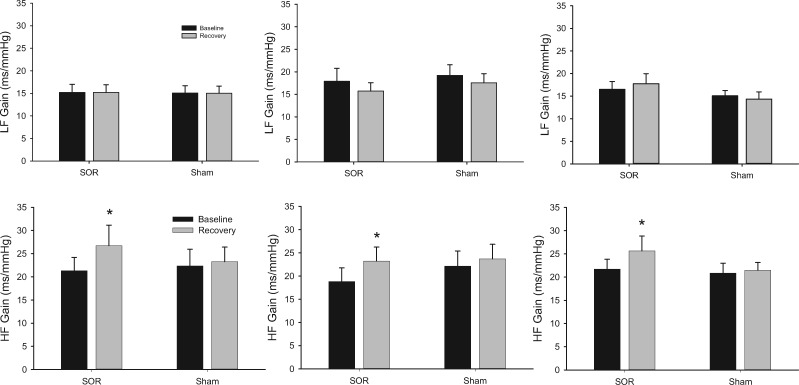
Low-frequency baroreflex sensitivity was not altered after pain in either condition regardless of type of pain (P = 0.785) (Figure 4). In contrast, high-frequency baroreflex sensitivity increased after pain and SOR but not after pain and sham (P = 0.031, ηp2 = 0.173) (Figure 4). Normalized low-frequency systolic blood pressure power spectral density trended toward differences between SOR and sham (P = 0.067, ηp2 = 0.147), but this was not different in the normalized high-frequency range (P = 0.178) (Table 2). Normalized low-frequency R-R interval power spectral density was different after pain between SOR and sham (P = 0.013, ηp2 = 0.180), and this trend was also observed in the high-frequency range (P = 0.076, ηp2 = 0.083) (Table 2). Finally, the ratio of low-frequency to high-frequency R-R interval spectral power was not different after pain between SOR and sham (P = 0.355) (Table 2).
Figure 4.
Response of low-frequency (LF) and high-frequency (HF) baroreflex sensitivity (ms/mmHg) during baseline and following (recovery) the CPT (left), PEMI (center), and PEMI + aspirin (right) protocols, where SOR = suboccipital release, Sham = yaw, and * denotes P ≤ 0.05.
Table 2.
Power spectral density and ratio of low-frequency to high-frequency power spectral density alterations before and after cold and ischemic pain
| A) Cold pressor test | ||||||
|---|---|---|---|---|---|---|
| Normalized Low-Frequency: Systolic Blood Pressure | Normalized High-Frequency: Systolic Blood Pressure | Normalized Low-Frequency: R-R Interval | Normalized Low-Frequency: R-R Interval | R-R Interval - Low-Frequency/High-Frequency | ||
| SOR | Baseline | 0.42 ± 0.03 | 0.19 ± 0.04 | 0.42 ± 0.05 | 0.41 ± 0.04 | 1.46 ± 0.33 |
| Recovery | 0.43 ± 0.02† | 0.20 ± 0.04 | 0.35 ± 0.04* | 0.40 ± 0.03† | 1.04 ± 0.17 | |
| Yaw | Baseline | 0.41 ± 0.03 | 0.17 ± 0.03 | 0.44 ± 0.04 | 0.39 ± 0.04 | 1.66 ± 0.41 |
| Recovery | 0.49 ± 0.03 | 0.18 ± 0.03 | 0.45 ± 0.04 | 0.35 ± 0.03 | 1.54 ± 0.20 | |
| B) Postexercise muscle ischemia | ||||||
|---|---|---|---|---|---|---|
| Normalized Low-Frequency: Systolic Blood Pressure | Normalized High-Frequency: Systolic Blood Pressure | Normalized Low-Frequency: R-R Interval | Normalized High-Frequency: R-R Interval | R-R Interval-Low-Frequency/High-Frequency | ||
| SOR | Baseline | 0.43 ± 0.03 | 0.21 ± 0.03 | 0.44 ± 0.04 | 0.37 ± 0.04 | 1.47 ± 0.25 |
| Recovery | 0.44 ± 0.03† | 0.25 ± 0.04 | 0.35 ± 0.04* | 0.45 ± 0.04† | 1.04 ± 0.22 | |
| Yaw | Baseline | 0.40 ± 0.03 | 0.20 ± 0.04 | 0.43 ± 0.03 | 0.40 ± 0.04 | 1.42 ± 0.27 |
| Recovery | 0.46 ± 0.04 | 0.22 ± 0.04 | 0.41 ± 0.04 | 0.41 ± 0.05 | 1.53 ± 0.42 | |
| C) Postexercise muscle ischemia + aspirin | ||||||
|---|---|---|---|---|---|---|
| Normalized Low-Frequency: Systolic Blood Pressure | Normalized High-Frequency: Systolic Blood Pressure | Normalized Low-Frequency: R-R Interval | Normalized High-Frequency: R-R Interval | R-R Interval-Low-Frequency/High-Frequency | ||
| SOR | Baseline | 0.47 ± 0.04 | 0.17 ± 0.02 | 0.43 ± 0.04 | 0.34 ± 0.04 | 1.88 ± 0.45 |
| Recovery | 0.39 ± 0.04† | 0.20 ± 0.04 | 0.38 ± 0.05* | 0.39 ± 0.04† | 1.39 ± 0.44 | |
| Yaw | Baseline | 0.46 ± 0.04 | 0.19 ± 0.04 | 0.45 ± 0.05 | 0.33 ± 0.04 | 1.92 ± 0.41 |
| Recovery | 0.45 ± 0.04 | 0.18 ± 0.03 | 0.39 ± 0.05 | 0.40 ± 0.05 | 1.46 ± 0.34 | |
|
| ||||||
SOR = suboccipital release.
Denotes difference compared with sham (P ≤ 0.05).
Denotes trend toward difference compared with sham (0.05 < P < 0.10).
Discussion
This study’s primary new finding is that SOR has the capacity to modulate pain-induced autonomic control and regulation, despite not altering the acute pain or hypertensive conditions caused by experimental pain. Baroreflex sensitivity, expressed as the cross-spectra transfer function gain between the R-R interval and systolic blood pressure, was accentuated in higher frequencies during SOR compared with sham following ischemic and cold pain. Additionally, dynamic control of the R-R interval and systolic blood pressure was affected or trended in both low- and high-frequency ranges. These data indicate the possibility of autonomic neural control and regulation of pain-induced sympathoexcitation to both the heart and vasculature that can be modulated by manual therapy.
Sympathetic activity increased during cold and ischemic pain in this study, as inferred by increased heart rate and mean arterial blood pressure. Our CPT and PEMI data were consistent with previous cardiovascular findings for CPT [17,18,31] and PEMI [19,20,32], and all subjects experienced subjective increases in pain. Thus, we successfully induced experimental pain and appropriate autonomic responses in participants. Heart rate decreased from minute 1 of pain during both SOR and sham in all protocols. This may indicate the possibility of “hands-on” interventions having a calming effect to a painful stimulus, but this is difficult to fully evaluate because we did not have a nontreatment pain control trial in the study.
SOR has been proposed to mitigate certain types of pain and affect the autonomic nervous system. We did not observe any effect of SOR on pain perception compared with sham. Henley et al. [15] identified that cervical myofascial release (SOR) attenuated the increase in heart rate variability caused by head-up tilt, leading the authors to conclude that vagal tone was increased. We extended observations to the more clinical sympathoexcitatory stimulus of pain rather than blood pooling and observed altered normalized R-R interval in the low frequency, trend in the high frequency, and no change in the ratio between low and high frequency. This low-frequency power spectral density change with SOR is novel and could indicate a more global autonomic system modulation of heart rate. Traditionally, high-frequency changes in R-R interval have been associated with the parasympathetic nervous system, and low-frequency changes with the sympathetic nervous system [14,33] however, this view is too simplistic [34,35]. We also extended the previous study [15] into a post-treatment period. This could have important clinical implications, as interventions should have some utility both during and in recovery from pain.
A substantial advantage of our experimental design was the ability to measure beat-by-beat arterial blood pressure to gain more insights compared with only heart rate variability, as the heart is under both sympathetic and parasympathetic control, whereas the vasculature is predominantly sympathetic. We observe a trend in normalized low-frequency systolic blood pressure power spectral density between SOR and sham post–pain perception, again indicating that dynamic control and regulation data provide additional information over static data [36]. Baroreflex sensitivity analysis did yield accentuation in higher frequencies in SOR compared with sham following ischemic and cold pain. These data indicate that SOR has the capacity to alter aspects of the baroreflex after pain, as evidenced by the input (systolic blood pressure)-to-output (R-R interval) gain of the reflex, despite no overall difference in the absolute heart rate or arterial blood pressure between SOR and sham. This autonomic reflex change is intriguing and could have an impact for autonomic disorders that also present with primary or secondary pain [37]. This baroreflex finding deserves further exploration into the level at which the pathway is altered.
This study used a sham manipulation, yaw head movements, to control for a potential treatment effect. Controls for manual manipulation are very difficult to create, as double-blinding is not possible [38]. Yaw head movements provide a mild semicircular canal stimulation and have previously been observed to have no effect on muscle or skin sympathetic nerve activity [21], even during heightened sympathoexcitatory states [22]. Thus, yaw head movements are an appropriate sham for the present study. In fact, polling participants after study completion revealed that the majority believed that the active yaw procedure was the manual therapy treatment and the more subtle SOR was the control. We did not intend to deceive participants but do believe that this is an ideal control.
The present study also tested a cyclooxygenase inhibitor for two reasons: 1) to investigate the interactive effects of a nonprescription prototypic nonsteroidal anti-inflammatory drug with manual treatment and 2) to investigate possible prostaglandin mechanism interactions associated with PEMI and baroreflexes. Previous studies testing prostaglandin [39] and pain biomarker [40–42] levels after manipulation and sympathetic responses to ischemic pain [19,43,44] have not yielded conclusive results. We did not observe any interactive effects with manual therapy and aspirin for static or dynamic cardiovascular responses. Subjective pain perceptions were slightly lower with aspirin treatment when compared with noncyclooxygenase inhibition conditions. Combined, these data indicate a lack of interaction between manual treatments and oral cyclooxygenase inhibition on pain perception and autonomic responses to pain.
Limitations
Despite the benefits in generalizability and transferability in these human-based studies, there are some limitations. First, although we tested cold and ischemic pain, we could not mimic musculoskeletal or headache pain. Thus, we cannot determine the role of SOR in those types of pain. Based on the current study, it is possible that autonomic control and regulation to pain in general are altered with SOR. We did not double-blind our aspirin treatment arm or assess plasma prostaglandin levels. A high dose of oral aspirin has previously been shown to decrease prostaglandin synthesis [45,46]. We did not observe an effect of aspirin on cardiovascular responses to pain, so it is doubtful that there was a placebo effect of the drug. Despite the lack of effect, we do think it is clinically important to have experimental arms that include nonprescription medications to investigate interactive effects with other treatments, should they exist. It is possible that there was a placebo effect of the manual therapy. However, we attempted to mask this with an active procedure (yaw) that has previously been identified to not alter sympathoexcitation. Based on polling, this masking was successful and perhaps could be a model for future studies assessing manual therapies that cannot be adequately blinded.
Conclusions
Our results indicate that most static ANS results (absolute heart rate and arterial blood pressure) were elevated by pain but not modulated by SOR. However, dynamic alterations in autonomic cardiovascular control were significantly affected by SOR compared with yaw after pain. The most prominent effect was an alteration in baroreflex sensitivity regardless of the type of pain (cold and ischemic). Baroreflexes contain both parasympathetic and sympathetic effector arms to alter arterial blood pressure [36]; the control and regulation of arterial blood pressure are fundamental autonomic homeostatic mechanisms, and a change indicates an alteration in regulation. SOR does not appear to modulate pain perception of either cold or ischemic origin. Thus, SOR has the capacity to modulate pain-induced autonomic control and regulation, despite not altering the acute pain or hypertensive conditions caused by experimental pain.
Acknowledgments
The authors would like to thank the research participants for their willing participation in study protocols and the investigators and support personnel at the Ohio Musculoskeletal and Neurological Institute.
Funding sources: This work was supported by the American Osteopathic Association (11-08-651). Additional personnel support was provided by the Osteopathic Heritage Foundation, Ohio University College of Osteopathic Medicine–Research and Scholarly Advancement Fellowship Program, and Marian University College of Osteopathic Medicine–Summer Scholars Fellowship Program. Current funding for the laboratory is provided by the National Institutes of Health (AR069912).
Conflicts of interest: No additional conflicts of interest declared.
References
- 1. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep 2015(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 2. Dutton M. Dutton's Orthopedic Examination, Evaluation and Intervention. 4th ed. New York: McGraw Hill Education; 2017. [Google Scholar]
- 3. Espi-Lopez GV, Rodriguez-Blanco C, Oliva-Pascual-Vaca A, Benitez-Martinez JC, Lluch E, Falla D. Effect of manual therapy techniques on headache disability in patients with tension-type headache. Randomized controlled trial. Eur J Phys Rehabil Med 2014;50(6):641–7. [PubMed] [Google Scholar]
- 4. Espi-Lopez GV, Ruescas-Nicolau MA, Nova-Redondo C, Benitez-Martinez JC, Dugailly PM, Falla D. Effect of soft tissue techniques on headache impact, disability, and quality of life in migraine sufferers: A pilot study. J Altern Complement Med 2018;24(11):1099–107. [DOI] [PubMed] [Google Scholar]
- 5. Kuchera ML. Applying osteopathic principles to formulate treatment for patients with chronic pain. J Am Osteopath Assoc 2007;107(10 Suppl 6):Es28–38. [PubMed] [Google Scholar]
- 6. Cutler MJ, Holland BS, Stupski BA, Gamber RG, Smith ML. Cranial manipulation can alter sleep latency and sympathetic nerve activity in humans: A pilot study. J Altern Complement Med 2005;11(1):103–8. [DOI] [PubMed] [Google Scholar]
- 7. Karason AB, Drysdale IP. Somatovisceral response following osteopathic HVLAT: A pilot study on the effect of unilateral lumbosacral high-velocity low-amplitude thrust technique on the cutaneous blood flow in the lower limb. J Manip Physiol Ther 2003;26(4):220–5. [DOI] [PubMed] [Google Scholar]
- 8. Nelson KE, Sergueef N, Glonek T. The effect of an alternative medical procedure upon low-frequency oscillations in cutaneous blood flow velocity. J Manip Physiol Ther 2006;29(8):626–36. [DOI] [PubMed] [Google Scholar]
- 9. Nelson KE, Sergueef N, Glonek T. Recording the rate of the cranial rhythmic impulse. J Am Osteopath Assoc 2006;106(6):337–41. [PubMed] [Google Scholar]
- 10. Purdy WR, Frank JJ, Oliver B. Suboccipital dermatomyotomic stimulation and digital blood flow. J Am Osteopath Assoc 1996;96(5):285–9. [DOI] [PubMed] [Google Scholar]
- 11. Giles PD, Hensel KL, Pacchia CF, Smith ML. Suboccipital decompression enhances heart rate variability indices of cardiac control in healthy subjects. J Altern Complement Med 2013;19(2):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curi ACC, Maior Alves AS, Silva JG. Cardiac autonomic response after cranial technique of the fourth ventricle (cv4) compression in systemic hypertensive subjects. J Bodyw Mov Ther 2018;22(3):666–72. [DOI] [PubMed] [Google Scholar]
- 13. Arroyo-Morales M, Olea N, Martinez M, Moreno-Lorenzo C, Diaz-Rodriguez L, Hidalgo-Lozano A. Effects of myofascial release after high-intensity exercise: A randomized clinical trial. J Manip Physiol Ther 2008;31(3):217–23. [DOI] [PubMed] [Google Scholar]
- 14.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 15. Henley CE, Ivins D, Mills M, Wen FK, Benjamin BA. Osteopathic manipulative treatment and its relationship to autonomic nervous system activity as demonstrated by heart rate variability: A repeated measures study. Osteopath Med Prim Care 2008;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheshire WP Jr, Goldstein DS. Autonomic uprising: The tilt table test in autonomic medicine. Clin Auton Res 2019;29(2):215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stancak A Jr, Yamamotova A, Kulls IP, Sekyra IV. Cardiovascular adjustments and pain during repeated cold pressor test. Clin Auton Res 1996;6(2):83–9. [DOI] [PubMed] [Google Scholar]
- 18. Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 1987;9(5):429–36. [DOI] [PubMed] [Google Scholar]
- 19. Ray CA, Secher NH, Mark AL. Modulation of sympathetic nerve activity during posthandgrip muscle ischemia in humans. Am J Physiol 1994;266(1 Pt 2):H79–83. [DOI] [PubMed] [Google Scholar]
- 20. Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol 2001;91(4):1679–86. [DOI] [PubMed] [Google Scholar]
- 21. Ray CA, Hume KM, Steele SL. Sympathetic nerve activity during natural stimulation of horizontal semicircular canals in humans. Am J Physiol 1998;275(4):R1274–8. [DOI] [PubMed] [Google Scholar]
- 22. Wilson TE, Kuipers NT, McHugh EA, Ray CA. Vestibular activation does not influence skin sympathetic nerve responses during whole body heating. J Appl Physiol 2004;97(2):540–4. [DOI] [PubMed] [Google Scholar]
- 23. Imholz BP, Wieling W, van Montfrans GA, Wesseling KH. Fifteen years experience with finger arterial pressure monitoring: Assessment of the technology. Cardiovasc Res 1998;38(3):605–16. [DOI] [PubMed] [Google Scholar]
- 24. Dyckman DJ, Sauder CL, Ray CA. Effects of short-term and prolonged bed rest on the vestibulosympathetic reflex. Am J Physiol Heart Circ Physiol 2012;302(1):H368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huskisson EC. Measurement of pain. Lancet (London, England) 1974;2(7889):1127–31. [DOI] [PubMed] [Google Scholar]
- 26. Negm AA, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout In: Katzung BG, ed. Basic & Clinical Pharmacology. New York: McGraw Hill Education; 2018. [Google Scholar]
- 27. O'Leary DS. Regional vascular resistance vs conductance: Which index for baroreflex responses? Am J Physiol 1991;260(2 Pt 2):H632–7. [DOI] [PubMed] [Google Scholar]
- 28. Cooke WH, Zhang R, Zuckerman JH, et al. Does nitric oxide buffer arterial blood pressure variability in humans? J Appl Physiol 2002;93(4):1466–70. [DOI] [PubMed] [Google Scholar]
- 29. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84(2):482–92. [DOI] [PubMed] [Google Scholar]
- 30. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol 2002;282(5):H1717–23. [DOI] [PubMed] [Google Scholar]
- 32. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 1937;89(4):372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: A review. Med Biol Eng Computing 2006;44(12):1031–51. [DOI] [PubMed] [Google Scholar]
- 34. Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol 2019;38(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 1995;25(6):1276–86. [DOI] [PubMed] [Google Scholar]
- 37. Low PA, Benarroch EE. Clinical Autonomic Disorders. 3rd ed Baltimore, MD: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 38. Henley CE, Wilson TE. Use of beat-to-beat cardiovascular variability data to determine the validity of sham therapy as the placebo control in osteopathic manipulative medicine research. J Am Osteopath Assoc 2014;114(11):860–6. [DOI] [PubMed] [Google Scholar]
- 39. Kokjohn K, Schmid DM, Triano JJ, Brennan PC. The effect of spinal manipulation on pain and prostaglandin levels in women with primary dysmenorrhea. J Manip Physiol Ther 1992;15(5):279–85. [PubMed] [Google Scholar]
- 40. Molins-Cubero S, Rodriguez-Blanco C, Oliva-Pascual-Vaca A, Heredia-Rizo AM, Bosca-Gandia JJ, Ricard F. Changes in pain perception after pelvis manipulation in women with primary dysmenorrhea: A randomized controlled trial. Pain Med 2014;15(9):1455–63. [DOI] [PubMed] [Google Scholar]
- 41. Licciardone JC, Kearns CM, Hodge LM, Bergamini MV. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: Results from the OSTEOPATHIC trial. J Am Osteopath Assoc 2012;112(9):596–605. [DOI] [PubMed] [Google Scholar]
- 42. Degenhardt BF, Kuchera ML. Osteopathic evaluation and manipulative treatment in reducing the morbidity of otitis media: A pilot study. J Am Osteopath Assoc 2006;106(6):327–34. [PubMed] [Google Scholar]
- 43. Cui J, McQuillan P, Momen A, et al. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol 2007;293(3):H1861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doerzbacher KJ, Ray CA. Muscle sympathetic nerve responses to physiological changes in prostaglandin production in humans. J Appl Physiol 2001;90(2):624–9. [DOI] [PubMed] [Google Scholar]
- 45. Ellis EF, Wright KF, Jones PS, Richardson DW, Ellis CK. Effect of oral aspirin dose on platelet aggregation and vascular prostacyclin (PGI2) synthesis in humans and rabbits. J Cardiovasc Pharmacol 1980;2(4):387–97. [DOI] [PubMed] [Google Scholar]
- 46. Collier HO. Prostaglandins and aspirin. Nature 1971;232(5305):17–9. [DOI] [PubMed] [Google Scholar]